Adhesion and Cohesion Strength of Phenol-Formaldehyde Resin Mixed with Different Types and Levels of Catalyst for Wood Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
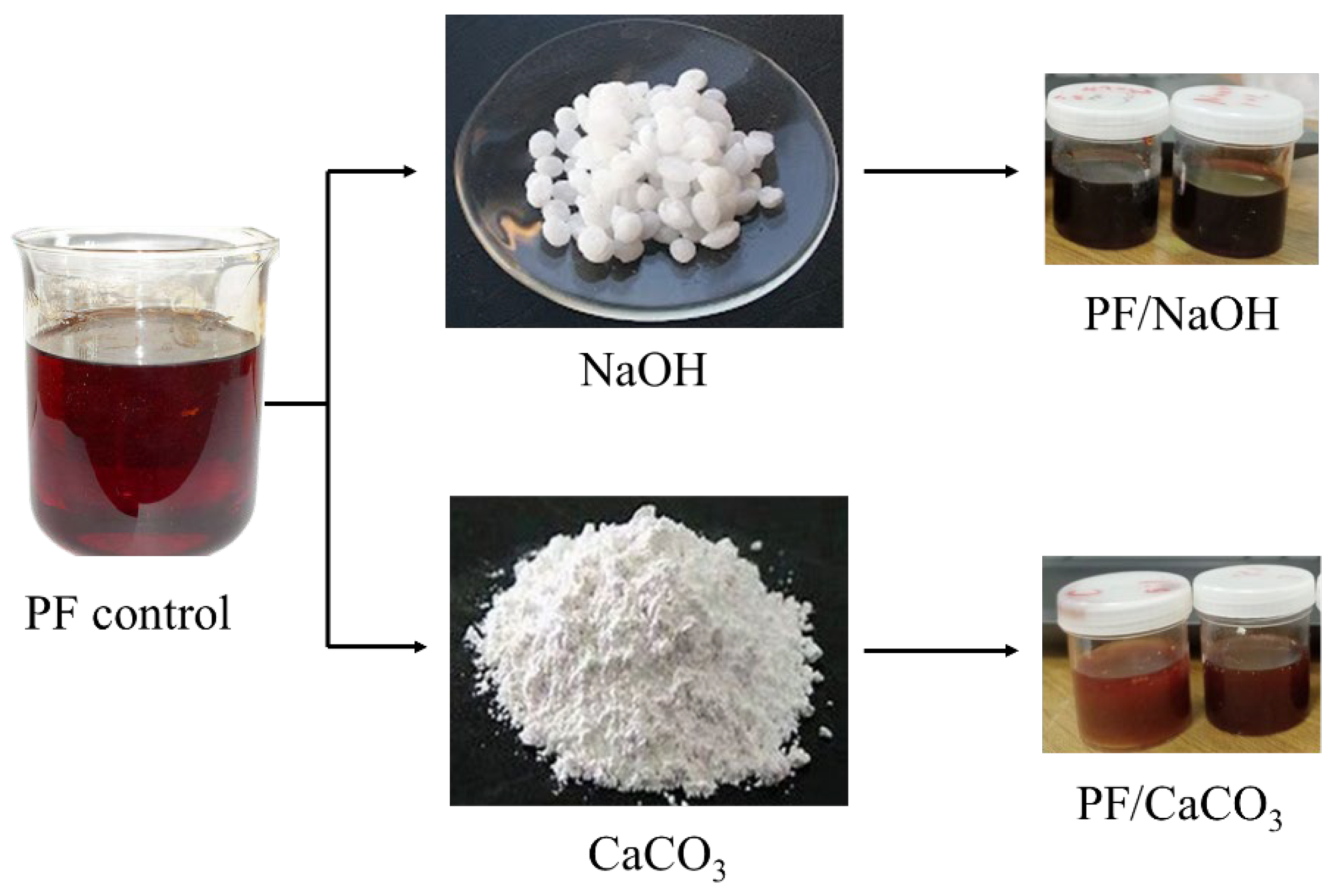
2.2. Adhesive Preparation
2.3. Characterization of Adhesives
2.3.1. Solids Content
2.3.2. Gelation Time
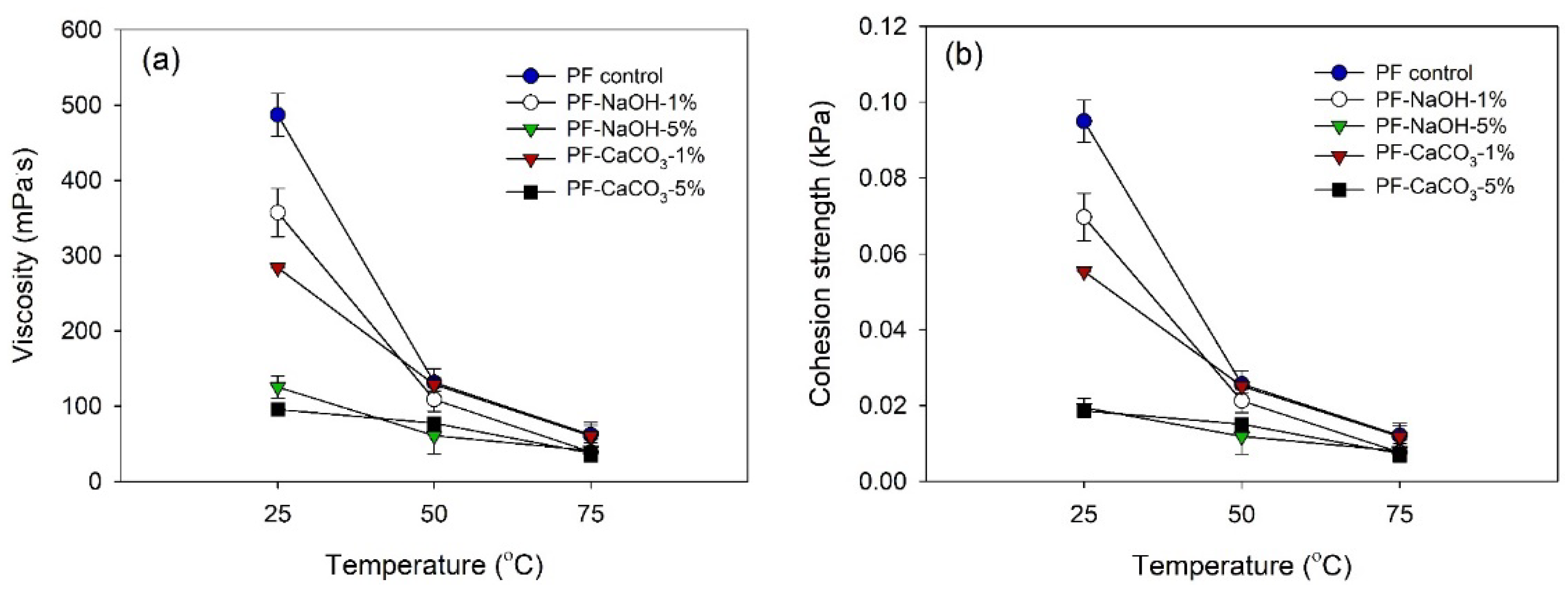
2.3.3. Viscosity
2.3.4. pH Value
2.3.5. Functional Group Analysis
2.3.6. Dynamic Mechanical Analysis
2.4. Fabrication and Evaluation of Plywood
2.5. Data Analysis
3. Results and Discussions
3.1. Characteristics of Adhesives
3.2. Functional Group Analysis
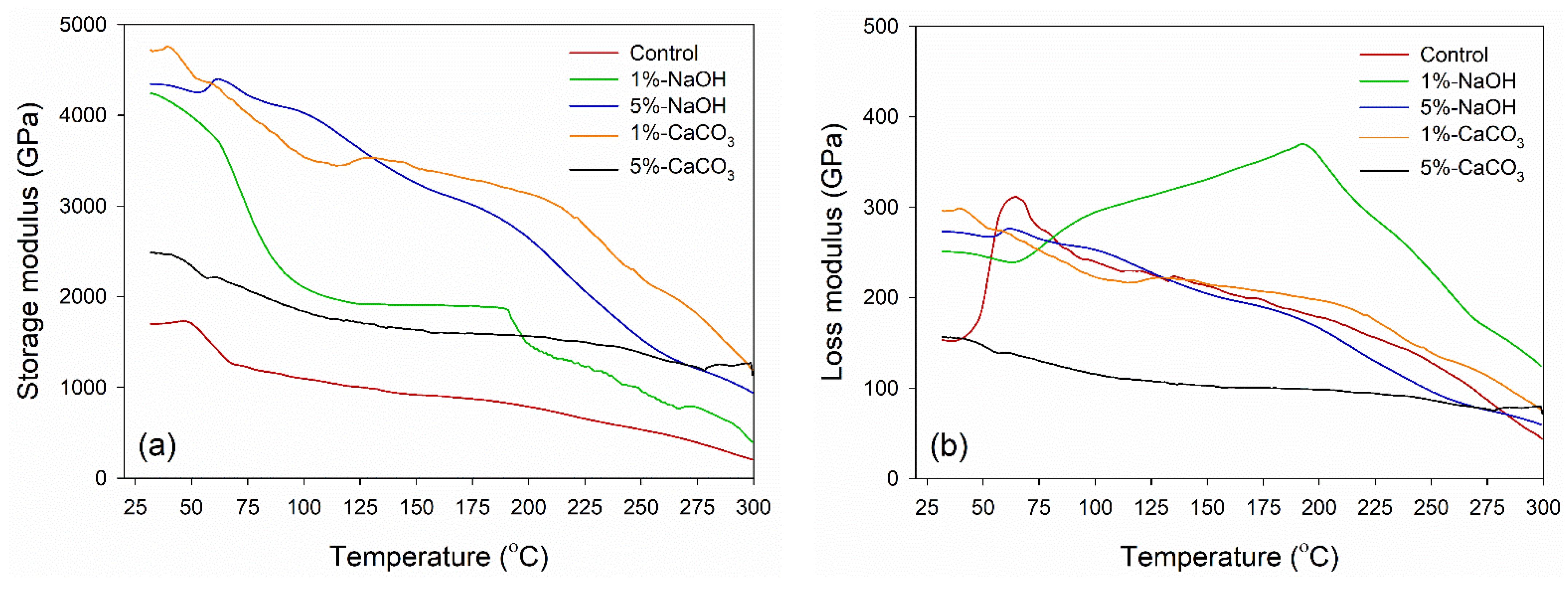
3.3. Dynamic Mechanical Analysis
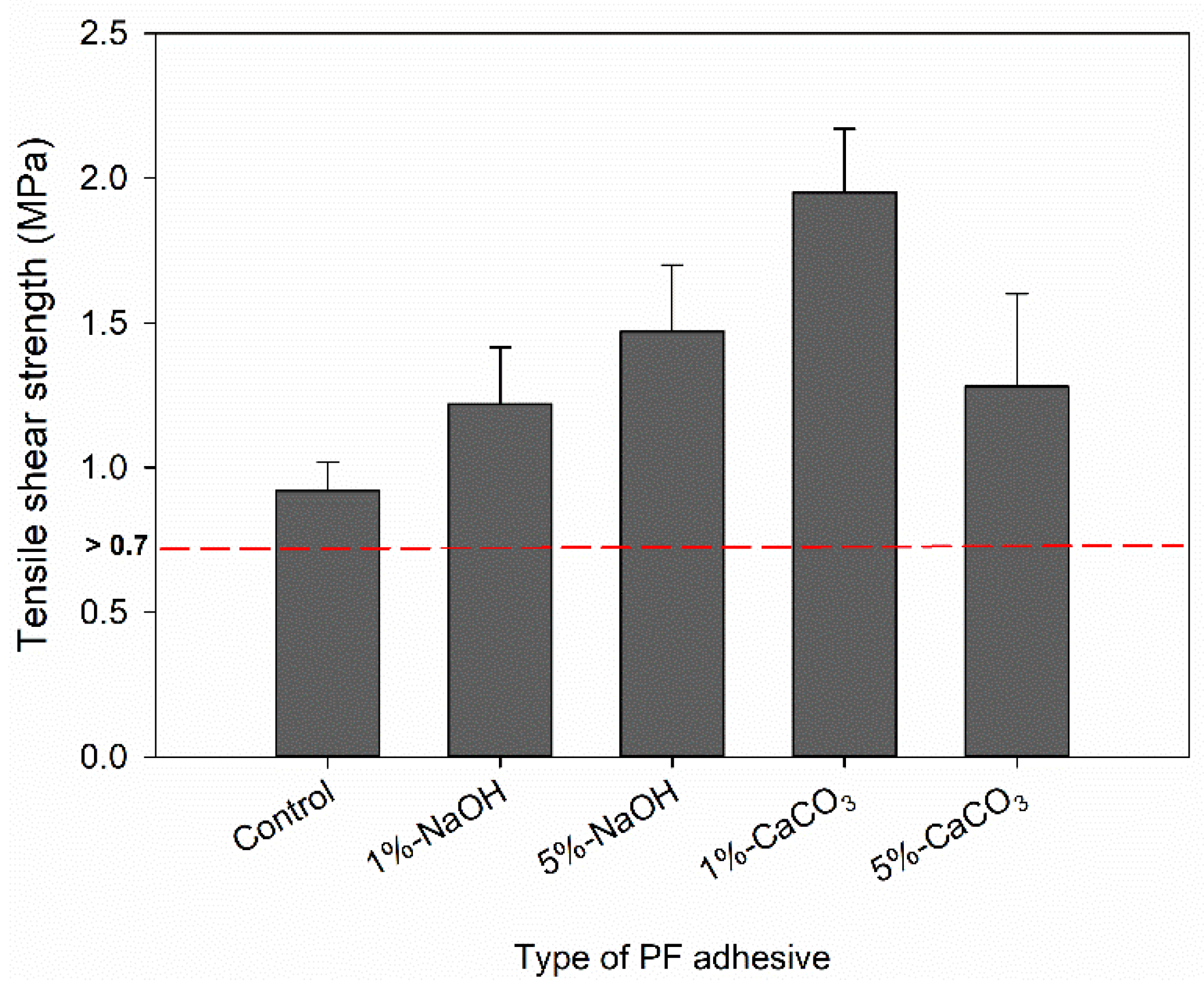
3.4. Tensile Shear Strength of Plywood
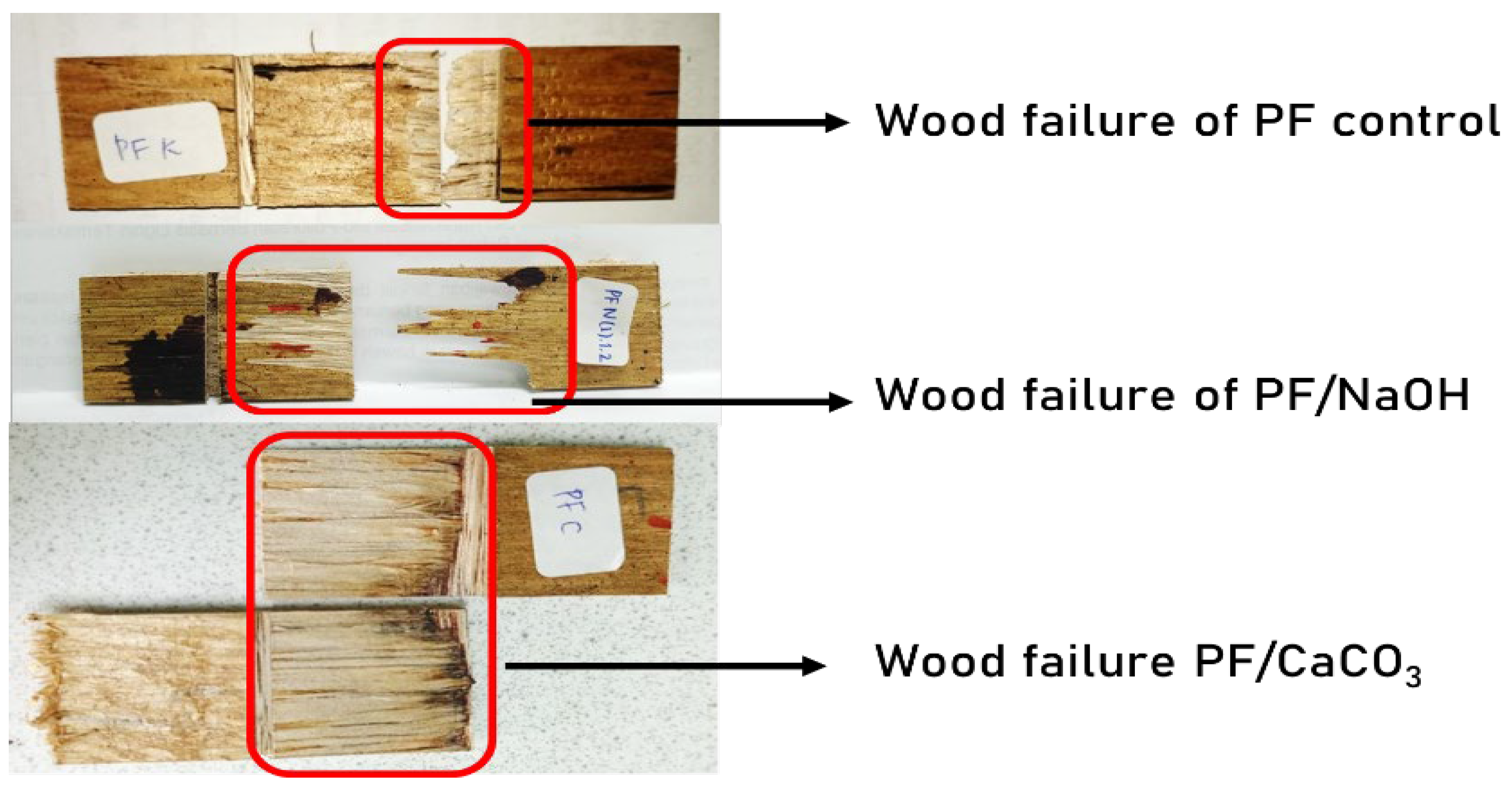
3.5. Wood Failure Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pizzi, A.; Mittal, K.L. Handbook of Adhesive Technology; CRC Press: Boca Raton, FL, USA, 2018; ISBN 1498736475. [Google Scholar]
- Mays, G.C.; Hutchinson, A.R. Adhesives in Civil Engineering; Cambridge University Press: Cambridge, UK, 1992; Volume 32. [Google Scholar]
- Fink, J.K. Reactive Polymers Fundamentals and Applications; British Library: Oxford, UK, 2017. [Google Scholar]
- Chai, Y.; Liu, J.; Zhao, Y.; Yan, N. Characterization of modified phenol formaldehyde resole resins synthesized in situ with various boron compounds. Ind. Eng. Chem. Res. 2016, 55, 9840–9850. [Google Scholar] [CrossRef]
- Hong, S.; Gu, Z.; Chen, L.; Zhu, P.; Lian, H. Synthesis of Phenol Formaldehyde (PF) Resin for Fast Manufacturing Laminated Veneer Lumber (LVL). Holzforschung 2018, 72, 745–752. [Google Scholar] [CrossRef]
- Berdnikova, P.V.; Zhizhina, E.G.; Pai, Z.P. Phenol-Formaldehyde Resins: Properties, Fields of Application, and Methods of Synthesis. Catal. Ind. 2021, 13, 119–124. [Google Scholar] [CrossRef]
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent Progress in Ultra-Low Formaldehyde Emitting Adhesive Systems and Formaldehyde Scavengers in Wood-Based Panels: A Review. Wood Mater. Sci. Eng. 2023, 18, 763–782. [Google Scholar] [CrossRef]
- Zhang, J.; Pizzi, A.; Li, J.; Zhang, W. MALDI-TOF MS Analysis of the Acceleration of the Curing of Phenol–Formaldehyde (PF) Resins Induced by Propylene Carbonate. Eur. J. Wood Wood Prod. 2015, 73, 135–138. [Google Scholar] [CrossRef]
- Park, B.D.; Riedl, B.; Hsu, E.W.; Shields, J. Application of Cure-Accelerated Phenol-Formaldehyde (PF) Adhesives for Three-Layer Medium Density Fiberboard (MDF) Manufacture. Wood Sci. Technol. 2001, 35, 311–323. [Google Scholar] [CrossRef]
- Lei, H.; Pizzi, A.; Du, G. Environmentally Friendly Mixed Tannin/Lignin Wood Resins. J. Appl. Polym. Sci. 2008, 107, 203–209. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, N.; Feng, M.W. Bark Extractives-Based Phenol–Formaldehyde Resins from Beetle-Infested Lodgepole Pine. J. Adhes. Sci. Technol. 2013, 27, 2112–2126. [Google Scholar] [CrossRef]
- Solt, P.; Rößiger, B.; Konnerth, J.; van Herwijnen, H.W.G. Lignin Phenol Formaldehyde Resoles Using Base-Catalysed Depolymerized Kraft Lignin. Polymers 2018, 10, 1162. [Google Scholar] [CrossRef] [Green Version]
- Duval, M.; Bloch, B.; Kohn, S. Analysis of Phenol-formaldehyde Resols by Gel Permeation Chromatography. J. Appl. Polym. Sci. 1972, 16, 1585–1602. [Google Scholar] [CrossRef]
- Steiner, P.R. Phenol–Formaldehyde Wood Adhesive Characterization by Proton Magnetic Resonance Spectroscopy. J. Appl. Polym. Sci. 1975, 19, 215–225. [Google Scholar] [CrossRef]
- Li, J.; Zhu, W.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Curing Properties of High- Ortho Phenol-formaldehyde Resins with Co-catalysis. J. Appl. Polym. Sci. 2019, 136, 47229. [Google Scholar] [CrossRef]
- SNI 06-4567-1998; Testing of Liquid Phenol Formaldehyde for Plywood Adhesive. Badan Standardisasi Nasional: Jakarta, Indonesia, 1998.
- Pari, R.; Abdurachman, A.; Santoso, A. Bonding Strength and Formaldehyde Emission of Rattan Laminated Board Using Tanin Formaldehyde Resin. J. Penelit. Has. Hutan 2019, 37, 33–41. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, D.; Qin, T.; Chu, F. Thermal Degradation and Stability of Accelerated-Curing Phenol-Formaldehyde Resin. Bioresources 2014, 9, 4063–4075. [Google Scholar] [CrossRef] [Green Version]
- Pizzi, A.; Stephanou, A. Phenol-Formaldehyde Wood Adhesives under Very Alkaline Conditions. Part II: Esters Curing Acceleration, Its Mechanism and Applied Results. Holzforschung 1994, 48, 150–156. [Google Scholar] [CrossRef]
- Park, B.; Riedl, B. 13C-NMR Study on Cure-accelerated Phenol–Formaldehyde Resins with Carbonates. J. Appl. Polym. Sci. 2000, 77, 841–851. [Google Scholar] [CrossRef]
- Poljanšek, I.; Krajnc, M. Poljanšek and Krajnc Characterization of Phenol-Formaldehyde Prepolymer Resins. Acta Chim. Slov. 2005, 52, 238. [Google Scholar]
- Lei, H.; Frazier, C.E. A Dynamic Mechanical Analysis Method for Predicting the Curing Behavior of Phenol-Formaldehyde Resin Adhesive. J. Adhes. Sci. Technol. 2015, 29, 981–990. [Google Scholar] [CrossRef]
- Menczel, J.D.; Prime, R.B. Thermal Analysis of Polymers: Fundamentals and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Abidin, W.N.S.N.Z.; Al-Edrus, S.S.O.; Hua, L.S.; Ghani, M.A.A.; Bakar, B.F.A.; Ishak, R.; Faisal, F.Q.A.; Sabaruddin, F.A.; Kristak, L.; Lubis, M.A.R.; et al. Properties of Phenol Formaldehyde-Bonded Layered Laminated Woven Bamboo Mat Boards Made from Gigantochloa scortechinii. Appl. Sci. 2023, 13, 47. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The Pyrolysis Mechanism of Phenol Formaldehyde Resin. Polym. Degrad. Stab. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]
- Wang, J.; Laborie, M.-P.G.; Wolcott, M.P. Kinetic Analysis of Phenol–Formaldehyde Bonded Wood Joints with Dynamical Mechanical Analysis. Thermochim. Acta 2009, 491, 58–62. [Google Scholar] [CrossRef]
- Böhm, R.; Hauptmann, M.; Pizzi, A.; Friedrich, C.; Laborie, M.-P. The Chemical, Kinetic and Mechanical Characterization of Tannin-Based Adhesives with Different Crosslinking Systems. Int. J. Adhes. Adhes. 2016, 68, 1–8. [Google Scholar] [CrossRef]
- Hassan, A.; Rahman, N.A.; Yahya, R. Extrusion and Injection-Molding of Glass Fiber/MAPP/Polypropylene: Effect of Coupling Agent on DSC, DMA, and Mechanical Properties. J. Reinf. Plast. Compos. 2011, 30, 1223–1232. [Google Scholar] [CrossRef]
- Maulana, M.I.; Fitrianum, F.; Noviyanti, D.; Audy, R.; Prasetia, D.; Maulana, S.; Lubis, M.A.R.; Hidayat, W.; Sari, R.K.; Febrianto, F.; et al. Effect of Pretreatment and Compaction Ratio on the Properties of Oriented Strand Board from Sengon (Paraserianthes Falcataria L. Nielsen) Wood. Wood Mater. Sci. Eng. 2023, 1–14. [Google Scholar] [CrossRef]


| Catalyst Type [6] |
|---|
| Sodium hydroxide, potassium hydroxide, lithium hydroxide Magnesium hydroxide, calcium hydroxide, barium hydroxide Sodium carbonate Calcium oxide, magnesium oxide Tertiary amines, triethylamine 2-Dimethylamino-2-methyl-1-propanol, 2-dimethylamino-2-hydroxymethyl-1,3-propanediol Tri(p-chlorophenyl)phosphine, triphenylphosphine Tetraalkylammonium hydroxide Aqueous ammonia Organic amines |
| Treatment Type | PF (g) | NaOH 20% (g) | CaCO3 20% (g) |
|---|---|---|---|
| Control * | 10 | - | - |
| NaOH-1% | 10 | 0.24 | - |
| NaOH-5% | 10 | 1.18 | - |
| CaCO3-1% | 10 | - | 0.24 |
| CaCO3-5% | 10 | - | 1.18 |
| Catalyst Types | Characteristics of Adhesive | ||||
|---|---|---|---|---|---|
| Solids Content (%) | Gelation Time | Viscosity (mPa.s, T = 25 °C) | pH | ||
| (min, T = 135 °C) | (min, T = 25 °C) | ||||
| Control | 47.4 ± 2.06 | 12.7 ± 1.71 | 60.5 ± 5.40 | 487.1 ± 28.34 | 13.4 ± 0.03 |
| NaOH 1% | 44.5 ± 0.28 | 6.1 ± 0.29 | 80.7 ± 6.70 | 357.4 ± 32.02 | 13.4 ± 0.08 |
| NaOH 5% | 40.9 ± 2.23 | 10.6 ± 0.55 | 130.4 ± 10.50 | 125.5 ± 14.60 | 13.7 ± 0.06 |
| CaCO3 1% | 44.1 ± 0.25 | 5.9 ± 0.44 | 135.6 ± 12.60 | 284.1 ± 0.26 | 13.2 ± 0.05 |
| CaCO3 5% | 40.6 ± 0.77 | 11.1 ± 1.18 | 160.2 ± 17.50 | 95.5 ± 0.37 | 13.6 ± 0.02 |
| Catalyst Type | Level | Average | |
|---|---|---|---|
| 1% | 5% | ||
| NaOH | 357.37 a * | 125.49 c | 241.43 |
| CaCO3 | 284.13 b | 95.51 d | 189.82 |
| Average | 320.75 | 110.50 | (+) |
| Treatment | Wood Failure (%) |
|---|---|
| PF-Control | 6.38 |
| PF-NaOH-1% | 26.52 |
| PF-NaOH-5% | 36.34 |
| PF- CaCO3-1% | 37.00 |
| PF- CaCO3-5% | 36.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitrianum, F.; Lubis, M.A.R.; Hadi, Y.S.; Sari, R.K.; Maulana, M.I.; Kristak, L.; Iswanto, A.H.; Mardawati, E.; Reh, R.; Sedliacik, J. Adhesion and Cohesion Strength of Phenol-Formaldehyde Resin Mixed with Different Types and Levels of Catalyst for Wood Composites. J. Compos. Sci. 2023, 7, 310. https://doi.org/10.3390/jcs7080310
Fitrianum F, Lubis MAR, Hadi YS, Sari RK, Maulana MI, Kristak L, Iswanto AH, Mardawati E, Reh R, Sedliacik J. Adhesion and Cohesion Strength of Phenol-Formaldehyde Resin Mixed with Different Types and Levels of Catalyst for Wood Composites. Journal of Composites Science. 2023; 7(8):310. https://doi.org/10.3390/jcs7080310
Chicago/Turabian StyleFitrianum, Fadilah, Muhammad Adly Rahandi Lubis, Yusuf Sudo Hadi, Rita Kartika Sari, Muhammad Iqbal Maulana, Lubos Kristak, Apri Heri Iswanto, Efri Mardawati, Roman Reh, and Jan Sedliacik. 2023. "Adhesion and Cohesion Strength of Phenol-Formaldehyde Resin Mixed with Different Types and Levels of Catalyst for Wood Composites" Journal of Composites Science 7, no. 8: 310. https://doi.org/10.3390/jcs7080310
APA StyleFitrianum, F., Lubis, M. A. R., Hadi, Y. S., Sari, R. K., Maulana, M. I., Kristak, L., Iswanto, A. H., Mardawati, E., Reh, R., & Sedliacik, J. (2023). Adhesion and Cohesion Strength of Phenol-Formaldehyde Resin Mixed with Different Types and Levels of Catalyst for Wood Composites. Journal of Composites Science, 7(8), 310. https://doi.org/10.3390/jcs7080310








