Adsorption of Acid Yellow 23 Dye on Organobentonite/Alginate Hydrogel Beads in a Fixed-Bed Column
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
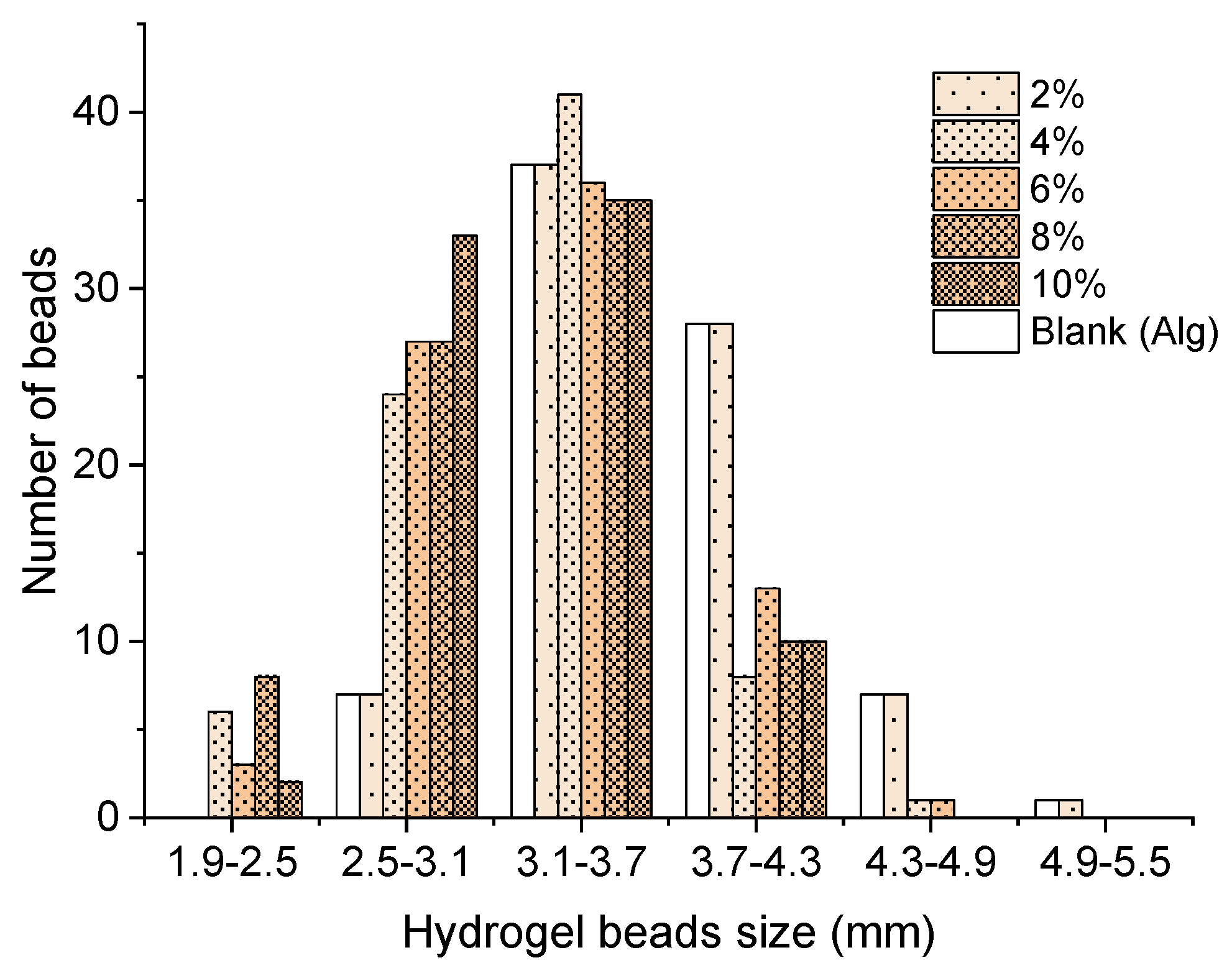
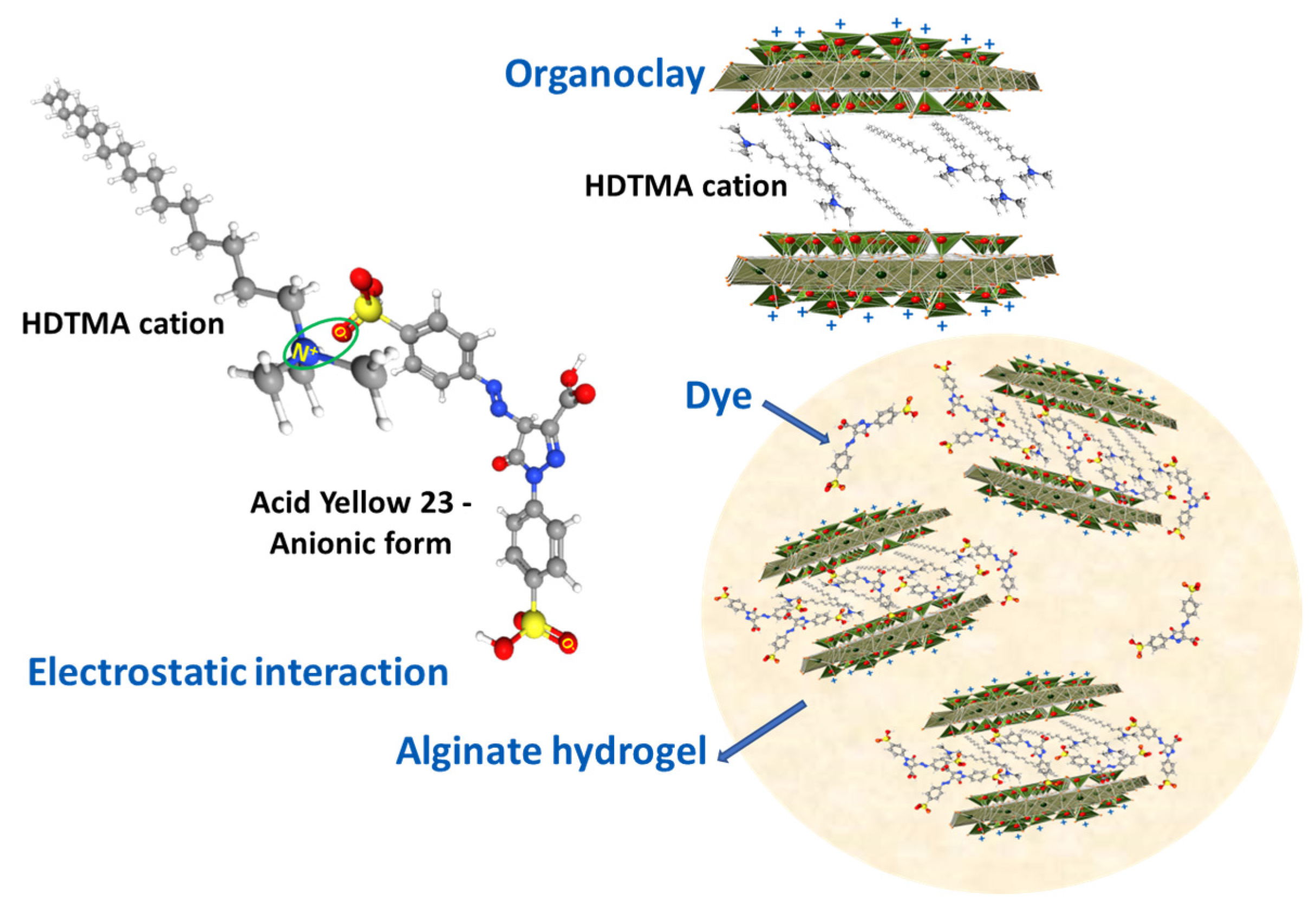
2.2. Preparation of the Organobentonite/Sodium Alginate Beads
2.3. Characterization of the Adsorbents
2.4. Batch Adsorption Tests
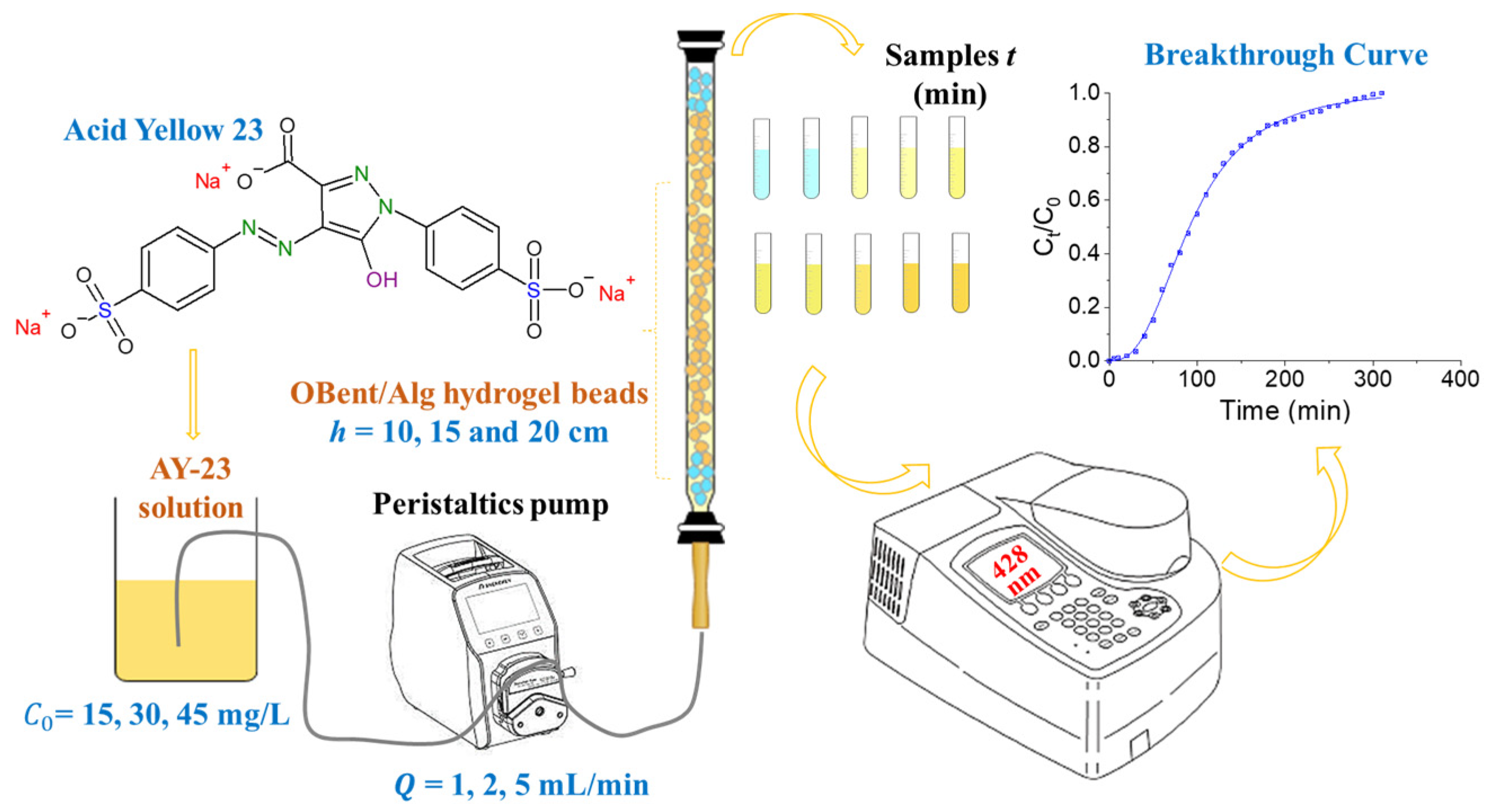
2.5. Fixed-Bed Column Adsorption Tests
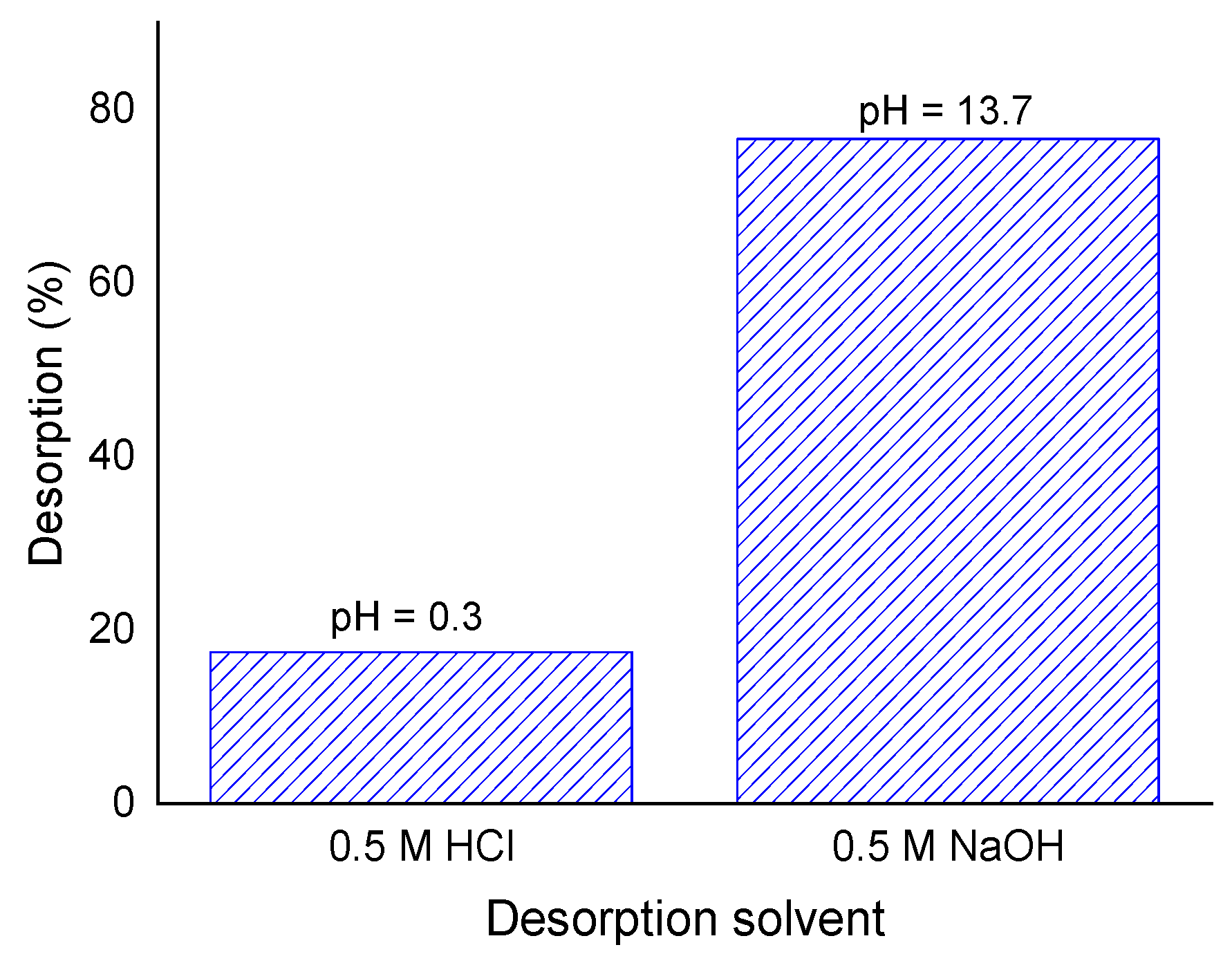
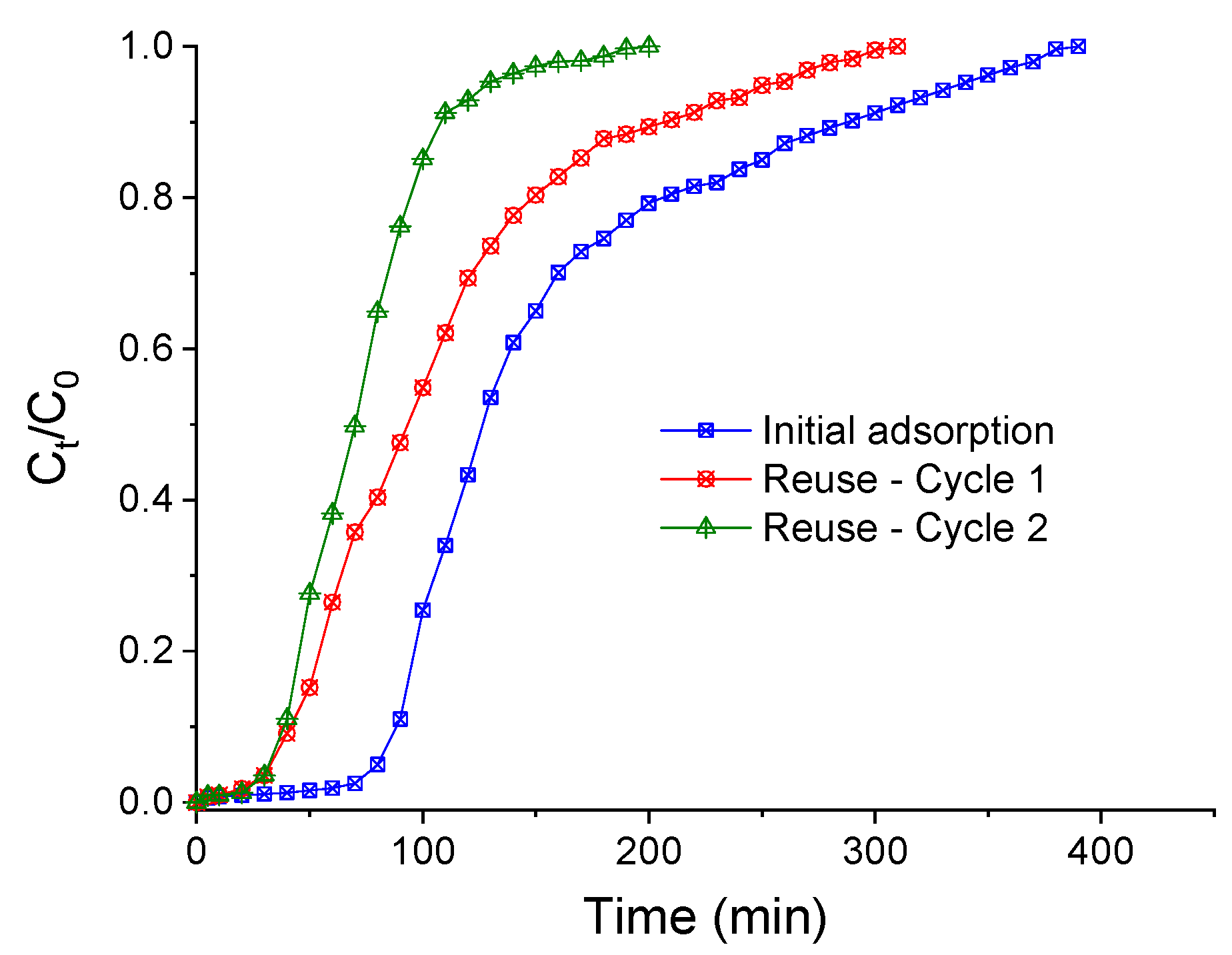
2.6. Desorption Studies and Reuse of the Adsorbent
2.7. Adsorption in Real Wastewater Matrix
3. Results and Discussion
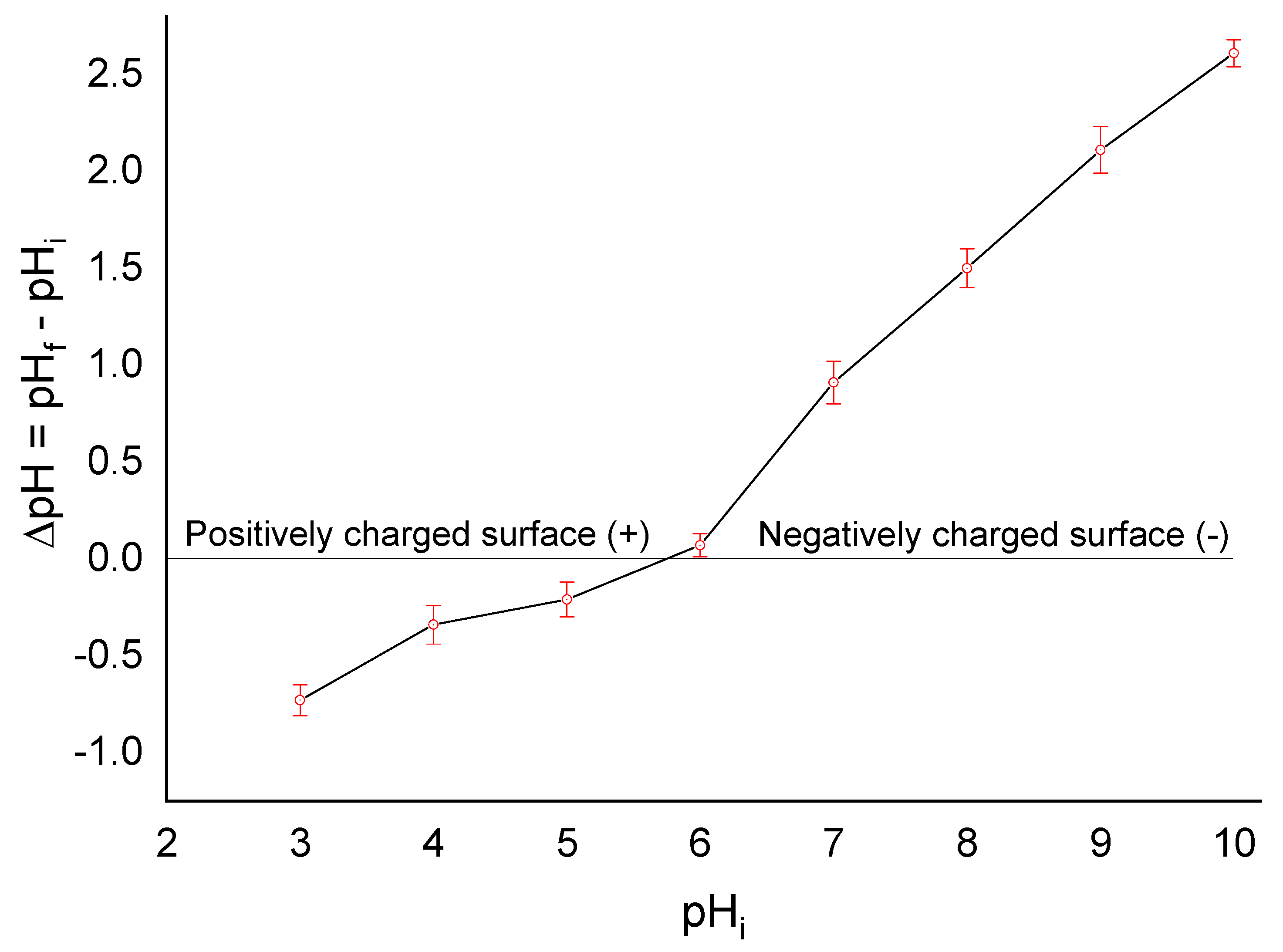
3.1. Adsorbent Characterization
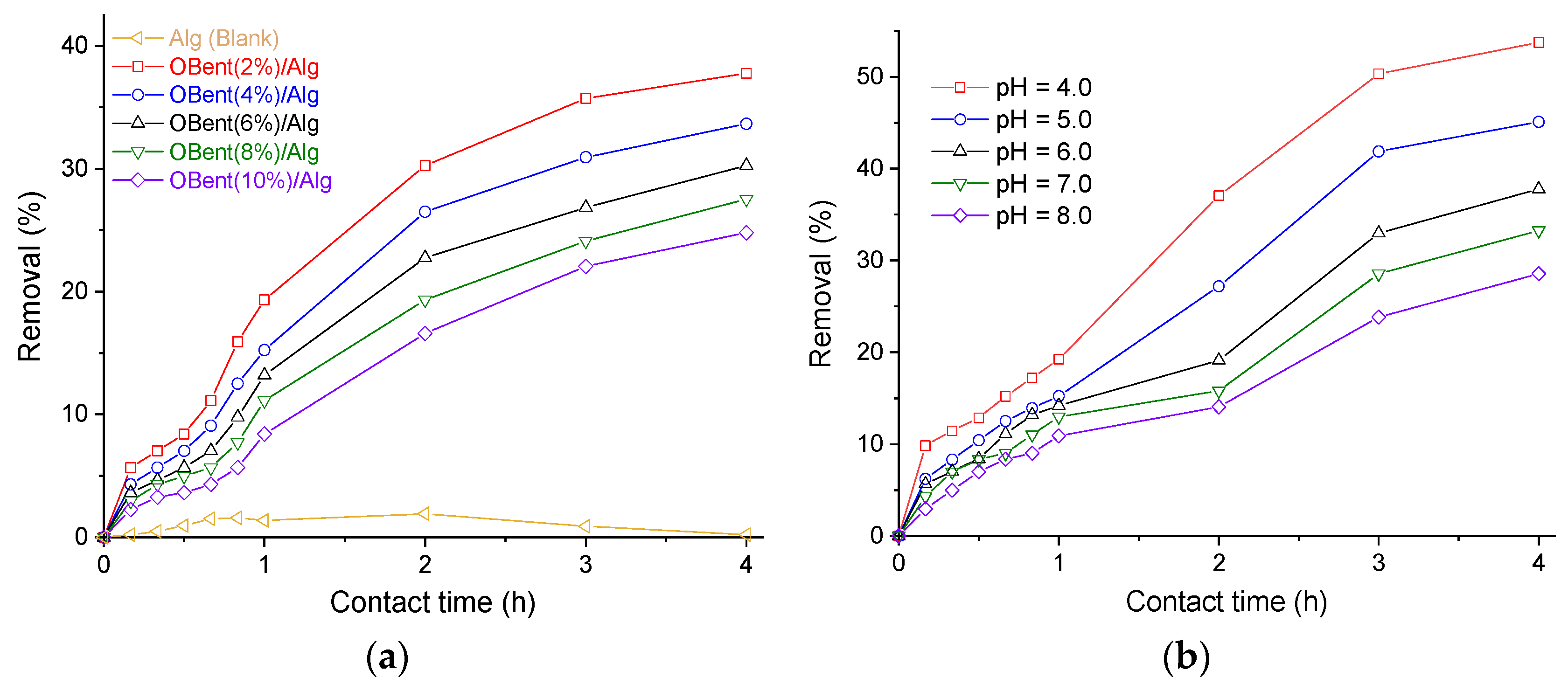
3.2. Batch Adsorption Tests
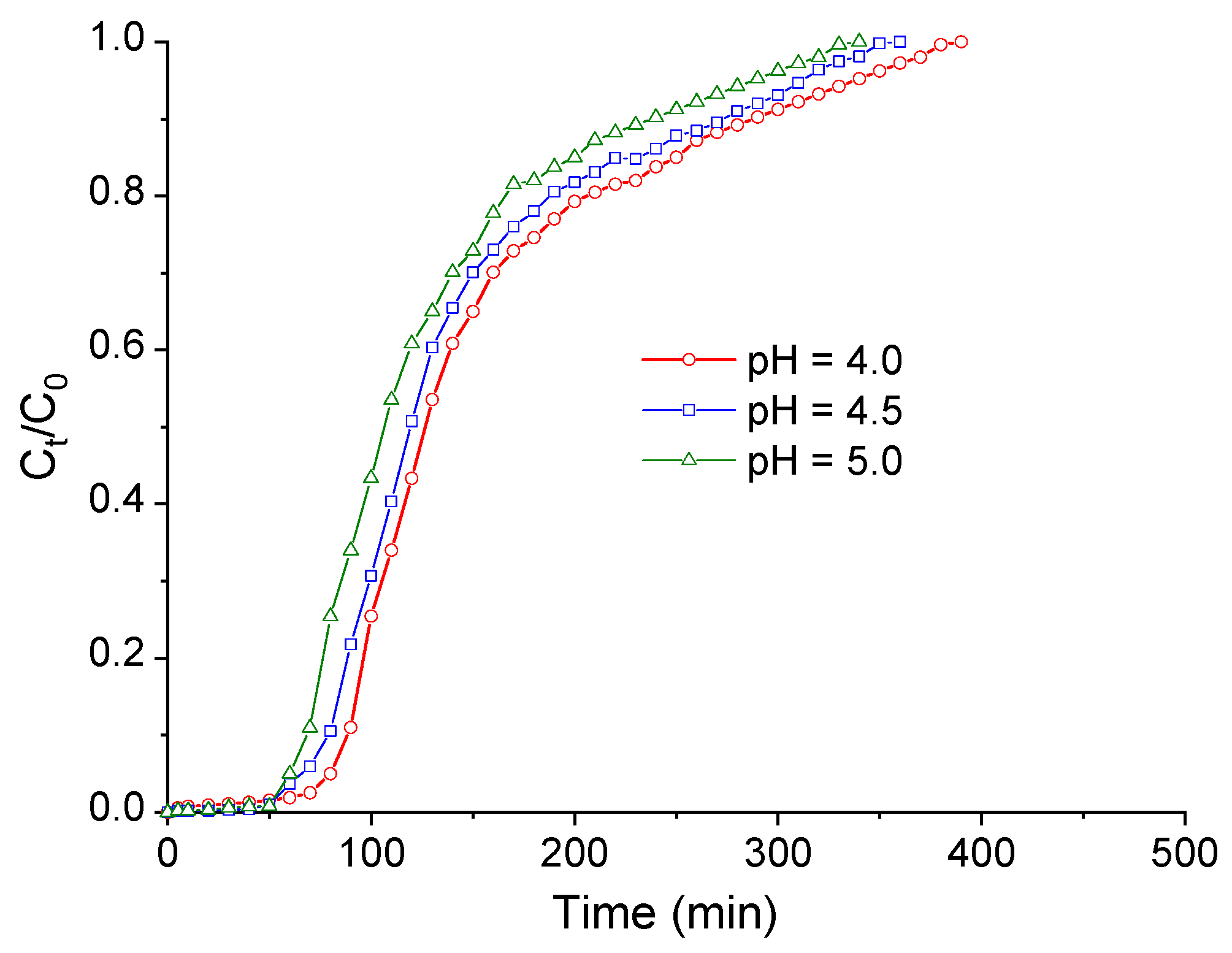
3.3. Fixed-Bed Column Adsorption Tests
3.4. Breakthrough Curve Modelling
| Adsorbent | Dye | Type | Process | , mg/g | Ref. |
|---|---|---|---|---|---|
| Alginate encapsulated pillared clay | Safranine | Cationic | Batch | 268.5 | [35] |
| Alginate/natural bentonite (1/3) | Methylene blue | Cationic | Batch | 550.92 | [44] |
| Congo red | Anionic | Batch | 111.07 | ||
| Alginate–organobentonite composite (1/3) | Methylene blue | Cationic | Batch | 670.54 | [45] |
| Organoclay–alginate matrix | Allura red | Anionic | Batch | 5.7–7.0 | [47] |
| Citrus peels–calcium alginate | Methylene blue | Cationic | Batch | 964.54 | [53] |
| Column | 31.45 | ||||
| Cellulose nanocrystal–alginate | Methylene blue | Cationic | Column | 255.5 | [54] |
| Activated bentonite/alginate composite | Methylene blue | Cationic | Batch | 780.59 | [75] |
| Crystal violet | Cationic | 546.53 | |||
| Activated carbon–bentonite–alginate | Methylene blue | Cationic | Batch | 756.97 | [76] |
| Organobentonite/alginate hydrogel | Acid Yellow 23 | Anionic | Column | 0.50 | This study |
cm | mL/min | mg/mL | 1/min | min | |||
|---|---|---|---|---|---|---|---|
| 10 | 1 | 0.030 | 0.0291 ± 0.0023 | 95.7365 ± 2.9960 | 0.9665 | 0.9655 | 0.0044 |
| 15 | 1 | 0.030 | 0.0263 ± 0.0020 | 140.2932 ± 3.3000 | 0.9685 | 0.9676 | 0.0047 |
| 20 | 1 | 0.030 | 0.0262 ± 0.0016 | 198.8837 ± 2.6672 | 0.9815 | 0.9811 | 0.0031 |
| 15 | 1 | 0.030 | 0.0263 ± 0.0020 | 140.2932 ± 3.3000 | 0.9685 | 0.9676 | 0.0047 |
| 15 | 2 | 0.030 | 0.0558 ± 0.0069 | 61.8320 ± 2.5204 | 0.9575 | 0.9558 | 0.0059 |
| 15 | 5 | 0.030 | 0.0568 ± 0.0138 | 21.3060 ± 4.1485 | 0.8177 | 0.8055 | 0.0171 |
| 15 | 1 | 0.015 | 0.0185 ± 0.0008 | 220.2752 ± 2.8097 | 0.9846 | 0.9843 | 0.0024 |
| 15 | 1 | 0.030 | 0.0263 ± 0.0020 | 140.2932 ± 3.3000 | 0.9685 | 0.9676 | 0.0047 |
| 15 | 1 | 0.045 | 0.0340 ± 0.0028 | 112.1453 ± 2.7188 | 0.9725 | 0.9717 | 0.0042 |
3.5. Desorption Studies and Adsorbent Reuse
3.6. Fixed-Bed Column Adsorption of a Real Wastewater Matrix
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, H.-Y.; Guo, H.; Shen, F.; Li, T.; Show, D.-Y.; Ling, M.; Yan, Y.-G.; Show, K.-Y.; Lee, D.-J. Anaerobic-aerobic treatment of high-strength and recalcitrant textile dyeing effluents. Bioresour. Technol. 2023, 379, 129060. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Gul, J.; Naqvi, S.R.; Ali, I.; Farooq, W.; Liaqat, R.; AlMohamadi, H.; Štěpanec, L.; Juchelková, D. Recent progress in microalgae-derived biochar for the treatment of textile industry wastewater. Chemosphere 2022, 306, 135565. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Khan, S.; Malik, A. Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ. Sci. Pollut. Res. 2018, 25, 4446–4458. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Ghanbary, F. A kinetic model for the decolorization of C.I. Acid Yellow 23 by Fenton process. J. Hazard. Mater. 2007, 148, 98–102. [Google Scholar] [CrossRef]
- Daneshvar, N.; Khataee, A.R.; Amani Ghadim, A.R.; Rasoulifard, M.H. Decolorization of C.I. Acid Yellow 23 solution by electrocoagulation process: Investigation of operational parameters and evaluation of specific electrical energy consumption (SEEC). J. Hazard. Mater. 2007, 148, 566–572. [Google Scholar] [CrossRef]
- Wu, L.; Lv, X.; Zhang, Y.; Xin, Q.; Zou, Y.; Li, X. Tartrazine exposure results in histological damage, oxidative stress, immune disorders and gut microbiota dysbiosis in juvenile crucian carp (Carassius carassius). Aquat. Toxicol. 2021, 241, 105998. [Google Scholar] [CrossRef]
- US FDA. How Safe Are Color Additives? U.S. Food and Drug Administration. 2021. Available online: https://www.fda.gov/consumers/consumer-updates/how-safe-are-color-additives (accessed on 7 July 2023).
- Georgiou, D.; Aivazidis, A.; Hatiras, J.; Gimouhopoulos, K. Treatment of cotton textile wastewater using lime and ferrous sulfate. Water Res. 2003, 37, 2248–2250. [Google Scholar] [CrossRef]
- Liang, C.-Z.; Sun, S.-P.; Li, F.-Y.; Ong, Y.-K.; Chung, T.-S. Treatment of highly concentrated wastewater containing multiple synthetic dyes by a combined process of coagulation/flocculation and nanofiltration. J. Membr. Sci. 2014, 469, 306–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Hu, Y.; Sun, S. Membrane technology in wastewater treatment enhanced by functional nanomaterials. J. Clean. Prod. 2018, 197, 339–348. [Google Scholar] [CrossRef]
- Hosny, N.M.; Gomaa, I.; Elmahgary, M.G. Adsorption of polluted dyes from water by transition metal oxides: A review. Appl. Surf. Sci. 2023, 15, 100395. [Google Scholar] [CrossRef]
- Osagie, C.; Othmani, A.; Ghosh, S.; Malloum, A.; Kashitarash Esfahani, Z.; Ahmadi, S. Dyes adsorption from aqueous media through the nanotechnology: Az review. J. Mater. Res. Technol. 2021, 14, 2195–2218. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for pollutants removal: A review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Colindres, P.; Yee-Madeira, H.; Reguera, E. Removal of Reactive Black 5 from aqueous solution by ozone for water reuse in textile dyeing processes. Desalination 2010, 258, 154–158. [Google Scholar] [CrossRef]
- Bener, S.; Bulca, Ö.; Palas, B.; Tekin, G.; Atalay, S.; Ersöz, G. Electrocoagulation process for the treatment of real textile wastewater: Effect of operative conditions on the organic carbon removal and kinetic study. Process Saf. Environ. Prot. 2019, 129, 47–54. [Google Scholar] [CrossRef]
- Modirshahla, N.; Behnajady, M.A.; Ghanbary, F. Decolorization and mineralization of C.I. Acid Yellow 23 by Fenton and photo-Fenton processes. Dyes Pigment. 2007, 73, 305–310. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Dhar, A.K.; Himu, H.A.; Bhattacharjee, M.; Mostufa, M.G.; Parvin, F. Insights on applications of bentonite clays for the removal of dyes and heavy metals from wastewater: A review. Environ. Sci. Pollut. Res. 2023, 30, 5440–5474. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, Q.; Zhou, Q.; Xi, Y.; Zhu, J.; He, H. Adsorbents based on montmorillonite for contaminant removal from water: A review. Appl. Clay Sci. 2016, 123, 239–258. [Google Scholar] [CrossRef]
- Belhouchat, N.; Zaghouane-Boudiaf, H.; Viseras, C. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl. Clay Sci. 2017, 135, 9–15. [Google Scholar] [CrossRef]
- Yílmaz, N.; Yapar, S. Adsorption properties of tetradecyl- and hexadecyl trimethylammonium bentonites. Appl. Clay Sci. 2004, 27, 223–228. [Google Scholar] [CrossRef]
- Kausar, A.; Iqbal, M.; Javed, A.; Aftab, K.; Nazli, Z.-i.-H.; Bhatti, H.N.; Nouren, S. Dyes adsorption using clay and modified clay: A review. J. Mol. Liq. 2018, 256, 395–407. [Google Scholar] [CrossRef]
- Luo, Z.; Gao, M.; Ye, Y.; Yang, S. Modification of reduced-charge montmorillonites by a series of Gemini surfactants: Characterization and application in methyl orange removal. Appl. Surf. Sci. 2015, 324, 807–816. [Google Scholar] [CrossRef]
- Mullassery, M.D.; Fernandez, N.B.; Anirudhan, T.S. Adsorptive removal of acid red from aqueous solutions by cationic surfactant-modified bentonite clay. Desalin. Water Treat. 2015, 56, 1929–1939. [Google Scholar] [CrossRef]
- Brito, D.F.; da Silva Filho, E.C.; Fonseca, M.G.; Jaber, M. Organophilic bentonites obtained by microwave heating as adsorbents for anionic dyes. J. Environ. Chem. Eng. 2018, 6, 7080–7090. [Google Scholar] [CrossRef]
- De Farias, M.B.; Silva, M.G.C.; Vieira, M.G.A. Adsorption of bisphenol A from aqueous solution onto organoclay: Experimental design, kinetic, equilibrium and thermodynamic study. Powder Technol. 2022, 395, 695–707. [Google Scholar] [CrossRef]
- Maia, G.S.; de Andrade, J.R.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of diclofenac sodium onto commercial organoclay: Kinetic, equilibrium and thermodynamic study. Powder Technol. 2019, 345, 140–150. [Google Scholar] [CrossRef]
- Park, Y.; Ayoko, G.A.; Kurdi, R.; Horváth, E.; Kristóf, J.; Frost, R.L. Adsorption of phenolic compounds by organoclays: Implications for the removal of organic pollutants from aqueous media. J. Colloid Interface Sci. 2013, 406, 196–208. [Google Scholar] [CrossRef]
- Elemen, S.; Akçakoca Kumbasar, E.P.; Yapar, S. Modeling the adsorption of textile dye on organoclay using an artificial neural network. Dyes Pigment. 2012, 95, 102–111. [Google Scholar] [CrossRef]
- Viseras, C.; Aguzzi, C.; Cerezo, P.; Bedmar, M.C. Biopolymer-clay nanocomposites for controlled drug delivery. Mater. Sci. Technol. 2008, 24, 1020–1026. [Google Scholar] [CrossRef]
- Chang, M.Y.; Kao, H.C.; Juang, R.S. Thermal inactivation and reactivity of beta-glucosidase immobilized on chitosan-clay composite. Int. J. Biol. Macromol. 2008, 43, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Lezehari, M.; Basly, J.-P.; Baudu, M.; Bouras, O. Alginate encapsulated pillared clays: Removal of a neutral/anionic biocide (pentachlorophenol) and a cationic dye (safranine) from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 366, 88–94. [Google Scholar] [CrossRef]
- Govan, J.R.W.; Fyfe, J.A.M.; Jarman, T.R. Isolation of alginate-producing mutants of Pseudomonas fluorescens, Pseudomonas putida and Pseudomonas mendocina. Microbiology 1981, 125, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Edathil, A.A.; Pal, P.; Banat, F. Alginate clay hybrid composite adsorbents for the reclamation of industrial lean methyldiethanolamine solutions. Appl. Clay Sci. 2018, 156, 213–223. [Google Scholar] [CrossRef]
- Russo, R.; Malinconico, M.; Santagata, G. Effect of cross-linking with calcium ions on the physical properties of alginate films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar] [CrossRef] [PubMed]
- Djebri, N.; Boutahala, M.; Chelali, N.-E.; Boukhalfa, N.; Zeroual, L. Enhanced removal of cationic dye by calcium alginate/organobentonite beads: Modeling, kinetics, equilibriums, thermodynamic and reusability studies. Int. J. Biol. Macromol. 2016, 92, 1277–1287. [Google Scholar] [CrossRef]
- Abou Taleb, M.F.; Hegazy, D.E.; Ismail, S.A. Radiation synthesis, characterization and dye adsorption of alginate–organophilic montmorillonite nanocomposite. Carbohydr. Polym. 2012, 87, 2263–2269. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Alcântara, A.C.S.; Pergher, S.B.C. Bionanocomposite systems based on montmorillonite and biopolymers for the controlled release of olanzapine. Mater. Sci. Eng. C 2017, 75, 1250–1258. [Google Scholar] [CrossRef]
- Bhat, S.D.; Aminabhavi, T.M. Novel sodium alginate–Na+MMT hybrid composite membranes for pervaporation dehydration of isopropanol, 1,4-dioxane and tetrahydrofuran. Sep. Purif. Technol. 2006, 51, 85–94. [Google Scholar] [CrossRef]
- Chen, H.-B.; Shen, P.; Chen, M.-J.; Zhao, H.-B.; Schiraldi, D.A. Highly efficient flame retardant polyurethane foam with alginate/clay aerogel coating. ACS Appl. Mater. Interfaces 2016, 8, 32557–32564. [Google Scholar] [CrossRef]
- Oussalah, A.; Boukerroui, A.; Aichour, A.; Djellouli, B. Cationic and anionic dyes removal by low-cost hybrid alginate/natural bentonite composite beads: Adsorption and reusability studies. Int. J. Biol. Macromol. 2019, 124, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, A.; Boukerroui, A. Removal of cationic dye using alginate–organobentonite composite beads. Euro-Mediterr. J. Environ. Integr. 2020, 5, 55. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, A. Efficient adsorption of methylene blue on an alginate-based nanocomposite hydrogel enhanced by organo-illite/smectite clay. Chem. Eng. J. 2013, 228, 132–139. [Google Scholar] [CrossRef]
- Saavedra-Labastida, E.; Díaz-Nava, M.C.; Illescas, J.; Muro, C. Comparison of the removal of an anionic dye from aqueous solutions by adsorption with organically modified clays and their composites. Water Air Soil Pollut. 2019, 230, 88. [Google Scholar] [CrossRef]
- Luo, W.; Huang, Q.; Antwi, P.; Guo, B.; Sasaki, K. Synergistic effect of ClO4− and Sr2+ adsorption on alginate-encapsulated organo-montmorillonite beads: Implication for radionuclide immobilization. J. Colloid Interface Sci. 2020, 560, 338–348. [Google Scholar] [CrossRef]
- Santoso, S.P.; Kurniawan, A.; Angkawijaya, A.E.; Shuwanto, H.; Warmadewanthi, I.D.A.A.; Hsieh, C.-W.; Hsu, H.-Y.; Soetaredjo, F.E.; Ismadji, S.; Cheng, K.-C. Removal of heavy metals from water by macro-mesoporous calcium alginate–exfoliated clay composite sponges. Chem. Eng. J. 2023, 452, 139261. [Google Scholar] [CrossRef]
- Macías-Quiroga, I.F.; Giraldo-Gómez, G.I.; Sanabria-González, N.R. Characterization of colombian clay and its potential use as adsorbent. Sci. World J. 2018, 2018, 5969178. [Google Scholar] [CrossRef]
- Shah, L.A.; da Silva Valenzuela, M.d.G.; Ehsan, A.M.; Valenzuela Díaz, F.R.; Khattak, N.S. Characterization of Pakistani purified bentonite suitable for possible pharmaceutical application. Appl. Clay Sci. 2013, 83–84, 50–55. [Google Scholar] [CrossRef]
- Otavo-Loaiza, R.A.; Sanabria-González, N.R.; Giraldo-Gómez, G.I. Tartrazine removal from aqueous solution by HDTMA-Br-modified colombian bentonite. Sci. World. J. 2019, 2019, 2042563. [Google Scholar] [CrossRef] [PubMed]
- Aichour, A.; Zaghouane-Boudiaf, H.; Mohamed Zuki, F.B.; Kheireddine Aroua, M.; Ibbora, C.V. Low-cost, biodegradable and highly effective adsorbents for batch and column fixed bed adsorption processes of methylene blue. J. Environ. Chem. Eng. 2019, 7, 103409. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Waeijen, H.A.; Berry, R.M.; Tam, K.C. Continuous flow adsorption of methylene blue by cellulose nanocrystal-alginate hydrogel beads in fixed bed columns. Carbohydr. Polym. 2016, 136, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Pan, J.; Li, Y.; Yang, Y.; Zhang, Z.; Nie, J.; Ma, J. Batch and continuous fixed-bed column adsorption of tetracycline by biochar/MOFs derivative covered with κ-carrageenan/calcium alginate hydrogels. J. Environ. Chem. Eng. 2022, 10, 107996. [Google Scholar] [CrossRef]
- Kutty, S.R.M.; Almahbashi, N.M.Y.; Nazrin, A.A.M.; Malek, M.A.; Noor, A.; Baloo, L.; Ghaleb, A.A.S. Adsorption kinetics of colour removal from palm oil mill effluent using wastewater sludge carbon in column studies. Heliyon 2019, 5, e02439. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Yarali, M.; Ebadi Amooghin, A. Comparison of adsorption properties of activated carbons with different crops residues as precursors for gold cyanide recovery: An Iranian gold industry guide. Iran. J. Chem. Chem. Eng. 2020, 39, 213–229. [Google Scholar] [CrossRef]
- Harripersadth, C.; Musonge, P. The dynamic behaviour of a binary adsorbent in a fixed bed column for the removal of Pb2+ ions from contaminated water bodies. Sustainability 2022, 14, 7662. [Google Scholar] [CrossRef]
- Han, R.; Wang, Y.; Zou, W.; Wang, Y.; Shi, J. Comparison of linear and nonlinear analysis in estimating the Thomas model parameters for methylene blue adsorption onto natural zeolite in fixed-bed column. J. Hazard. Mater. 2007, 145, 331–335. [Google Scholar] [CrossRef]
- Omitola, O.B.; Abonyi, M.N.; Akpomie, K.G.; Dawodu, F.A. Adams-Bohart, Yoon-Nelson, and Thomas modeling of the fix-bed continuous column adsorption of amoxicillin onto silver nanoparticle-maize leaf composite. Appl. Water Sci. 2022, 12, 94. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Abbas, A.; Sallam, A.S.; Usman, A.R.A.; Al-Wabel, M.I. Organoclay-based nanoparticles from montmorillonite and natural clay deposits: Synthesis, characteristics, and application for MTBE removal. Appl. Clay Sci. 2017, 142, 21–29. [Google Scholar] [CrossRef]
- Xi, Y.; Ding, Z.; He, H.; Frost, R.L. Structure of organoclays—An X-ray diffraction and thermogravimetric analysis study. J. Colloid Interface Sci. 2004, 277, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Sundarrajan, P.; Eswaran, P.; Marimuthu, A.; Subhadra, L.B.; Kannaiyan, P. One pot synthesis and characterization of alginate stabilized semiconductor sanoparticles. Bull. Korean Chem. Soc. 2012, 33, 3218–3224. [Google Scholar] [CrossRef]
- Shameem, A.; Devendran, P.; Siva, V.; Venkatesh, K.S.; Manikandan, A.; Bahadur, S.A.; Nallamuthu, N. Dielectric investigation of NaLiS nanoparticles ñoaded on alginate polymer matrix synthesized by single pot microwave irradiation. J. Inorg. Organomet. Polym. Mater. 2018, 28, 671–678. [Google Scholar] [CrossRef]
- Da Silva Fernandes, R.; de Moura, M.R.; Glenn, G.M.; Aouada, F.A. Thermal, microstructural, and spectroscopic analysis of Ca2+ alginate/clay nanocomposite hydrogel beads. J. Mol. Liq. 2018, 265, 327–336. [Google Scholar] [CrossRef]
- Kragović, M.; Stojmenović, M.; Petrović, J.; Loredo, J.; Pašalić, S.; Nedeljković, A.; Ristović, I. Influence of alginate encapsulation on point of zero charge (pHpzc) and thermodynamic properties of the natural and Fe(III)—Modified zeolite. Procedia Manuf. 2019, 32, 286–293. [Google Scholar] [CrossRef]
- Pandi, K.; Viswanathan, N. Synthesis of alginate bioencapsulated nano-hydroxyapatite composite for selective fluoride sorption. Carbohydr. Polym. 2014, 112, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Ho, Y.-S. Removal of lead (II) ions from synthetic and real effluents using immobilized Pinus sylvestris sawdust: Adsorption on a fixed-bed column. J. Hazard. Mater. 2005, 123, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Bhatti, H.N.; Nausheen, S.; Sadaf, S.; Ashfaq, M. Batch and fixed bed adsorption study for the removal of Drimarine Black CL-B dye from aqueous solution using a lignocellulosic waste: A cost affective adsorbent. Ind. Crops Prod. 2013, 50, 568–579. [Google Scholar] [CrossRef]
- Hu, Q.; Xie, Y.; Zhang, Z. Modification of breakthrough models in a continuous-flow fixed-bed column: Mathematical characteristics of breakthrough curves and rate profiles. Sep. Purif. Technol. 2020, 238, 116399. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, Q.; Yang, D.; Liu, H. Prediction of breakthrough curves in a fixed-bed column based on normalized Gudermannian and error functions. J. Mol. Liq. 2021, 323, 115061. [Google Scholar] [CrossRef]
- Shanmugam, D.; Alagappan, M.; Rajan, R.K. Bench-scale packed bed sorption of Cibacron blue F3GA using lucrative algal biomass. Alex. Eng. J. 2016, 55, 2995–3003. [Google Scholar] [CrossRef]
- Manja, K.; Majda Sfiligoj, S. Polymer nanocomposite hydrogels for water purification. In Nanocomposites; Farzad, E., Ed.; IntechOpen: Rijeka, Croatia, 2012; Chapter 7; pp. 161–185. [Google Scholar]
- Aichour, A.; Zaghouane-Boudiaf, H. Synthesis and characterization of hybrid activated bentonite/alginate composite to improve its effective elimination of dyes stuff from wastewater. Appl. Water Sci. 2020, 10, 146. [Google Scholar] [CrossRef]
- Benhouria, A.; Islam, M.A.; Zaghouane-Boudiaf, H.; Boutahala, M.; Hameed, B.H. Calcium alginate–bentonite–activated carbon composite beads as highly effective adsorbent for methylene blue. Chem. Eng. J. 2015, 270, 621–630. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of gas adsorption kinetics I. A theoretical model for respirator cartridge service life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y.; Dundar, M. Adsorption of chromium(VI) on pomace—An olive oil industry waste: Batch and column studies. J. Hazard. Mater. 2006, 138, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Mitra, T.; Singha, B.; Bar, N.; Das, S.K. Removal of Pb(II) ions from aqueous solution using water hyacinth root by fixed-bed column and ANN modeling. J. Hazard. Mater. 2014, 273, 94–103. [Google Scholar] [CrossRef]
- Padmesh, T.V.N.; Vijayaraghavan, K.; Sekaran, G.; Velan, M. Biosorption of acid blue 15 using fresh water macroalga Azolla filiculoides: Batch and column studies. Dyes Pigment. 2006, 71, 77–82. [Google Scholar] [CrossRef]
- Saadi, Z.; Saadi, R.; Fazaeli, R. Fixed-bed adsorption dynamics of Pb (II) adsorption from aqueous solution using nanostructured γ-alumina. J. Nanostruct. Chem. 2013, 3, 48. [Google Scholar] [CrossRef]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Özcan, A.; Ömeroğlu, Ç.; Erdoğan, Y.; Özcan, A.S. Modification of bentonite with a cationic surfactant: An adsorption study of textile dye Reactive Blue 19. J. Hazard. Mater. 2007, 140, 173–179. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Congo red adsorption from aqueous solutions by using chitosan hydrogel beads impregnated with nonionic or anionic surfactant. Bioresour. Technol. 2009, 100, 3862–3868. [Google Scholar] [CrossRef] [PubMed]
- Gkika, D.A.; Mitropoulos, A.C.; Kyzas, G.Z. Why reuse spent adsorbents? The latest challenges and limitations. Sci. Total Environ. 2022, 822, 153612. [Google Scholar] [CrossRef] [PubMed]
- Parimelazhagan, V.; Jeppu, G.; Rampal, N. Continuous fixed-bed column studies on Congo red dye adsorption-desorption using free and immobilized Nelumbo nucifera leaf adsorbent. Polymers 2022, 14, 54. [Google Scholar]


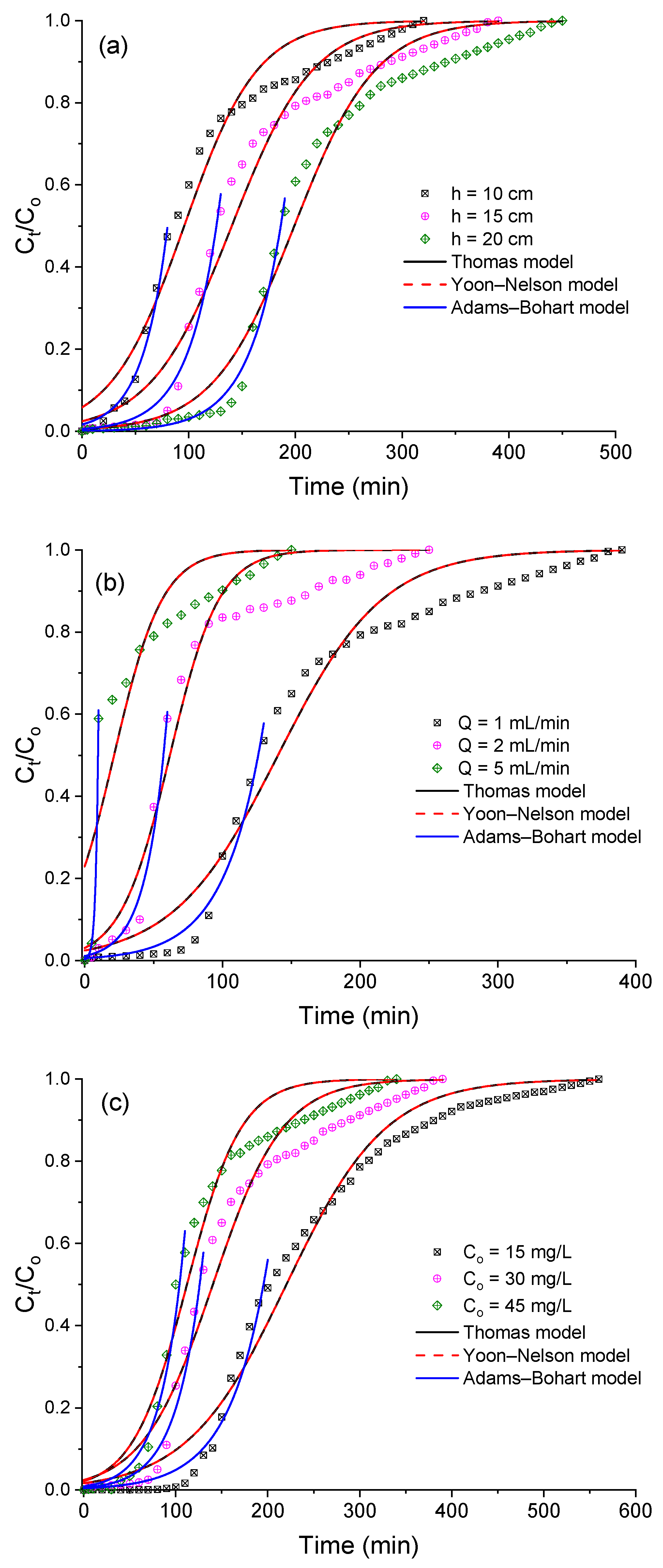


| Model–Equation | Parameters | Ref. |
|---|---|---|
| Thomas Equation (10) | = influent dye concentration, mg/L = effluent dye concentration (mg/L) at time t time, min = Thomas rate constant, mL/mg min = adsorption capacity, mg/g = volumetric flow rate, mL/min = adsorbent amount, g | [59] |
| Yoon–Nelson Equation (11) | = Yoon–Nelson rate constant, 1/min = time required for = 0.5, min | [60] |
| Adams–Bohart Equation (12) | = Adams–Bohart rate constant, L/mg min = adsorption capacity per unit volume of the bed, mg/L = height of the bed, cm = linear flow velocity, cm/min | [60] |
| Parameter a | OBent(2%)/Alg | OBent(4%)/Alg | OBent(6%)/Alg | OBent(10%)/Alg |
|---|---|---|---|---|
| Mass, mg | 38.8 ± 0.4 | 42.7 ± 0.4 | 48.3 ± 0.3 | 63.9 ± 0.5 |
| Water content, % | 94.3 ± 0.5 | 93.6 ± 0.5 | 92.1 ± 0.6 | 91.8 ± 0.9 |
| Swelling capacity, % | 133.3 ± 0.7 | 133.3 ± 0.6 | 133.3 ± 0.8 | 133.3 ± 0.5 |
cm | g | mL/min | mg/L | min | min | mL | min | mg | mg/g | mg | mg/L | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 6.55 | 1 | 30 | 45.030 | 274.843 | 274.843 | 320 | 3.280 | 0.416 | 9.625 | 22.994 | 34.168 |
| 15 | 10.10 | 1 | 30 | 88.333 | 337.909 | 337.909 | 390 | 4.648 | 0.460 | 11.737 | 20.871 | 39.724 |
| 20 | 13.65 | 1 | 30 | 147.500 | 395.004 | 395.004 | 450 | 6.343 | 0.485 | 13.501 | 18.120 | 46.981 |
| 15 | 10.10 | 1 | 30 | 88.333 | 337.909 | 337.909 | 390 | 4.648 | 0.460 | 11.701 | 20.871 | 39.724 |
| 15 | 10.10 | 2 | 30 | 40.000 | 204.934 | 409.868 | 250 | 3.864 | 0.383 | 15.003 | 27.171 | 25.758 |
| 15 | 10.10 | 5 | 30 | 5.512 | 125.699 | 628.494 | 150 | 0.530 | 0.053 | 22.505 | 34.967 | 2.355 |
| 15 | 10.10 | 1 | 15 | 138.483 | 449.401 | 449.401 | 560 | 3.483 | 0.345 | 8.404 | 10.940 | 41.469 |
| 15 | 10.10 | 1 | 30 | 88.333 | 337.909 | 337.909 | 390 | 4.648 | 0.460 | 11.707 | 20.871 | 39.724 |
| 15 | 10.10 | 1 | 45 | 69.057 | 287.910 | 287.910 | 340 | 5.622 | 0.557 | 15.306 | 33.615 | 36.745 |
cm | mL/min | mg/mL | mL/mg min | mg/g | |||
|---|---|---|---|---|---|---|---|
| 10 | 1 | 0.030 | 0.9684 ± 0.0758 | 0.3640 ± 0.0114 | 0.9655 | 0.9665 | 0.0044 |
| 15 | 1 | 0.030 | 0.8748 ± 0.0671 | 0.4169 ± 0.0098 | 0.9676 | 0.9685 | 0.0047 |
| 20 | 1 | 0.030 | 0.8738 ± 0.0540 | 0.4558 ± 0.0061 | 0.9811 | 0.9815 | 0.0031 |
| 15 | 1 | 0.030 | 0.8748 ± 0.0671 | 0.4169 ± 0.0098 | 0.9676 | 0.9685 | 0.0047 |
| 15 | 2 | 0.030 | 1.8612 ± 0.2300 | 03675 ± 0.0150 | 0.9558 | 0.9575 | 0.0059 |
| 15 | 5 | 0.030 | 1.8962 ± 0.4603 | 0.3163 ± 0.0616 | 0.8055 | 0.8177 | 0.0171 |
| 15 | 1 | 0.015 | 1.2320 ± 0.0567 | 0.3273 ± 0.0042 | 0.9843 | 0.9846 | 0.0024 |
| 15 | 1 | 0.030 | 0.8748 ± 0.0671 | 0.4169 ± 0.0098 | 0.9676 | 0.9685 | 0.0047 |
| 15 | 1 | 0.045 | 0.7548 ± 0.0671 | 0.4998 ± 0.0121 | 0.9717 | 0.9725 | 0.0042 |
cm | cm/min | mL/min | mg/mL | mL/mg min | mg/L | |||
|---|---|---|---|---|---|---|---|---|
| 10 | 0.884 | 1 | 0.030 | 0.0014 ± 9.6 × 10−5 | 256.0382 ± 4.8154 | 0.9851 | 0.9833 | 4.58 × 10−4 |
| 15 | 0.884 | 1 | 0.030 | 0.0012 ± 1.3 × 10−4 | 257.1605 ± 4.4398 | 0.9661 | 0.9635 | 1.17 × 10−3 |
| 20 | 0.884 | 1 | 0.030 | 0.0011 ± 6.4 × 10−5 | 275.0142 ± 2.5432 | 0.9851 | 0.9768 | 5.57 × 10−4 |
| 15 | 0.884 | 1 | 0.030 | 0.0012 ± 1.0 × 10−4 | 257.1605 ± 4.4398 | 0.9661 | 0.9635 | 1.17 × 10−3 |
| 15 | 1.768 | 2 | 0.030 | 0.0022 ± 2.5 × 10−4 | 238.7197 ± 5.2533 | 0.9743 | 0.9701 | 1.36 × 10−3 |
| 15 | 4.421 | 5 | 0.030 | 0.0179 ± 4.6 × 10−4 | 96.5801 ± 0.2316 | 0.9999 | 0.9999 | 8.19 × 10−6 |
| 15 | 0.884 | 1 | 0.015 | 0.0016 ± 1.3 × 10−4 | 197.8971 ± 3.1616 | 0.9576 | 0.9555 | 1.23 × 10−3 |
| 15 | 0.884 | 1 | 0.030 | 0.0012 ± 1.3 × 10−4 | 257.1605 ± 4.4398 | 0.9661 | 0.9635 | 1.17 × 10−3 |
| 15 | 0.884 | 1 | 0.045 | 0.0086 ± 7.4 × 10−5 | 323.3084 ± 5.4846 | 0.9716 | 0.9690 | 1.26 × 10−3 |
| Test | min | min | mL | min | mg | mg/g | mg | mg/L | % |
|---|---|---|---|---|---|---|---|---|---|
| Initial adsorption | 88.333 | 337.909 | 337.909 | 390 | 4.648 | 0.460 | 11.737 | 20.871 | 39.724 |
| Reuse–Cycle 1 | 41.402 | 252.871 | 252.873 | 310 | 3.046 | 0.302 | 9.300 | 24.734 | 31.750 |
| Reuse–Cycle 2 | 38.588 | 128.599 | 128.599 | 200 | 1.539 | 0.152 | 6.001 | 34.691 | 25.645 |
| Parameter | Standard Method (SM) | Wastewater Sample (before Adsorption) | Wastewater Sample (after Adsorption) |
|---|---|---|---|
| pH | 4500-H+ B | 7.4 ± 0.1 | 7.5 ± 0.2 |
| Conductivity (μS/cm) | 2510-B | 510 ± 10 | 303 ± 8 |
| COD (mg O2/L) | 5220-D | 825 ± 35 | 527 ± 14 |
| BOD5 (mg O2/L) | 5210-B | 348 ± 21 | 269 ± 11 |
| TOC, mg C/L | 5310-B | 318 ± 12 | 235 ± 8 |
| Acid Yellow 23, mg/L | 11.37 ± 0.03 | <0.13 * |
| Test | min | min | mL | mg | mg/g | mg | mg/L | % |
|---|---|---|---|---|---|---|---|---|
| Initial | 170.498 | 503.768 | 503.768 | 3.451 | 0.342 | 7.618 | 8.272 | 45.301 |
| Duplicate | 169.337 | 498.462 | 498.462 | 3.383 | 0.335 | 7.595 | 8.499 | 44.543 |
| Triplicate | 169.139 | 496.838 | 496.838 | 3.355 | 0.332 | 7.583 | 8.509 | 44.244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Arenas, D.; Macías-Quiroga, I.F.; Dávila-Arias, M.T.; Vélez-Upegui, J.J.; Sanabria-González, N.R. Adsorption of Acid Yellow 23 Dye on Organobentonite/Alginate Hydrogel Beads in a Fixed-Bed Column. J. Compos. Sci. 2023, 7, 362. https://doi.org/10.3390/jcs7090362
Rivera-Arenas D, Macías-Quiroga IF, Dávila-Arias MT, Vélez-Upegui JJ, Sanabria-González NR. Adsorption of Acid Yellow 23 Dye on Organobentonite/Alginate Hydrogel Beads in a Fixed-Bed Column. Journal of Composites Science. 2023; 7(9):362. https://doi.org/10.3390/jcs7090362
Chicago/Turabian StyleRivera-Arenas, Daniela, Iván F. Macías-Quiroga, María T. Dávila-Arias, Jorge J. Vélez-Upegui, and Nancy R. Sanabria-González. 2023. "Adsorption of Acid Yellow 23 Dye on Organobentonite/Alginate Hydrogel Beads in a Fixed-Bed Column" Journal of Composites Science 7, no. 9: 362. https://doi.org/10.3390/jcs7090362







