Effects of Low-Velocity-Impact on Facesheet-Core Debonding of Natural-Core Composite Sandwich Structures—A Review of Experimental Research
Abstract
1. Introduction
2. Composites Usage, Environmental Impact and Recyclable Composites
- Legislation promoting end-of-life recycling of vehicle parts, such as the European Guideline 2000/53/EG which sets a target that 85% of a vehicle’s weight should be recyclable by 2005 and 95% by 2015 [6].
- Large increase in composite use in the automotive and aviation industries, due to the need to reduce vehicle weight, fuel consumption, and CO2 emissions, and therefore reduce operating costs and comply with legislation to reduce emissions, such as the Kyoto Protocol [6]. In particular, the proportion of composites used in aircraft has grown from 10–12% (1990—Airbus A330 and Boeing 777) to over 50% by weight (2010s—Boeing 787 and Airbus A350) in just 20 years [7,8].
- Massive growth of the wind industry as the technology matures and costs have decreased: from 6.4 GW in 1996 to 837 GW in 2021 [9]. Tower height, turbine blade, and rotor diameters have also greatly increased, as shown in Figure 1. Wind turbine blades also traditionally use balsa core sandwich composites, which is covered more in detail in Section 4.

3. Natural Materials in Composites
Wind Turbine Blades
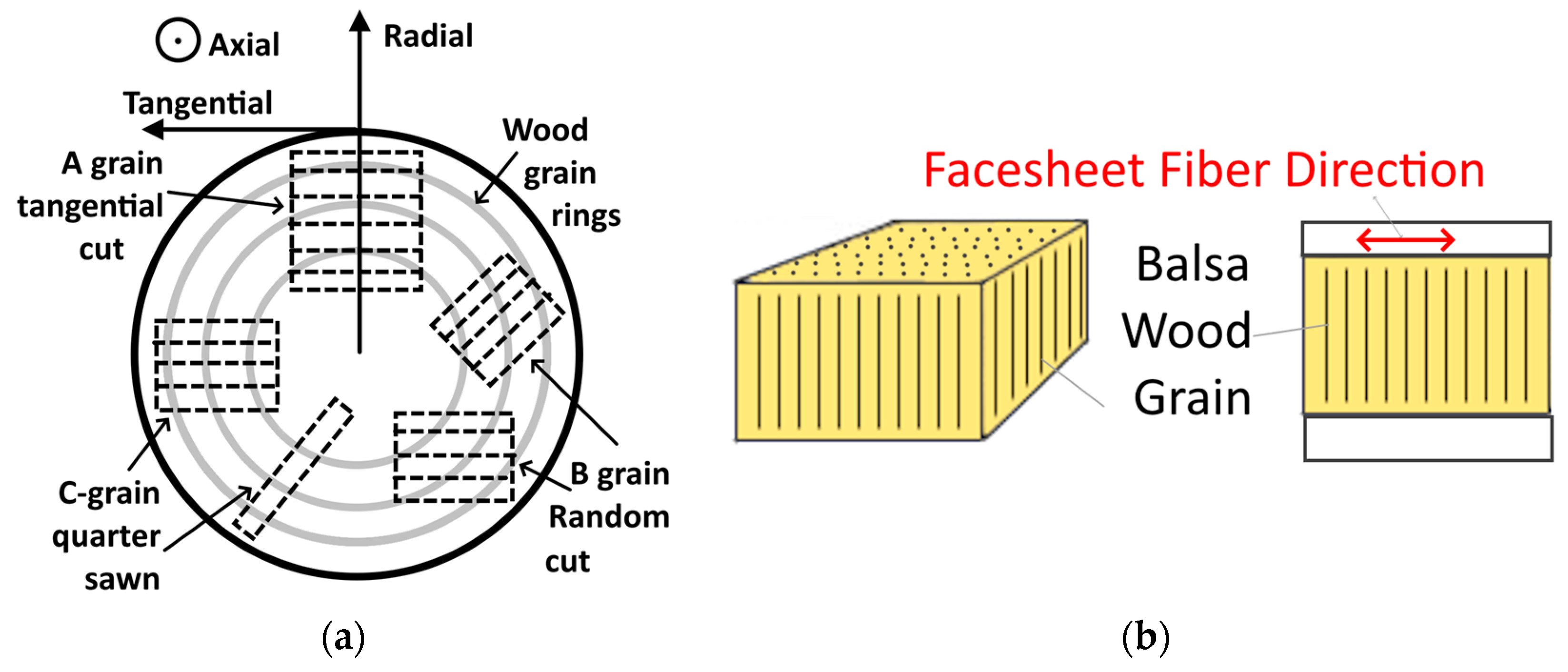
4. Balsa
4.1. Balsa Wood Applications
4.2. Coated Balsa for Reduced Resin Uptake
5. Cork and Cork Agglomerates

5.1. Effect of Low-Velocity Impact on Non-Sandwich Cork Agglomerates
5.2. Co-Cured Cork Agglomerates
6. Comparison of Cork Agglomerates and Balsa
7. Sandwich Composite Manufacturing Processes
Vacuum Assisted Resin Infusion for Large Composite Structures
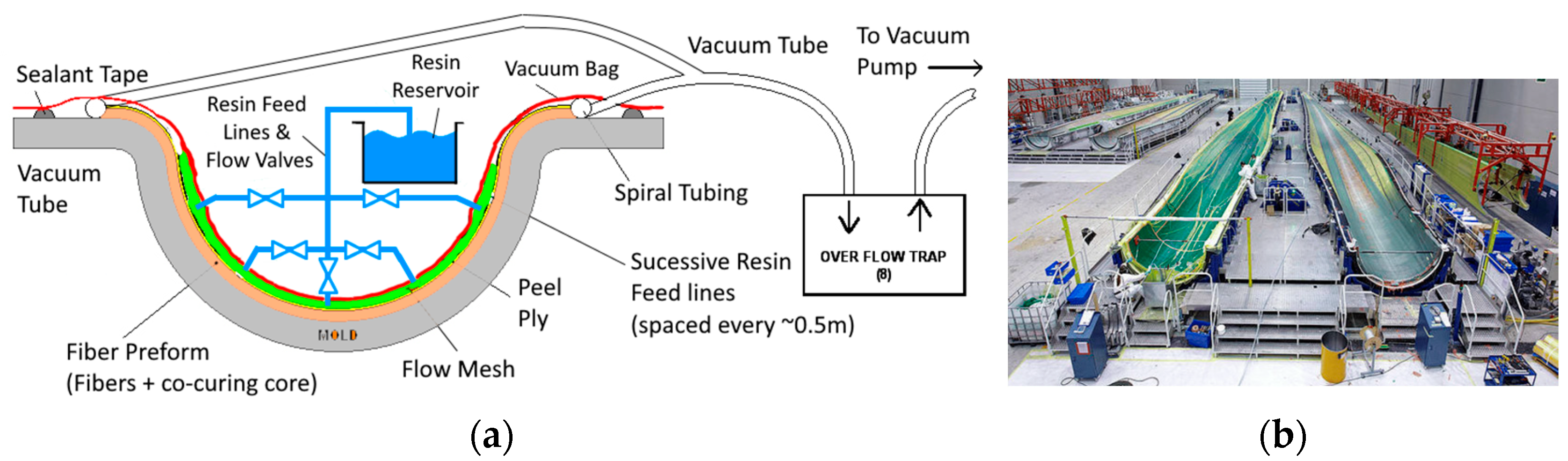
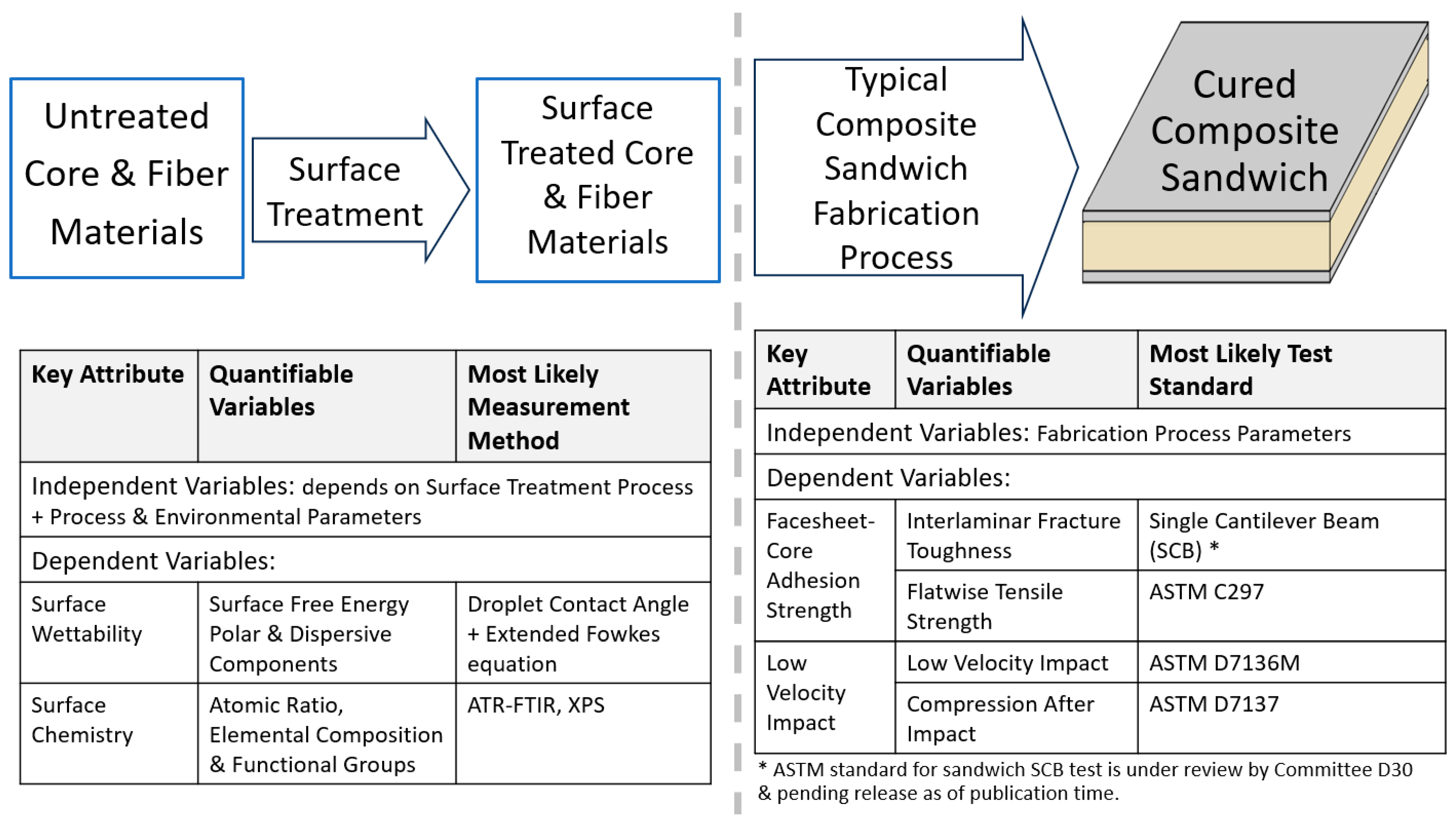
8. Importance of Adhesion in Composites and Sandwich Structures
9. Methods of Boosting Adhesion—Surface and Bulk Treatment of Materials
9.1. Surface Free Energy
9.2. Measuring Surface Free Energy
Deriving Surface Free Energy from Contact Angle Measurements
9.3. Surface-Free Energies of Various Materials
9.4. Need for Surface Treatments on Natural Fibers
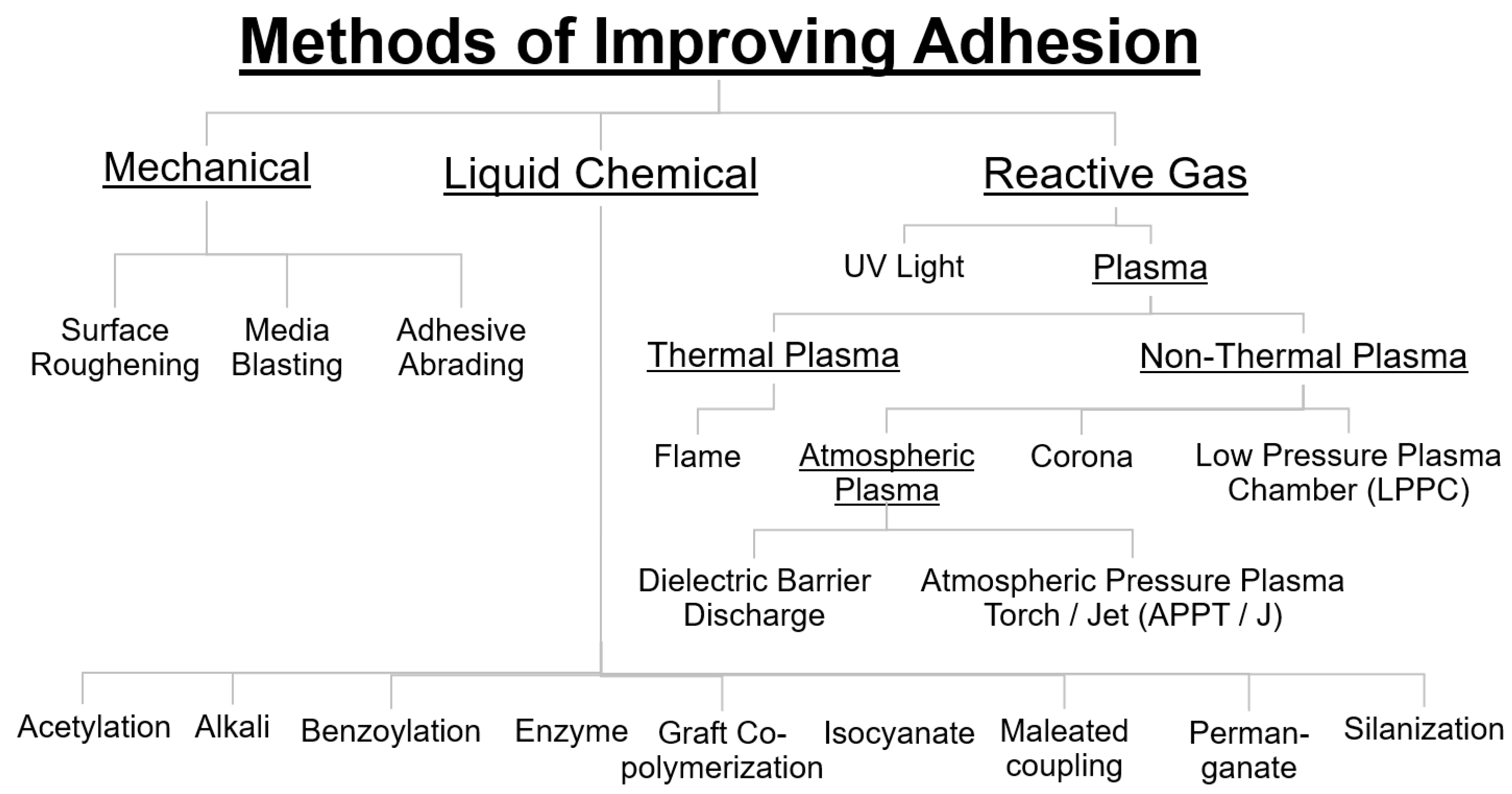
9.5. Methods to Improve Adhesion: Bulk and Surface Treatments
9.5.1. Mechanical Surface Treatments—Surface Roughening
9.5.2. Surface Chemical Activation
9.5.3. Surface Modification of Glass and Carbon Fibers
9.6. Liquid Chemical Treatments
9.6.1. Liquid Chemical Treatments of Cork and Balsa
9.6.2. Suitability of Liquid Chemical Treatments in a Composite Processing Environment
9.7. Reactive Gas Surface Treatments—Thermal and Nonthermal Plasma and UV for Surface Activation
9.7.1. UV Light Treatment
- Ozone generation: O2 + hv (184.9 nm) → O * + O *;
- Ozonolysis: O3 + hv (253.7 nm) → O * + O2;Contaminants + hv (200–300 nm) → Ions, free radicals, excited, and neutral molecules;
- Contaminant destruction and removal: Hydrocarbons and nitrogen-containing species react with excited substances and free radicals and are decomposed into simpler volatile materials H2O, CO2, and NOx.
9.7.2. Thermal Plasmas: Flame Treatment
9.7.3. Non-Thermal Plasma Treatments
9.8. Reactive Gas Surface Treatments of Composites Materials
| Fiber/Core/Who | Treatment Method | Key Findings |
|---|---|---|
| Cork sheets 200 × 100 × 12 mm * [95] | Atmospheric Pressure Plasma Treatment—
| Treated vs. untreated cork: CA: Increased polar (23 vs. 0 mN/m), dispersive (31 vs. 18 mN/m) and total surface energy (54 vs. 18 mN/m). ATR-FTIR: Increases in OH, ArC-O-C-al and ArC-H peaks Pull-off test: Decrease in pull-off strength from 1.45 to 1.3 MPa. APPT specimens fail due to cohesive failure of cork, while untreated specimens show adhesive failure in different areas. Peel Test: 34% increase in peel strength |
| Cork sheets 200 × 100 × 12 mm * [95] | Low-Pressure Plasma Chamber—
| Treated vs. untreated cork: CA: Increased polar (18 vs. 0 mN/m), dispersive (30 vs. 18 mN/m), and total surface energy (48 vs. 18 mN/m). ATR-FTIR: Similar to APPT-treated cork as shown above. Pull-off test: Decrease in pull-off strength from 1.45 to 1.41 MPa. LPPC specimens fail due to cohesive failure of cork, while untreated specimens fail due to adhesive failure at different areas. Peel Test: 15% increase in peel strength |
| Cork [97] | DBD plasma treatment—No mention of Frequency and voltage, as well as if any working gas used. Power—750 W, velocity of 5m min−1. Number of passes varied for dosage 1 pass—300 W min m−2. 2 pass—600 W min m−2. 4 pass—1200 W min m−2. 8 pass—2400 W min m−2. 16 pass—4800 W min m−2. Droplet Contact Angle Measurements conducted using water, glycerol, and diidomethane. No adhesion of resins or other materials to cork was carried out in this paper. Mechanical testing of plasma-treated cork was also not carried out in this paper. | Treated vs. untreated: DBD plasma treatment increases cork surface energy significantly. Calculated SFE based on CA: 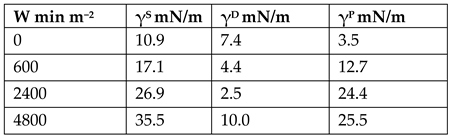 ATIR/FTIR: Untreated vs. 2400 W: Increase in 1735 cm−1 (carbonyl), 1357 (C-N) peaks EDS (15 kV voltage, Si(Li) detector,): Increase of O/C ratio from 0.33 to 0.36 DSC and SEM: Did not detect any major structural change in cork material after plasma treatment. |
| Cured Carbon fiber prepregs [119] | Adhesive bonding of CF Prepregs into single lap-joint specimens. Toray T800S/3900-2B prepreg, nominal thk 0.19 mm, 8 plies in quasi isotropic layup—already cured at 180 °C and 0.64 MPa for 2 h. After curing, demold and wipe surface clean with MEK solvent before flame treatment. LPG flame (1000 °C) treatment process (ITRO) applied on CFRP plates: 1, 6, or 12 passes. To avoid overheating, plates left to cool for 1 min after every 2 passes. Control: Plate surface sanded before bonding Bonding: Within 24 h after ITRO, sandwich film adhesive between two CFRP panels and cure in autoclave for 2 h at 177 °C and 0.31 MPa. | ITRO causes formation of SiO2 film and oxygen-containing functional groups, improving adhesion across the adhesive–substrate interface. No ATR-FTIR or contact angle measurements done. XPS: % of O and Si increase steadily with number of passes, % of C and F decreases steadily. Max apparent shear strength at 12 passes (18 MPa) compared to untreated (7 MPa) and sanded (19 MPa). Dominant failure mode changes from interfacial failure to substrate failure as the number of passes increases. |
| Glass Fibers [136] | Glass Fiber Reinforced Laminated Veneer Lumber Composites made by Hot Pressing Materials: Poplar Veneer and Plain weave E-glass 500 g/m2 Adhesive: phenol formaldehyde (PF) Custom DBD Air Plasma Setup Treatment carried out on Glass Fibers Speed: 8 m/min Power levels: 1.5, 3, 4.5, 6 kW Hot press parameters: Pressing speed 1.5 min/mm at 130 °C and pressure 1.2 MPa | Optimal results found at 4.5 kW power level. Overtreatment occurs at 6 kW, Fractographic analysis shows bare GF fiber surface exposed (excessive etching) and residual resin exists independently of fiber (undesirable). Up to 4.5 kW, Fracture model changes with power level from interfacial failure to combination of resin interlayer and interface failure 4.5 kW compared to untreated specimens:
|
| Aramid Fibers (Twaron) [91] (Figure 13) | Clean with acetone at room temperature before DBD plasma treatment DBD Plasma parameters: Steel Electrode ⌀ 4.7 cm Dielectric barrier: 1 mm thick Quartz Discharge power (power density) 143.5 W (27.6 W/cm3) Discharge gas (pressure): Air (1atm) Treatment levels: Untreated, 6 s, 12 s, 18 s Adhesion of PPESK thermoplastic resin to fibers after treatment. | 12 s found to be optimal treatment time, overtreatment occurs at 18 s. SFE is highest at 18 s, while XPS and SEM data show optimum at 12 s and deterioration at 18 s. 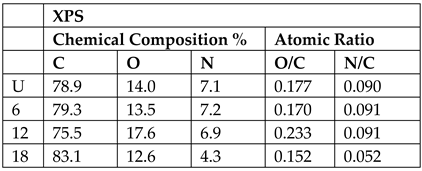 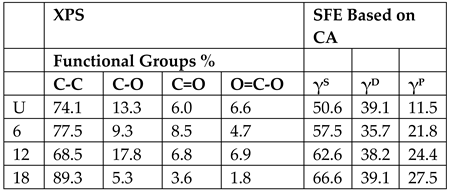 Mechanical tests not done. |
- It is possible to use reactive gas surface treatments to boost polar components of surface free energy levels of glass fibers, carbon fibers, cured thermoset, and thermoplastic fiber laminates and cork core materials.
- For secondarily bonded composite samples, failures occur due to substrate failure rather than adhesive failures.
- It is possible to overtreat specimens, and therefore an optimal limit should be found for each material and process.
- Deriving Surface Free Energy based on Contact Angle measurements is not able to detect overtreatment. Therefore, other methods like XPS, SEM, and ATR-FTIR also need to be used to determine an optimal level of treatment.
10. Considerations for Choosing Appropriate Surface Treatment Methods for a Composites Processing Environment
- Environmentally friendly—Minimal or no chemical usage.
- Safety.
- No ignition hazard—composites processing facilities use large quantities of potentially flammable resins and solvents like acetone.
- Minimal or no toxicity.
- Compact—does not require much space.
- Ease of use.
- Inexpensive.
- Ability to process samples of large area.
- Effective in terms of improving adhesion and/or removing contaminants such as dirt and moisture.
- Fast—low processing time.
- No requirement for processing complex geometry.
11. Composite Laminates and Sandwich Composite Failure Modes
- Matrix cracking parallel to the fibers.
- Delamination/Debonding between layers due to interlaminar stress.
- Fibers in tension fiber breakage and in compression fiber buckling.
Delamination and Facesheet-Core Debonding
12. Susceptibility of Fiber and Sandwich Composites to Impact Damage
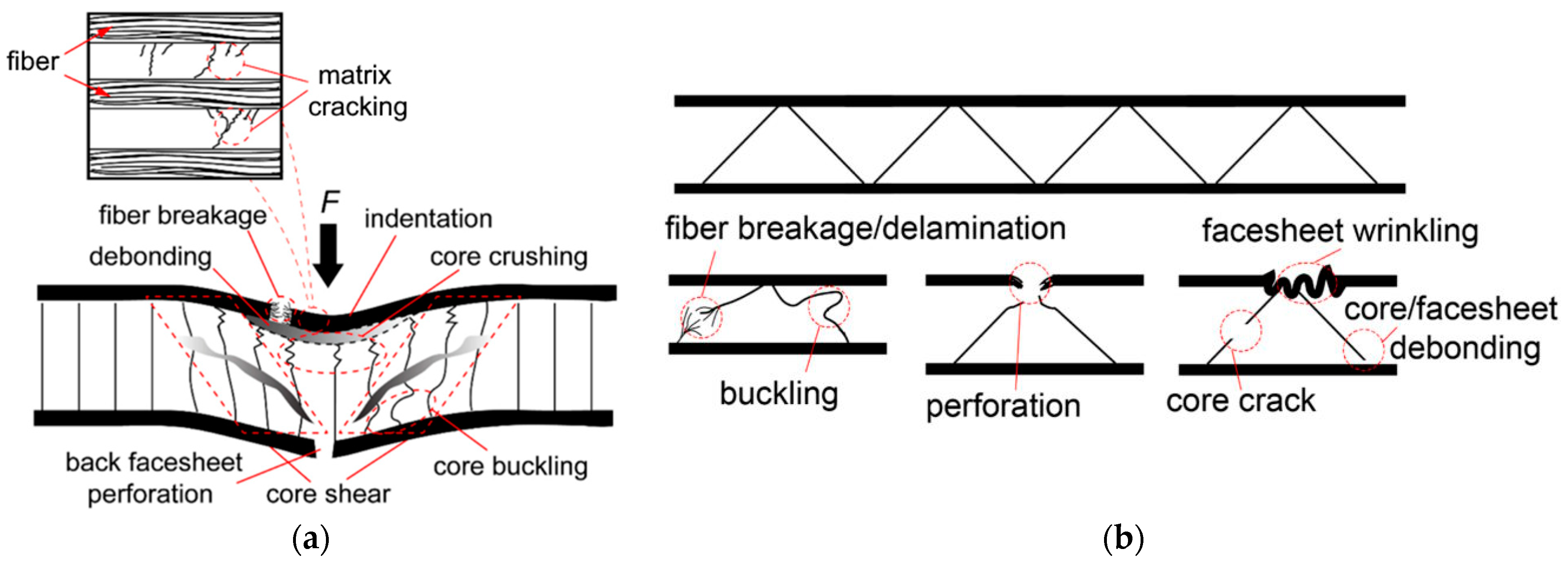
12.1. Different Types of Failure Modes on Composites
12.2. Facesheet-Core Debonding and Barely Visible Impact Damage (BVID)
12.3. Effects of Low and High Impact Velocities on Barely-Visible Impact Damage (BVID)
12.4. Experimental Results of Low-Velocity Impact (LVI) on Balsa Core Composite Sandwich Structures
| Who/Fabrication | Test Method | Key Findings |
|---|---|---|
| [155] Co-cured vacuum bagging
| NS, Instron Dynatup 9250HV DWT ⌀ 50.8 mm HI mass 7.7 kg Energy levels—17 J, 26 J, 35 J Specimen size 100 × 100 × 11.5 m, exposed opening ⌀ 76.2 mm Also done: CAI on damaged area, NS. Cst rate 0.05 mm/s in thickness direction with ⌀ 20.5 mm steel cylinder | 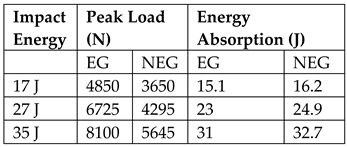 Above table shows that EGB core sandwich can withstand higher impact loads but has less energy absorption than regular balsa cores. EGB core sandwich retained higher CAI residual strength than regular balsa core. 17 J impact energy causes large (40–52%) reduction in residual strength for both EGB and Regular core sandwich. |
| [156] Method: Co-cured VARI, FSNT: 11.5 mm:
| NS, CEAST Fractovis Plus, exposed opening ⌀ 76.2 mm ⌀ 12.7 mm HI with mass 5 kg | See Figure 17 Balsa more prone to debonding than PVC as balsa has poor interface with glass/epoxy. Balsa has a linear elastic response until the point of initial localized failure, followed by drop in stress level as buckling occurs in weakest sites. |
| [55] Method: Co-cured vacuum bagging, FSNT: 35.53–36.97 mm
| ASTM D7136, Rosand IFW 5 HV DWT Impact energies: 50, 100, 150, 200, 300 J Specimen size 150 × 150 mm2 Also done:
|  Poor adhesion between balsa and Glass/epoxy layer—facesheet is easily ripped off from the core material during quasi-static indentation. PVC and NL20: Load increases linearly until rupture of 1st facesheet around 12–13 kN and 10 mm displacement. Authors remark that Cork behaves similarly to a piece of rubber or spring in that it almost regains its original shape after compressive force is released. |
| [157] Method: SB under vacuum, FSNT: 28.2 mm
| NS, DWT model NG Impact energies: 7.8–108 J Specimen size 150 × 150 mm2 Also done:
| Minimum energy required for debonding to occur: UD + Balsa: 15 J, Woven + Balsa: 9 J UD + PVC: 30–35 J UD + Al honeycomb: 9 J Balsa core sandwiches perform well under static loading, but are weak at impact loading as low fracture toughness along grain direction leads to catastrophic core failure and thus debonding at non-impacted facesheet. PVC core Sandwiches tend to absorb more energy through indentation and are more stable than balsa cores. Debonding tends to occur at impacted Facesheet. |
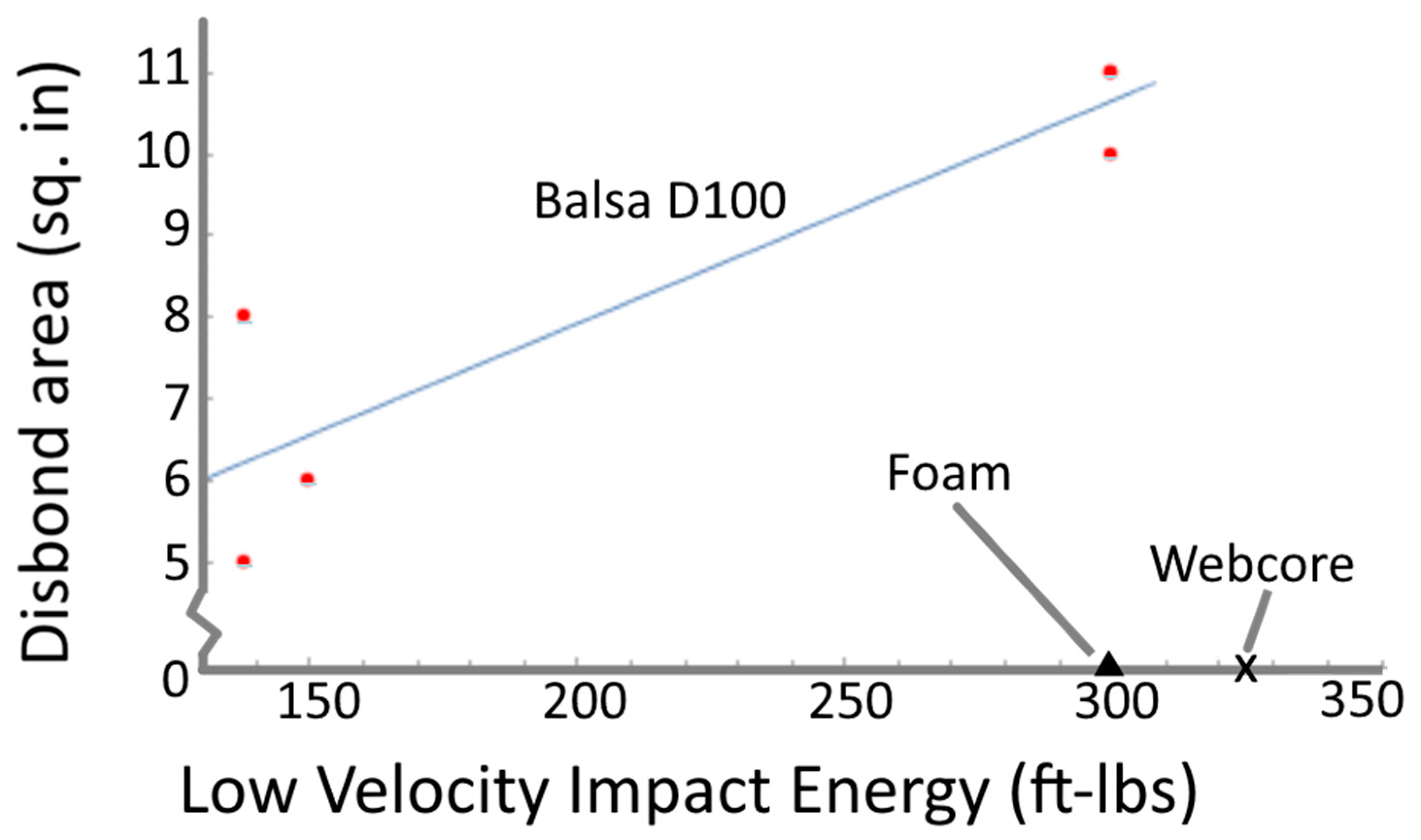
| [158] Co-cured SCRIMP (patented VARI derivative method) Each Facesheet: E-glass 810 g/m2 Woven Roving, (number of layers and direction not given) nominal thk 3 mm Core: 1″ thk. Either
Some samples have intentionally created facesheet-core disbond | NS, DWT model not given, ⌀ 101.4 mm spherical steel impactor, height 6 ft, 25 and 50 lbs (energy levels—150 ft-lbs (203.4 J) and 300 ft-lbs (407.7 J) Thereafter: CAI, NS. End loading of plates at 0.1 in/min rate | Balsa showed higher compression after impact capacity, better local crushing and local shearing but lesser resistance to disbonds than foam. All Foam specimens did not exhibit any delamination or core crushing, but balsa exhibited delamination for both impact damage and release agents. Balsa compression after impact load capacity is dependent on delamination size—higher size means less load capacity. It does not matter whether the delamination is due to impact or existing disbonds. Webcore did not experience any delaminations due to impact. Refer to Figure 18 for graph on disbond area |
| [159] Method: SB with hot press machine:
| NS, CEAST Fractovis Plus 7526.000, exposed opening ⌀ 76.2 mm ⌀ 12.7 mm HI with mass 4.926 kg | Main damage modes: fiber fractures at top and bottom facesheets, delaminations between thermoplastic facesheets, transverse fractures of balsa wood core. Refer to Figure 19 Except for the first 5 impacts, the maximum contact force of the sandwich composite specimens decreases with each subsequent impact Number of repeated 10 J impacts required to fracture laminate: 38 (15 mm), 98 (25 mm) |
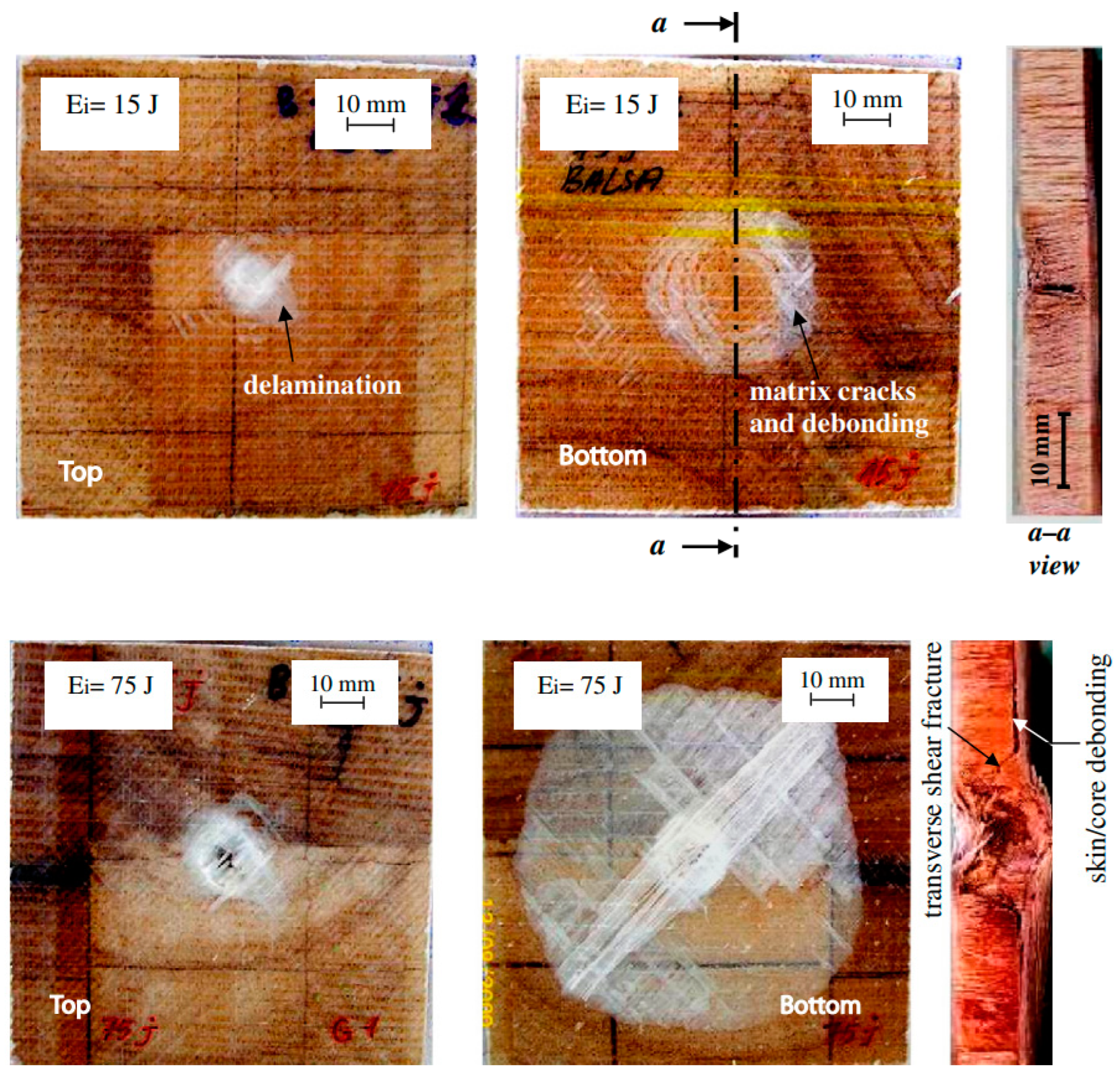
- Debonding can occur for balsa core sandwiches even at low LVI energy levels, and severity increases with increasing LVI energy levels [159].
- Delamination is the dominant damage mode, particularly at higher impact energy levels [156].
- Delamination occurs more significantly at the non-impacted than the impacted side for balsa core sandwiches for all cases and impact energies. This is in contrast to PVC foam which is more prone to front facesheet debonding [156].
12.5. Low Velocity Impact of Cork Core Sandwich Structures
12.6. Effects of Surface Treatment on Low-Velocity Impact (LVI) Damage on Laminates
13. Conclusions
- Surface Treatment of Core Materials—balsa, and Amorim Corecork cork core
- Effects of surface treatment on co-curing sandwich composite fabrication process –Vacuum Assisted Resin Infusion and Vacuum Bagging
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Equation Name | Equation | Remarks |
|---|---|---|
| Cassie/Cassie Baxter Equation [166,167] | cos θc = f1 cos θ1 – f2, with f1 + f2 ≥ 1 where:
| Typically used for superhydrophobic surfaces, and surfaces manufactured using lithography, micromachining, etching, or similar techniques. Not commonly used for surface treatment of composite or natural materials. Simplified form cos θc = f1 cos θ – (1 – f) is valid for flat-topped pillar geometry without any penetration of the liquid. |
| Van Oss Acid-Base Equation [87,168] | According to the Lewis acid-base theory, polar interactions occur when an electron acceptor (+) is attracted to an electron donor (−). These form the geometric mean. γSL is then substituted into Young’s Equation to obtain | Since 2 of the Van Oss theory’s 3 parameters deal with polar interaction, this theory works best for organometallic surfaces, inorganic surfaces, and surfaces containing ions. Not often used in practice, largely because of very limited choice of test liquids with known basic and acidic fractions. |
| Young’s Equation [87,88] | where:
| Valid for 3 phase systems in thermodynamic equilibrium for ideal (smooth and chemically homogenous) solids and pure liquids. Not to be confused with the Young Laplace fit, which describes the sustained capillary pressure difference at the interface between two static fluids. |
| Zisman Equation [87,169] | The surface energy of a solid is based on the highest surface tension liquid (real or imaginary) that completely wets out the solid, i.e., gives a contact angle of 0°: e.g., for Polyethylene (PE):  Then draw a best fit line of the cosine of contact angle against liquid surface tension (mN/m) of the liquids with non-zero contact angles, and see where the best fit line intersects the y = 0 mark. That intersection marks the highest surface tension liquid, and thus the surface free energy of the solid. | Not used for surface treatment of natural materials. Only measures 1 parameter—the overall surface tension of the solid surface. At least 5 liquids are required to conduct measurements. Generally, Zisman theory works best for non-polar surfaces, such as polyethylene or polypropylene. Unsuitable for polar surfaces. Since surface treatment typically increases the polar component of surface free energy, it is not used for assessing effectiveness of surface treatment. |
References
- Mishnaevsky, L.; Branner, K.; Petersen, H.N.; Beauson, J.; McGugan, M.; Sørensen, B.F. Materials for Wind Turbine Blades: An Overview. Materials 2017, 10, 1285. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.; Madenci, E. Experimental investigation of low-velocity impact characteristics of sandwich composites. Compos. Struct. 2000, 50, 239–247. [Google Scholar] [CrossRef]
- Castanie, B.; Bouvet, C.; Ginot, M. Review of composite sandwich structure in aeronautic applications. Compos. Part C Open Access 2020, 1, 100004. [Google Scholar] [CrossRef]
- Vicard, C.; De Almeida, O.; Cantarel, A.; Bernhart, G. Experimental study of polymerization and crystallization kinetics of polyamide 6 obtained by anionic ring opening polymerization of ε-caprolactam. Polymer 2017, 132, 88–97. [Google Scholar] [CrossRef]
- Zhang, J.; Chevali, V.S.; Wang, H.; Wang, C.-H. Current status of carbon fibre and carbon fibre composites recycling. Compos. Part B Eng. 2020, 193, 108053. [Google Scholar] [CrossRef]
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Compos. Part B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Marsh, G. Composites in commercial jets. Reinf. Plast. 2015, 59, 190–193. [Google Scholar] [CrossRef]
- Griffiths, B. Boeing Sets Pace for Composite Usage in Large Civil Aircraft. Available online: https://www.compositesworld.com/articles/boeing-sets-pace-for-composite-usage-in-large-civil-aircraft (accessed on 30 May 2022).
- Global Wind Energy Council. A Gust of Growth in China Makes 2020 a Record Year for Wind Energy. Available online: https://gwec.net/a-gust-of-growth-in-china-makes-2020-a-record-year-for-wind-energy/ (accessed on 1 May 2023).
- Krauklis, A.E.; Karl, C.W.; Gagani, A.I.; Jørgensen, J.K. Composite Material Recycling Technology—State-of-the-Art and Sustainable Development for the 2020s. J. Compos. Sci. 2021, 5, 28. [Google Scholar] [CrossRef]
- Beauson, J.; Laurent, A.; Rudolph, D.P.; Pagh Jensen, J. The complex end-of-life of wind turbine blades: A review of the European context. Renew. Sustain. Energy Rev. 2022, 155, 111847. [Google Scholar] [CrossRef]
- Bošnjaković, M.; Katinić, M.; Santa, R.; Marić, D. Wind Turbine Technology Trends. Appl. Sci. 2022, 12, 8653. [Google Scholar] [CrossRef]
- Ferrell, M. Why Are Floating Wind Turbines So Huge? Available online: https://undecidedmf.com/why-are-floating-wind-turbines-so-huge/ (accessed on 10 December 2022).
- Rasmussen, B. Fragments of wind turbine blades await burial at the Casper Regional Landfill in Wyoming. Bloom. Green 2020. [Google Scholar]
- Hunt, A. Jetphotos. Available online: https://www.jetphotos.com/photo/10343202 (accessed on 1 November 2023).
- Sanjay, M.; Arpitha, G.; Naik, L.L.; Gopalakrishna, K.; Yogesha, B. Applications of natural fibers and its composites: An overview. Nat. Resour. 2016, 7, 108–114. [Google Scholar] [CrossRef]
- Azwa, Z.N.; Yousif, B.F.; Manalo, A.C.; Karunasena, W. A review on the degradability of polymeric composites based on natural fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef]
- Venkateshwaran, N.; Elaya Perumal, A.; Arunsundaranayagam, D. Fiber surface treatment and its effect on mechanical and visco-elastic behaviour of banana/epoxy composite. Mater. Des. 2013, 47, 151–159. [Google Scholar] [CrossRef]
- Suddell, B.C.; Evans, W.J. Natural Fiber Composites in Automotive Applications; CRC Press: Boca Raton, FL, USA, 2005; pp. 231–259. [Google Scholar]
- Stewart, R. Automotive composites offer lighter solutions. Reinf. Plast. 2010, 54, 22–28. [Google Scholar] [CrossRef]
- Wilkinson, S. The Miraculous Mosquito: De Havilland’s versatile Wooden Wonder racked up an admirable combat record within a remarkably short timespan. In Aviation History; HistoryNet LLC: Arlington, VA, USA, 2015. [Google Scholar]
- Alfredo Rodriguez Zunino, M.N.S.T.F. Gone with the Wind: China’s Balsa Wood Consumption is Exposing Flaws in Peru’s Forest Regulations & Enforcement Regime. In Forest Trends Association; Forest Trends Association: Washington, DC, USA, 2022; p. 27. [Google Scholar]
- Shohag, M.A.S.; Hammel, E.C.; Olawale, D.O.; Okoli, O.I. Damage mitigation techniques in wind turbine blades: A review. Wind Eng. 2017, 41, 185–210. [Google Scholar] [CrossRef]
- Lee, J.-K.; Park, J.-Y.; Oh, K.-Y.; Ju, S.; Lee, J.-S. Transformation algorithm of wind turbine blade moment signals for blade condition monitoring. Renew. Energy 2015, 79, 209–218. [Google Scholar] [CrossRef]
- Argus Media Group. Ecuador Balsa Wood Exports for Wind Turbines Decline. 2022. Available online: https://www.argusmedia.com/en/news/2350426-ecuador-balsa-wood-exports-for-wind-turbines-decline (accessed on 1 November 2023).
- Borrega, M.; Ahvenainen, P.; Serimaa, R.; Gibson, L. Composition and structure of balsa (Ochroma pyramidale) wood. Wood Sci. Technol. 2015, 49, 403–420. [Google Scholar] [CrossRef]
- Toubia, E.A.; Emami, S.; Klosterman, D. Degradation mechanisms of balsa wood and PVC foam sandwich core composites due to freeze/thaw exposure in saline solution. J. Sandw. Struct. Mater. 2019, 21, 990–1008. [Google Scholar] [CrossRef]
- 3A-Composites. BALTEK®® SealX: Closing the Gap on foam Core Material; 3A Composites: Steinhausen, Switzerland, 2020. [Google Scholar]
- Corelite. CoreLite Unveils Next-Generation PC11 Pro Coating for BALSASUD®® Core. Available online: https://www.corelitecomposites.com/blogs/post/CoreLite-Unveils-Next-Generation-PC11-Pro-Coating-for-BALSASUD%C2%AE-Core (accessed on 10 December 2022).
- Gurit. Balsaflex Lite. Available online: https://gurit.com/wp-content/uploads/2023/01/Core-Lite-flyer_v1_web.pdf (accessed on 30 March 2023).
- Purnawati, R.; Febrianto, F.; J Wistara, I.N.; Nikmatin, S.; Hidayat, W.; Lee, S.H.; Kim, N.H. Physical and Chemical Properties of Kapok (Ceiba pentandra) and Balsa (Ochroma pyramidale) Fibers. J. Korean Wood Sci. Technol. 2018, 46, 393–401. [Google Scholar] [CrossRef]
- Galos, J.; Das, R.; Sutcliffe, M.P.; Mouritz, A.P. Review of balsa core sandwich composite structures. Mater. Des. 2022, 221, 111013. [Google Scholar] [CrossRef]
- PT Sibalsa Indonesia Balsa. End Grain Balsa—Sibalsa. Available online: https://sibalsa.id/end-grain-balsa/ (accessed on 1 November 2023).
- Rogers, C. Core Material Reference. Available online: https://explorecomposites.com/materials-library/core-ref/ (accessed on 30 May 2023).
- Ma, W.; Elkin, R. Sandwich Structural Composites: Theory and Practice; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Wang, Z.; Jia, C.; Xiang, H.; Zhu, M. Palladium Nanoparticle-Loaded Mesostructural Natural Woods for Efficient Water Treatment. Polymers 2023, 15, 658. [Google Scholar] [CrossRef] [PubMed]
- Ardian, M. Lighter, Stiffer & Stronger: Sandwich Composites. Available online: https://sutd-composites.blogspot.com/2023/12/lighter-stiffer-stronger-sandwich.html (accessed on 1 November 2023).
- Taylor, D. The Dawn of the Age of Plastics. Available online: https://undark.org/2019/02/22/dawn-age-plastics/ (accessed on 1 November 2023).
- ThermalCork Solutions. Cork Insulation FAQs. Available online: https://thermalcorksolutions.com/cork-insulation-faqs/ (accessed on 1 November 2023).
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Antunes, A.; Pereira, J.; Paiva, N.; Ferra, J.; Martins, J.; Carvalho, L.; Barros-Timmons, A.; Magalhães, F.D. Effects of resin content on mechanical properties of cork-based panels bound with melamine-urea-formaldehyde and polyurethane binders. Int. J. Adhes. Adhes. 2020, 101, 102632. [Google Scholar] [CrossRef]
- Sergi, C.; Sarasini, F.; Russo, P.; Vitiello, L.; Barbero, E.; Sanchez-Saez, S.; Tirillò, J. Effect of temperature on the low-velocity impact response of environmentally friendly cork sandwich structures. J. Sandw. Struct. Mater. 2021, 24, 1099–1121. [Google Scholar] [CrossRef]
- Sergi, C.; Sarasini, F.; Tirillò, J. The Compressive Behavior and Crashworthiness of Cork: A Review. Polymers 2022, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.; Lopes, P.; Mateus, N.; de Freitas, V. Cork, a Natural Choice to Wine? Foods 2022, 11, 2638. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H. The Rationale behind Cork Properties: A Review of Structure and Chemistry. BioResources 2015, 10. [Google Scholar] [CrossRef]
- Pereira, H. Introduction. In Cork; Pereira, H., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 1–3. [Google Scholar]
- APCOR. Composite Agglomerates. Available online: https://www.apcor.pt/en/cork/processing/industrial-path/composite-agglomerates/ (accessed on 1 November 2023).
- Flor-Montalvo, F.J.; Martínez-Cámara, E.; García-Alcaraz, J.L.; Jiménez-Macías, E.; Latorre-Biel, J.-I.; Blanco-Fernández, J. Environmental Impact Analysis of Natural Cork Stopper Manufacturing. Agriculture 2022, 12, 636. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Bellat, J.-P.; Gougeon, R.D.; Karbowiak, T. Mechanical properties of agglomerated cork stoppers for sparkling wines: Influence of adhesive and cork particle size. Compos. Struct. 2018, 203, 789–796. [Google Scholar] [CrossRef]
- Correia, J.M.D.; Serra, G.F.; Alves de Sousa, R.J.; Pereira, A.B.; Fernandes, F.A.O. Expanded (Black) Cork for the Development of an Eco-Friendly Surfboard: Environmental Impact and Mechanical Properties. Sustainability 2022, 14, 668. [Google Scholar] [CrossRef]
- Ptak, M.; Kaczynski, P.; Fernandes, F.A.O.; de Sousa, R.J.A. Assessing impact velocity and temperature effects on crashworthiness properties of cork material. Int. J. Impact Eng. 2017, 106, 238–248. [Google Scholar] [CrossRef]
- Sanchez-Saez, S.; García-Castillo, S.K.; Barbero, E.; Cirne, J. Dynamic crushing behaviour of agglomerated cork. Mater. Des. 2015, 65, 743–748. [Google Scholar] [CrossRef]
- Arteiro, A.; Reis, A.L.M.A.; Nóvoa, P.J.R.O.; Silva, L.F.M.; Zupan, M.; Marques, A.T. Low velocity impact and flexural performance of sandwich structures with cork and polymer foam cores. Ciência Tecnol. Dos Mater. 2013, 25, 79–84. [Google Scholar] [CrossRef]
- Castilho, T.; Sutherland, L.; Soares, C.G. Impact resistance of marine sandwich composites. In Maritime Technology and Engineering; Taylor & Francis Group: London, UK, 2015; pp. 607–618. [Google Scholar]
- Ivañez, I.; Sánchez-Saez, S.; Garcia-Castillo, S.K.; Barbero, E.; Amaro, A.; Reis, P.N.B. High-velocity impact behaviour of damaged sandwich plates with agglomerated cork core. Compos. Struct. 2020, 248, 112520. [Google Scholar] [CrossRef]
- Silva, J.M.; Devezas, T.C.; Silva, A.; Gil, L.; Nunes, C.; Franco, N. Exploring the use of Cork Based Composites for Aerospace Applications. Mater. Sci. Forum 2010, 636–637, 260–265. [Google Scholar] [CrossRef]
- Walsh, J.; Kim, H.-I.; Suhr, J. Low velocity impact resistance and energy absorption of environmentally friendly expanded cork core-carbon fiber sandwich composites. Compos. Part A Appl. Sci. Manuf. 2017, 101, 290–296. [Google Scholar] [CrossRef]
- Amorim Cork Composites. Corecork NL20 Datasheet. Available online: https://amorimcorkcomposites.com/media/4203/mds-nl20-en.pdf (accessed on 1 November 2023).
- 3A Composites. BALTEK SB Balsa TDS. Available online: https://www.3accorematerials.com/uploads/documents/TDS-BALTEK-SB-E_1106.pdf (accessed on 1 June 2023).
- Corelite. Balsasud Product Data Sheet. Available online: https://www.corelitecomposites.com/files/BALSASUD%20Core%20Data%20Sheet.pdf (accessed on 1 November 2023).
- Gurit, A.G. Gurit Balsaflex Balsa Wood Core Material Full General Datasheet. Available online: http://www.guritbalsa.com/images/documents/balsaflex/Balsaflex-TDS.pdf (accessed on 1 June 2023).
- Schmid Fuertes, T.A.; Kruse, T.; Körwien, T.; Geistbeck, M. Bonding of CFRP primary aerospace structures—Discussion of the certification boundary conditions and related technology fields addressing the needs for development. Compos. Interfaces 2015, 22, 795–808. [Google Scholar] [CrossRef]
- Dillingham, R.G. Composite bond inspection. In Structural Integrity and Durability of Advanced Composites; Beaumont, P.W.R., Soutis, C., Hodzic, A., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 695–706. [Google Scholar]
- Seemann, W.H., I; Tunis, G.C., III; Perrella, A.P.; Haraldsson, R.K.; Everitt, W.E.; Pearson, E.A. Large Composite Structures Incorporating a Resin Distribution Network. U.S. Patent 5,721,034, 24 February 1998. [Google Scholar]
- FibreGlast. Vacuum Infusion Complete Guide. Available online: https://www.fibreglast.com/product/vacuum-infusion-Guide/Learning_Center (accessed on 1 June 2023).
- Cairns, D.; Skramstad, J.; Mandell, J. Evaluation of hand lay-up and resin transfer molding in composite wind turbine blade structures. In Proceedings of the 20th 2001 ASME Wind Energy Symposium, Bozeman, MT, USA, 11–14 January 1999. [Google Scholar]
- Loos, A.C. Low-cost fabrication of advanced polymeric composites by resin infusion processes. Adv. Compos. Mater. 2001, 10, 99–106. [Google Scholar] [CrossRef]
- Modi, D.; Correia, N.; Johnson, M.; Long, A.; Rudd, C.; Robitaille, F. Active control of the vacuum infusion process. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1271–1287. [Google Scholar] [CrossRef]
- Di Tomasso, C.; József Gombos, Z.; Summerscales, J. Styrene emissions during gel-coating of composites. J. Clean. Prod. 2014, 83, 317–328. [Google Scholar] [CrossRef]
- Cortés, E.; Sánchez, F.; O’Carroll, A.; Madramany, B.; Hardiman, M.; Young, T.M. On the Material Characterisation of Wind Turbine Blade Coatings: The Effect of Interphase Coating–Laminate Adhesion on Rain Erosion Performance. Materials 2017, 10, 1146. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, M. Polymer surface treatment and coating technologies. In Handbook of Manufacturing Engineering and Technology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 99–124. [Google Scholar]
- Ellsworth-Adhesives. Webinar: How Plasma and Flame Surface Treating Improve Adhesive Bonding. 2018. Available online: https://www.ellsworth.com/resources/insights/webinars/how-plasma-and-flame-surface-treating-improve-adhesive-bonding/ (accessed on 1 May 2023).
- Zille, A.; Oliveira, F.R.; Souto, A.P. Plasma treatment in textile industry. Plasma Process. Polym. 2015, 12, 98–131. [Google Scholar] [CrossRef]
- Lewarchik, R. A Guide to Providing Initial and Long-Lasting Coating Adhesion. Available online: https://www.ulprospector.com/knowledge/10127/pc-a-guide-to-providing-perfect-coating-adhesion/ (accessed on 30 May 2023).
- Schuster, J.M.; Schvezov, C.E.; Rosenberger, M.R. Analysis of the Results of Surface Free Energy Measurement of Ti6Al4V by Different Methods. Procedia Mater. Sci. 2015, 8, 732–741. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Abe, Y. Determination of dispersive and nondispersive components of the surface free energy of glass fibers. Colloid Polym. Sci. 1989, 267, 637–642. [Google Scholar] [CrossRef]
- Cordeiro, N.; Neto, C.P.; Gandini, A.; Belgacem, M.N. Characterization of the Cork Surface by Inverse Gas Chromatography. J. Colloid Interface Sci. 1995, 174, 246–249. [Google Scholar] [CrossRef]
- Brighton Science. Common Surface Energy Tests: Dyne Inks; Brighton Science: Cinncinati, OH, USA, 2016. [Google Scholar]
- Watts, J.F. Surface Characterization and Its Role in Adhesion Science and Technology. In Handbook of Adhesion Technology; da Silva, L.F.M., Öchsner, A., Adams, R.D., Eds.; Springer International Publishing: New York, NY, USA, 2018; pp. 197–226. [Google Scholar]
- Brighton Science. Manufacturer’s Roadmap to Eliminating Adhesion Issues in Production: The Path to Overcoming Adhesion Challenges; Brighton Science: Cinncinati, OH, USA, 2022. [Google Scholar]
- Förch, R.; Schönherr, H.; Jenkins, A.T.A. Surface Design: Applications in Bioscience and Nanotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Lauren, S. Contact Angle—What Is It and How Do You Measure It? Available online: https://content.biolinscientific.com/contact-angle-what-is-it-and-how-to-measure-it (accessed on 15 October 2023).
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- CR—For Krüss GmbH. So You Want to Measure Surface Energy? In Models for Surface Free Energy Calculation; Krüss GmbH: Hamburg, Germany, 1999. [Google Scholar]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Wu, S. Calculation of interfacial tension in polymer systems. J. Polym. Sci. Part C: Polym. Symp. 1971, 34, 19–30. [Google Scholar] [CrossRef]
- FT—For Krüss GmbH. Practical Contact Angle Measurement (5). Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjl8NuQmL6DAxV4slYBHZpCA_QQFnoECAoQAQ&url=http%3A%2F%2Fing.univaq.it%2Ffioravanti%2Fchimica%2FCorso%2520Chimica%2520delle%2520Superfici%2520ed%2520Interfasi%2FLaboratorio%2FReferences%2520laboratorio%2FKruss_01.pdf&usg=AOvVaw1wmShPZb3EQn5hMC4LsPL5&opi=89978449 (accessed on 15 October 2023).
- Young, T. III. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Brighton Science. What Is the Difference between Surface Free Energy and Surface Energy? Available online: https://www.brighton-science.com/blog/what-is-the-difference-between-surface-free-energy-and-surface-energy (accessed on 30 May 2023).
- Liu, L.; Huang, Y.D.; Zhang, Z.Q.; Jiang, Z.X.; Wu, L.N. Ultrasonic treatment of aramid fiber surface and its effect on the interface of aramid/epoxy composites. Appl. Surf. Sci. 2008, 254, 2594–2599. [Google Scholar] [CrossRef]
- Jia, C.; Chen, P.; Liu, W.; Li, B.; Wang, Q. Surface treatment of aramid fiber by air dielectric barrier discharge plasma at atmospheric pressure. Appl. Surf. Sci. 2011, 257, 4165–4170. [Google Scholar] [CrossRef]
- Gindl, M.; Tschegg, S. Significance of the Acidity of Wood to the Surface Free Energy Components of Different Wood Species. Langmuir 2002, 18, 3209–3212. [Google Scholar] [CrossRef]
- Pukánszky, B.; Fekete, E. Adhesion and Surface Modification. In Mineral Fillers in Thermoplastics I: Raw Materials and Processing; Jancar, J., Fekete, E., Hornsby, P.R., Jancar, J., Pukánszky, B., Rothon, R.N., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 109–153. [Google Scholar]
- Lee, Y.-S.; Lee, B.-K. Surface properties of oxyfluorinated PAN-based carbon fibers. Carbon 2002, 40, 2461–2468. [Google Scholar] [CrossRef]
- Abenojar, J.; Barbosa, A.Q.; Ballesteros, Y.; del Real, J.C.; da Silva, L.F.M.; Martínez, M.A. Effect of surface treatments on natural cork: Surface energy, adhesion, and acoustic insulation. Wood Sci. Technol. 2014, 48, 207–224. [Google Scholar] [CrossRef]
- Gomes, C.M.C.P.S.; Fernandes, A.C.; de Almeida, B.d.J.V.S. The Surface Tension of Cork from Contact Angle Measurements. J. Colloid Interface Sci. 1993, 156, 195–201. [Google Scholar] [CrossRef]
- Silva, E.A.; Oliveira, F.R.; Carmo, S.N.; Steffens, F.; Souto, A.P. Characterization of natural cork agglomerate functionalised by plasma treatment. In Proceedings of the International Conference on Natural Fibers, Guimarães, Portugal, 9–11 June 2013. [Google Scholar]
- Park, S.-J.; Jin, J.-S. Effect of silane coupling agent on mechanical interfacial properties of glass fiber-reinforced unsaturated polyester composites. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 55–62. [Google Scholar] [CrossRef]
- Lancaster, J.F. 3—The use of adhesives for making structural joints. In Metallurgy of Welding, 6th ed.; Lancaster, J.F., Ed.; Woodhead Publishing: Sawston, UK, 1999; pp. 54–84. [Google Scholar]
- Thomas, R.; Sinturel, C.; Thomas, S.; Sadek El Akiaby, E.M. Introduction, in Micro- and Nanostructured Epoxy/Rubber Blends; Wiley-VCH: Weinheim, Germany, 2014; pp. 1–30. [Google Scholar]
- Bellmann, C.; Caspari, A.; Albrecht, V.; Doan, T.T.L.; Mäder, E.; Luxbacher, T.; Kohl, R. Electrokinetic properties of natural fibres. Colloids Surf. A Physicochem. Eng. Asp. 2005, 267, 19–23. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.S.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Ferreira, D.P.; Cruz, J.; Fangueiro, R. Chapter 1—Surface modification of natural fibers in polymer composites. In Green Composites for Automotive Applications; Koronis, G., Silva, A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 3–41. [Google Scholar]
- Enercon Industries Corp. Corona vs. Plasma. Available online: https://www.enerconind.com/plasma-treating/library/tech-papers-articles/corona-vs-plasma.aspx (accessed on 30 May 2023).
- Petersen, H.N. Investigation of Sizing—From Glass Fibre Surface to Composite Interface; Technical University of Denmark, Kongens: Lyngby, Denmark, 2017. [Google Scholar]
- Tiwari, S.; Bijwe, J. Surface treatment of carbon fibers-a review. Procedia Technol. 2014, 14, 505–512. [Google Scholar] [CrossRef]
- Park, S.-J.; Meng, L.-Y. Surface Treatment and Sizing of Carbon Fibers. In Carbon Fibers; Springer: Dordrecht, The Netherlands, 2015; pp. 101–133. [Google Scholar]
- Thomason, J.L. Glass fibre sizing: A review. Compos. Part A Appl. Sci. Manuf. 2019, 127, 105619. [Google Scholar] [CrossRef]
- Seboka, N. Jute reinforced PLA bio composite for the production of ceiling fan blades. Indian Text. J. 2021, 74–82. [Google Scholar]
- Godara, S.S. Effect of chemical modification of fiber surface on natural fiber composites: A review. Mater. Today Proc. 2019, 18, 3428–3434. [Google Scholar]
- Faruk, O.; Bledzki, A.K.; Fink, H.-P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Ben Abdallah, F.; Ben Cheikh, R.; Baklouti, M.; Denchev, Z.; Cunha, A.M. Effect of surface treatment in cork reinforced composites. J. Polym. Res. 2010, 17, 519–528. [Google Scholar] [CrossRef]
- Tran, M.Q.; Ho, K.K.C.; Kalinka, G.; Shaffer, M.S.P.; Bismarck, A. Carbon fibre reinforced poly(vinylidene fluoride): Impact of matrix modification on fibre/polymer adhesion. Compos. Sci. Technol. 2008, 68, 1766–1776. [Google Scholar] [CrossRef]
- He, J.M.; Huang, Y.D. Effect of silane-coupling agents on interfacial properties of CF/PI composites. J. Appl. Polym. Sci. 2007, 106, 2231–2237. [Google Scholar] [CrossRef]
- Bassyouni, M.; Gutub, S.A. Materials Selection Strategy and Surface Treatment of Polymer Composites for Wind Turbine Blades Fabrication. Polym. Polym. Compos. 2013, 21, 463–472. [Google Scholar] [CrossRef]
- Barnes, B.K.; Ouro-Koura, H.; Derickson, J.; Lebarty, S.; Omidokun, J.; Bane, N.; Suleiman, O.; Omagamre, E.; Fotouhi, M.J.; Ogunmolasuyi, A. Plasma generation by household microwave oven for surface modification and other emerging applications. Am. J. Phys. 2021, 89, 372–382. [Google Scholar] [CrossRef]
- Nehra, V.; Kumar, A.; Dwivedi, H. Atmospheric non-thermal plasma sources. Int. J. Eng. 2008, 2, 53–68. [Google Scholar]
- Henniker-Plasma. Let’s Talk About… How Long Plasma Treatment Lasts? Henniker Plasma. 2020. Available online: https://plasmatreatment.co.uk/knowledge-base/knowledge-articles/142-let-s-talk-about-how-long-plasma-treatment-lasts (accessed on 27 December 2022).
- Takeda, T.; Yasuoka, T.; Hoshi, H.; Sugimoto, S.; Iwahori, Y. Effectiveness of flame-based surface treatment for adhesive bonding of carbon fiber reinforced epoxy matrix composites. Compos. Part A Appl. Sci. Manuf. 2019, 119, 30–37. [Google Scholar] [CrossRef]
- Nasiru, M.M.; Frimpong, E.B.; Muhammad, U.; Qian, J.; Mustapha, A.T.; Yan, W.; Zhuang, H.; Zhang, J. Dielectric barrier discharge cold atmospheric plasma: Influence of processing parameters on microbial inactivation in meat and meat products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2626–2659. [Google Scholar] [CrossRef] [PubMed]
- Fröhling, A.; Durek, J.; Schnabel, U.; Ehlbeck, J.; Bolling, J.; Schlüter, O. Indirect plasma treatment of fresh pork: Decontamination efficiency and effects on quality attributes. Innov. Food Sci. Emerg. Technol. 2012, 16, 381–390. [Google Scholar] [CrossRef]
- Ha, J.-W.; Kang, D.-H. Simultaneous near-infrared radiant heating and UV radiation for inactivating Escherichia coli O157: H7 and Salmonella enterica serovar Typhimurium in powdered red pepper (Capsicum annuum L.). Appl. Environ. Microbiol. 2013, 79, 6568–6575. [Google Scholar] [CrossRef]
- Guan, W.; Fan, X.; Yan, R. Effects of UV-C treatment on inactivation of Escherichia coli O157: H7, microbial loads, and quality of button mushrooms. Postharvest Biol. Technol. 2012, 64, 119–125. [Google Scholar] [CrossRef]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, V.M.; Jubinville, E.; Rodríguez-López, M.I.; Trudel-Ferland, M.; Bouchard, S.; Jean, J. Inactivation of foodborne viruses by UV light: A review. Foods 2021, 10, 3141. [Google Scholar] [CrossRef] [PubMed]
- Kohli, R. Chapter 9—Applications of UV-Ozone Cleaning Technique for Removal of Surface Contaminants. In Developments in Surface Contamination and Cleaning: Applications of Cleaning Techniques; Kohli, R., Mittal, K.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 355–390. [Google Scholar]
- Vig, J.R. UV/ozone cleaning of surfaces. J. Vac. Sci. Technol. A 1985, 3, 1027–1034. [Google Scholar] [CrossRef]
- Takacs, G.A.; Miri, M.J.; Kovach, T. Vacuum UV Surface Photo-Oxidation of Polymeric and Other Materials for Improving Adhesion: A Critical Review. Prog. Adhes. Adhes. 2021, 6, 559–585. [Google Scholar]
- Strobel, M.; Jones, V.; Lyons, C.S.; Ulsh, M.; Kushner, M.J.; Dorai, R.; Branch, M.C. A comparison of corona-treated and flame-treated polypropylene films. Plasmas Polym. 2003, 8, 61–95. [Google Scholar] [CrossRef]
- Flynn Burner Corp. Flame Plasma Surface Treating—What’s it All About. In Proceedings of the Tappi PLACE, St. Louis, MO, USA, 16–19 September 2007. [Google Scholar]
- Farris, S.; Pozzoli, S.; Biagioni, P.; Duó, L.; Mancinelli, S.; Piergiovanni, L. The fundamentals of flame treatment for the surface activation of polyolefin polymers—A review. Polymer 2010, 51, 3591–3605. [Google Scholar] [CrossRef]
- Strobel, M.; Branch, M.C.; Ulsh, M.; Kapaun, R.S.; Kirk, S.; Lyons, C.S. Flame surface modification of polypropylene film. J. Adhes. Sci. Technol. 1996, 10, 515–539. [Google Scholar] [CrossRef]
- Bárdos, L.; Baránková, H. Plasma processes at atmospheric and low pressures. Vacuum 2008, 83, 522–527. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Thierry Corp. Atmospheric Plasma vs. Low Pressure Plasma. Available online: https://www.thierry-corp.com/plasma-knowledgebase/atmospheric-plasma-vs-low-pressure-plasma (accessed on 30 May 2023).
- Zhang, W.; Yang, P.; Cao, Y.; Yu, P.; Chen, M.; Zhou, X. Evaluation of fiber surface modification via air plasma on the interfacial behavior of glass fiber reinforced laminated veneer lumber composites. Constr. Build. Mater. 2020, 233, 117315. [Google Scholar] [CrossRef]
- Richardson, M.O.W.; Wisheart, M.J. Review of low-velocity impact properties of composite materials. Compos. Part A Appl. Sci. Manuf. 1996, 27, 1123–1131. [Google Scholar] [CrossRef]
- Vadakke, V.; Carlsson, L.A. Experimental investigation of compression failure of sandwich specimens with face/core debond. Compos. Part B Eng. 2004, 35, 583–590. [Google Scholar] [CrossRef]
- Vitale, J.P.; Francucci, G.; Xiong, J.; Stocchi, A. Failure mode maps of natural and synthetic fiber reinforced composite sandwich panels. Compos. Part A Appl. Sci. Manuf. 2017, 94, 217–225. [Google Scholar] [CrossRef]
- Zhu, S.; Chai, G.B. Effect of adhesive in sandwich panels subjected to low-velocity impact. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2011, 225, 171–181. [Google Scholar] [CrossRef]
- Goswami, S.; Becker, W. The effect of facesheet/core delamination in sandwich structures under transverse loading. Compos. Struct. 2001, 54, 515–521. [Google Scholar] [CrossRef]
- Abrate, S. Impact on Laminated Composite Materials. Appl. Mech. Rev. 1991, 44, 155–190. [Google Scholar] [CrossRef]
- Kueh, A.B.H.; Abo Sabah, S.H.; Qader, D.N.; Drahman, S.H.; Amran, M. Single and repetitive low-velocity impact responses of sandwich composite structures with different skin and core considerations: A review. Case Stud. Constr. Mater. 2023, 18, e01908. [Google Scholar] [CrossRef]
- Burlayenko, V.N.; Sadowski, T. Linear and Nonlinear Dynamic Analyses of Sandwich Panels with Face Sheet-to-Core Debonding. Shock Vib. 2018, 2018, 5715863. [Google Scholar] [CrossRef]
- Ismail, K.I.; Sultan, M.T.H.; Shah, A.U.M.; Jawaid, M.; Safri, S.N.A. Low velocity impact and compression after impact properties of hybrid bio-composites modified with multi-walled carbon nanotubes. Compos. Part B Eng. 2019, 163, 455–463. [Google Scholar] [CrossRef]
- Cantwell, W.J.; Morton, J. The impact resistance of composite materials—a review. Composites 1991, 22, 347–362. [Google Scholar] [CrossRef]
- Robinson, P.; Davies, G. Impactor mass and specimen geometry effects in low velocity impact of laminated composites. Int. J. Impact Eng. 1992, 12, 189–207. [Google Scholar] [CrossRef]
- Davies, G.; Robinson, P. Predicting failure by debonding/delamination. In Debonding/Delamination of Composites 28 p (SEE N 93-21507 07-24); AGARD: Gárdony, Hungary, 1992. [Google Scholar]
- Ismail, M.F.; Sultan, M.T.H.; Hamdan, A.; Shah, A.U.M.; Jawaid, M. Low velocity impact behaviour and post-impact characteristics of kenaf/glass hybrid composites with various weight ratios. J. Mater. Res. Technol. 2019, 8, 2662–2673. [Google Scholar] [CrossRef]
- Adams, D. Impact Testing of Composite Materials. 2012. Available online: https://www.compositesworld.com/articles/impact-testing-of-composite-materials (accessed on 1 September 2023).
- ASTM International. ASTM D7136/D7136M-15; ASTM D7136/D7136M-15 Standard Test Method for Measuring the Damage Resistance of a Fiber-Reinforced Polymer Matrix Composite to a Drop-Weight Impact Event. American Society for Testing Materials: West Conshohocken, PA, USA, 2015.
- ASTM International. ASTM D7766/D7766M-16; ASTM D7766/D7766M-16 Standard Practice for Damage Resistance Testing of Sandwich Constructions. American Society for Testing Materials: West Conshohocken, PA, USA, 2016.
- Mitrevski, T.; Marshall, I.H.; Thomson, R.; Jones, R.; Whittingham, B. The effect of impactor shape on the impact response of composite laminates. Compos. Struct. 2005, 67, 139–148. [Google Scholar] [CrossRef]
- Mitrevski, T.; Marshall, I.H.; Thomson, R. The influence of impactor shape on the damage to composite laminates. Compos. Struct. 2006, 76, 116–122. [Google Scholar] [CrossRef]
- Abdalslam, S. Impact Damage Analysis of Balsa Wood Sandwich Composite Materials. Master’s Thesis, Graduate School of Wayne State University, Detroit, MI, USA, 2013. [Google Scholar]
- Atas, C.; Sevim, C. On the impact response of sandwich composites with cores of balsa wood and PVC foam. Compos. Struct. 2010, 93, 40–48. [Google Scholar] [CrossRef]
- Daniel, I.M.; Abot, J.L.; Schubel, P.M.; Luo, J.J. Response and Damage Tolerance of Composite Sandwich Structures under Low Velocity Impact. Exp. Mech. 2012, 52, 37–47. [Google Scholar] [CrossRef]
- Kelly, J.; Hoyns, D. Effect of Impact on the Compression Response of Large Composite Sandwich Plates; Woodhead Publishing: Sawston, UK, 2000; pp. 330–336. [Google Scholar]
- Ozdemir, O.; Oztoprak, N.; Kandas, H. Single and repeated impact behaviors of bio-sandwich structures consisting of thermoplastic face sheets and different balsa core thicknesses. Compos. Part B Eng. 2018, 149, 49–57. [Google Scholar] [CrossRef]
- Cantwell, W.; Davies, P. A study of skin-core adhesion in glass fibre reinforced sandwich materials. Appl. Compos. Mater. 1996, 3, 407–420. [Google Scholar] [CrossRef]
- Dimassi, M.A.; John, M.; Herrmann, A.S. Investigation of the temperature dependent impact behaviour of pin reinforced foam core sandwich structures. Compos. Struct. 2018, 202, 774–782. [Google Scholar] [CrossRef]
- Ostapiuk, M.; Bieniaś, J.; Surowska, B. Analysis of the bending and failure of fiber metal laminates based on glass and carbon fibers. Sci. Eng. Compos. Mater. 2018, 25, 1095–1106. [Google Scholar] [CrossRef]
- Pärnänen, T.; Kanerva, M.; Sarlin, E.; Saarela, O. Debonding and impact damage in stainless steel fibre metal laminates prior to metal fracture. Compos. Struct. 2015, 119, 777–786. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Wang, H. Research Progress on Interlaminar Failure Behavior of Fiber Metal Laminates. Adv. Polym. Technol. 2020, 2020, 3097839. [Google Scholar] [CrossRef]
- Ferro, C. Wind Turbine Propellers Boost Balsawood Sales. Available online: https://en.unav.edu/web/global-affairs/las-helices-de-los-aerogeneradores-disparan-las-ventas-de-madera-de-balsa. (accessed on 30 May 2023).
- Milne, A.; Amirfazli, A. The Cassie equation: How it is meant to be used. Adv. Colloid Interface Sci. 2012, 170, 48–55. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Chaudhury, M.K.; Good, R.J. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Zisman, W.A. Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution. In Contact Angle, Wettability, and Adhesion, Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1964; Volume 43, pp. 1–51. [Google Scholar]





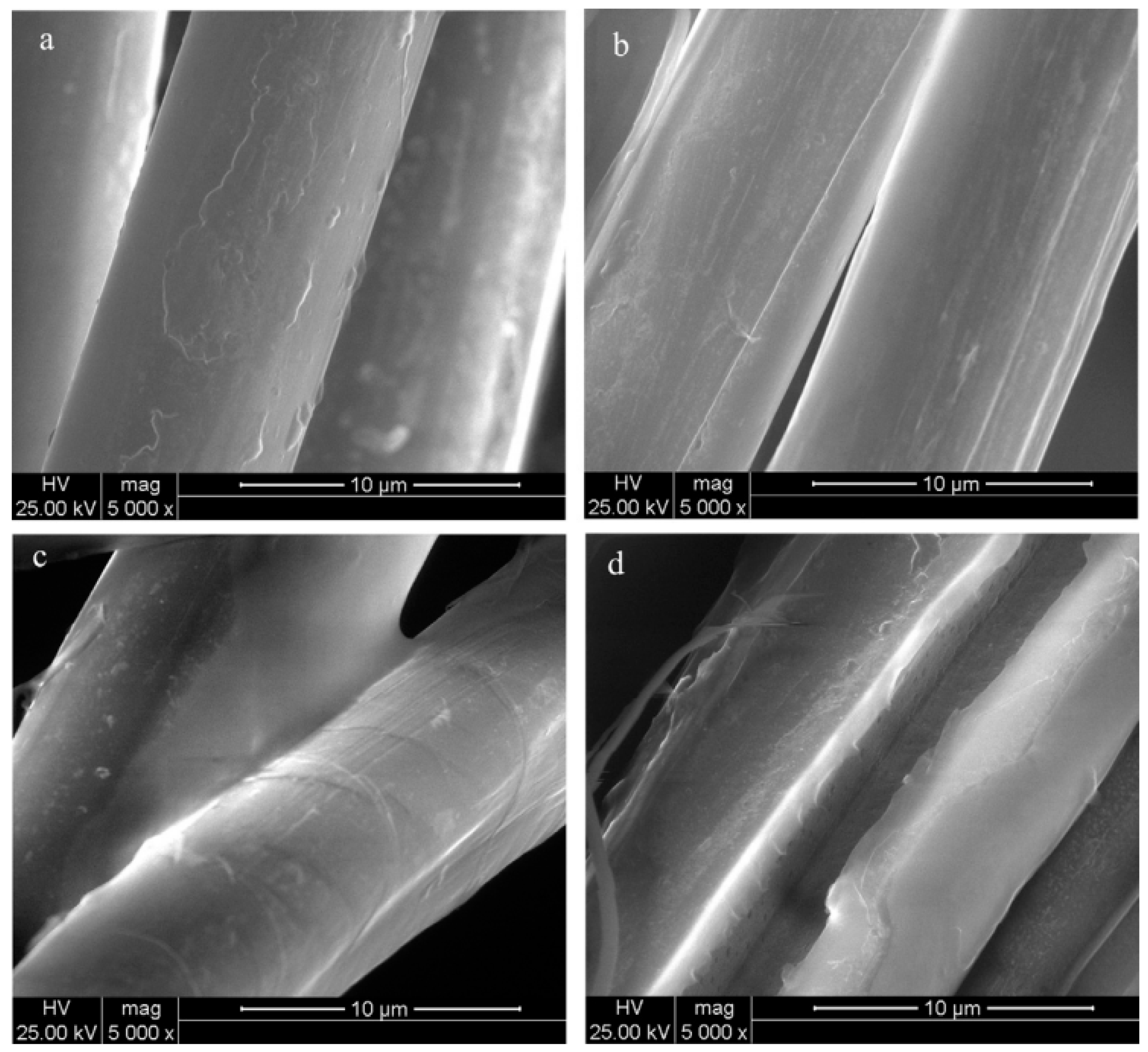


| Coating | Baltek Al600/10 | Baltek SealX | Balsaflex Lite | Corelite PC11 | Corelite PC11 Pro |
|---|---|---|---|---|---|
| Type of Panel | FP 25 mm thk | RP, thickness not given | Balsaflex 150 155 kg/m3 FP 25 mm thk | “Typical balsa sheet of non-coated wood core”, FP, thk NG | |
| Untreated balsa Resin uptake | 3500 g/m2 | Not given | 3800 g/m2 | 1900 g/m2 | 1900 g/m2 |
| Treated balsa resin uptake | 1800 g/m2 | Not given | 2100 g/m2 | 1020 g/m2 | 450 g/m2 |
| Claimed max % reduction | 50% | 80% | 40% | 46% | 76% |
| Amorim Corecork | Corelite | 3AComposites | Gurit Balsaflex | |||||
|---|---|---|---|---|---|---|---|---|
| Grade | Standard | NL10 | NL20 | Balsasud UL | Baltek SB 100 | 110 | 150 | |
| Property/Unit | ||||||||
| Uncured Properties | ||||||||
| Density/kgm−3 | # ISO 7322 [61], * ASTM C271 [62], ! NS | 120–180 # | 170–235 # | 100 * | 148 * | 110–125, avg 110 ! | 135–176, avg 155 ! | |
| Resin Uptake/kgm−3 | - | 270 | 170 | NG | NG | NG | NG | |
| Compressive Strength/MPa | # ASTM C365 [63], * ISO 844 [64] | 0.3 # | 0.5 # | 6.53 * | 9.2 * | 8.3 * | 13.0 * | |
| Comp. Modulus/MPa | ASTM C365 | 5.1 | 6 | 2036 | 2526 * | 2130 # | 3518 # | |
| Shear Strength/MPa | ASTM C273 [62] | 0.9 | 0.9 | 1.88 | 2.6 | 2.0 # | 2.8 # | |
| Shear Modulus/MPa | ASTM C273 | 5.9 | 5.9 | 110 | 187 | 103 # | 163 # | |
| Tensile Strength/MPa | ISO 7322 | >0.2 # | >0.4 | 7.5 # | NG | NG | NG | |
| Tensile Modulus/MPa | ASTM C297 | NG | NG | 2238 | NG | NG | NG | |
| CTE @ RT/10−6 per °C | ASTM E831-06 [65] | 110 | 110 | NG | NG | NG | NG | |
| Thermal Conductivity W/m.K | ISO 8301 | 0.0408 | 0.0507 | 0.048 * | 0.066 * | NG | NG | |
| Cured Properties | ||||||||
| Resin absorption, g/m2 mm | - | 270 | 170 | NG | NG | NG | NG | |
| Moisture Absorption % | ASTM C272 [66] | <4 | <4 | NG | NG | NG | NG | |
| Method | Processes | Total No. of Processes | Joining Mechanism |
|---|---|---|---|
| Co curing | 2 fiber facesheets and core cured in the same mold, in the same process | 1 | Chemical cross-linking |
| Co bonding | 1 facesheet and core cured together, another facesheet cured separately, then both joined together with adhesives | 3 | Adhesive bonding |
| Secondary bonding | Each core and facesheet is cured separately, then 3 joined together with adhesives. | 4 | Adhesive bonding |
| Wettability Classification | Contact Angle and Remarks |
|---|---|
| Strongly Hydrophilic | Close to 0° |
| Less Strong hydrophilic | ≤90° |
| Hydrophobic | 90–150° |
| Superhydrophobic | Above 150°. Water droplets simply rest on the surface without actually wetting to any significant extent. |
| Equation Name | Equation | Remarks |
|---|---|---|
| Extended Fowkes/OWRK Equation [82,83,84] | where:
| Also referred to as the Owens–Wendt–Rable–Kaelble (OWRK) or geometric mean method. Requires a minimum of 1 polar and 1 dispersive fluid. Mathematically equivalent to the Owens Wendt method, but only requires a minimum of 2 liquid measurements to find the polar and dispersive components of the solid’s surface free energy. |
| Fowkes Equation [84,85] | Based on Young’s Equation Fowkes defines γSL by the equation below: The Extended Fowkes method is typically used in practical calculations. | Fowkes equation interprets the dispersive and polar solid-liquid interactions as the geometric mean of the dispersive (nonpolar) and polar component of surface free energy, without elaborating in more detail. |
| Owens Wendt Equation [83,84] | Droplet measurements of at least 5 different liquids are needed. Plot a best fit linear graph of , using the droplet measurements. Surface Free Energy polar and dispersive Components can be found by using the best fit line Gradient , and y intercept | Mathematically equivalent to the Extended Fowkes Equation shown above. Based on Good’s & Young’s Equation. Requires a minimum of 5 different liquids. Extended Fowkes is often preferred over Owens Wendt as it requires much less experimental data collection (2 vs. 5 liquids). |
| Wu Equation [82,86] | where:
| Also referred to as the harmonic mean method. Based on Wu’s findings where OWRK method underestimated the polar interactions in molten polymers. Requires a minimum of 1 polar and 1 dispersive fluid. Wu equation is best used for polymers with low surface free energies of up to 30–40 mN/m. |
| Young’s Equation [84,87,88] | where:
| Valid for 3 phase systems in thermodynamic equilibrium for ideal (smooth and chemically homogenous) solids and pure liquids. Not to be confused for the Young Laplace fit, which describes the sustained capillary pressure difference at the interface between two static fluids. |
| Substrate Materials (Who) | (mN/m) | (mN/m) | γS (mN/m) |
|---|---|---|---|
| Aramid Fibers % [90] | 34.3 | 8.5 | 42.8 |
| Aramid (Twaron) Fibers ! [91] | 39.1 | 11.5 | 50.6 |
| Balsa [92] | 42.33 | 1.73 | 44.07 |
| CaCO3 $ [93] | 54.5 | 153.4 | 207.9 |
| Carbon Fiber, PAN based [94] | 21.4 | 0.0 | 21.4 |
| Cork ! [95]—OWRK method on cork, likely agglomerate sheet | 18 | ~0 | 18 |
| Cork ! [96]—First Quality Cork, measurements taken on surface perpendicular to tangential direction, taken at 24 °C | 25 (GM) 22 (HM) | 6 (GM) 11 (HM) | 31 (GM) 33 (HM) |
| Cork $ [77]—Cork powder (Champcork Company) ground to 25 mesh, type of cork not specified | 38 @ 40 °C 35 @ 50 °C 34 @ 60 °C 31 @ 70 °C | Not mentioned in paper | Not Applicable |
| Cork ! [97]—Cork agglomerate laminated with a polymer membrane | 7.4 | 3.5 | 10.9 |
| E-Glass Fibers ! [98]—E-glass fibers surface treated with silane coupling agent γ-methacryloxypropyltrimethoxysilane | 31.5 @ 0.0% 29 @ 0.1% 28.5 @ 0.2% 28 @ 0.3% ~27.5 @ 0.4% 27.5 @ 0.5% 31.5 @ 0.8% | 2.5 @ 0.0% 9.5 @ 0.1% 11 @ 0.2% 12.5 @ 0.3% 15 @ 0.4% 14 @ 0.5% 5 @ 0.8% | 34 @ 0.0% 38.5 @ 0.1% 39.5 @ 0.2% 40.5 @ 0.3% 43 @ 0.4% 42 @ 0.5% 5 @ 0.8% |
| E-Glass Fibers [98]—untreated | 26 @ 0.0% | 5 | 31.5 |
| E-Glass Fibers [98]—PVA sizing | 26.5 | 7 | 33.5 |
| E-Glass Fibers [98]—polyester sizing | 24 | 13 | 37 |
| E-Glass Fibers [98]—epoxy sizing | 29.5 | 9.5 | 28 |
| PET bulk material [99] | 38.4 | 2.7 | 41.1 |
| PMMA ! bulk material [93] | 34.3 | 5.8 | 40.1 |
| PP ! bulk material [93] | 32.5 | 0.9 | 33.4 |
| PVC ! bulk material [99] | 39.7 | 2.2 | 41.9 |
| SiO2 # [93] | 94.7 | 163.0 | 257.7 |
| Talc # [93] | 49.3 | 90.1 | 139.4 |
| Typical Reagents | Main Purpose | Remarks | |
|---|---|---|---|
| Acetylation [103] | Acetyl groups (CH3COO−) | Improve adhesion to nonpolar matrix materials (e.g., PP) by increasing hydrophobicity—replace polar OH groups near cell wall with less polar acetyl groups | |
| Alkali Treatments [103] | NaOH |
Increase number of reactive sites | OH functional groups on fibers replaced with O-Na groups |
| Benzoylation [102] | Benzoyl chloride (C6HCOCl) | Decrease hydrophilicity and moisture absorption of natural fibers—replace polar OH groups near cell wall with less polar benzoyl groups | Normally used in conjunction with alkali treatment |
| Enzyme [111] | Various Enzymes | Enzymes digest waxy layer, modify fiber itself. | Tensile strength increases of 5–45% reported in abaca fibers. |
| Graft Copolymerization [102] | Various | Grafting of monomers onto cellulose backbone of natural fibers, such as vinyl and allyl ethers | |
| Isocyanate [102] | −N=C=O functional group | Acts as a coupling agent—Forms strong covalent bond with hydroxyl groups in cellulose and lignins | |
| Maleated coupling [111] | Maleic anhydride | Modifies both fiber surface and polymeric matrix to improve interfacial bonding and mechanical properties. | Often used on natural fibers |
| Permanganate treatment [102] | KMnO4 solution in acetone | MnO3 ion formation leads to cellulose radical, boosts adhesion through free radical reactions with OH groups. | |
| Silanization [102] | Silane (SiH4) compounds | Silane acts as a coupling agent—which binds to matrix as well as hydroxyl groups in the fiber. | Commonly used on glass and natural fibers |
| Fiber/Core/Who | Treatment Method | Key findings |
|---|---|---|
| Cork sheets 200 × 100 × 12 mm * [95] | Silanization: dip cork in silane solution (1% aminopropyltriethoxysilane in 50 vol% ethanol/water solution adjusted to pH 8) for 30 min, then use compressed air to remove fluid and the dry cork for 3 h in oven. Fabrication—secondary bonding with epoxy. | Treated vs. untreated cork: Reduced total (10 vs. 18 mN/m) and dispersive (9 vs. 18 mN/m) surface energy. Peel test strength—13% lower. Increased Young’s modulus and reduced damping. |
| Cork granules [112] | Silanization: immerse in silane-methanol or silane-acetone solution for 30 min, then dry in the oven at 60 °C for 3 h. Then mix 5 wt% with PP and extrude | Treated vs. untreated: Contact angle: increase by 15° (methanol) and 20° (acetone). Charpy (ASTM D6110) impact energy: No change. Tensile stress: +6% |
| Cork granules [112] | Alkaline treatment with NaOH: immerse cork granules for 1 h in NaOH-distilled water of varying concentrations. Then mix 5 wt% with PP and extrude | Treated vs. untreated: Contact angle: <1° change in both polar and dispersive, Mechanical properties: tensile strength—4–6%, tensile modulus—4–8% |
| Carbon Fiber [113] | Oxidation treatment: Immerse fibers for 5 h in boiling HNO3 solution | Increase in surface oxygen and nitrogen content and increased surface energy of fibers |
| Carbon Fiber [114] | Silanization: Soak for 8 h in 1 mMol silane solution for 8 h at room temp, then wash off surface residues with deionized water, then dry at 110 °C for 10 min, then cool in vacuum desiccator | |
| Jute Fabric [109] | Alkali + Silane: Fabric dipped in 5% NaOH solution for 1 h, then wash with 1% HCl solution for neutralization then wash with distilled water. Then dried in oven at 70 °C for 3 h. Then soak fibers for 45 min in solution of 0.5 wt% silane coupling agent [3-(2-aminoethyl amino) propyl trimethoxysilane] in acetone. | Laminates made from Alkali + Silane treated fibers exhibited 25% higher ILSS than laminates with untreated fibers |
| Glass and Carbon Fibers [115] | Silanization Surface Treatment and fabrication process:
| Treated vs. untreated cured laminates: Glass Fiber: 8–10% higher Tensile strength and Young’s modulus. Attributed to the primary amine in APS Silane reacting with epoxy, plus formation of H-bonds between silanol group and oxide surfaces in silica, which leads to M-O-Si oxane bond with the fiber. Carbon Fiber: Decreases of up to 17% in tensile strength. Attributed to the absence of reactivity and oxane bond formation between silane and CF. |
| Name | Reactions | Description |
|---|---|---|
| Etching | AB + Csolid → A + BCvapour | Material erosion |
| Adsorption | Mg + S → Ms Rg − S → Rs | Molecules (Mg) or radicals (Rg) from a plasma come in contact with a surface (S) exposed to the plasma and are adsorbed on surfaces |
| Deposition | AB → A + Bsolid | Thin film formation |
| Recombination | S − A + A → S + A2 S − R + R1 → S + M | Atoms (A) or radicals (R) from the plasma can react with the species already adsorbed on the surface to combine and form a compound. |
| Metastable de-excitation | S + A * → A | Excited species (denoted by *) collide with a solid surface and return to the ground state. |
| Sputtering | S − B + A+ → S+ + B+ + A | Positive ions accelerated from the plasma towards the surface with sufficient energy can remove an atom from the surface. |
| Polymerization | Rg + Rs → Ps Mg + Rs → Ps | Radicals in the plasma (Rg) can react with radicals adsorbed on the surface (Rs) and form polymers (Ps). |
| LPPC | Atmospheric Plasma, Corona, and DBD | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Aspect | Metals | Fiber Composites |
|---|---|---|
| Detectability | Easy to detect as damage is typically starts on the impacted surface [142] | Difficult to detect. This is often referred to as Barely Visible Impact Damage (BVID) as damage often occurs as internal delamination or on non-impacted surface but rarely shows on impacted surface. |
| Energy absorption by plastic deformation | High due to ductility of metal. Often large strains of >10% at constant yield stress before work hardening occurs. | Low. Most composites are brittle, thus cannot absorb much energy by plastic deformation. |
| Who/Fabricated Sample | Impact Test Method | Key Findings (U—Unimpacted) |
|---|---|---|
| [56] SB under pressure, epoxy adhesive, FSNT 10.5 mm
| ASTM D7136, Imatek DWT Drop height 0.8 m, impact velocity 4 ms−1, Impact energy 23 J Also done: ASTM C393 4PB | Cork has better impact performance than PMI foam: Cork (vs. PMI foam): Impactor rebounds (versus no rebound) and higher peak force levels (3 vs. 2kN)—indicating cork shows rapid response to transient loads. Much less visible damage area—slight superficial dimple (vs. perforation of core material and destruction of core foam). |
| [53] SB: Compress with weights without vacuum bagging, SikaForce 7888 L10 (VP) PU adhesive, FSNT 15 mm
| NS, Rosand IFW 5 HV DWT Specimen size 60 × 60 mm2 Impact energies: 10, 15, 20, 25, 30, 40 J Also done: ASTM C393 4PB | Flexural: Both cork cores exhibit lower flexural stiffness than thermoplastic foams Impact: Cork has less damage than thermoplastic foams
|
| [54] Method: Co-cured vacuum bagging, FSNT: 35.53–36.97 mm
| ASTM D7136, Rosand IFW 5 HV Impact energies: 50, 100, 150, 200, 300 J Specimen size 150 × 150 mm2 Also done:
|  NL20 shows complete separation of non-impacted facesheet during impact, likely due to higher strain. Cork laminates have potential for applications with impact requirements, with the downside of lower stiffness and higher weight than balsa and thermoplastic foams. Both NL20 and Balsa show debonding at the non-impacted facesheet and core. Correlation of QSI and impact tests: PVC, NL20, and Balsa behave similarly: QSI max force is 150% of max force during LVI. NL10—QSI max force 66% of impact max load. |
| [161] Method: Co-cured VARI
| NS, Custom drop-weight machine, HI 25.4 mm (mass not given). Flat sandwich panels of size 330 × 200 mm clamped at 2 edges with steel bars Impact energies—39 and 53 J Post LVI, C-scan, and visual inspection of panels to detect damage. | High energy absorption and rebound effect of cork significantly reduces energy absorbed by the rest of the structure. Damage: VSD—Visible Small dent, VFR—Visible Facesheet Rupture, PGD—permanent global deformation, CSC—Core shear cracks, NC—No core shear cracks detected visually or by C-scan  |
| [55] Comparison of composite structures subjected to post-LVI ballistic impact. Structures compared are monolithic Texipreg E-glass epoxy and SB E-glass sandwich with cork core. Monolithic: [0/90]8, thk 2.6 mm Sandwich: Each Facesheet: [0/90]4, thk 1.3 mm Core: 2 mm Corecork SB done by autoclave consolidation | NS, CEAST Fractovis Plus, ⌀ 20 mm HI with mass 5.62 kg Impact energies: 25 and 40 J High velocity (HV) (78–201 m/s) impact tests conducted on LVI impacted specimens, projectile residual velocity after perforation measured | Perforation did not occur for both impact energies in either material. Matrix cracks were the first damage introduced by transverse impact load. Cork Sandwich (C) vs. Monolithic (M): LVI Peak Loads: Slightly higher (higher is better, implies less damage) LVI absorbed energy: At 25 J: roughly equal. At 40 J: −30%. (lower is better, implies less damage) Post perforation projectile residual velocity after ballistic impact @ 200 m/s: C: −6%, 25 J: −14%, 40 J: −20%. (lower is better, implies better energy absorption) Lambert-Jonas Ballistic limit / areal limit density: C: 4%, 25 J: 10%, 40 J: 25% (higher is better) |
| [42] comparing effect of different temperatures on LVI performance of SB sandwich structures. SB process: Apply bio-epoxy Elan-tech ADH 46.46 and cure for 24 h under constant load Facesheets: Hot Compression Molded Polypropylene (PP) (2.2 + 0.1 mm thk) and Polypropylene Compatibilized (PPC) (2.1 + 0.1 mm thk), +4 layers of 360 kg/m3 twill Flax/Basalt hybrid fabric. Core—15 mm thk, using either Divinycell HP-130 PVC Closed Cell Foam 130 kg/m3, Corecork NL 10 and NL 25 | ASTM D7136 HI 12.7 mm, 12.055 kg for Impact energies: 32.5 J, 65 J, 97.5 J, 130 J Specimen size 150 × 150 mm2 Impact energy needed to cause perforation is measured. | Embrittlement occurs in both PVC and cork synthetic cores at −40 °C, leading to a larger crack on sample back face Average PI at 32.5 J and 65 J: Cork NL10 and NL25 display less PI depth than HP-130 at both 32.5 J and 65 J. Perforation energy in J (higher is better): 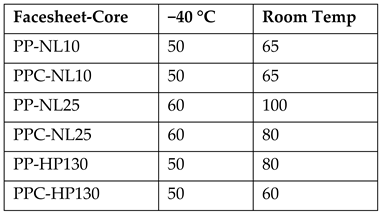 |
| [57] Comparison of LVI performance of cork and PMI foam sandwich. SB: carried out at “uniform pressure”, FSNT—9.7 mm
| ASTM D7136, Instron Dynatup 9200, HI ⌀ 12.7 mm Impact energies: 10 J Specimen size 150 × 150 mm2 Also done:
| Cork has longer average impact time than PMI foam (13 ms vs. 9.5 ms) Both sandwiches exhibit similar planar damage areas but cork has much smaller average dent depth than PMI foam (3.4 vs. 7.1 mm). See Figure 20. Rebound: HI rebounds past original impact point for cork, HI does not for PMI. Damage:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ong, M.; Silva, A. Effects of Low-Velocity-Impact on Facesheet-Core Debonding of Natural-Core Composite Sandwich Structures—A Review of Experimental Research. J. Compos. Sci. 2024, 8, 23. https://doi.org/10.3390/jcs8010023
Ong M, Silva A. Effects of Low-Velocity-Impact on Facesheet-Core Debonding of Natural-Core Composite Sandwich Structures—A Review of Experimental Research. Journal of Composites Science. 2024; 8(1):23. https://doi.org/10.3390/jcs8010023
Chicago/Turabian StyleOng, Michael, and Arlindo Silva. 2024. "Effects of Low-Velocity-Impact on Facesheet-Core Debonding of Natural-Core Composite Sandwich Structures—A Review of Experimental Research" Journal of Composites Science 8, no. 1: 23. https://doi.org/10.3390/jcs8010023
APA StyleOng, M., & Silva, A. (2024). Effects of Low-Velocity-Impact on Facesheet-Core Debonding of Natural-Core Composite Sandwich Structures—A Review of Experimental Research. Journal of Composites Science, 8(1), 23. https://doi.org/10.3390/jcs8010023








