Ternary Composite of Polymer, Fullerene and Fluorinated Multi-Walled Carbon Nanotubes as the Active Layer of Organic Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fluorinated Nanotubes and Their Suspensions
2.2. Scanning Electron Microscopy
2.3. UV/Vis Absorption Spectra
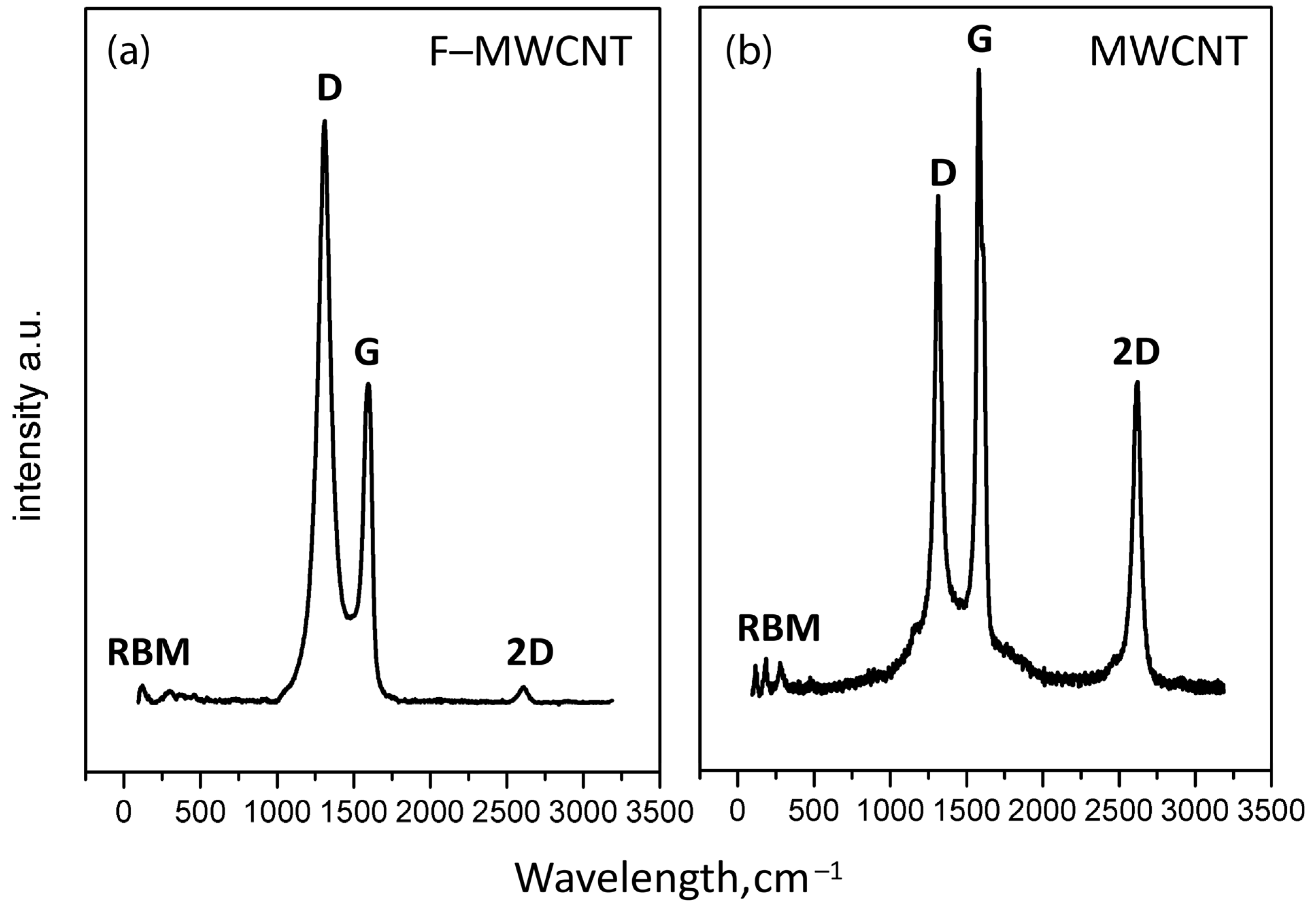
2.4. Raman Spectroscopy
2.5. Device Fabrication and Characterization
2.6. Photo-CELIV Measurements
2.7. Cyclic Voltammetry
2.8. Atomic Force Microscopy
2.9. Degradation of OPV Devices
2.10. Light-Induced EPR
3. Results and Discussion
3.1. Characterization of Fluorinated MWCNTs
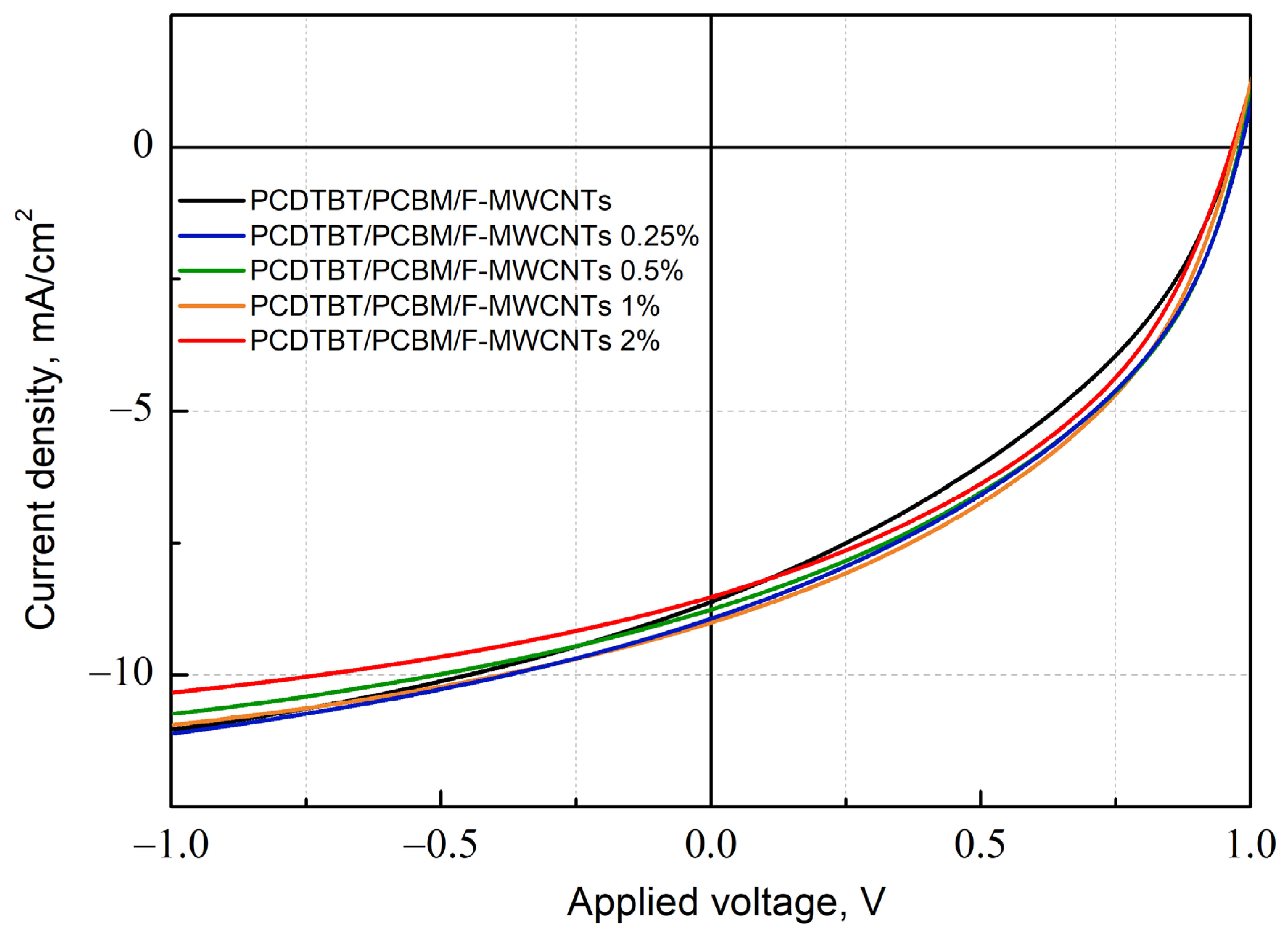
3.2. Effect of Fluorinated MWCNTs Additive on OPV Device Performance
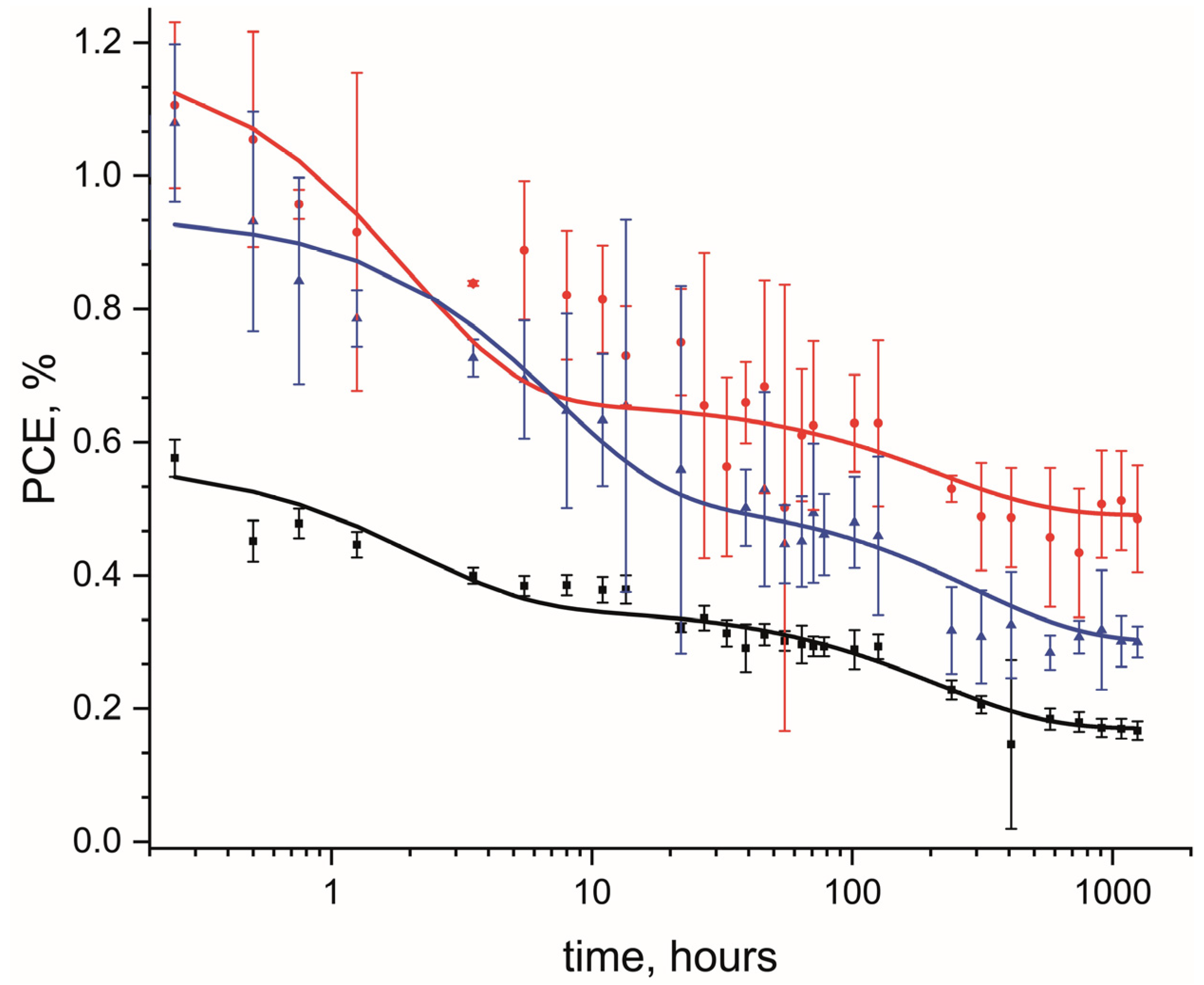
3.3. The Effect of the Addition of Fluorinated MWCNTs on Long-Term Stability of OPV Devices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, W.; Qi, F.; Peng, Z.; Lin, F.R.; Jiang, K.; Zhong, C.; Kaminsky, W.; Guan, Z.; Lee, C.; Marks, T.J.; et al. Achieving 19% Power Conversion Efficiency in Planar-Mixed Heterojunction Organic Solar Cells Using a Pseudosymmetric Electron Acceptor. Adv. Mater. 2022, 34, 2202089. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Zhang, S.; Chen, Z.; Xu, Y.; Cui, Y.; Zhang, T.; Ren, J.; Qin, J.; Hong, L.; Hao, X.; et al. Reduced Non-Radiative Charge Recombination Enables Organic Photovoltaic Cell Approaching 19% Efficiency. Joule 2021, 5, 2408–2419. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Y.; Yao, H.; Bi, P.; Hong, L.; Zhang, J.; Zu, Y.; Zhang, T.; Qin, J.; Ren, J.; et al. Single-Junction Organic Photovoltaic Cell with 19% Efficiency. Adv. Mater. 2021, 33, 2102420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lin, F.R.; Qi, F.; Heumüller, T.; Distler, A.; Egelhaaf, H.-J.; Li, N.; Chow, P.C.Y.; Brabec, C.J.; Jen, A.K.-Y.; et al. Renewed Prospects for Organic Photovoltaics. Chem. Rev. 2022, 122, 14180–14274. [Google Scholar] [CrossRef] [PubMed]
- Deibel, C.; Strobel, T.; Dyakonov, V. Role of the Charge Transfer State in Organic Donor-Acceptor Solar Cells. Adv. Mater. 2010, 22, 4097–4111. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, C.; Sin, D.H.; Park, J.H.; Cho, K. Recent Advances in Morphology Optimization for Organic Photovoltaics. Adv. Mater. 2018, 30, 1800453. [Google Scholar] [CrossRef]
- Thompson, B.C.; Fréchet, J.M.J. Polymer–Fullerene Composite Solar Cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef]
- Kim, Y.; Park, B.N. Electrical Signature of Ultrasound-Induced Anisotropic Self-Assembly of Poly(3-Hexylthiophene) (P3HT) during Channel Formation. Appl. Sci. 2020, 10, 6886. [Google Scholar] [CrossRef]
- Duan, L.; Uddin, A. Progress in Stability of Organic Solar Cells. Adv. Sci. 2020, 7, 1903259. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.-K.; Lin, F.R.; Jen, A.K.-Y. Improving the Stability of Organic Solar Cells: From Materials to Devices. Sol. RRL 2023, 7, 2300531. [Google Scholar] [CrossRef]
- Yan, L.; Ma, C.-Q. Degradation of Polymer Solar Cells: Knowledge Learned from the Polymer:Fullerene Solar Cells. Energy Technol. 2021, 9, 2000920. [Google Scholar] [CrossRef]
- Doumon, N.Y.; Wang, G.; Chiechi, R.C.; Koster, L.J.A. Relating Polymer Chemical Structure to the Stability of Polymer: Fullerene Solar Cells. J. Mater. Chem. C 2017, 5, 6611–6619. [Google Scholar] [CrossRef]
- Ciammaruchi, L.; Oliveira, R.; Charas, A.; Tulus; von Hauff, E.; Polino, G.; Brunetti, F.; Hansson, R.; Moons, E.; Krassas, M.; et al. Stability of Organic Solar Cells with PCDTBT Donor Polymer: An Interlaboratory Study. J. Mater. Res. 2018, 33, 1909–1924. [Google Scholar] [CrossRef]
- Rivaton, A.; Tournebize, A.; Gaume, J.; Bussière, P.-O.; Gardette, J.-L.; Therias, S. Photostability of Organic Materials Used in Polymer Solar Cells: Photostability of Polymer Solar Cells. Polym. Int. 2014, 63, 1335–1345. [Google Scholar] [CrossRef]
- Blazinic, V.; Ericsson, L.K.E.; Muntean, S.A.; Moons, E. Photo-Degradation in Air of Spin-Coated PC60BM and PC70BM Films. Synth. Met. 2018, 241, 26–30. [Google Scholar] [CrossRef]
- Yamilova, O.R.; Martynov, I.V.; Brandvold, A.S.; Klimovich, I.V.; Balzer, A.H.; Akkuratov, A.V.; Kusnetsov, I.E.; Stingelin, N.; Troshin, P.A. What Is Killing Organic Photovoltaics: Light-Induced Crosslinking as a General Degradation Pathway of Organic Conjugated Molecules. Adv. Energy Mater. 2020, 10, 1903163. [Google Scholar] [CrossRef]
- Kim, T.; Younts, R.; Lee, W.; Lee, S.; Gundogdu, K.; Kim, B.J. Impact of the Photo-Induced Degradation of Electron Acceptors on the Photophysics, Charge Transport and Device Performance of All-Polymer and Fullerene–Polymer Solar Cells. J. Mater. Chem. A 2017, 5, 22170–22179. [Google Scholar] [CrossRef]
- de Zerio, A.D.; Müller, C. Glass Forming Acceptor Alloys for Highly Efficient and Thermally Stable Ternary Organic Solar Cells. Adv. Energy Mater. 2018, 8, 1702741. [Google Scholar] [CrossRef]
- Fraga Domínguez, I.; Distler, A.; Lüer, L. Stability of Organic Solar Cells: The Influence of Nanostructured Carbon Materials. Adv. Energy Mater. 2017, 7, 1601320. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Yu, L.; Batmunkh, M.; Shapter, J.G. Recent Advances in Applications of Sorted Single-Walled Carbon Nanotubes. Adv. Funct. Mater. 2019, 29, 1902273. [Google Scholar] [CrossRef]
- Muchuweni, E.; Mombeshora, E.T.; Martincigh, B.S.; Nyamori, V.O. Recent Applications of Carbon Nanotubes in Organic Solar Cells. Front. Chem. 2022, 9, 733552. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, S.; Wang, W.; Zhang, X.; Zhou, W.; Xie, S.; Liu, H. Recent Advances in Structure Separation of Single-Wall Carbon Nanotubes and Their Application in Optics, Electronics, and Optoelectronics. Adv. Sci. 2022, 9, 2200054. [Google Scholar] [CrossRef] [PubMed]
- Shastry, T.A.; Hartnett, P.E.; Wasielewski, M.R.; Marks, T.J.; Hersam, M.C. Ternary Polymer–Perylenediimide–Carbon Nanotube Photovoltaics with High Efficiency and Stability under Super-Solar Irradiation. ACS Energy Lett. 2016, 1, 548–555. [Google Scholar] [CrossRef]
- Salim, T.; Lee, H.-W.; Wong, L.H.; Oh, J.H.; Bao, Z.; Lam, Y.M. Semiconducting Carbon Nanotubes for Improved Efficiency and Thermal Stability of Polymer-Fullerene Solar Cells. Adv. Funct. Mater. 2016, 26, 51–65. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-Nanotube-Based Thermoelectric Materials and Devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.; Konidakis, I.; Arjmand, M.; Sundararaj, U.; Fuge, R.; Liebscher, M.; Hampel, S.; Klaus, M.; Serpetzoglou, E.; Stratakis, E.; et al. Nitrogen-Doped Carbon Nanotube/Polypropylene Composites with Negative Seebeck Coefficient. J. Compos. Sci. 2020, 4, 14. [Google Scholar] [CrossRef]
- El-Moussawi, Z.; Nourdine, A.; Flandin, L. A Key Progress in Introducing Single Walled Carbon Nanotubes to Photovoltaic Devices. Appl. Nanosci. 2022, 12, 2277–2290. [Google Scholar] [CrossRef]
- Kobeleva, E.S.; Uvarov, M.N.; Kravets, N.V.; Ponomarev, S.A.; Gurova, O.A.; Okotrub, A.V.; Kazantzev, M.S.; Degtyarenko, K.M.; Kulik, L.V. Fluorinated Carbon Nanotubes as Nonvolatile Additive to the Active Layer of Polymer/Fullerene Solar Cells. Fuller. Nanotub. Carbon Nanostructures 2023, 31, 464–473. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Gorodetskiy, D.V.; Gusel’nikov, A.V.; Kondranova, A.M.; Bulusheva, L.G.; Korabovska, M.; Meija, R.; Erts, D. Distribution of Iron Nanoparticles in Arrays of Vertically Aligned Carbon Nanotubes Grown by Chemical Vapor Deposition. Materials 2022, 15, 6639. [Google Scholar] [CrossRef]
- Juška, G.; Arlauskas, K.; Viliūnas, M.; Kočka, J. Extraction Current Transients: New Method of Study of Charge Transport in Microcrystalline Silicon. Phys. Rev. Lett. 2000, 84, 4946–4949. [Google Scholar] [CrossRef]
- Gagne, R.R.; Koval, C.A.; Lisensky, G.C. Ferrocene as an Internal Standard for Electrochemical Measurements. Inorg. Chem. 1980, 19, 2854–2855. [Google Scholar] [CrossRef]
- Kamalakannan, R.; Ganesan, K.; Ilango, S.; Thirumurugan, N.; Singh, V.N.; Kamruddin, M.; Mehta, B.R.; Tyagi, A.K. The Role of Structural Defects on the Transport Properties of a Few-Walled Carbon Nanotube Networks. Appl. Phys. Lett. 2011, 98, 192105. [Google Scholar] [CrossRef]
- Mihailetchi, V.D.; Koster, L.J.A.; Blom, P.W.M. Effect of Metal Electrodes on the Performance of Polymer:Fullerene Bulk Heterojunction Solar Cells. Appl. Phys. Lett. 2004, 85, 970–972. [Google Scholar] [CrossRef]
- Kobeleva, E.S.; Popov, A.A.; Baranov, D.S.; Uvarov, M.N.; Nevostruev, D.A.; Degtyarenko, K.M.; Gadirov, R.M.; Sukhikh, A.S.; Kulik, L.V. Origin of Poor Photovoltaic Performance of Bis(Tetracyanoantrathiophene) Non-Fullerene Acceptor. Chem. Phys. 2021, 546, 111162. [Google Scholar] [CrossRef]
- Popov, A.A.; Uvarov, M.N.; Kulik, L.V. Mode of Action of the Third Component in Ternary Organic Photovoltaic Blend PBDB-T/ITIC:PC70BM Revealed by EPR Spectroscopy. Synth. Met. 2021, 277, 116783. [Google Scholar] [CrossRef]
- Marumoto, K.; Takeuchi, N.; Ozaki, T.; Kuroda, S. ESR Studies of Photogenerated Polarons in Regioregular Poly(3-Alkylthiophene)–Fullerene Composite. Synth. Met. 2002, 129, 239–247. [Google Scholar] [CrossRef]
- Lukina, E.A.; Uvarov, M.N.; Kulik, L.V. Charge Recombination in P3HT/PC70BM Composite Studied by Light-Induced EPR. J. Phys. Chem. C 2014, 118, 18307–18314. [Google Scholar] [CrossRef]
- Tachiya, M.; Seki, K. Theory of Bulk Electron-Hole Recombination in a Medium with Energetic Disorder. Phys. Rev. B 2010, 82, 085201. [Google Scholar] [CrossRef]
- Ho, C.H.Y.; Dong, Q.; Yin, H.; Leung, W.W.K.; Yang, Q.; Lee, H.K.H.; Tsang, S.W.; So, S.K. Impact of Solvent Additive on Carrier Transport in Polymer:Fullerene Bulk Heterojunction Photovoltaic Cells. Adv. Mater. Interfaces 2015, 2, 1500166. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, C.; Qiu, B.; Liu, W.; So, S.K.; Mainville, M.; Leclerc, M.; Shoaee, S.; Neher, D.; Zou, Y. Effects of Energetic Disorder in Bulk Heterojunction Organic Solar Cells. Energy Environ. Sci. 2022, 15, 2806–2818. [Google Scholar] [CrossRef]




| Element | Weight, % | Atomic Weight, % |
|---|---|---|
| C | 54.79 | 66.38 |
| O | 3.01 | 2.74 |
| F | 39.73 | 30.43 |
| Br | 2.47 | 0.45 |
| Voc, V | Jsc, mA/cm2 | FF, % | PCE, % | |
|---|---|---|---|---|
| PCDTBT/PC60BM | 0.983 0.977 ± 0.006 | 8.61 8.50 ± 0.20 | 38.1 37.5 ± 0.5 | 3.18 3.11 ± 0.09 |
| PCDTBT/PC60BM/F-MWCNTs 0.25% | 0.983 0.981 ± 0.002 | 8.93 8.90 ± 0.10 | 40.9 40.0 ± 1.0 | 3.59 3.50 ± 0.10 |
| PCDTBT/PC60BM/F-MWCNTs 0.5% | 0.980 0.979 ± 0.002 | 8.76 8.80 ± 0.10 | 41.7 41.2 ± 0.7 | 3.59 3.54 ± 0.07 |
| PCDTBT/PC60BM/F-MWCNTs 1% | 0.977 0.973 ± 0.004 | 9.00 8.90 ± 0.20 | 41.9 41.0 ± 0.9 | 3.67 3.50 ± 0.10 |
| PCDTBT/PC60BM/F-MWCNTs 2% | 0.966 0.965 ± 0.002 | 8.52 8.30 ± 0.20 | 42.0 41.6 ± 0.6 | 3.46 3.30 ± 0.10 |
| PCDTBT/PC60BM/MW0 1% | 0.897 0.890 ± 0.010 | 7.44 7.40 ± 0.20 | 41.3 40.6 ± 0.5 | 2.75 2.69 ± 0.08 |
| Active Layer | tmax, us | µ, cm2/Vs |
|---|---|---|
| PCDTBT/PC60BM | 2.5 | 7.1 × 10−5 |
| PCDTBT/PC60BM/F-MWCNTs 0.25% | 3.0 | 4.9 × 10−5 |
| PCDTBT/PC60BM/F-MWCNTs 0.5% | 2.3 | 8.4 × 10−5 |
| PCDTBT/PC60BM/F-MWCNTs 1% | 3.3 | 4.1 × 10−5 |
| PCDTBT/PC60BM/F-MWCNTs 2% | 2.9 | 5.4 × 10−5 |
| Active Layer | A1/A2 | t1, h | t2, h | PCE∞, % |
|---|---|---|---|---|
| PCDTBT/PC60BM | 1.3 | 1.7 | 210 | 0.16 |
| PCDTBT/PC60BM/F-MWCNTs 0.5% | 1.0 | 9.9 | 305 | 0.22 |
| PCDTBT/PC60BM/F-MWCNTs 1% | 1.7 | 7.1 | 293 | 0.29 |
| PCDTBT/PC60BM/F-MWCNTs 2% | 0.6 | 2.0 | 214 | 0.45 |
| PCDTBT/PC60BM/MWCNTs 1% | 2.2 | 0.7 | 223 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobeleva, E.S.; Uvarov, M.N.; Kravets, N.V.; Kulikova, A.V.; Zinovyev, V.A.; Gurova, O.A.; Sysoev, V.I.; Kondranova, A.M.; Kazantsev, M.S.; Degtyarenko, K.M.; et al. Ternary Composite of Polymer, Fullerene and Fluorinated Multi-Walled Carbon Nanotubes as the Active Layer of Organic Solar Cells. J. Compos. Sci. 2024, 8, 3. https://doi.org/10.3390/jcs8010003
Kobeleva ES, Uvarov MN, Kravets NV, Kulikova AV, Zinovyev VA, Gurova OA, Sysoev VI, Kondranova AM, Kazantsev MS, Degtyarenko KM, et al. Ternary Composite of Polymer, Fullerene and Fluorinated Multi-Walled Carbon Nanotubes as the Active Layer of Organic Solar Cells. Journal of Composites Science. 2024; 8(1):3. https://doi.org/10.3390/jcs8010003
Chicago/Turabian StyleKobeleva, Elena S., Mikhail N. Uvarov, Natalia V. Kravets, Aina V. Kulikova, Vladimir A. Zinovyev, Olga A. Gurova, Vitalii I. Sysoev, Anastasiya M. Kondranova, Maxim S. Kazantsev, Konstantin M. Degtyarenko, and et al. 2024. "Ternary Composite of Polymer, Fullerene and Fluorinated Multi-Walled Carbon Nanotubes as the Active Layer of Organic Solar Cells" Journal of Composites Science 8, no. 1: 3. https://doi.org/10.3390/jcs8010003
APA StyleKobeleva, E. S., Uvarov, M. N., Kravets, N. V., Kulikova, A. V., Zinovyev, V. A., Gurova, O. A., Sysoev, V. I., Kondranova, A. M., Kazantsev, M. S., Degtyarenko, K. M., Matveeva, A. G., & Kulik, L. V. (2024). Ternary Composite of Polymer, Fullerene and Fluorinated Multi-Walled Carbon Nanotubes as the Active Layer of Organic Solar Cells. Journal of Composites Science, 8(1), 3. https://doi.org/10.3390/jcs8010003







