Synthesis of a New Composite Material Derived from Cherry Stones and Sodium Alginate—Application to the Adsorption of Methylene Blue from Aqueous Solution: Process Parameter Optimization, Kinetic Study, Equilibrium Isotherms, and Reusability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Composite Preparation
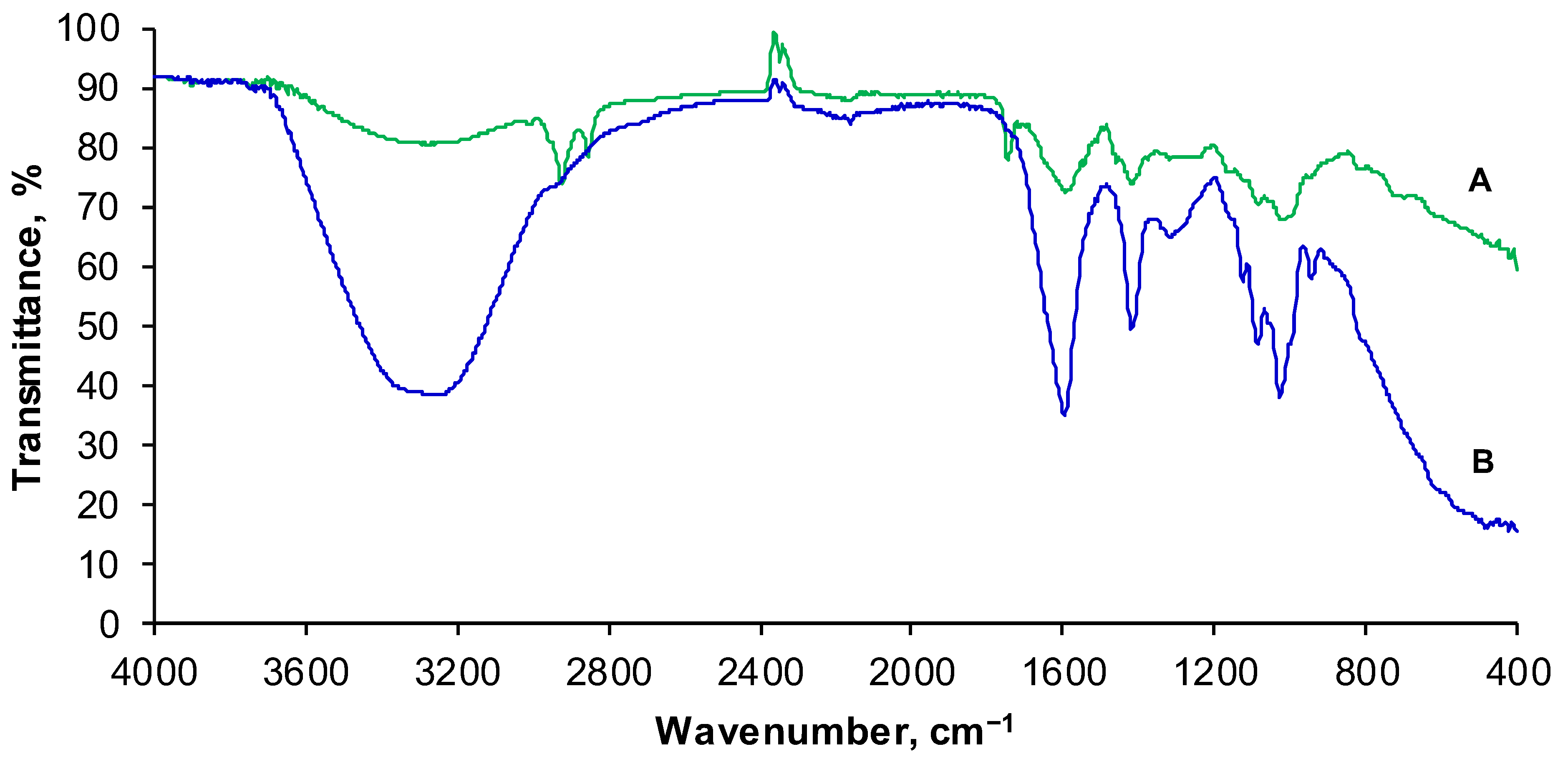
2.3. Composite Characterization
2.4. Analytical Method
2.5. Optimization of Adsorption Parameters
2.6. Kinetic and Equilibrium Isotherms Assessment
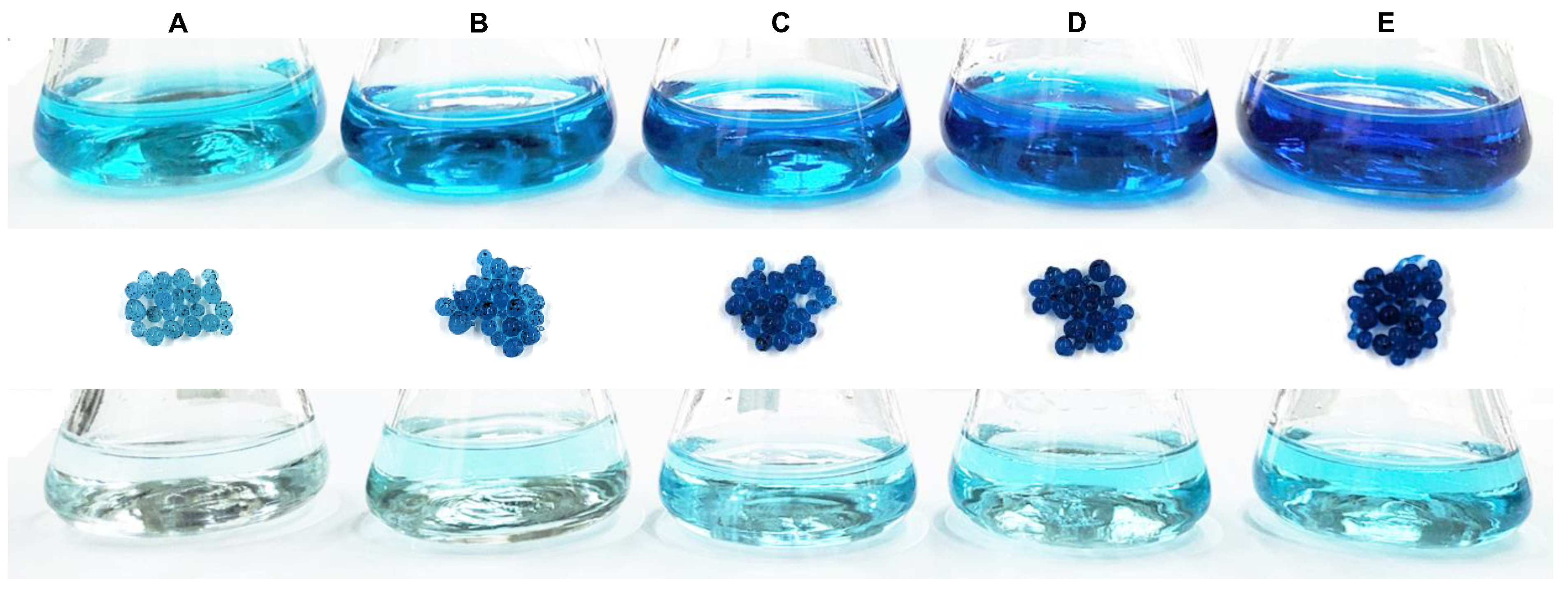
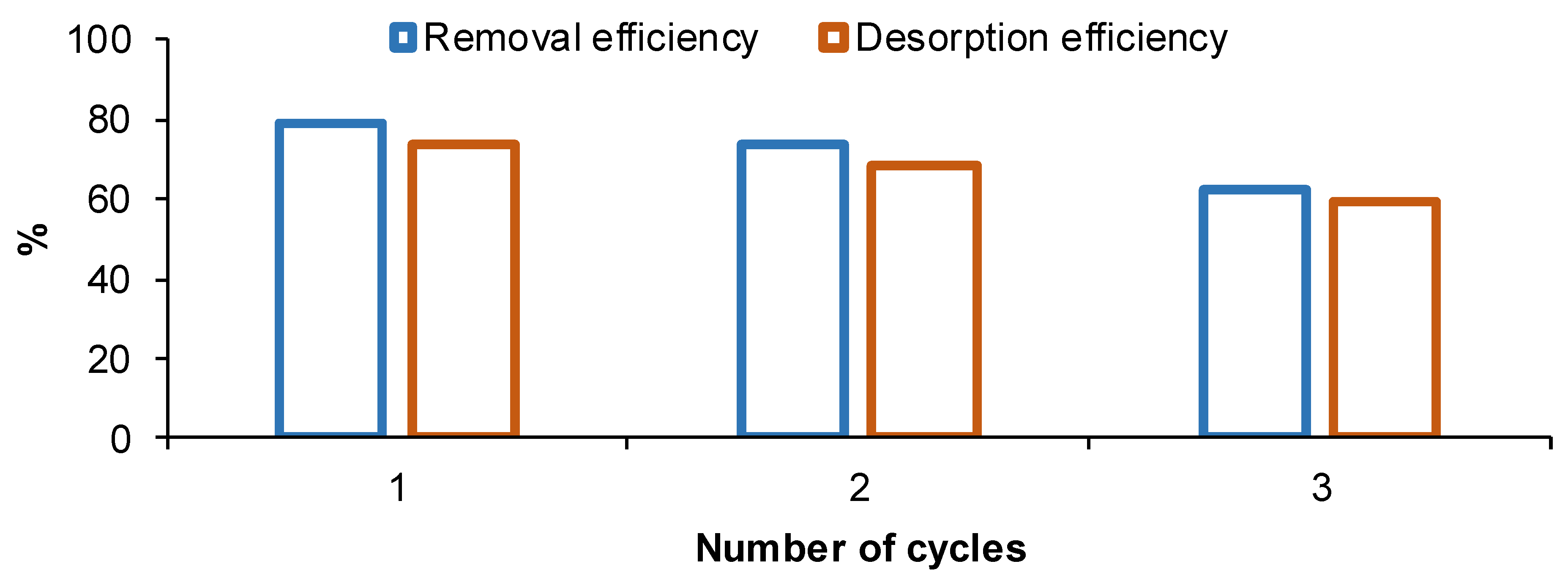
2.7. Composite Regeneration and Reusability
2.8. Statistical Analysis
3. Results and Discussion
3.1. Composite Preparation and Characterization
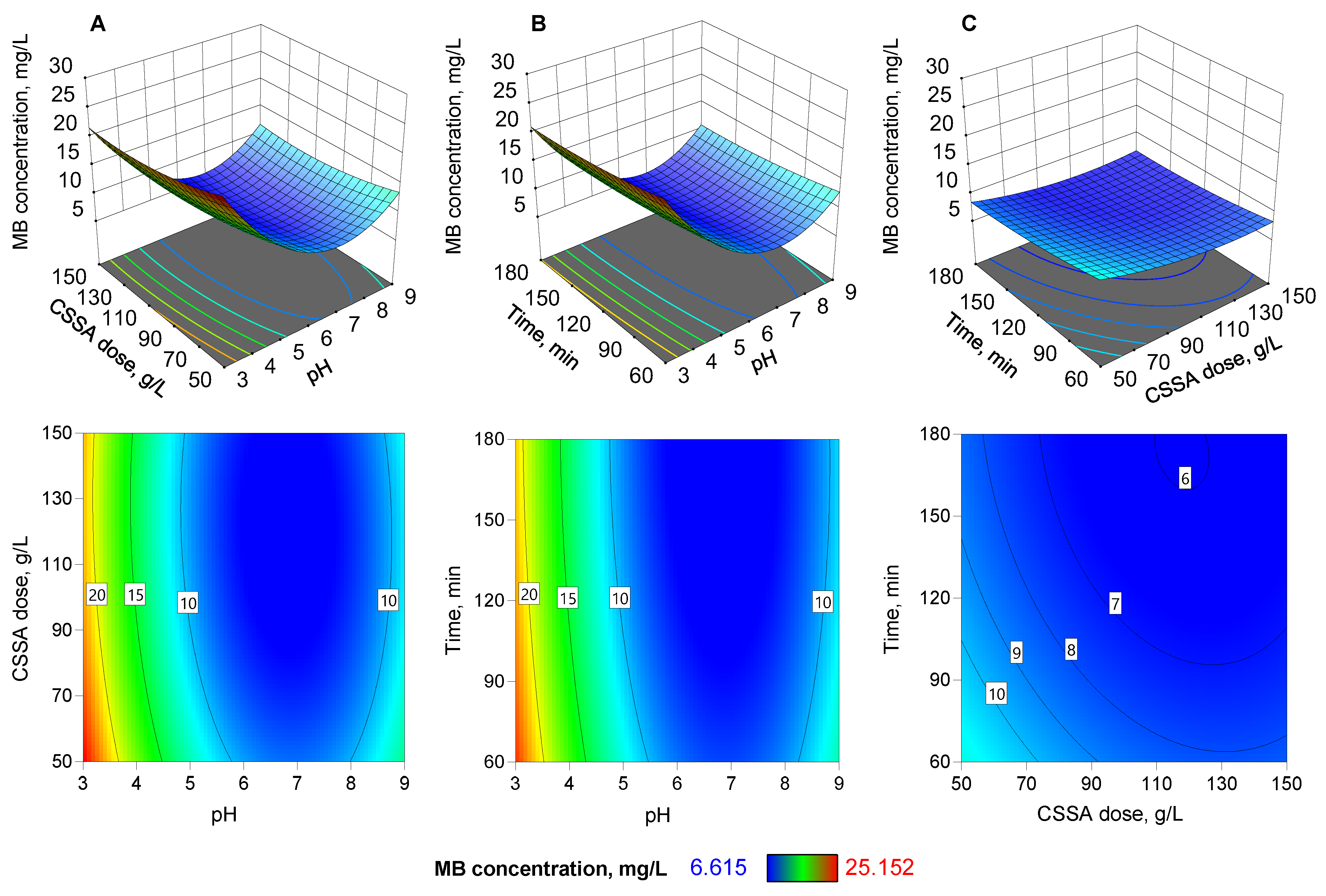
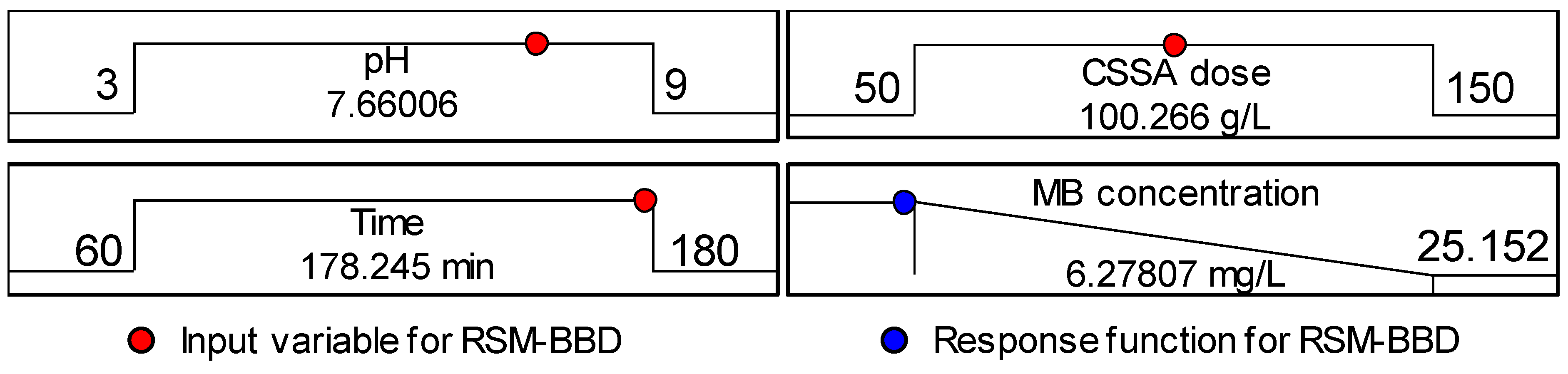
3.2. Adsorption Parameters Optimization by Box-Behnken Design
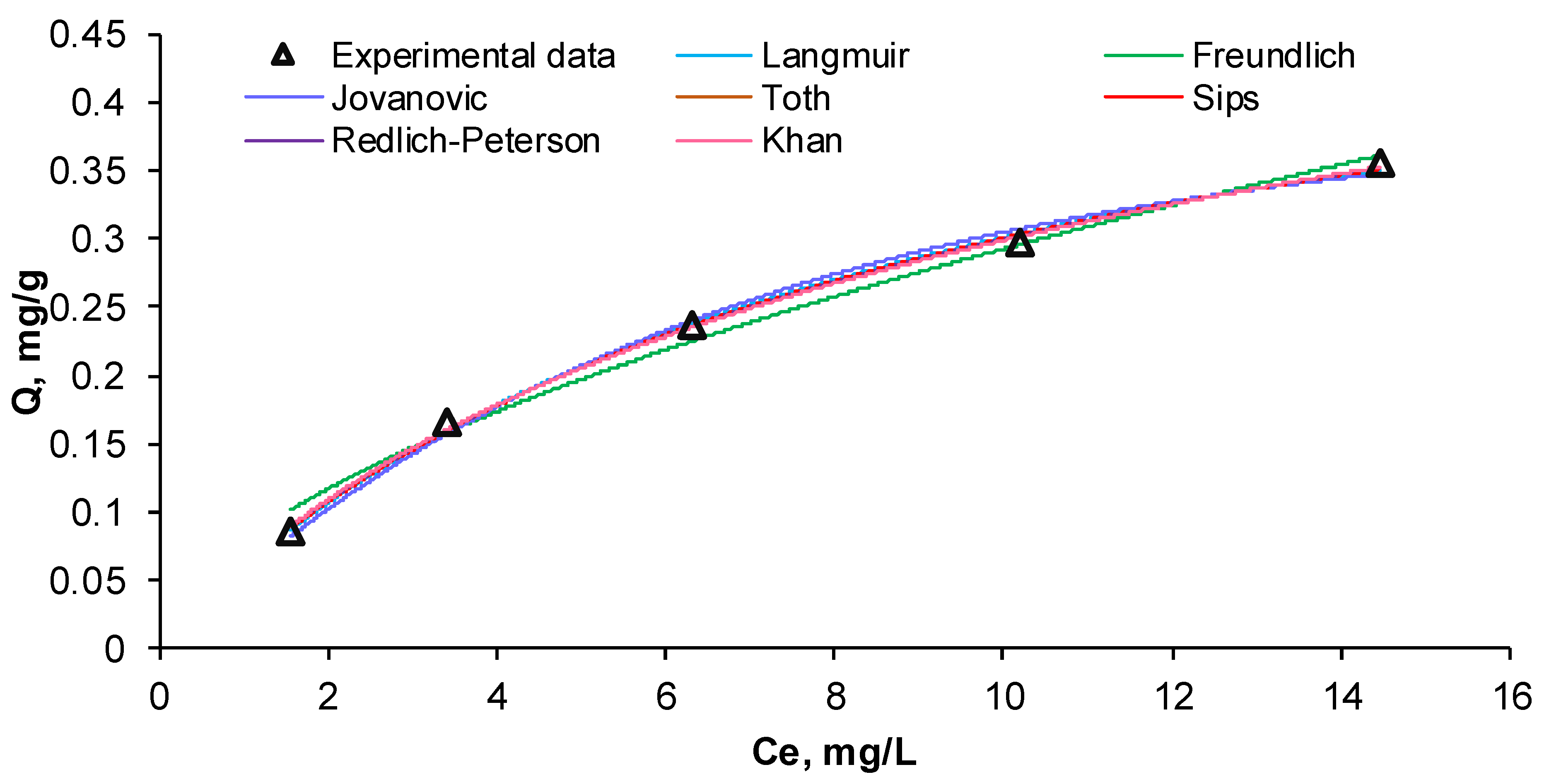
3.3. Kinetic and Equilibrium Isotherms Study
3.3.1. Kinetic Analysis
3.3.2. Equilibrium Isotherms Analysis
3.4. Adsorbent Regeneration and Reusability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J.S. Nanotechnology in wastewater management: A new paradigm towards wastewater treatment. Molecules 2021, 26, 1797. [Google Scholar] [CrossRef]
- Salazar-Sogamoso, L.M.; Gómez-García, M.-Á.; Dobrosz-Gómez, I. Comparative life cycle assessment of sequential chemical and electrochemical processes for the treatment of industrial textile wastewater. J. Solid State Electrochem. 2024. [Google Scholar] [CrossRef]
- Zhang, T.; Payne, K.; Zhang, J.; Purswani, P.; Karpyn, Z.; Wang, M. Hybrid ion exchange and biological processes for water and wastewater treatment: A comprehensive review of process applications and mathematical modeling. Rev. Environ. Sci. Bio/Technol. 2024, 23, 163–188. [Google Scholar] [CrossRef]
- Gaioto, F.C.; Matheus, M.C.; de Souza-Chaves, B.M.; Campos, J.C.; Barra, T.A.; de Almeida Azevedo, D.; Bassin, J.P.; Dezotti, M. Treatment of a pesticide-containing wastewater by biological and physicochemical processes: Seeking the best conditions towards reuse. Braz. J. Chem. Eng. 2023. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef]
- Alhasan, H.S.; Omran, A.R.; Al Mahmud, A.; Mady, A.H.; Thalji, M.R. Toxic Congo red dye photodegradation employing green synthesis of zinc oxide nanoparticles using gum arabic. Water 2024, 16, 2202. [Google Scholar] [CrossRef]
- Melhi, S.; Alqadami, A.A.; Alosaimi, E.H.; Ibrahim, G.M.; El-Gammal, B.; Bedair, M.A.; Elnaggar, E.M. Effective removal of Malachite Green dye from water using low-cost porous organic polymers: Adsorption kinetics, isotherms, and reusability studies. Water 2024, 16, 1869. [Google Scholar] [CrossRef]
- González-Rodríguez, L.; Yáñez, O.; Mena- Ulecia, K.; Hidalgo-Rosa, Y.; García- Carmona, X.; Ulloa- Tesser, C. Exploring the adsorption of five emerging pollutants on activated carbon: A theoretical approach. J. Environ. Chem. Eng. 2024, 12, 112911. [Google Scholar] [CrossRef]
- Khnifira, M.; Boumya, W.; Atarki, J.; Sadiq, M.; Achak, M.; Bouich, A.; Barka, N.; Abdennouri, M. Experimental, DFT and MD simulation combined studies for the competitive adsorption of anionic and cationic dyes on activated carbon in an aqueous medium. J. Mol. Struct. 2024, 1310, 138247. [Google Scholar] [CrossRef]
- Domergue, L.; Cimetière, N.; Giraudet, S.; Cloirec, P.L. Adsorption onto granular activated carbons of a mixture of pesticides and their metabolites at trace concentrations in groundwater. J. Environ. Chem. Eng. 2022, 10, 108218. [Google Scholar] [CrossRef]
- Sanz-Santos, E.; Gutiérrez-Sánchez, P. Multicomponent and continuous adsorption of neonicotinoid pesticides identified in the EU Watch Lists onto mesoporous and biogenic activated carbon. Sep. Purif. Technol. 2024, 346, 127514. [Google Scholar] [CrossRef]
- Zhu, X.; He, M.; Sun, Y.; Xu, Z.; Wan, Z.; Hou, D.; Alessi, D.S.; Tsang, D.C.W. Insights into the adsorption of pharmaceuticals and personal care products (PPCPs) on biochar and activated carbon with the aid of machine learning. J. Hazard. Mater. 2022, 423, 127060. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, V.; Pillai, R.; Balan, R.; Vijayaraghavan, R.; Rangaraj, L.; Revathi, V. Nanoarchitectonics of hydrothermal carbonized sugarcane juice-derived biochar for high supercapacitance and dye adsorption performance. Nano-Struct. Nano-Objects 2024, 39, 101249. [Google Scholar] [CrossRef]
- Xu, S.; Yang, J.; Marrakchi, F.; Wei, M.; Liu, Y.; Xiao, Y.; Li, C.; Wang, S. Macro- and micro-algae-based carbon composite for pharmaceutical wastewater treatment: Batch adsorption and mechanism study. Process Saf. Environ. Prot. 2023, 176, 641–652. [Google Scholar] [CrossRef]
- Sayed, K.; Wan Mohtar, W.H.M.; Hanafiah, Z.M.; Wan-Mohtar, W.A.A.Q.I.; Abd Manan, T.S.B.; Mohamad Sharif, S.A.B. Simultaneous enhanced removal of pharmaceuticals and hormone from wastewaters using series combinations of ultra-violet irradiation, bioremediation, and adsorption technologies. J. Water Process Eng. 2024, 57, 104589. [Google Scholar] [CrossRef]
- Chu, T.T.H.; Tran, T.M.N.; Pham, M.T.; Viet, N.M.; Thi, H.P. Magnesium oxide nanoparticles modified biochar derived from tea wastes for enhanced adsorption of o-chlorophenol from industrial wastewater. Chemosphere 2023, 337, 139342. [Google Scholar] [CrossRef]
- Saleh, H.M.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M. Design, preparation, and characterization of polycaprolactone–chitosan nanofibers via electrospinning techniques for efficient methylene blue removal from aqueous solutions. J. Compos. Sci. 2024, 8, 68. [Google Scholar] [CrossRef]
- Rivera-Arenas, D.; Macías-Quiroga, I.F.; Dávila-Arias, M.T.; Vélez-Upegui, J.J.; Sanabria-González, N.R. Adsorption of acid yellow 23 dye on organobentonite/alginate hydrogel beads in a fixed-bed column. J. Compos. Sci. 2023, 7, 362. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAOSTAT). Crops and Livestock Products: Cherries; United Nations: New York, NY, USA, 2022. [Google Scholar]
- Soares Mateus, A.R.; Pena, A.; Sendón, R.; Almeida, C.; Nieto, G.A.; Khwaldia, K.; Sanches Silva, A. By-products of dates, cherries, plums and artichokes: A source of valuable bioactive compounds. Trends Food Sci. Technol. 2023, 131, 220–243. [Google Scholar] [CrossRef]
- Abuşka, M.; Şevik, S.; Kayapunar, A. Comparative energy and exergy performance investigation of forced convection solar air collectors with cherry stone/powder. Renew. Energy 2019, 143, 34–46. [Google Scholar] [CrossRef]
- Nowicki, P.; Kaźmierczak, M.; Wiśniewska, M. Adsorption of organic pollutants on carbonaceous adsorbents prepared by direct activation of sweet cherry stones. ChemPhysChem 2024, 25, e202300859. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Arana, J.M.R.; Mazzoco, R.R. Adsorption studies of methylene blue and phenol onto black stone cherries prepared by chemical activation. J. Hazard. Mater. 2010, 180, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, R.; Nowicki, P.; Kaźmierczak, J.; Kuszyńska, I.; Goscianska, J.; Przepiórski, J. Comparison of the effects of different chemical activation methods on properties of carbonaceous adsorbents obtained from cherry stones. Chem. Eng. Res. Des. 2014, 92, 1187–1191. [Google Scholar] [CrossRef]
- Koohestani, M.; Salem, A.; Salem, S. Decolorization of corn syrup in a dynamic system by activated carbon bed: Microwave-assisted impregnation of H3PO4 over cherry and date stones for efficient purification. Sustain. Chem. Pharm. 2023, 36, 101298. [Google Scholar] [CrossRef]
- Pawar, R.R.; Lalhmunsiama; Gupta, P.; Sawant, S.Y.; Shahmoradi, B.; Lee, S.-M. Porous synthetic hectorite clay-alginate composite beads for effective adsorption of methylene blue dye from aqueous solution. Int. J. Biol. Macromol. 2018, 114, 1315–1324. [Google Scholar] [CrossRef]
- Mallick, S.; Barik, D.; Pradhan, N. Commelina erecta, L. biomass-silver nanoparticle composite as a heterogeneous catalyst for methylene blue degradation. Appl. Catal. O Open 2024, 192, 206939. [Google Scholar] [CrossRef]
- Bushra, R.; Mohamad, S.; Alias, Y.; Jin, Y.; Ahmad, M. Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: A review. Microp. Mesoporous Mater. 2021, 319, 111040. [Google Scholar] [CrossRef]
- Jiang, Q.; Han, Z.; Li, W.; Ji, T.; Yuan, Y.; Zhang, J.; Zhao, C.; Cheng, Z.; Wang, S. Adsorption properties of heavy metals and antibiotics by chitosan from larvae and adult Trypoxylus dichotomus. Carbohydr. Polym. 2022, 276, 118735. [Google Scholar] [CrossRef]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef]
- Guo, H.; Qin, Q.; Chang, J.-S.; Lee, D.-J. Modified alginate materials for wastewater treatment: Application prospects. Bioresour. Technol. 2023, 387, 129639. [Google Scholar] [CrossRef] [PubMed]
- Doyo, A.N.; Kumar, R.; Barakat, M.A. Recent advances in cellulose, chitosan, and alginate based biopolymeric composites for adsorption of heavy metals from wastewater. J. Taiwan Inst. Chem. Eng. 2023, 151, 105095. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, K.; Bai, B. A critical review of sodium alginate-based composites in water treatment. Carbohydr. Polym. 2024, 331, 121850. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, X.; Zhao, J.; Ling, G.; Zhang, P. Emerging adsorbents: Applications of sodium alginate/graphene oxide composite materials in wastewater treatment. J. Water Process Eng. 2024, 59, 105100. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, X.; Chen, N.; Cui, X.; Yu, H.; Feng, Y.; Xing, D.; He, W. Efficient removal of hexavalent chromium from wastewater using a novel sodium alginate-biochar composite adsorbent. J. Water Process Eng. 2024, 64, 105655. [Google Scholar] [CrossRef]
- Akin, K.; Ugraskan, V.; Isik, B.; Cakar, F. Adsorptive removal of crystal violet from wastewater using sodium alginate-gelatin-montmorillonite ternary composite microbeads. Int. J. Biol. Macromol. 2022, 223, 543–554. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Owens, G.; Chen, Z. Recovery of rare earth elements from mine wastewater using alginate microspheres encapsulated with zeolitic imidazolate framework-8. J. Hazard. Mater. 2024, 471, 134435. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Wang, A.; Yuan, H.; Chi, Y. Adsorption behavior and mechanism of Cu(II) by sodium alginate/carboxymethylcellulose/magnesium hydroxide (SC-MH) hydrogel. Int. J. Biol. Macromol. 2024, 277, 134046. [Google Scholar] [CrossRef]
- Cai, Z.; Zhou, W.; Chen, W.; Huang, R.; Zhang, R.; Sheng, L.; Shi, M.; Hu, Y.; Huang, C.; Chen, Y. Preparation and properties of cationic starch-carrageenan-sodium alginate hydrogels with pH and temperature sensitivity. Food Chem. 2024, 459, 140272. [Google Scholar] [CrossRef]
- Venegas-Gómez, A.; Gómez-Corzo, M.; Macías-García, A.; Carrasco-Amador, J.P. Charcoal obtained from cherry stones in different carbonization atmospheres. J. Environ. Chem. Eng. 2020, 8, 103561. [Google Scholar] [CrossRef]
- Angin, D. Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel 2014, 115, 804–811. [Google Scholar] [CrossRef]
- Pradhan, A.C.; Paul, A.; Rao, G.R. Sol-gel-cum-hydrothermal synthesis of mesoporous Co-Fe@Al2O3−MCM-41 for methylene blue remediation. J. Chem. Sci. 2017, 129, 381–395. [Google Scholar] [CrossRef]
- Raiyaan, G.I.D.; Khalith, S.B.M.; Sheriff, M.A.; Arunachalam, K.D. Bio-adsorption of methylene blue dye using chitosan-extracted from Fenneropenaeus indicus shrimp shell waste. J. Aquac. Mar. Biol. 2021, 10, 146–150. [Google Scholar]
- Nasiruddin Khan, M.; Sarwar, A. Determination of points of zero charge of natural and treated adsorbents. Surf. Rev. Lett. 2007, 14, 461–469. [Google Scholar] [CrossRef]
- Karimifard, S.; Alavi Moghaddam, M.R. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640-641, 772–797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Jing, K.; Liu, H. Facile construction of pH-responsive graphene oxide-based aerogel and its efficient and selective adsorption performance toward methylene blue. Chem. Eng. Res. Des. 2024, 208, 1–14. [Google Scholar] [CrossRef]
- Abu Musa, M.; Chowdhury, S.; Biswas, S.; Nur Alam, S.M.; Parvin, S.; Abdus Sattar, M. Removal of aqueous methylene blue dye over Vallisneria Natans biosorbent using artificial neural network and statistical response surface methodology analysis. J. Mol. Liq. 2024, 393, 123624. [Google Scholar] [CrossRef]
- Zein, R.; Satrio Purnomo, J.; Ramadhani, P.; Safni; Alif, M.F.; Putri, C.N. Enhancing sorption capacity of methylene blue dye using solid waste of lemongrass biosorbent by modification method. Arab. J. Chem. 2023, 16, 104480. [Google Scholar] [CrossRef]
- Shiferraw, B.T.; Mengesha, D.N.; Kim, H. Enhanced adsorption capacity of phosphoric acid-modified montmorillonite clay and ground coffee waste-derived carbon-based functional composite beads for the effective removal of methylene blue. Colloids Surf. Physicochem. Eng. Asp. 2024, 695, 134243. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Duru Kamacı, U.; Kamacı, M. Hydrogel beads based on sodium alginate and quince seed nanoparticles for the adsorption of methylene blue. Inorg. Chem. Commun. 2024, 160, 111919. [Google Scholar] [CrossRef]
- Allahkarami, E.; Allahkarami, E.; Heydari, M.; Azadmehr, A.; Maghsoudi, A. Assessment of chromite ore wastes for methylene blue adsorption: Isotherm, kinetic, thermodynamic studies, ANN, and statistical physics modeling. Chemosphere 2024, 358, 142098. [Google Scholar] [CrossRef] [PubMed]
- Chaima, H.; Eddine, B.C.; Faouzia, B.; Rana, H.; Aymene Salah, B.; Gil, A.; Imene, B.-A.; Ferhat, D.; Riadh, B.; Mokhtar, B. Adsorptive removal of cationic dye from aqueous solutions using activated carbon prepared from Crataegus monogyna/sodium alginate/polyaniline composite beads: Experimental study and molecular dynamic simulation. J. Mol. Liq. 2024, 408, 125372. [Google Scholar] [CrossRef]
- Dalmaz, A.; SİVrİKaya ÖZak, S. Methylene blue dye efficient removal using activated carbon developed from waste cigarette butts: Adsorption, thermodynamic and kinetics. Fuel 2024, 372, 132151. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, D.; Zhang, G.; Cai, C.; Zhang, C.; Qiu, G.; Zheng, K.; Wu, Z. Adsorption of methylene blue from aqueous solution onto multiporous palygorskite modified by ion beam bombardment: Effect of contact time, temperature, pH and ionic strength. Appl. Clay Sci. 2013, 83–84, 137–143. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Bensalah, J. Removal of the textile dyes by a resin adsorbent polymeric: Insight into optimization, kinetics and isotherms adsorption phenomenally. Inorg. Chem. Commun. 2024, 161, 111975. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Akdemir Evrendilek, G.; Zerrouq, F.; Lairini, S. Recent advances in adsorption kinetic models: Their application to dye types. Arab. J. Chem. 2021, 14, 103031. [Google Scholar] [CrossRef]
- Waghmare, C.; Ghodmare, S.; Ansari, K.; Alfaisal, F.M.; Alam, S.; Khan, M.A.; Ezaier, Y. Adsorption of methylene blue dye onto phosphoric acid-treated pomegranate peel adsorbent: Kinetic and thermodynamic studies. Desalin. Water Treat. 2024, 318, 100406. [Google Scholar] [CrossRef]
- Loutfi, M.; Mariouch, R.; Mariouch, I.; Belfaquir, M.; ElYoubi, M.S. Adsorption of methylene blue dye from aqueous solutions onto natural clay: Equilibrium and kinetic studies. Mater. Today 2023, 72, 3638–3643. [Google Scholar] [CrossRef]
- Boughrara, L.; Zaoui, F.; Guezzoul, M.h.; Sebba, F.Z.; Bounaceur, B.; Kada, S.O. New alginic acid derivatives ester for methylene blue dye adsorption: Kinetic, isotherm, thermodynamic, and mechanism study. Int. J. Biol. Macromol. 2022, 205, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wen, H.; Zheng, T.; Liu, X.; Wang, Z.; Tian, S.; Fan, H.; Chen, Y.; Zhao, H.; Wang, Y. Engineering sodium alginate-SiO2 composite beads for efficient removal of methylene blue from water. Int. J. Biol. Macromol. 2023, 239, 124279. [Google Scholar] [CrossRef] [PubMed]
- Yetgin, S.; Amlani, M. Agricultural low-cost waste adsorption of methylene blue and modelling linear isotherm method versus nonlinear prediction. Clean Technol. Environ. Pol. 2024. [Google Scholar] [CrossRef]
- Farhat, Z.; Kumar, A.; Das, C. Fabrication of used-tea embedded alginate beads for cationic dye remediation: Synergistic effect of surface adsorption and intraparticle diffusion. Surf. Interfaces 2024, 51, 104601. [Google Scholar] [CrossRef]
- Chu, K.H.; Hashim, M.A.; Debord, J.; Harel, M.; Salvestrini, S.; Bollinger, J.-C. The Jovanović adsorption isotherm in water contaminant research: Unmasking spurious versions and spotlighting the real thing. Chem. Eng. Sci. 2023, 281, 119127. [Google Scholar] [CrossRef]
- Mechati, F.; Djilani, C.; Bougdah, N.; Messikh, N.; Boussaha, E.; Moumen, A.; Bouchalta, C.; Medjram, M.S. Adsorption of methylene blue onto activated carbon prepared under N2/microwave radiation supported cobalt: Kinetics, isotherms, and thermodynamics studies. Desalin. Water Treat. 2023, 284, 288–300. [Google Scholar] [CrossRef]
- Bazan-Wozniak, A.; Nosal-Wiercińska, A.; Yilmaz, S.; Pietrzak, R. Chitin-based porous carbons from Hermetia illucens fly with large surface area for efficient adsorption of methylene blue; adsorption mechanism, kinetics and equilibrium studies. Measurement 2024, 226, 114129. [Google Scholar] [CrossRef]
- Hui, Y.; Liu, R.; Lan, J.; Sun, T.; Xu, A. Recyclable chitosan adsorbent: Facile functionalization strategy, excellent removal capacity of dyes and adsorption mechanism. Chemosphere 2024, 359, 142291. [Google Scholar] [CrossRef]
- Watwe, V.; Kulkarni, S.; Kulkarni, P. Development of dried uncharred leaves of Ficus benjamina as a novel adsorbent for cationic dyes: Kinetics, isotherm, and batch optimization. Ind. Crops Prod. 2023, 195, 116449. [Google Scholar] [CrossRef]
- Thakur, S.; Pandey, S.; Arotiba, O.A. Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr. Polym. 2016, 153, 34–46. [Google Scholar] [CrossRef]
- Brazil, T.R.; Sousa, É.M.L.; dos Anjos, E.G.R.; Moura, N.K.; Rocha, L.S.; Calisto, V.; Gonçalves, M.; Rezende, M.C. Producing efficient adsorbents from kraft lignin for the temoval of contaminants from water—A full factorial design. Water 2024, 16, 1838. [Google Scholar] [CrossRef]
- Holilah; Asranudin; El Messaoudi, N.; Ulfa, M.; Hamzah, A.; Hamid, Z.A.A.; Ramadhani, D.V.; Suryanegara, L.; Mahardika, M.; Melenia, A.T.; et al. Fabrication a sustainable adsorbent nanocellulose-mesoporous hectorite bead for methylene blue adsorption. Case Stud. Chem. Environ. Eng. 2024, 10, 100850. [Google Scholar] [CrossRef]
- Aksu Demirezen, D.; Demirezen Yılmaz, D.; Yıldız, Y.Ş. Magnetic chitosan/calcium alginate double-network hydrogel beads: Preparation, adsorption of anionic and cationic surfactants, and reuse in the removal of methylene blue. Int. J. Biol. Macromol. 2023, 239, 124311. [Google Scholar] [CrossRef] [PubMed]
- Pishdadi-Aghdarreh, F.; Norouzbeigi, R.; Velayi, E. Simultaneous adsorption of methylene blue cationic dye and paraquat herbicide on activated lightweight expanded clay aggregate (LECA) as a sustainable and recyclable adsorbent. Appl. Clay Sci. 2024, 256, 107432. [Google Scholar] [CrossRef]





| Factors | Variation Levels | ||
|---|---|---|---|
| Coded | Uncoded | Low (−1) | High (+1) |
| X1 | pH | 3 | 9 |
| X2 | CSSA dose, g/L | 50 | 150 |
| X3 | Time, min | 60 | 180 |
| Kinetic Model | Equation | |
|---|---|---|
| Pseudo-first-order | (4) | |
| Pseudo-second-order | (5) | |
| Intraparticle diffusion | (6) | |
| , 0 ≤ t ≤ t1 | (7) | |
| , t1 ≤ t ≤ t2 | (8) | |
| Bangham | (9) | |
| Equilibrium Isotherm | Non-Linear Equation | |
|---|---|---|
| Langmuir | (10) | |
| Freundlich | (11) | |
| Jovanovic | (12) | |
| Toth | (13) | |
| Sips | (14) | |
| Redlich–Peterson | (15) | |
| Khan | (16) | |
| Run Order | X1 | X2 | X3 | MB Blue Final Concentration, mg/L | |
|---|---|---|---|---|---|
| Experimental Value | Predicted Value | ||||
| 1 | 6 | 100 | 120 | 6.778 | 6.79 |
| 2 | 9 | 100 | 180 | 10.759 | 10.80 |
| 3 | 3 | 150 | 120 | 21.554 | 21.65 |
| 4 | 3 | 100 | 180 | 21.174 | 21.13 |
| 5 | 9 | 100 | 60 | 12.875 | 12.92 |
| 6 | 6 | 100 | 120 | 6.788 | 6.79 |
| 7 | 6 | 50 | 180 | 8.421 | 8.47 |
| 8 | 6 | 100 | 120 | 6.795 | 6.79 |
| 9 | 6 | 150 | 180 | 6.615 | 6.57 |
| 10 | 6 | 50 | 60 | 11.784 | 11.83 |
| 11 | 6 | 150 | 60 | 8.381 | 8.33 |
| 12 | 3 | 100 | 60 | 24.175 | 24.13 |
| 13 | 3 | 50 | 120 | 25.152 | 25.15 |
| 14 | 9 | 50 | 120 | 13.666 | 13.57 |
| 15 | 9 | 150 | 120 | 11.672 | 11.68 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 619.93 | 9 | 68.88 | 10,265.12 | <0.0001 |
| X1 | 232.02 | 1 | 232.02 | 34,576.67 | <0.0001 |
| X2 | 14.58 | 1 | 14.58 | 2173.20 | <0.0001 |
| X3 | 13.12 | 1 | 13.12 | 1955.60 | <0.0001 |
| X1X2 | 0.6432 | 1 | 0.6432 | 95.85 | 0.0002 |
| X1X3 | 0.1958 | 1 | 0.1958 | 29.18 | 0.0029 |
| X2X3 | 0.6376 | 1 | 0.6376 | 95.02 | 0.0002 |
| X12 | 357.13 | 1 | 357.13 | 53,221.34 | <0.0001 |
| X22 | 7.13 | 1 | 7.13 | 1061.99 | <0.0001 |
| X32 | 1.44 | 1 | 1.44 | 214.25 | <0.0001 |
| Residual | 0.0336 | 5 | 0.0067 | ||
| Lack of Fit | 0.0334 | 3 | 0.0111 | 152.54 | 0.0065 |
| Pure Error | 0.0001 | 2 | 0.0001 | ||
| Cor Total | 619.97 | 14 |
| Kinetic | Parameters | Values | Sum of Squares | Residual Sum of Squares | R2 | Adj. R2 |
|---|---|---|---|---|---|---|
| MB initial concentration: 10 mg/L | ||||||
| Experimental data | Qe, mg/g | 0.0844 | ||||
| Pseudo-first-order | Qe, mg/g | 0.0828 | 0.1323 | 0.000014 | 0.7640 | 0.7509 |
| k1, min−1 | 0.2433 | |||||
| Pseudo-second-order | Qe, mg/g | 0.0844 | 0.1324 | 0.000009 | 0.9848 | 0.9839 |
| k2, g/(mg·min) | 6.3750 | |||||
| Intraparticle diffusion (Equation (6)) | ki, mg/(g/min−1/2) | 0.0007 | 0.0003 | 0.000226 | 0.6323 | 0.6119 |
| Ci, mg/g | 0.0720 | |||||
| Intraparticle diffusion (Equations (7) and (8)) | ki1, mg/(g/min−1/2) | 0.0181 | 0.0025 | 0.000716 | 0.8684 | 0.8529 |
| ki2, mg/(g/min−1/2) | 0.0002 | |||||
| Bangham | kB, mL/(g/L) | 0.0644 | 0.1323 | 0.000099 | 0.8387 | 0.8297 |
| ϑ, dimensionless | 0.0479 | |||||
| MB initial concentration: 20 mg/L | ||||||
| Experimental data | Qe, mg/g | 0.1656 | ||||
| Pseudo-first-order | Qe, mg/g | 0.1628 | 0.5172 | 0.000280 | 0.7849 | 0.7730 |
| k1, min−1 | 0.3074 | |||||
| Pseudo-second-order | Qe, mg/g | 0.1652 | 0.5174 | 0.000033 | 0.9742 | 0.9728 |
| k2, g/(mg·min) | 4.8066 | |||||
| Intraparticle diffusion (Equation (6)) | ki, mg/(g/min−1/2) | 0.0010 | 0.0008 | 0.000483 | 0.6295 | 0.6090 |
| Ci, mg/g | 0.1473 | |||||
| Intraparticle diffusion (Equations (7) and (8)) | ki1, mg/(g/min−1/2) | 0.0324 | 0.0012 | 0.000658 | 0.7048 | 0.6702 |
| ki2, mg/(g/min−1/2) | 0.0007 | |||||
| Bangham | kB, mL/(g/L) | 0.1358 | 0.5172 | 0.000227 | 0.8256 | 0.8159 |
| ϑ, dimensionless | 0.0348 | |||||
| MB initial concentration: 30 mg/L | ||||||
| Experimental data | Qe, mg/g | 0.2367 | ||||
| Pseudo-first-order | Qe, mg/g | 0.2328 | 1.0504 | 0.000443 | 0.8776 | 0.8708 |
| k1, min−1 | 0.2693 | |||||
| Pseudo-second-order | Qe, mg/g | 0.2365 | 1.0508 | 0.000074 | 0.9793 | 0.9781 |
| k2, g/(mg·min) | 2.7981 | |||||
| Intraparticle diffusion (Equation (6)) | ki, mg/(g/min−1/2) | 0.0016 | 0.0020 | 0.001600 | 0.5587 | 0.5342 |
| Ci, mg/g | 0.2080 | |||||
| Intraparticle diffusion (Equations (7) and (8)) | ki1, mg/(g/min−1/2) | 0.0533 | 0.0182 | 0.005804 | 0.8429 | 0.8245 |
| ki2, mg/(g/min−1/2) | 0.0012 | |||||
| Bangham | kB, mL/(g/L) | 0.1896 | 1.0500 | 0.000883 | 0.7564 | 0.7428 |
| ϑ, dimensionless | 0.0390 | |||||
| MB initial concentration: 40 mg/L | ||||||
| Experimental data | Qe, mg/g | 0.2977 | ||||
| Pseudo-first-order | Qe, mg/g | 0.2934 | 1.6589 | 0.000609 | 0.9164 | 0.9117 |
| k1, min−1 | 0.2412 | |||||
| Pseudo-second-order | Qe, mg/g | 0.2985 | 1.6594 | 0.000110 | 0.9848 | 0.9839 |
| k2, g/(mg·min) | 1.9127 | |||||
| Intraparticle diffusion (Equation (6)) | ki, mg/(g/min−1/2) | 0.0021 | 0.0035 | 0.003730 | 0.4879 | 0.4595 |
| Ci, mg/g | 0.2598 | |||||
| Intraparticle diffusion (Equations (7) and (8)) | ki1, mg/(g/min−1/2) | 0.0603 | 0.0349 | 0.012444 | 0.8084 | 0.7860 |
| ki2, mg/(g/min−1/2) | 0.0002 | |||||
| Bangham | kB, mL/(g/L) | 0.2345 | 1.6576 | 0.002200 | 0.6979 | 0.6811 |
| ϑ, dimensionless | 0.0422 | |||||
| MB initial concentration: 50 mg/L | ||||||
| Experimental data | Qe, mg/g | 0.3552 | ||||
| Pseudo-first-order | Qe, mg/g | 0.3513 | 2.3805 | 0.000556 | 0.9453 | 0.9422 |
| k1, min−1 | 0.2443 | |||||
| Pseudo-second-order | Qe, mg/g | 0.3572 | 2.3808 | 0.000296 | 0.9708 | 0.9692 |
| k2, g/(mg·min) | 1.6499 | |||||
| Intraparticle diffusion (Equation (6)) | ki, mg/(g/min−1/2) | 0.0024 | 0.0046 | 0.005550 | 0.4529 | 0.4239 |
| Ci, mg/g | 0.3128 | |||||
| Intraparticle diffusion (Equations (7) and (8)) | ki1, mg/(g/min−1/2) | 0.0801 | 0.0382 | 0.009936 | 0.8722 | 0.8572 |
| ki2, mg/(g/min−1/2) | 0.0006 | |||||
| Bangham | kB, mL/(g/L) | 0.2834 | 2.3776 | 0.0034 | 0.6604 | 0.6415 |
| ϑ, dimensionless | 0.0404 | |||||
| Equilibrium Isotherm | Parameters | Values | Standard Error | Sum of Squares | Residual Sum of Squares | R2 | Adj. R2 |
|---|---|---|---|---|---|---|---|
| Experimental data | Qe, mg/g | 0.3552 | 0.0005 | ||||
| Langmuir | QL, mg/g | 0.5567 | 0.0223 | 0.3053 | 0.000089 | 0.9980 | 0.9973 |
| KL, L/mg | 0.1177 | 0.0096 | |||||
| Freundlich | KF, (mg/g)(L/mg)1/nF | 0.0785 | 0.0076 | 0.3049 | 0.000488 | 0.9892 | 0.9857 |
| nF, dimensionless | 1.7504 | 0.1305 | |||||
| Jovanovic | QJ, mg/g | 0.3929 | 0.0175 | 0.3052 | 0.000235 | 0.9948 | 0.9931 |
| KJ, L/g | 0.1495 | 0.0143 | |||||
| Toth | QTo, mg/g | 0.4310 | 0.1826 | 0.3053 | 0.000070 | 0.9984 | 0.9969 |
| KTo, L/mg | 0.1497 | 0.0640 | |||||
| nTo, dimensionless | 0.8867 | 0.1582 | |||||
| Sips | QS, mg/g | 0.5766 | 0.0668 | 0.3053 | 0.000084 | 0.9981 | 0.9962 |
| KS, L/mg | 0.0991 | 0.0505 | |||||
| nS, dimensionless | 0.9344 | 0.1777 | |||||
| Redlich–Peterson | KR, L/g | 0.0727 | 0.0131 | 0.3053 | 0.000070 | 0.9984 | 0.9969 |
| αR, 1/mg | 0.1853 | 0.1261 | |||||
| nR, dimensionless | 0.8870 | 0.1588 | |||||
| Khan | QK, mg/g | 0.3459 | 0.1922 | 0.3053 | 0.000063 | 0.9986 | 0.9972 |
| KK, L/mg | 0.2024 | 0.1359 | |||||
| nK, dimensionless | 0.7736 | 0.2077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoraș, C.-G.; Simion, A.-I. Synthesis of a New Composite Material Derived from Cherry Stones and Sodium Alginate—Application to the Adsorption of Methylene Blue from Aqueous Solution: Process Parameter Optimization, Kinetic Study, Equilibrium Isotherms, and Reusability. J. Compos. Sci. 2024, 8, 402. https://doi.org/10.3390/jcs8100402
Grigoraș C-G, Simion A-I. Synthesis of a New Composite Material Derived from Cherry Stones and Sodium Alginate—Application to the Adsorption of Methylene Blue from Aqueous Solution: Process Parameter Optimization, Kinetic Study, Equilibrium Isotherms, and Reusability. Journal of Composites Science. 2024; 8(10):402. https://doi.org/10.3390/jcs8100402
Chicago/Turabian StyleGrigoraș, Cristina-Gabriela, and Andrei-Ionuț Simion. 2024. "Synthesis of a New Composite Material Derived from Cherry Stones and Sodium Alginate—Application to the Adsorption of Methylene Blue from Aqueous Solution: Process Parameter Optimization, Kinetic Study, Equilibrium Isotherms, and Reusability" Journal of Composites Science 8, no. 10: 402. https://doi.org/10.3390/jcs8100402






