Bond Strength and Corrosion Protection Properties of Hot-Dip Galvanized Prestressing Reinforcement in Normal-Strength Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used
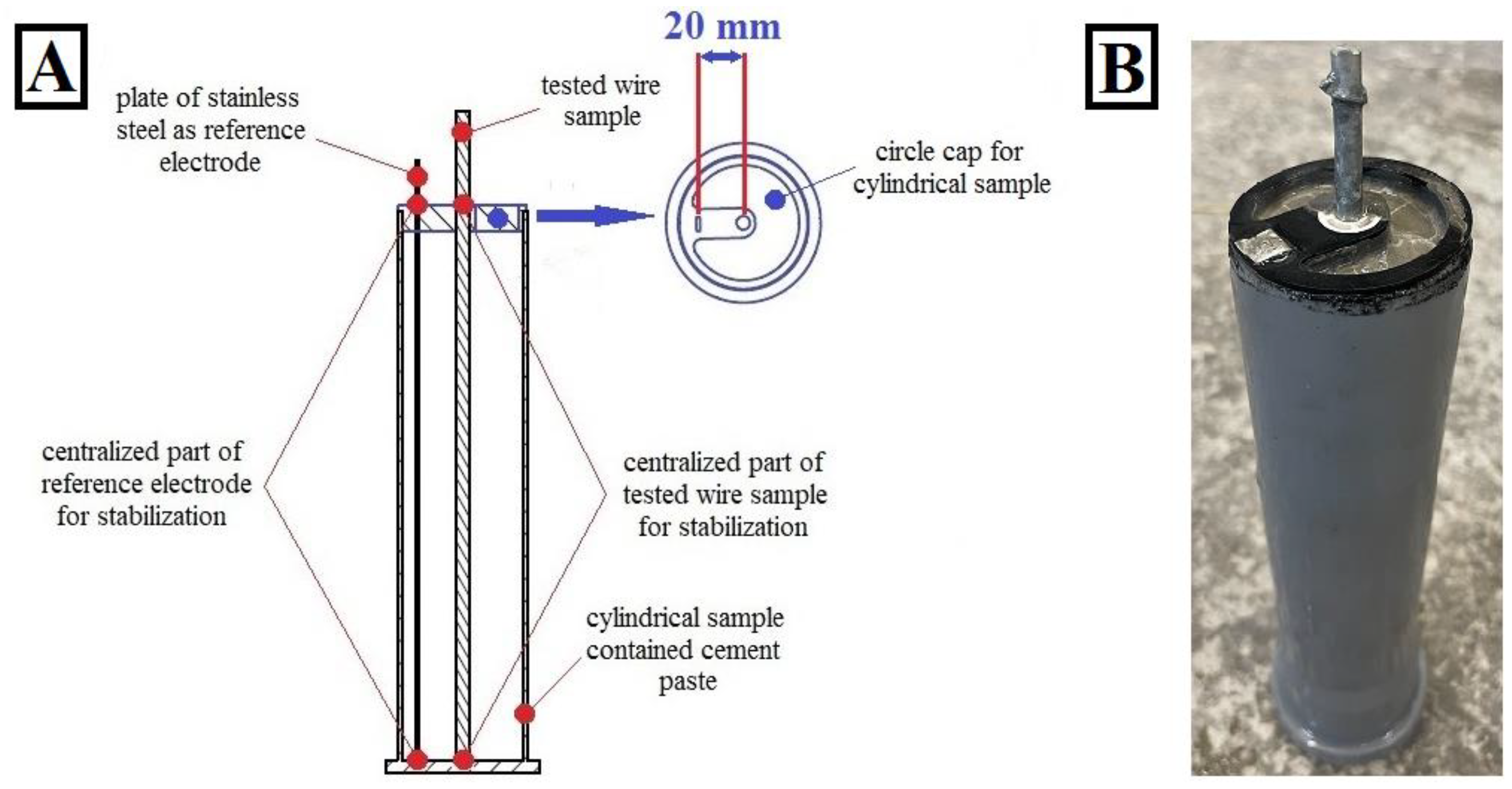
2.2. Corrosion Investigations
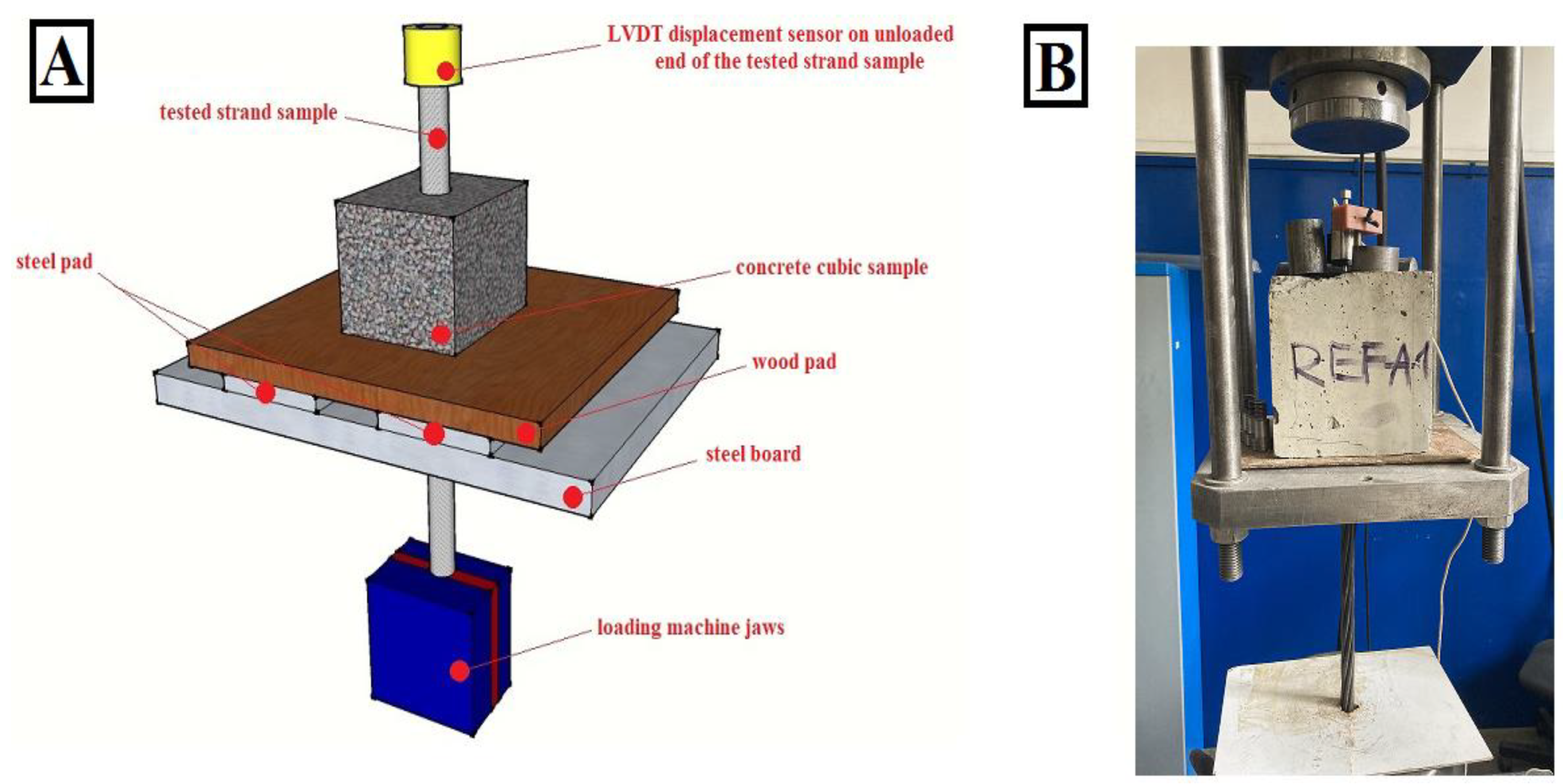
2.3. Bond Strength Investigation
2.4. Scanning Electron Microscopy
2.5. Roughness Investigations of Prestressing Reinforcement
2.6. X-ray Diffraction of Corrosion Products
3. Results and Discussion
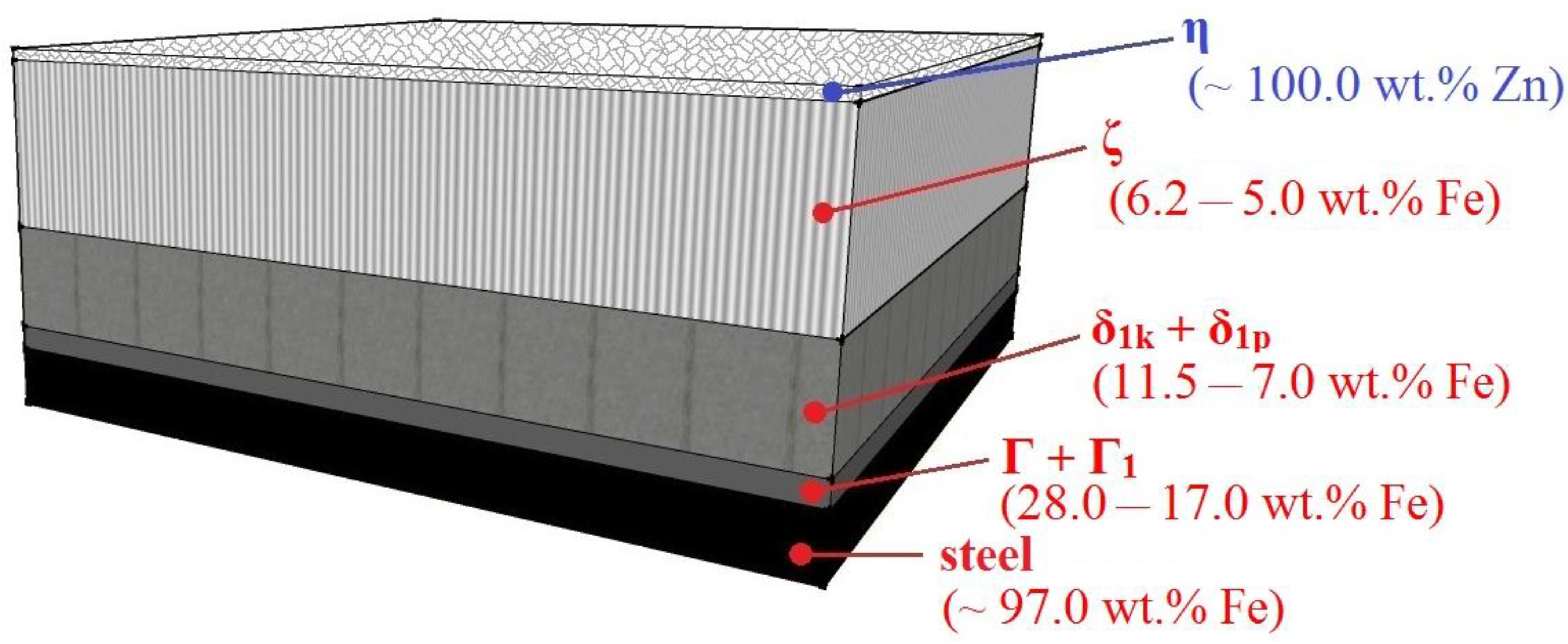
3.1. Structure of HDG Coating
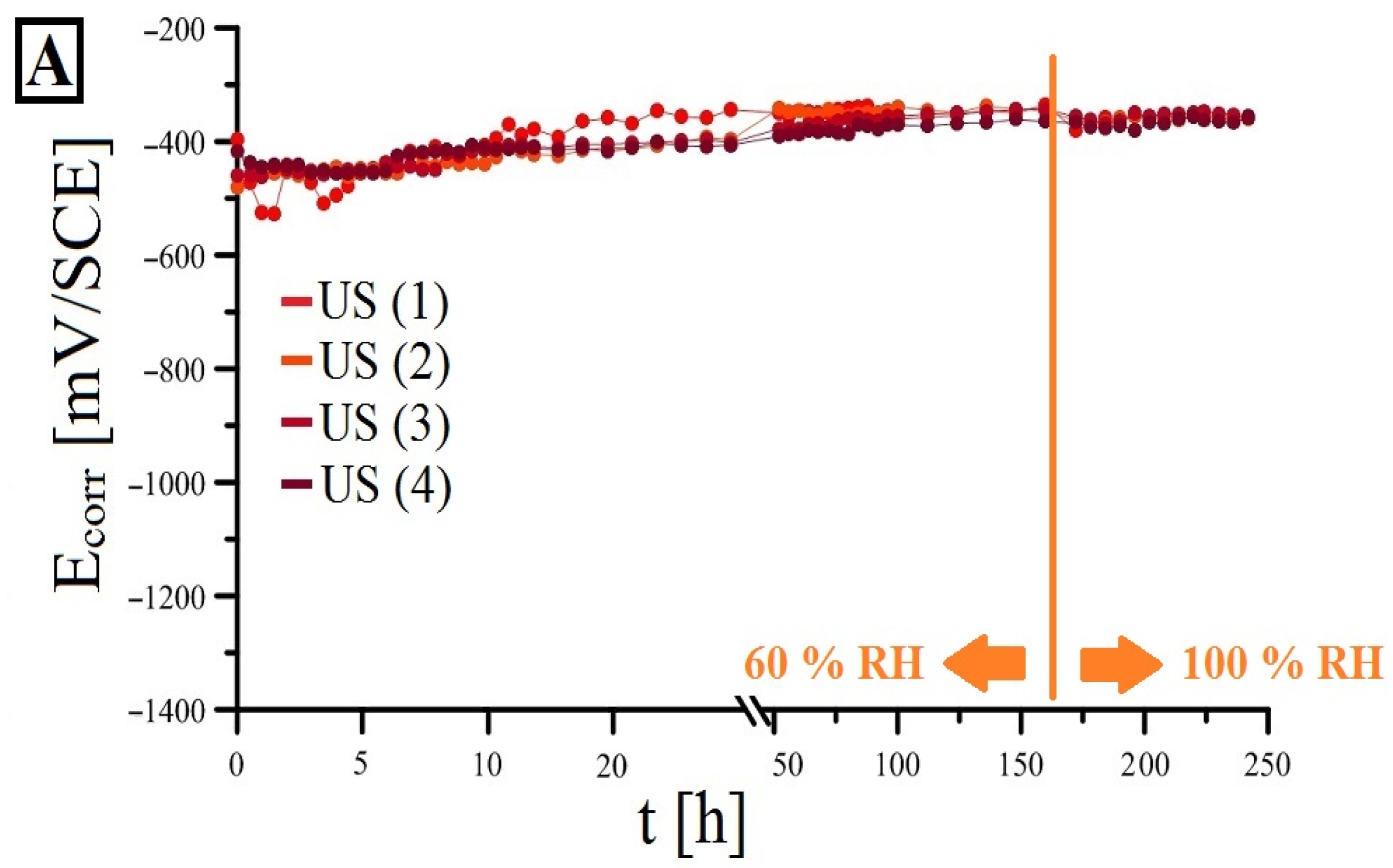
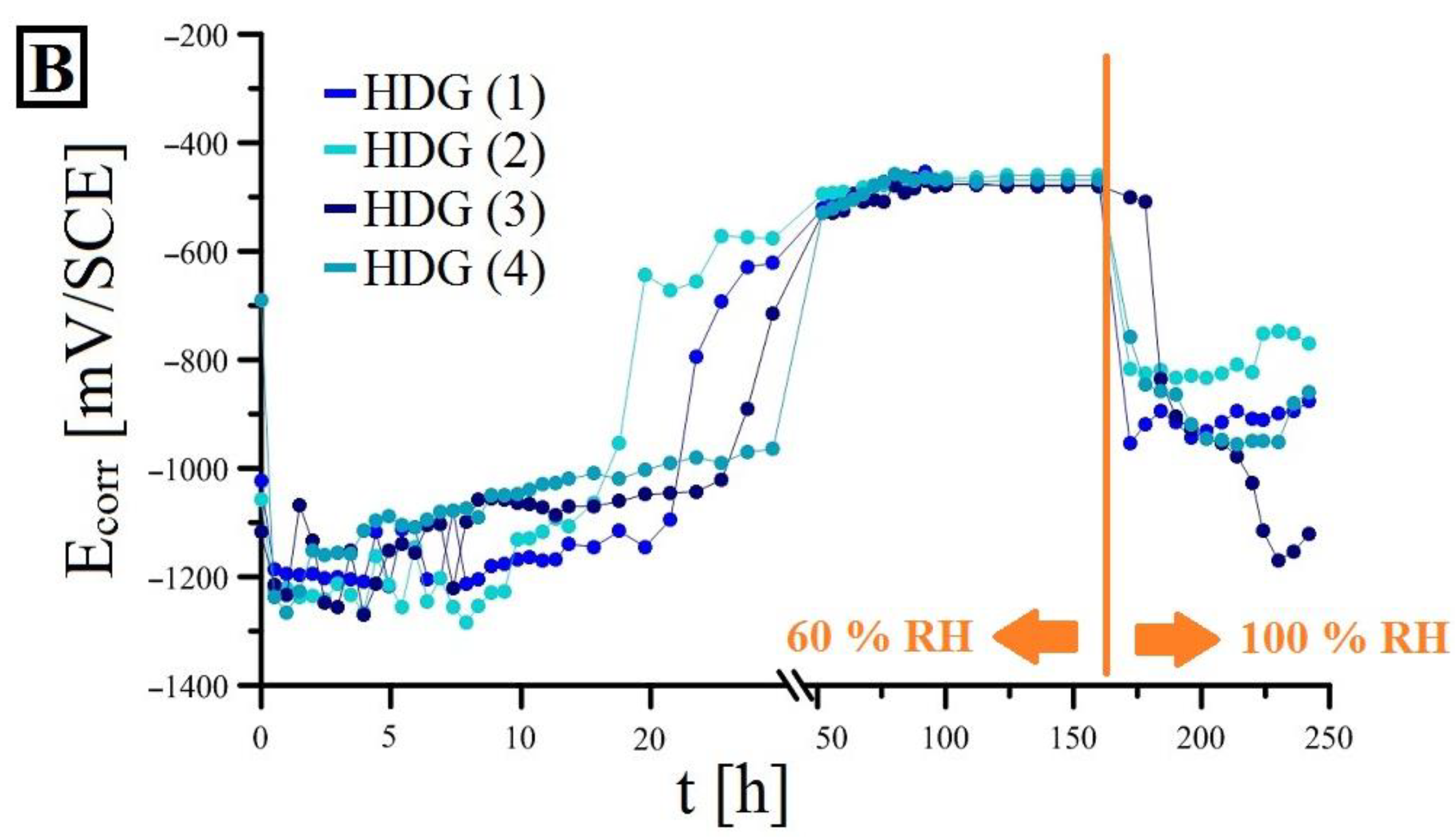
3.2. Open-Circuit Potential Measurements
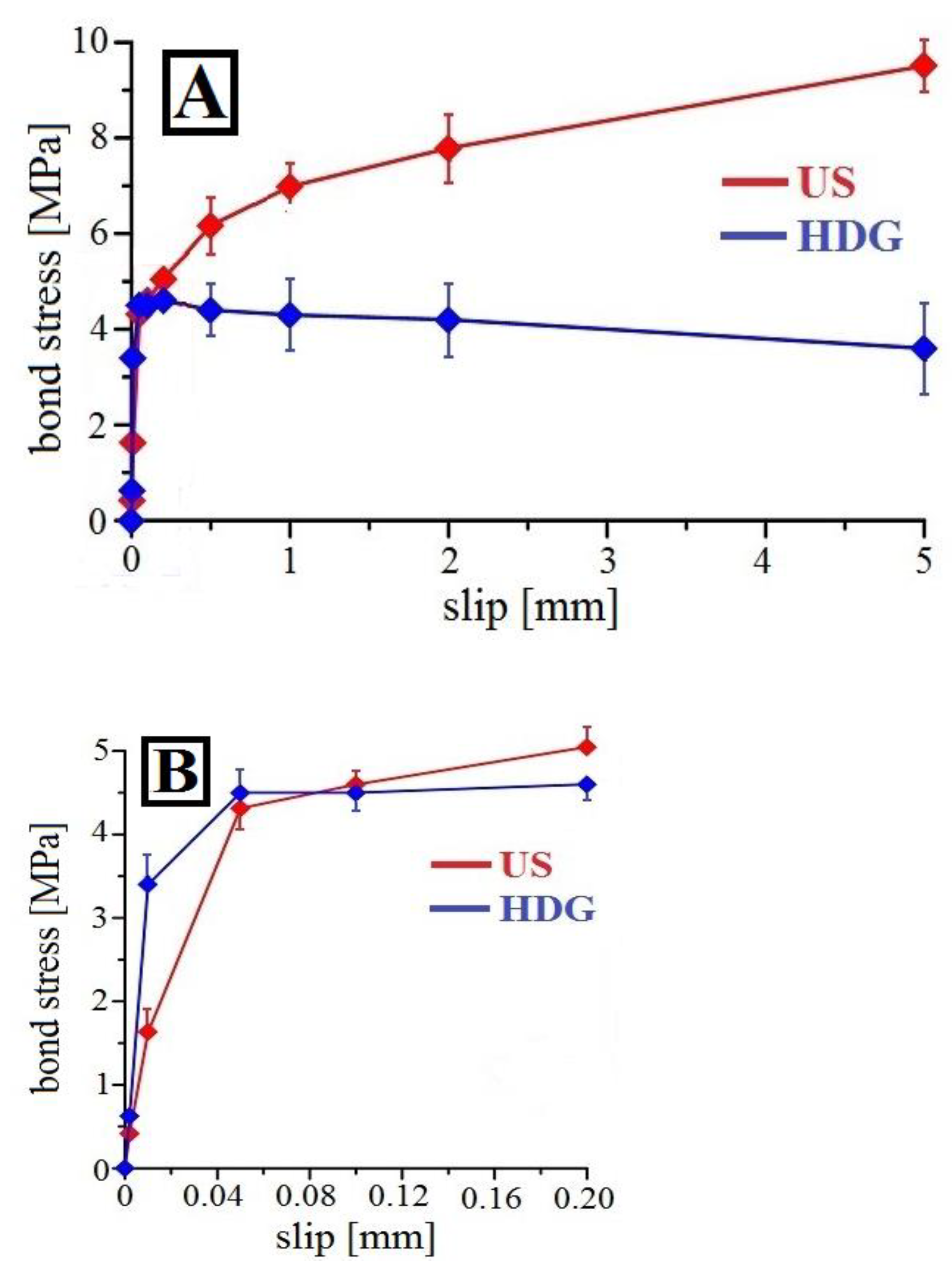
3.3. Bond Strength
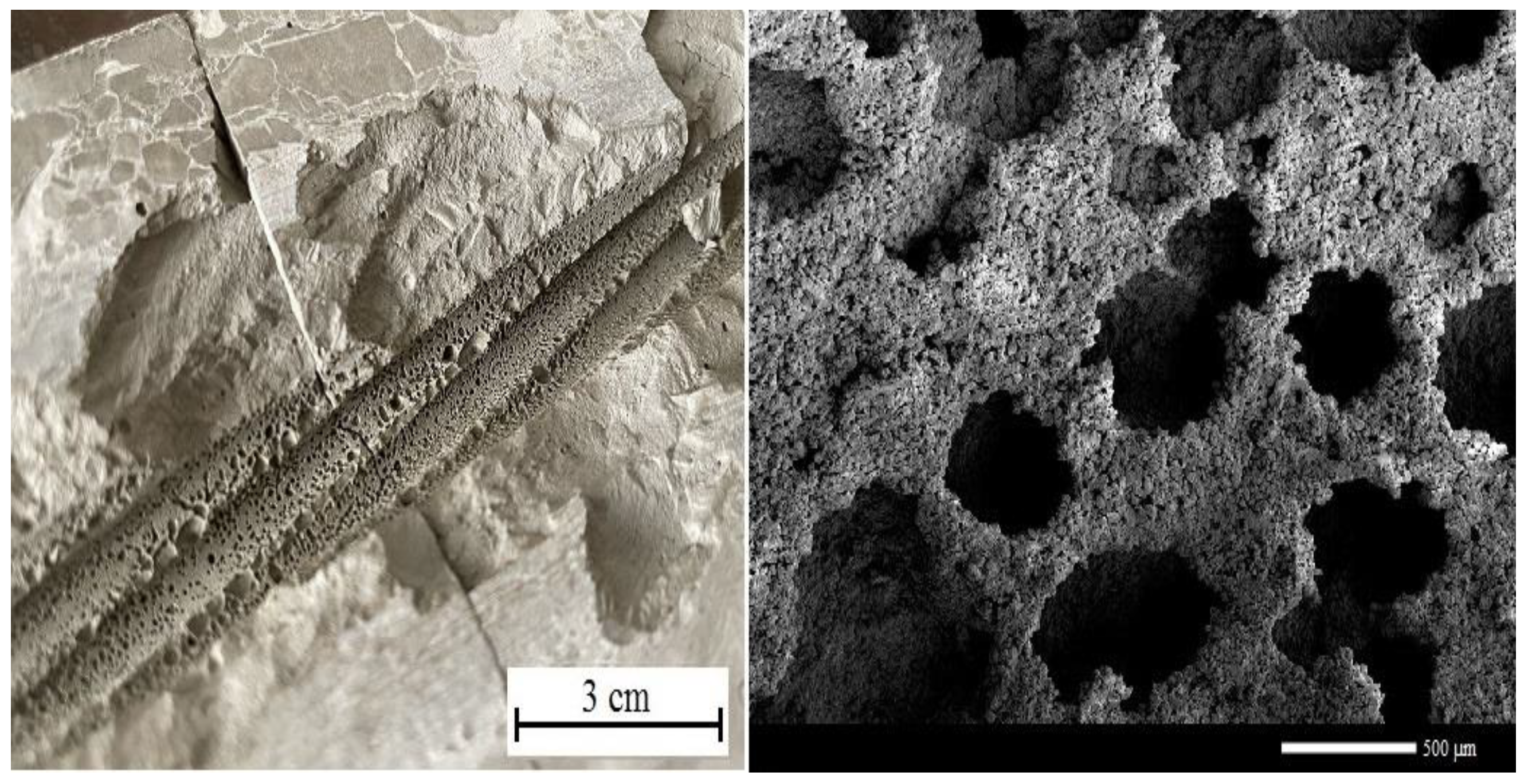
3.4. Cement Paste Porosity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borges da Fonsêca, M.S.D.A.; Meira, G.R. Accelerated chloride-induced corrosion of hot-dipped galvanized reinforcements and its influence on the bond strength to concrete. Constr. Build. Mater. 2024, 426, 136123. [Google Scholar] [CrossRef]
- Al-Negheimish, A.; Hussain, R.R.; Alhozaimy, A.; Singh, D.D.N. Corrosion performance of hot-dip galvanized zinc-aluminium coated steel rebars in comparison to the conventional pure zinc coated rebars in concrete environment. Constr. Build. Mater. 2021, 274, 121921. [Google Scholar] [CrossRef]
- Xie, W.; Li, J.; Li, Y. Electrochemical corrosion behavior of carbon steel and hot dip galvanized steel in simulated concrete pore solution with different pH values. Mater. Sci. 2017, 23, 280–284. [Google Scholar]
- Fagueira, R.B.; Silva, C.J.R.; Pereira, E.V. Hybrid sol-gel coatings for corrosion protection of hot-dip galvanized steel in alkaline medium. Surf. Coat. Technol. 2015, 265, 191–204. [Google Scholar] [CrossRef]
- Zheng, H.; Dai, J.G.; Poon, C.S.; Li, W. Influence of a superplasticizer on initial corrosion of galvanized steel bars in concrete pore solution. J. Mater. Civ. Eng. 2021, 33, 04021113. [Google Scholar] [CrossRef]
- Pokorný, P.; Pernicová, R.; Tej, P.; Kolísko, J. Changes of bond strength properties of hot-dip galvanized plain bars with cement paste after 1 year of curing. Constr. Build. Mater. 2019, 226, 920–931. [Google Scholar] [CrossRef]
- Gallego, A. Comparison between concrete-black steel and concrete-galvanized steel bond via the pull-out test supplied with acoustic emission. In Proceedings of the European Working Group on Acoustic Emission, Berlin, Germany, 15–17 September 2004; pp. 761–767. [Google Scholar]
- Hamad, B.S.; Jumaa, G.K. Bond strength of hot-dip galvanized hooked bars in high strength concrete structures. Constr. Build. Mater. 2008, 22, 2042–2052. [Google Scholar] [CrossRef]
- Belaïd, F.; Arliguie, G.; François, R. Porous structure of ITZ around galvanized and ordinary steel reinforced. Cem. Concr. Res. 2001, 31, 1561–1566. [Google Scholar] [CrossRef]
- Fratesi, R.; Moriconi, G.; Coppola, L. The Influence of Steel Galvanization on Rebars Behaviour in Concrete. In Corrosion of Reinforcement in Concrete Construction; The Royal Society of Chemistry: London, UK, 1996; pp. 630–640. [Google Scholar]
- Arenas, M.A.; Casado, C.; Nobel-Pujol, V.; Damborenea, J. Influence of the conversion coating on the corrosion of galvanized steel. Cem. Concr. Compos. 2006, 28, 267–275. [Google Scholar] [CrossRef]
- Bikulčius, G. Corrosion behaviour in alkaline media of steel with various conversion coatings in concrete. J. Appl. Chem. 2003, 76, 1809–1813. [Google Scholar]
- Sanchez, M.; Alonso, M.C.; Cecilio, P.; Montemor, M.F.; Andrade, C. Electrochemical and analytical assessment of galvanized steel reinforcement pre-treated with Ce and La salts under alkaline media. Cem. Concr. Compos. 2006, 28, 256–266. [Google Scholar] [CrossRef]
- Pokorný, P.; Kouřil, M. Predicted corrosion performance of organofunctional silane coated steel reinforcement for concrete structures: An overview. Buildings 2024, 14, 1756. [Google Scholar] [CrossRef]
- Prüfbericht. Bundesanstalt für Materialforschung und-Prüfung, Prüfung von einem Nachbehandlungsmittel an Feurverzinktem Betonstahl; BAM: Bam, Iran, 2018; pp. 1–6. [Google Scholar]
- King, D. Pre-Stressed Concrete Structure with Galvanized Reinforcement. U.S. Patent US 10,753,095 B2, 25 August 2020. [Google Scholar]
- EN 206 + A2. Concrete—Specification, Performance, Production and Conformity. ÚNMZ: Prague, Czech Republic, 2020.
- Hájková, K.; Šmilauer, V.; Jendele, L.; Červenka, J. Prediction of reinforcement corrosion due to chloride ingress and its effects on serviceability. Eng. Struct. 2018, 174, 768–777. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chou, J.-S. Bayesian-optimized deep learning model to segment deteroriation patterns underneath bridge decks photographed by unmanned aerial vehicle. Autom. Constr. 2023, 146, 104666. [Google Scholar] [CrossRef]
- Morgese, M.; Ansari, F.; Domaneschi, M.; Cimellaro, G.P. Post-collapse analysis of Morandi’s Polcevera viaduct in Genoa Italy. J. Civ. Struct. Health Monit. 2020, 10, 69–85. [Google Scholar] [CrossRef]
- Dorsten, V.; Hunt, F.F.; Preston, H.K. Epoxy coated seven-wire strand for pre-stressed concrete. Pre-Stress. Concr. Inst. 1984, 29, 120–129. [Google Scholar]
- Andrade, C.; Macias, A. Galvanized Reinforcement in Concrete, Surface Coatings-2; Wilson, A.D., Nicholson, J.W., Prosser, H.J., Eds.; Elsevier Applied Science Publishers Ltd.: Amsterdam, The Netherlands, 1988; pp. 137–182. [Google Scholar]
- Bellezze, T.; Malavolta, M.; Quaranta, A.; Ruffini, N.; Roventi, G. Corrosion behaviour in concrete of tree differently galvanized steel bars. Cem. Concr. Compos. 2006, 28, 246–255. [Google Scholar] [CrossRef]
- Sistonen, E. Service Life of Hot Dip Galvanized Reinforcement Bars in Carbonated and Chloride Contaminated Concrete. Ph.D. Thesis, Structural Enginnering and Building Technology, Helsinki University of Technology, Helsinki, Finland, 2000. [Google Scholar]
- Vontorová, J.; Mohyla, P.; Kreislová, K. Quality of zinc coating formed on structural steel by hot-dip galvanizing after surface contamination. Coatings 2024, 14, 493. [Google Scholar] [CrossRef]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar]
- Okamoto, N.L.; Yasuhara, A.; Inui, H. Order-disorder structure of the δ1k phase in the Fe-Zn system determined by scanning transmission electron microscopy. Acta Mater. 2014, 81, 345–357. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Tanaka, K. Structure refinement of the δ1p phase in the Fe-Zn system by single crystal X-ray diffraction combined with scanning transmission electron microscopy. Acta Crystallogr. 2014, 70, 275–282. [Google Scholar]
- Yu, J.; Liu, J.; Zhou, W.; Zhang, J.; Wu, J. Cross-sectional TEM observation of iron—Zinc intermetallic Γ and Γ1 phases in commercial galvannealed IF steel sheets. Mater. Des. 2007, 28, 249–253. [Google Scholar] [CrossRef]
- Maahn, E.; Sorensen, B. The Influence of microstructure on the corrosion properties of hot–dip galvanized reinforcement in concrete. Corrosion 1986, 42, 187–196. [Google Scholar] [CrossRef]
- Bird, C.E. The influence of minor constituents in Portland cement on the behavior of galvanized steel in concrete. Corros. Prev. Control. 1964, 17–21. [Google Scholar]
- Lee, H.H.; Hiam, D. Corrosion resistance of galvannealed steel. Corrosion 1989, 45, 852–856. [Google Scholar] [CrossRef]
- prEN 10138-3; Pre-Stressing Steels—Part 3: Strand. European Committee for Standardization: Brussel, Belgium.
- Available online: https://wiegel.de/cs/standorte/cenkov/ (accessed on 18 September 2024).
- Kondraivendhan, B.; Bhattacharjee, B. Strength and w/c ratio relationship of cement based materials though pore features. Mater. Charact. 2016, 122, 124–129. [Google Scholar] [CrossRef]
- Kurda, R.; De Brito, J.; Silvestre, J.D. Water absorption and electrical resistivity of concrete with recycled concrete aggregates and fly ash. Cem. Concr. Compos. 2019, 95, 169–182. [Google Scholar] [CrossRef]
- Song, X.; Wu, Y.; Gu, X.; Chen, C. Bond behaviour of reinforcing steel bars in early age concrete. Constr. Build. Mater. 2015, 94, 209–217. [Google Scholar] [CrossRef]
- RILEM RC6; Bond Test for Reinforcement Steel. 2: Pull-Out Test. E & FN SPON: Paris, France, 1983.
- ASTM C234-91a; Standard Test Method for Comparing Concretes on the Basis of the Bond Developed with Reinforcing Steel. ASTM International: West Conshohocken, PA, USA, 1991.
- Matsumoto, K.; Wang, T.; Hayashi, D.; Nagai, K. Investigation on the pull-out behavior of deformed bars in cracked reinforced concrete. J. Adv. Concr. Technol. 2016, 14, 573–589. [Google Scholar] [CrossRef]
- Pistofidis, N.; Vourlias, G.; Konidaris, S.; Pavlidou, E.; Stergiou, A.; Stergioudis, G. Microstructure of zinc hot-dip galvanized coatings used for corrosion protection. Mater. Lett. 2006, 60, 786–789. [Google Scholar] [CrossRef]
- Khabaz, A. Analysis of sliding mechanism of straight steel fibers in concrete and determine the effect of friction. Arch. Civ. Mech. Eng. 2017, 17, 599–608. [Google Scholar] [CrossRef]
- Jiang, C.; Wu, Y.-F.; Dai, M.-J. Degradation of steel-to-concrete bond due to corrosion. Constr. Build. Mater. 2018, 158, 1073–1080. [Google Scholar] [CrossRef]
- Blumenau, M.; Norden, M.; Friedel, F.; Peters, K. Reactive wetting during hot-dip galvanizing of high manganese alloyed steel. Surf. Coat. Technol. 2011, 205, 3319–3327. [Google Scholar] [CrossRef]
- Persson, D.; Thierry, D.; Karlsson, O. Corrosion and corrosion products of hot dipped galvanized steel during long term atmospheric exposure at different sites world-wide. Corros. Sci. 2017, 126, 152–165. [Google Scholar] [CrossRef]
- Alvarenga, E.d.A.; Lins, V.F.C. Atmospheric corrosion evaluation of electrogalvanized, hot-dip galvanized and galvannealed intersticial free steels using accelerated field and cyclic tests. Surf. Coat. Technol. 2016, 306, 428–438. [Google Scholar] [CrossRef]
- Carpio, J.; Casado, J.A.; Álvarez, J.A.; Gutiérrez-Solana, F. Environmental factors in failure during structural steel hot-dip galvanizing. Eng. Fail. Anal. 2009, 16, 585–595. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, J.; Meng, H. A review of recent developments in coating systems for hot dip galvanized steel. Front. Mater. 2020, 7, 74. [Google Scholar] [CrossRef]
- Andrade, C.; Holst, J.D.; Nürnberger, U.; Whiteley, J.D.; Woodman, N. Protection System for Reinforcement; CEB-Bulletin D’Information: Lousanne, Switzerland, 1992. [Google Scholar]
- Macías, A.; Andrade, C. Corrosion rate of galvanized steel immersed in saturated solutions of Ca(OH)2 in the pH range 12-13.8. Br. Corros. J. 1983, 18, 82–87. [Google Scholar] [CrossRef]
- Kania, H.; Mendala, J.; Kozuba, J.; Saternus, M. Development of bath chemical composition for batch hot-dip galvanizing—A review. Materials 2020, 13, 4168. [Google Scholar] [CrossRef]
- Bondareva, O.S.; Melnikov, A.A. Effect of silicon content in steel on the hot-dip zinc coating microstructure formation. IOP Conf. Ser. Mater. Sci. Eng. 2016, 156, 012015. [Google Scholar] [CrossRef]
- Yeomans, S.R. Galvanized Steel Reinforcement in Concrete, 2nd ed.; Elsevier: Canberra, Australia, 2004. [Google Scholar]
- Santos, P. Influencia de la Estructura Metalográfica y del tipo de Cemento en la Corrosion de Armaduras Galvanizadas. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 1986. [Google Scholar]
- Valcarce, M.B.; Vázquez, M. Carbon steel passivity examined in alkaline solutions: The effect of chloride and nitrite ions. Electrochim. Acta 2008, 53, 5007–5015. [Google Scholar] [CrossRef]
- Elsener, B. Macrocell corrosion of steel in concrete—Implications for corrosion monitoring. Cem. Concr. Compos. 2002, 24, 65–72. [Google Scholar] [CrossRef]
- Lu, P.; Kursten, B.; Macdonald, D.D. Deconvolution of the partial anodic and cathodic processes during the corrosion of carbon steel in concrete pore solution under simulated anoxic conditions. Electrochim. Acta 2014, 143, 312–323. [Google Scholar] [CrossRef]
- Eichler, T.; Isecke, B.; Bäßler, R. Investigations on the re-passivation of carbon steel in chloride containing concrete in consequence of cathodic polarization. Mater. Corros. 2009, 60, 119–129. [Google Scholar] [CrossRef]
- Williamson, J.; Isgor, O.B. The effect of simulated concrete pore solution composition and chlorides on the electronic properties of passive films on carbon steel rebar. Corros. Sci. 2016, 106, 82–95. [Google Scholar] [CrossRef]
- Freire, L.; Nóvoa, X.R.; Montemor, M.F.; Carmezim, M.J. Study of passive films formed on mild steel in alkaline media by the application of anodic potentials. Mater. Chem. Phys. 2009, 114, 962–972. [Google Scholar] [CrossRef]
- Volpi, E.; Olietti, A.; Stefanoni, M.; Trasatti, S.P. Electrochemical characterization of mild steel in alkaline solutions simulating concrete environment. J. Electroanal. Chem. 2015, 736, 38–46. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Y.; Liu, L.; Pu, Q.; Jiang, L.; Chu, H.; Luo, Y.; Liu, Q.; Cai, H. Use of XPS for quantitative evaluation of tensile-stress-induced degradation of passive film on carbon steel in simulated concrete pore solutions. Constr. Build. Mater. 2021, 274, 121779. [Google Scholar] [CrossRef]
- Macías, A.; Andrade, C. Corrosion of galvanized steel reinforcements in alkaline solutions. Part 1: Electrochemical results. Br. Corros. J. 1987, 22, 113–118. [Google Scholar] [CrossRef]
- Macías, A.; Andrade, C. Corrosion of galvanized steel reinforcements in alkaline solutions. Part 2: SEM study and identification of corrosion products. Br. Corros. J. 1987, 22, 119–129. [Google Scholar] [CrossRef]
- Menzel, K. Zur Korrosion von verzinktem Stahl in Kontakt mit Beton–IWB (Mitteilungen); Universität Stuttgart: Stuttgart, Germany, 1992. [Google Scholar]
- Wienerová, K.; Kouřil, M.; Stoulil, J. Koroze a ochrana zinkované oceli v prostředí betonu. Koroze Ochr. Mater. 2010, 54, 148–154. [Google Scholar]
- Macias, A.; Andrade, C. Stability of the calcium hydroxyzincate protective layer developer on galvanized reinforcements after a further increase of the pH value. Mater. Constr. 1986, 36, 19–28. [Google Scholar]
- Macias, A.; Andrade, C. Galvanized steel behaviour in Ca(OH)2 saturated solution containing SO4 ions. Cem. Concr. Res. 1987, 17, 307–316. [Google Scholar] [CrossRef]
- González, J.A.; Andrade, C. Effect of carbonation, chlorides and relative ambient humidity on the corrosion of galvanized rebars embedded in concrete. Br. Corros. J. 1982, 17, 21–28. [Google Scholar] [CrossRef]
- Andrade, C.; Macias, A. Influencia del contenido en álcalis de los cementos sobre la corrosión de armaduras galvanizadas. Mater. Constr. 1981, 184, 83–93. [Google Scholar] [CrossRef]
- Yeomans, S.R. Galvanized steel reinforcement—A prespect view, conference. In Proceedings of the Real World Concrete–Symposium of RN Swamy, Milwaukee, WI, USA, 4–9 June 1995; American Concrete Institute: Farmington Hills, MI, USA, 1995; pp. 57–70. [Google Scholar]
- Yeomans, S.R. Comparative Studies of Galvanized and Epoxy Coated Steel Reinforcement in Concrete; Research Report, No. R103; The University of New South Wales: Canberra, Australia, 1991; pp. 1–15. [Google Scholar]
- Gong, Z.; Gao, Y.; Huang, X. Influence of surface roughness on the morphology of interfacial layer and on out-brust formation during hot-dip galvanizing. SN Appl. Sci. 2020, 2, 550. [Google Scholar] [CrossRef]
- Kaboli, S.; McDermid, J.R. Effect of process variables on the grain size and crystallographic texture of hot dip galvanized coatings. Metall. Mater. Trans. A 2014, 45, 3938–3953. [Google Scholar] [CrossRef]
- Baran, E.; Akis, T.; Yesilmen, S. Pull-out behavior of pre-stressing strands in steel fiber reinforced concrete. Constr. Build. Mater. 2012, 28, 362–371. [Google Scholar] [CrossRef]
- Mohandoss, P.; Pillai, R.G.; Gettu, R. Determining bond strength of seven-wire strands in pre-stressed concrete. Structures 2021, 33, 2413–2423. [Google Scholar] [CrossRef]
- Sahamitmongkol, R.; Kishi, T. Tension stiffening effect and bonding characteristics of chemically pre-stressed concrete under tension. Mater. Struct. 2011, 44, 455–474. [Google Scholar] [CrossRef]
- Kurdowski, W. Cement and Concrete Chemistry; Springer: London, UK, 2014. [Google Scholar]
- Al Khalaf, M.N.; Page, C.L. Steel/mortar interfaces: Microstructural features and mode of failure. Cem. Concr. Res. 1979, 9, 197–207. [Google Scholar] [CrossRef]
- Nie, R.; Huang, Y.; Li, X.; Sun, H.; Li, D.; Ying, J. Bond of epoxy-coated reinforcement to seawater coral aggregate concrete. Ocean. Eng. 2020, 208, 107350. [Google Scholar] [CrossRef]
- Chang, Y.; Qin, S.; Huang, M.; Hu, D.; Yang, H.; Li, S. Analytical model of the bond stress-slip relationship for reinforced concrete due to splitting failure. Constr. Build. Mater. 2021, 287, 123025. [Google Scholar] [CrossRef]
- Lieber, W.; Gebauer, J. Einbau von Zink in Calciumsilicathydrate. Zement-Klak-Gips 1969, 4, 161–164. [Google Scholar]
- Asavapisit, S.; Fowler, G. Solution chemistry during cement hydratation in the presence of metal hydroxide wastes. Cem. Concr. Res. 1997, 27, 1249–1260. [Google Scholar] [CrossRef]
- Mellado, A.; Borrachero, M.V.; Soriano, L.; Paya, J.; Monzo, J. Immobilization of Zn(II) in portland cement pastes. J. Therm. Anal. Calorim. 2013, 112, 1377–1389. [Google Scholar] [CrossRef]
- Tashiro, C.; Oba, J. The effect of Cr2O3, Cu(OH)2, ZnO and PbO on the compressive strength and the hydrates of the hardened C3A paste. Cem. Concr. Res. 1979, 9, 253–258. [Google Scholar] [CrossRef]
- Tan, Z.Q. The Effect of Galvanized Steel Corrosion on Integrity of Concrete. Master’s Thesis, Applied Science in Civil Engineering, University of Waterloo, Waterloo, ON, Canada, 2007. [Google Scholar]
- Rovnaníková, P.; Bayer, P. Změny v mikrostruktuře cementového tmelu na styku s pozinkovanou výztuží v čase. In Proceedings of the Konference AKI, Bystřice nad Pernštejnem, Czech Republic, 2003; pp. 19–20. [Google Scholar]
- Rovnaníková, P.; Bayer, P. Study of the interfacial transition zone between cement paste and zinc coated steel reinforcement. In Proceedings of the 13th International Conference Corrosion of Underground Structures, Kosice, Slovakia, 27–28 May 2003; pp. 65–70. [Google Scholar]
- Rovnaníková, P.; Bayer, P. Role of the physical-chemical methods for evaluation of the relation between galvanized steel-cement stone. CERM 2004, 469–474. [Google Scholar]
- Lai, D.L.; Jiang, Y.B.; Wang, Y.Q.; Kong, G.; Che, C.S. Improved corrosion resistance of galvanized steel with a zinc phosphate coating in alkaline solution. Int. J. Electrochem. Sci. 2020, 15, 4853–4868. [Google Scholar] [CrossRef]
- Kong, G.; Liu, L.; Lu, J.; Che, C.; Zhong, Z. Study on lanthanum salt conversion coating modified with citric acid on hot dip galvanized steel. J. Rare Earths 2010, 28, 461–465. [Google Scholar] [CrossRef]
- Bailey, R.W.; Ridge, H.G. Corrosion of metals in buildings. Chem. Ind. 1957, 1222–1227. [Google Scholar]
- Burchett, K.R. Metal Treatment to Prevent Corrosion and Blemishes in Metal Reinforced Concrete Structures. U.S. Patent 3619441, 9 November 1971. [Google Scholar]













| Compound | C | Mn | Si | P | S | N | Fe |
|---|---|---|---|---|---|---|---|
| Content (wt. %) | 0.81 | 0.72 | 0.26 | 0.008 | 0.009 | 0.006 | balance |
| Property | Value and Unit |
|---|---|
| Ultimate tensile strength | 1860 MPa |
| Nominal diameter | 15.7 mm |
| Nominal density | ~1172 g/m |
| Yield strength (0.1%) | 246 kN |
| Young’s modulus | ~195 GPa |
| Maximum elongation | 3.5% |
| Compound | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|
| content (wt. %) | 64.2 | 19.5 | 4.7 | 3.2 | 1.3 | 3.2 | 0.78 | 0.09 |
| Mixture | Portland Cement (kg) | Water (L) | Gravel (kg) | Sand (kg) |
|---|---|---|---|---|
| w/c: 0.35 | 14 | 4.9 | 0 | 0 |
| Admixture | Content (kg/m3) | Note |
|---|---|---|
| Cement (CEM I 42.5 R) | 365 | Pure Portland cement |
| Aggregate | 900 | Fraction 0/4—fine sand |
| 585 | Fraction 4/8 | |
| 285 | Fraction 8/16 | |
| Mixture (w/c) | 0.55 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokorný, P.; Chobotský, T.; Prodanovic, N.; Steinerová, V.; Hurtig, K. Bond Strength and Corrosion Protection Properties of Hot-Dip Galvanized Prestressing Reinforcement in Normal-Strength Concrete. J. Compos. Sci. 2024, 8, 407. https://doi.org/10.3390/jcs8100407
Pokorný P, Chobotský T, Prodanovic N, Steinerová V, Hurtig K. Bond Strength and Corrosion Protection Properties of Hot-Dip Galvanized Prestressing Reinforcement in Normal-Strength Concrete. Journal of Composites Science. 2024; 8(10):407. https://doi.org/10.3390/jcs8100407
Chicago/Turabian StylePokorný, Petr, Tomáš Chobotský, Nikola Prodanovic, Veronika Steinerová, and Karel Hurtig. 2024. "Bond Strength and Corrosion Protection Properties of Hot-Dip Galvanized Prestressing Reinforcement in Normal-Strength Concrete" Journal of Composites Science 8, no. 10: 407. https://doi.org/10.3390/jcs8100407
APA StylePokorný, P., Chobotský, T., Prodanovic, N., Steinerová, V., & Hurtig, K. (2024). Bond Strength and Corrosion Protection Properties of Hot-Dip Galvanized Prestressing Reinforcement in Normal-Strength Concrete. Journal of Composites Science, 8(10), 407. https://doi.org/10.3390/jcs8100407





