In Situ PANI–Graphite Nanochain-like Structures and Their Application as Supercapacitive Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
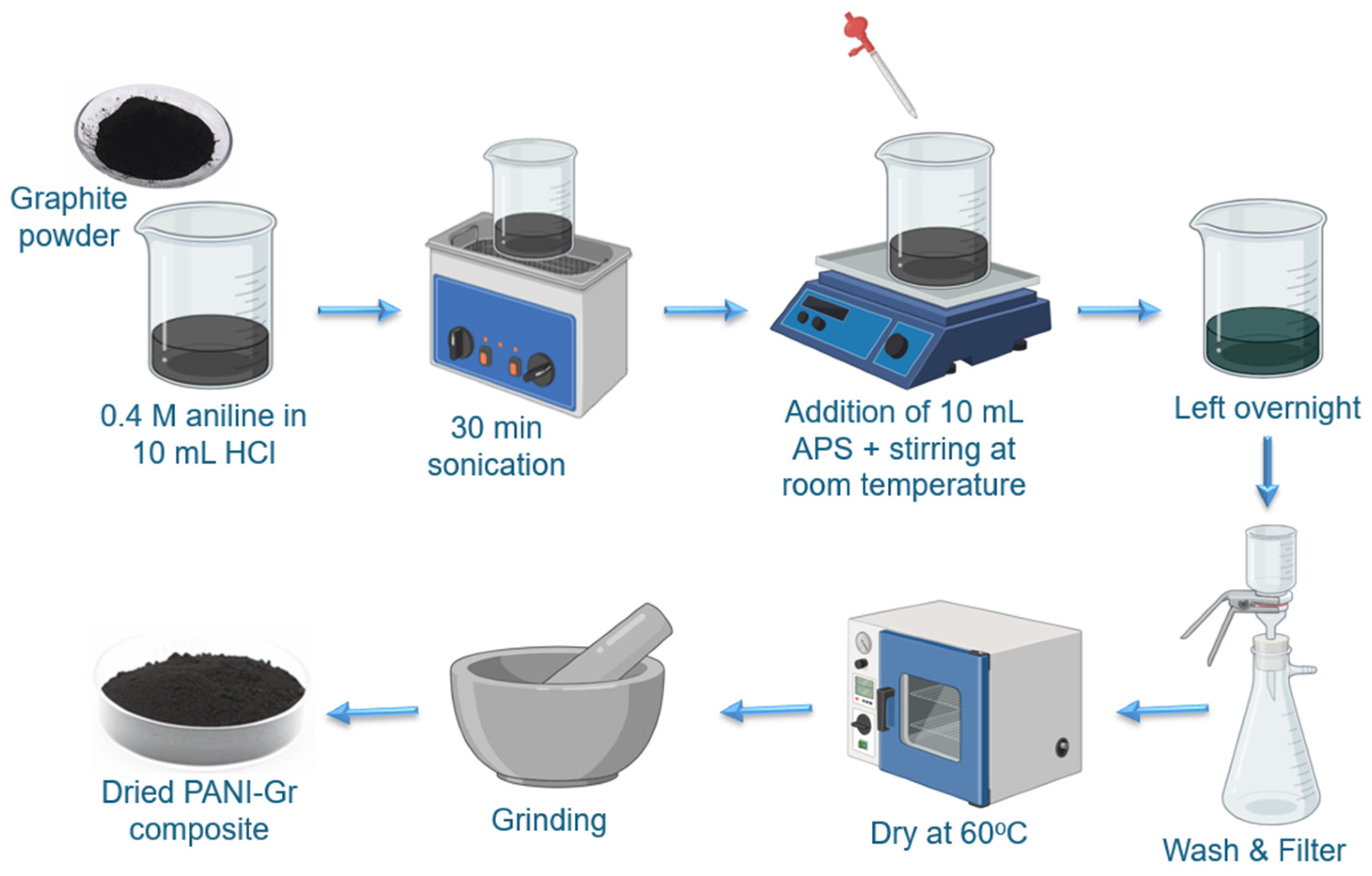
2.2. Composite Materials Preparation
2.3. Characterization
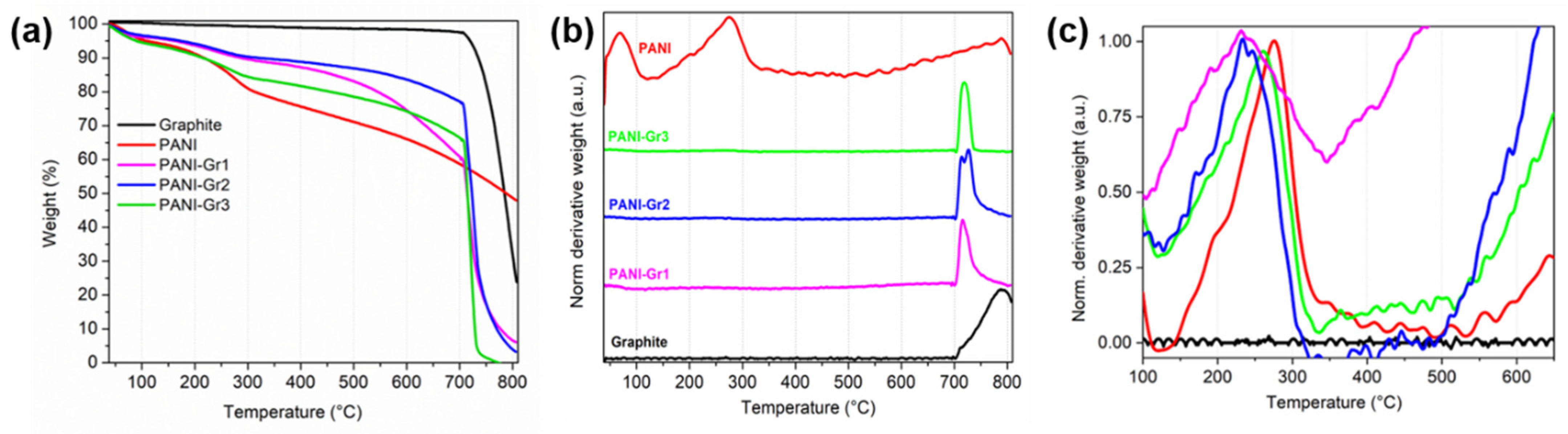
3. Results
| Sample | Aniline: Graphite Weight Ratio | PANI Content (wt. %) | Rod Length (nm) | Rod Diameter (nm) | Surface Area (m2/g) | Pore Volume(cm3/g) |
|---|---|---|---|---|---|---|
| Graphite | - | 0 | - | - | 4.42 | 0.009 |
| PANI | - | 100 | - | - | 9.86 * | 0.083 * |
| PANI-Gr1 | 1:1 | 50 | 546 ± 136 | 136 ± 40 | 11.37 | 0.016 |
| PANI-Gr2 | 2:1 | 66 | 383 ± 150 | 148 ± 23 | 11.87 | 0.011 |
| PANI-Gr3 | 3:1 | 75 | 973 ± 276 | 178 ± 9 | 16.68 | 0.014 |
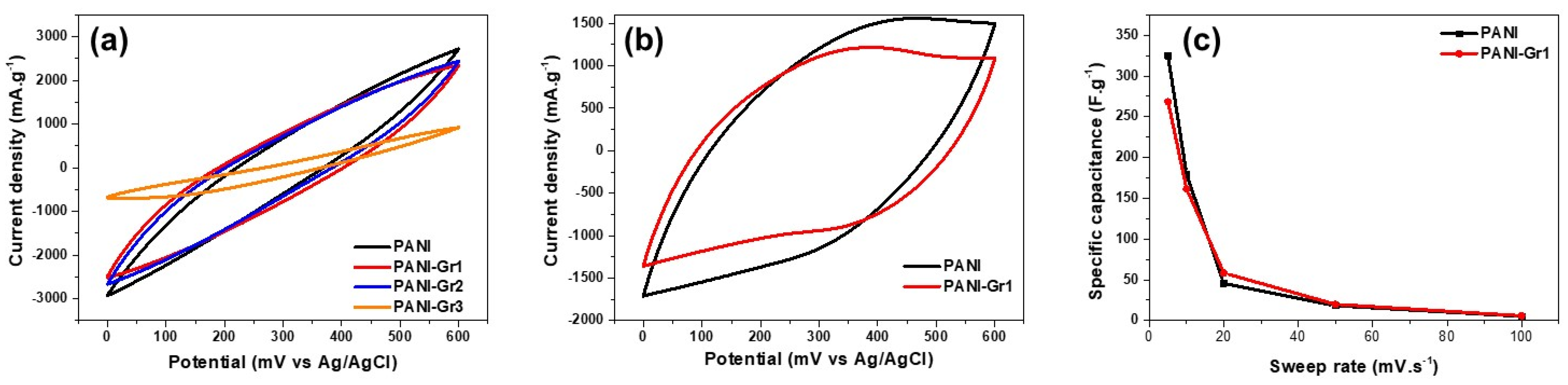
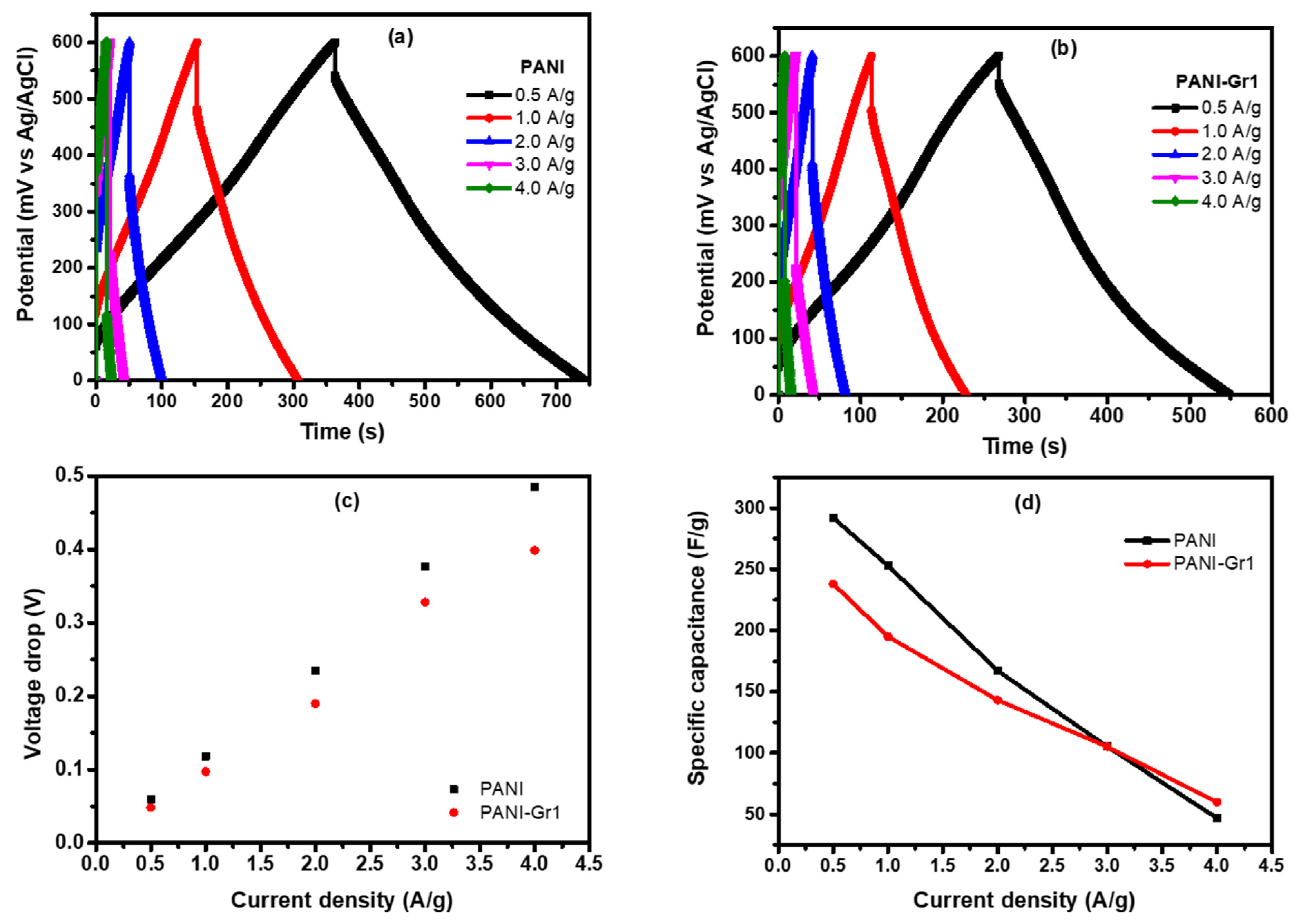
Electrochemical Studies of PANI-Gr Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. C 2021, 7, 39. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, S.-X.; Wang, Q.; Tan, M.-X.; Wang, D.-Y.; Yu, Z.-Q.; Luo, S.-H.; Zhang, Y.-H.; Liu, X. Walnut Septum-Derived Hierarchical Porous Carbon for Ultra-High-Performance Supercapacitors. Rare Met. 2022, 41, 2280–2291. [Google Scholar] [CrossRef]
- Kayode, S.E.; González, F.J. Treatment of Biowaste for Electrodes in Energy Storage Applications: A Brief Review. J. Compos. Sci. 2023, 7, 127. [Google Scholar] [CrossRef]
- Qing, Y.; Jiang, Y.; Lin, H.; Wang, L.; Liu, A.; Cao, Y.; Sheng, R.; Guo, Y.; Fan, C.; Zhang, S.; et al. Boosting the supercapacitor performance of activated carbon by constructing overall conductive networks using graphene quantum dots. J. Mater. Chem. A 2019, 7, 6021–6027. [Google Scholar] [CrossRef]
- Shokry, A.; Karim, M.; Khalil, M.; Ebrahim, S.; El Nady, J. Supercapacitor Based on Polymeric Binary Composite of Polythiophene and Single-Walled Carbon Nanotubes. Sci. Rep. 2022, 12, 11278. [Google Scholar] [CrossRef]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2021, 36, 56–76. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Sayed, E.T. Carbon-Based Materials for Supercapacitors: Recent Progress, Challenges and Barriers. Batteries 2023, 9, 19. [Google Scholar] [CrossRef]
- Majumdar, D.; Maiyalagan, T.; Jiang, Z. Recent Progress in Ruthenium Oxide-Based Composites for Supercapacitor Applications. ChemElectroChem 2019, 6, 4343–4372. [Google Scholar] [CrossRef]
- Ahmad, R.; Shah, M.A. Hydrothermally Synthesised Nickel Oxide Nanostructures on Nickel Foam and Nickel Foil for Supercapacitor Application. Ceram. Int. 2023, 49, 6470–6478. [Google Scholar] [CrossRef]
- Radhakanth, S.; Singhal, R. In–Situ Synthesis of MnO Dispersed Carbon Nanofibers as Binder-Free Electrodes for High-Performance Supercapacitors. Chem. Eng. Sci. 2023, 265, 118224. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, H.; Li, Y.; Cui, D.; Fan, Z.; Xue, C. PANI-Co3O4 with Excellent Specific Capacitance as an Electrode for Supercapacitors. Ceram. Int. 2021, 47, 8433–8440. [Google Scholar] [CrossRef]
- Yuan, K.; Gao, T.-J.; Yang, Y.; Luo, W.; Li, S.; Zhang, C.-Y.; Xu, J.-X.; Li, N.; Zhu, Y.-R. Template Sacrificial Controlled Synthesis of Hierarchical Nanoporous Carbon@NiCo2S4 Microspheres for High-Performance Hybrid Supercapacitors. Rare Met. 2023, 42, 2643–2657. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, M.; Lou, H.; Hu, Y.; Sun, X.; Peng, H. Conjugated Polymers for Flexible Energy Harvesting and Storage. Adv. Mater. 2018, 30, 1704261. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.; Jewell, D.; Chen, G.Z. Carbon nanotube and conducting polymer composites for supercapacitors. Prog. Nat. Sci. 2008, 18, 777–788. [Google Scholar] [CrossRef]
- Lota, K.; Khomenko, V.; Frackowiak, E. Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes composites. J. Phys. Chem. Solids 2004, 65, 295–301. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Chu, Q.; Wang, Z.; Zhang, F.; Wang, S. Theoretical and experimental specific capacitance of polyaniline in sulfuric acid. J. Power Sources 2009, 190, 578–586. [Google Scholar] [CrossRef]
- Hassan, H.; Iqbal, M.W.; Afzal, A.M.; Asghar, M.; Aftab, S. Enhanced the Performance of Zinc Strontium Sulfide-Based Supercapattery Device with the Polyaniline Doped Activated Carbon. J. Solid State Electrochem. 2023, 27, 125–137. [Google Scholar] [CrossRef]
- Ajay, K.M.; Dinesh, M.N.; Byatarayappa, G.; Radhika, M.G.; Kathyayini, N.; Vijeth, H. Electrochemical Investigations on Low Cost KOH Activated Carbon Derived from Orange-Peel and Polyaniline for Hybrid Supercapacitors. Inorg. Chem. Commun. 2021, 127, 108523. [Google Scholar] [CrossRef]
- Akbar, A.R.; Saleem, A.; Rauf, A.; Iqbal, R.; Tahir, M.; Peng, G.; Khan, A.S.; Hussain, A.; Ahmad, M.; Akhtar, M.; et al. Integrated MnO2/PEDOT Composite on Carbon Cloth for Advanced Electrochemical Energy Storage Asymmetric Supercapacitors. J. Power Sources 2023, 579, 233181. [Google Scholar] [CrossRef]
- Himadri Reddy, P.C.; Amalraj, J.; Ranganatha, S.; Patil, S.S.; Chandrasekaran, S. A Review on Effect of Conducting Polymers on Carbon-Based Electrode Materials for Electrochemical Supercapacitors. Synth. Met. 2023, 298, 117447. [Google Scholar] [CrossRef]
- Javed, M.S.; Khan, A.J.; Hanif, M.; Nazir, M.T.; Hussain, S.; Saleem, M.; Raza, R.; Yun, S.; Liu, Z. Engineering the Performance of Negative Electrode for Supercapacitor by Polyaniline Coated Fe3O4 Nanoparticles Enables High Stability up to 25,000 Cycles. Int. J. Hydrogen Energy 2021, 46, 9976–9987. [Google Scholar] [CrossRef]
- González, F.J.; Montesinos, A.; Araujo-Morera, J.; Verdejo, R.; Hoyos, M. ‘In-Situ’ Preparation of Carbonaceous Conductive Composite Materials Based on PEDOT and Biowaste for Flexible Pseudocapacitor Application. J. Compos. Sci. 2020, 4, 87. [Google Scholar] [CrossRef]
- Ates, M.; Serin, M.A.; Calisskan, S. Electrochemical supercapacitors of PANI/MWCNT, PEDOT/MWCNT and PANI-co-EDOT/MWCNT nanocomposites. Polym. Bull. 2019, 76, 3207–3231. [Google Scholar] [CrossRef]
- . Sun, M.; Wang, G.; Yang, C.; Jiang, H.; Li, C. A graphene/carbon nanotube@π-conjugated polymer nanocomposite for high-performance organic supercapacitor electrodes. J. Mater. Chem. A 2015, 3, 3880–3890. [Google Scholar] [CrossRef]
- Maddu, A.; Nugroho, R.A.; Rustami, E.; Arjo, S.; Hidayat, M. Synthesis of Graphene/Polyaniline Nanocomposite for Supercapacitor Electrodes. J. Phys. Conf. Ser. 2019, 1171, 12043. [Google Scholar] [CrossRef]
- Qi, W.; Lv, R.; Na, B.; Liu, H.; He, Y.; Yu, N. Nanocellulose-Assisted Growth of Manganese Dioxide on Thin Graphite Papers for High-Performance Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2018, 6, 4739–4745. [Google Scholar] [CrossRef]
- Sun, X.-T.; Wan, Y.; Wang, B.; Xu, Q.; Teng, X.-L.; Liu, H.-Y.; Wang, Y.-J.; Guo, S.-W.; Wu, C.-H.; Hu, H.; et al. Laser Irradiation of Graphite Foils as Robust Current Collectors for High-Mass Loaded Electrodes of Supercapacitors. Rare Met. 2022, 41, 4094–4103. [Google Scholar] [CrossRef]
- Sharma, K.; Pareek, K.; Rohan, R.; Kumar, P. Flexible supercapacitor based on three-dimensional cellulose/graphite/polyaniline composite. Int. J. Energy Res. 2019, 43, 604–611. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, W.; Zhi, X.; Zhai, H.J. Remarkably enhanced performances of polyaniline/electrochemically surface-treated graphite electrodes with optimal charge transfer pathways for flexible supercapacitor application. J. Power Sources 2018, 402, 311–319. [Google Scholar] [CrossRef]
- Yao, B.; Yuan, L.; Xiao, X.; Zhang, J.; Qi, Y.; Zhou, J.; Zhou, J.; Hu, B.; Chen, W. Paper-based solid-state supercapacitors with pencil-drawing graphite/polyaniline networks hybrid electrodes. Nano Energy 2013, 2, 1071–1078. [Google Scholar] [CrossRef]
- Melih, B.A.; Emirhan, A.; Ozge, G.; Semih, G.; Sahin, Y. Construction of Phthalocyanine-Titanium Dioxide/Graphene/Polyaniline Composite Electrodes by Electrochemical Method for Supercapacitor Applications. ECS J. Solid State Sci. Technol. 2023, 12, 031008. [Google Scholar] [CrossRef]
- González, F.; Tiemblo, P.; Hoyos, M. In-Situ Approaches for the Preparation of Polythiophene-Derivative Cellulose Composites with High Flexibility and Conductivity. Appl. Sci. 2019, 9, 3371. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. Preparation of a conducting polymer (IUPAC technical report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef]
- Patil, D.S.; Pawar, S.A.; Devan, R.S.; Gang, M.G.; Ma, Y.R.; Kim, J.H.; Patil, P.S. Electrochemical supercapacitor electrode material based on polyacrylic acid/polypyrrole/silver composite. Electrochim. Acta 2013, 105, 569–577. [Google Scholar] [CrossRef]
- Mo, Z.; Shi, H.; Chen, H.; Niu, G.; Zhao, Z.; Wu, Y. Synthesis of graphite nanosheets/polyaniline nanorods composites with ultrasonic and conductivity. J. Appl. Polym. Sci. 2009, 112, 573–578. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Yang, Z.; Wang, Z.; Zhang, F.; Wang, S. A novel strategy for the synthesis of polyaniline nanostructures with controlled morphology. React. Funct. Polym. 2008, 68, 1435–1440. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Zhang, X.; Wang, Z. Assembly of polyaniline nanostructures. Macromol. Rapid Commun. 2007, 28, 84–87. [Google Scholar] [CrossRef]
- Tsagkalias, I.S.; Manios, T.K.; Achilias, D.S. Effect of graphene oxide on the reaction kinetics of methyl methacrylate in situ radical polymerization via the bulk or solution technique. Polymers 2017, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Wang, G. Polyaniline Nanofibers with a High Specific Surface Area and an Improved Pore Structure for Supercapacitors. J. Power Sources 2015, 294, 16–21. [Google Scholar] [CrossRef]
- Ayuningsih, A.; Budi, S.; Paristiowati, M.; Fahdiran, R.; Handoko, E.; Sugihartono, I. Aniline Concentration-Dependent Surface Area of Emeraldine Salt Polyaniline. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1098, 062068. [Google Scholar] [CrossRef]
- El-Shazly, A.H.; Elkady, M.; Abdelraheem, A. Investigating the Adsorption Behavior of Polyaniline and Its Clay Nanocomposite towards Ammonia Gas. Polymers 2022, 14, 4533. [Google Scholar] [CrossRef] [PubMed]
- Arvizu, M.A.; González, F.J.; Romero-Galarza, A.; Rodríguez-Varela, F.J.; Garcia, C.R.; Garcia-Lobato, M.A. Symmetric Supercapacitors of PANI Coated RuO2 /TiO2 Macroporous Structures Prepared by Electrostatic Spray Deposition. J. Electrochem. Soc. 2022, 169, 020564. [Google Scholar] [CrossRef]
- Li, N.; Hou, Z.; Liang, S.; Cao, Y.; Liu, H.; Hua, W.; Wei, C.; Kang, F.; Wang, J.-G. Highly Flexible MnO2@polyaniline Core-Shell Nanowire Film toward Substantially Expedited Zinc Energy Storage. Chem. Eng. J. 2023, 452, 139408. [Google Scholar] [CrossRef]
- Yelil Arasi, A.; Juliet Latha Jeyakumari, J.; Sundaresan, B.; Dhanalakshmi, V.; Anbarasan, R. The structural properties of Poly(aniline)-Analysis via FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 74, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ding, Y.; Zhang, J. Uniform rice-like nanostructured polyanilines with highly crystallinity prepared in dodecylbenzene sulfonic acid micelles. Mater. Chem. Phys. 2008, 112, 322–324. [Google Scholar] [CrossRef]
- Nikzad, L.; Alibeigi, S.; Vaezi, M.R.; Yazdani, B.; Rahimipour, M.R. Synthesis of a graphite-polyaniline nanocomposite and evaluation of its electrochemical properties. Chem. Eng. Technol. 2009, 32, 861–866. [Google Scholar] [CrossRef]
- Shao, L.; Chang, X.; Zhang, Y.; Huang, Y.; Yao, Y.; Guo, Z. Graphene oxide cross-linked chitosan nanocomposite membrane. Appl. Surf. Sci. 2013, 280, 989–992. [Google Scholar] [CrossRef]
- Zaghib, K.; Song, X.; Kinoshita, K. Thermal Analysis of the Oxidation of Natural Graphite: Isothermal Kinetic Studies. Thermochim. Acta 2001, 371, 57–64. [Google Scholar] [CrossRef]
- Farivar, F.; Yap, P.L.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameters. J. Carbon Res. 2021, 7, 41. [Google Scholar] [CrossRef]
- Sui, X.; Chu, Y.; Xing, S.; Yu, M.; Liu, C. Self-organization of spherical PANI/TiO2 nanocomposites in reverse micelles. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 103–107. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Mudila, H.; Awasthi, K.; Kumar, V. Synthesis and thermal analysis of polyaniline (PANI). J. Phys. Conf. Ser. 2020, 1531, 012108. [Google Scholar] [CrossRef]
- Du, X.; Xu, Y.; Xiong, L.; Bai, Y.; Zhu, J.; Mao, S. Polyaniline with high crystallinity degree: Synthesis, structure, and electrochemical properties. J. Appl. Polym. Sci. 2014, 131, 40827. [Google Scholar] [CrossRef]
- Du, J.; Li, Y.; Zhong, Q.; Yang, J.; Xiao, J.; Chen, D.; Li, W. Boosting the Utilization and Electrochemical Performances of Polyaniline by Forming a Binder-Free Nanoscale Coaxially Coated Polyaniline/Carbon Nanotube/Carbon Fiber Paper Hierarchical 3D Microstructure Composite as a Supercapacitor Electrode. ACS Omega 2020, 5, 22119–22130. [Google Scholar] [CrossRef] [PubMed]
- Bhoyate, S.; Ranaweera, C.K.; Zhang, C.; Morey, T.; Hyatt, M.; Kahol, P.K.; Gupta, R.K. Eco-Friendly and High Performance Supercapacitors for Elevated Temperature Applications Using Recycled Tea Leaves. Glob. Chall. 2017, 1, 1700063. [Google Scholar] [CrossRef] [PubMed]
- Jeyaranjan, A.; Sakthivel, T.S.; Neal, C.J.; Seal, S. Scalable ternary hierarchical microspheres composed of PANI/ rGO/CeO2 for high performance supercapacitor applications. Carbon 2019, 151, 192–202. [Google Scholar] [CrossRef]
- Pan, C.; Gu, H.; Dong, L. Synthesis and electrochemical performance of polyaniline @MnO2/graphene ternary composites for electrochemical supercapacitors. J. Power Sources 2016, 303, 175–181. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, B.; She, W.; Gao, S.; Wang, J.; Wang, Y.; Wang, K.; Li, H.; Han, L. Simultaneously Achieving High Energy and Power Density for Ultrafast-Charging Supercapacitor Built by a Semi-Graphitic Hierarchical Porous Carbon Nanosheet and a High-Voltage Alkaline Aqueous Electrolyte. J. Power Sources 2021, 506, 230103. [Google Scholar] [CrossRef]
- Wu, T.-M.; Lin, Y.-W.; Liao, C.-S. Preparation and characterization of polyaniline/multi-walled carbon nanotube composites. Carbon 2005, 43, 734–740. [Google Scholar] [CrossRef]
- Elnaggar, E.M.; Kabel, K.I.; Farag, A.A.; Al-Gamal, A.G. Comparative study on doping of polyaniline with graphene and multi-walled carbon nanotubes. J. Nanostruct. Chem. 2017, 7, 75–83. [Google Scholar] [CrossRef]
- Kim, J.W.; Augustyn, V.; Dunn, B. The Effect of Crystallinity on the Rapid Pseudocapacitive Response of Nb2O5. Adv. Energy Mater. 2012, 2, 141–148. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Sirimanne, D.C.U.; Kularatna, N.; Arawwawala, N. Electrical Performance of Current Commercial Supercapacitors and Their Future Applications. Electronics 2023, 12, 2465. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Vinu, A.; Lokhande, C.D. Stable nanostructured polyaniline electrode for supercapacitor application. Electrochim. Acta 2011, 56, 9482–9487. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, S.; Zhang, J.; Guo, P.; Zheng, J.; Zhao, X.S. Enhancement of Electrochemical Performance of Macroporous Carbon by Surface Coating of Polyaniline. Chem. Mater. 2010, 22, 1195–1202. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, Y. Enhanced electrochemical performance of carbon quantum dots-polyaniline hybrid. J. Power Sources 2017, 337, 54–64. [Google Scholar] [CrossRef]
- Li, L.; Raji, A.-R.O.; Fei, H.; Yang, Y.; Samuel, E.L.G.; Tour, J.M. Nanocomposite of Polyaniline Nanorods Grown on Graphene Nanoribbons for Highly Capacitive Pseudocapacitors. ACS Appl. Mater. Interfaces 2013, 5, 6622–6627. [Google Scholar] [CrossRef]
- Li, J.; Ren, Y.; Ren, Z.; Wang, S.; Qiu, Y.; Yu, J. Aligned polyaniline nanowires grown on the internal surface of macroporous carbon for supercapacitors. J. Mater. Chem. A 2015, 3, 23307–23315. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Q.; Zhang, X.; Peng, K. A novel 3D conductive network-based polyaniline/graphitic mesoporous carbon composite electrode with excellent electrochemical performance. J. Power Sources 2018, 401, 278–286. [Google Scholar] [CrossRef]


| Sample | Potential Range (V) | Electrolyte | Current Density (A·g−1) | Specific Energy (Wh·kg−1) | Specific Power (W·kg−1) | Reference |
|---|---|---|---|---|---|---|
| PANI | −0.1–0.8 | 1 M H2SO4 | 1.0 mA.cm−2 | 9.88 | 96 | [66] |
| PANI-DOMC | −0.2–0.8 | 2 M H2SO4 | 0.5 | 49 | 182 | [67] |
| CQDs-PANI/CFs | 0–0.6 | 1 M H2SO4 | 1.0 | 36.9 | 300 | [68] |
| PANI/GNRs | 0–0.8 | 1 M H2SO4 | 0.25 | 7.56 | 3149 | [69] |
| PANI/MC | 0–1.0 | 1 M H2SO4 | 1.0 | 19 | 500 | [70] |
| PANI/GMC | −0.2–0.8 | 1 M H2SO4 | 1.0 | 24.64 | 250 | [71] |
| PANI-Gr | 0–0.6 | 0.1 M H2SO4 | 0.5 | 12 | 150 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kayode, S.E.; Awobifa, O.S.; Garcia-Lobato, M.A.; Rosas, M.T.; Hoyos, M.; González, F.J. In Situ PANI–Graphite Nanochain-like Structures and Their Application as Supercapacitive Electrodes. J. Compos. Sci. 2024, 8, 200. https://doi.org/10.3390/jcs8060200
Kayode SE, Awobifa OS, Garcia-Lobato MA, Rosas MT, Hoyos M, González FJ. In Situ PANI–Graphite Nanochain-like Structures and Their Application as Supercapacitive Electrodes. Journal of Composites Science. 2024; 8(6):200. https://doi.org/10.3390/jcs8060200
Chicago/Turabian StyleKayode, Samuel E., Olaolu S. Awobifa, Marco A. Garcia-Lobato, María Téllez Rosas, Mario Hoyos, and Francisco J. González. 2024. "In Situ PANI–Graphite Nanochain-like Structures and Their Application as Supercapacitive Electrodes" Journal of Composites Science 8, no. 6: 200. https://doi.org/10.3390/jcs8060200







