Multifunctional Anatase–Silica Photocatalytic Material for Cements and Concretes
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials for Obtaining ASPMs
2.2. Raw Materials for Obtaining Cement Stone and Fine-Grained Concrete
2.3. ASPMs Synthesis Technique
2.4. Preparation of Cement Paste, Cement–Sand Mortar and the Concrete Mix
2.5. Research Methods
2.5.1. Methods of ASPM Research
2.5.2. Methods of Studying the Cement System
2.5.3. The Determination of Photocatalytic Activity and the Self-Cleaning Ability of the Materials
3. Results and Discussions
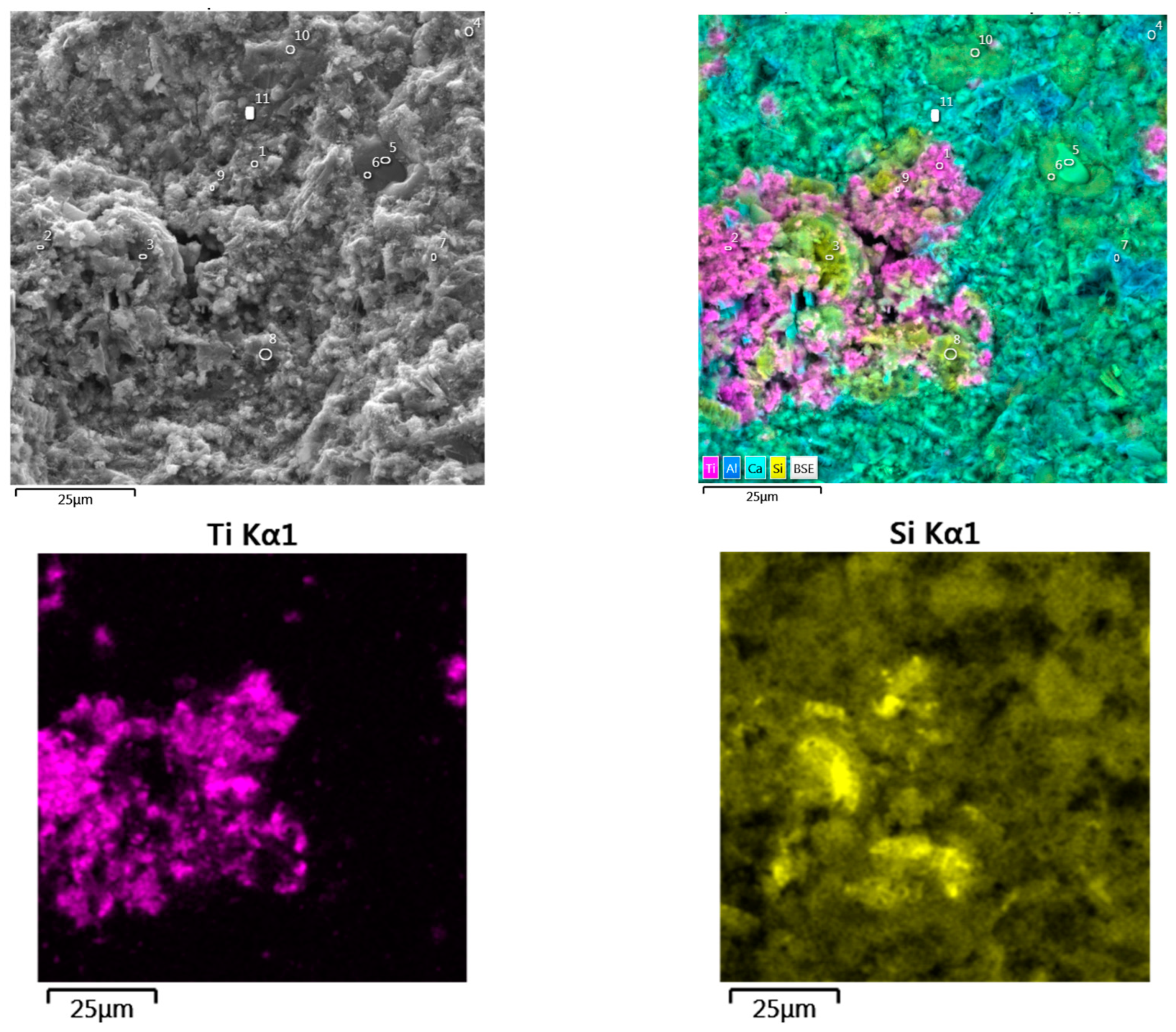
3.1. The properties of the Anatase–Silica Photocatalytic Material
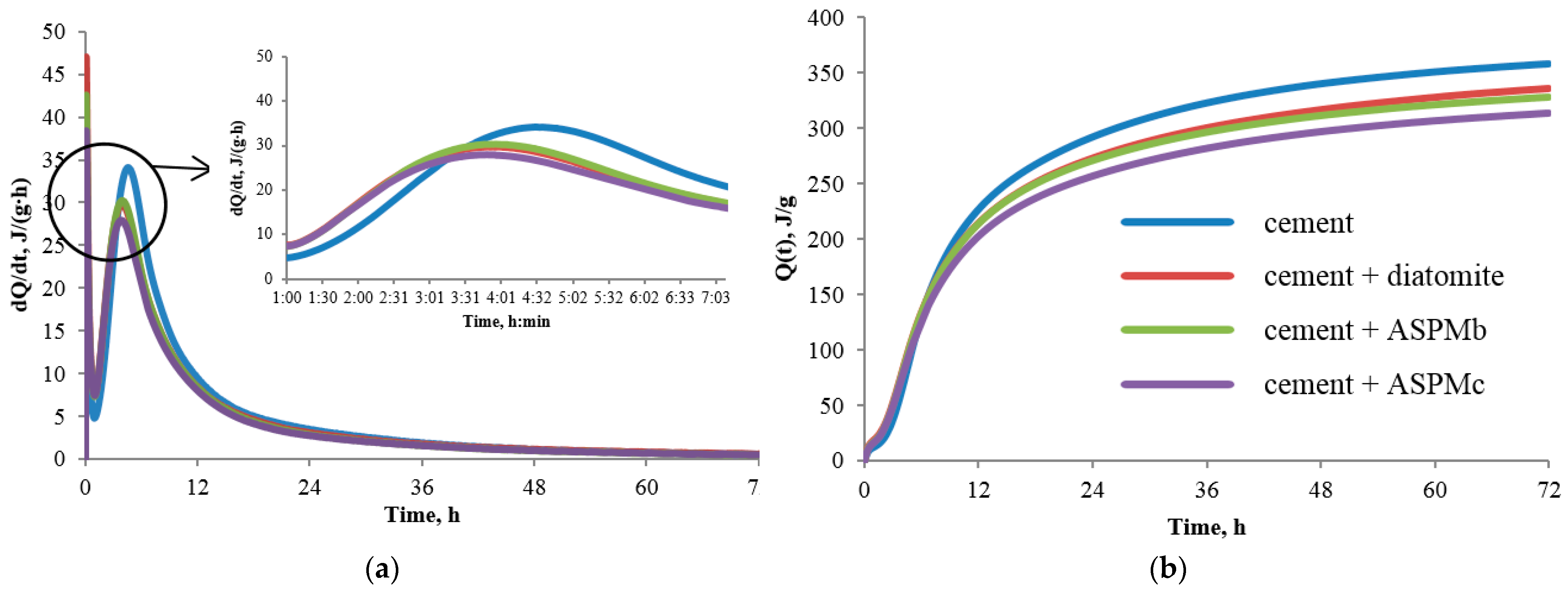
3.2. The properties of the Cement System with the Multifunctional Anatase–Silica Additive
- 15% of diatomite—3 h 55 min;
- 15% of ASPMb—3 h 57 min;
- 15% of ASPMc—3 h 48 min.
3.3. The Self-Cleaning Ability of Fine-Grained Concrete with the Multifunctional Anatase–Silica Additive
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, Y.; Wu, Q.; Meng, H.; Zhang, Y.; Cao, C. Recent advances in photocatalytic self-cleaning performances of TiO2-based building materials. RSC Adv. 2023, 13, 20584–20597. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, P.; Haghighat, F. TiO2-based photocatalytic oxidation process for indoor air VOCs removal: A comprehensive review. Build. Environ. 2024, 249, 111108. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Pal, S.; Ul Haq, E.; Licciulli, A. Nanocrystalline TiO2–diatomite composite catalysts: Effect of crystallization on the photocatalytic degradation of rhodamine B. Appl. Catal. A Gen. 2014, 485, 157–162. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Hashisho, Z.; Sun, Z.; Zheng, S.; Zhong, L. Adsorption and photocatalytic degradation performances of TiO2/diatomite composite for volatile organic compounds: Effects of key parameters. Appl. Surf. Sci. 2020, 525, 146633. [Google Scholar] [CrossRef]
- Zuo, R.; Du, G.; Zhang, W.; Liu, L.; Liu, Y.; Mei, L.; Li, Z. Photocatalytic Degradation of Methylene Blue Using TiO2 Impregnated Diatomite. Adv. Mater. Sci. Eng. 2014, 2014, 170148. [Google Scholar] [CrossRef]
- Bengotni, L.; Trari, B.; Lebeau, B.; Michelin, L.; Josien, L.; Bengueddach, A.; Hamacha, R. Effect of diatomite addition on crystalline phase formation of TiO2 and photocatalytic degradation of MDMA. New J. Chem. 2021, 45, 13463–13474. [Google Scholar] [CrossRef]
- Bellardita, M.; Addamo, M.; Di Paola, A.; Marcì, G.; Palmisano, L.; Cassar, L.; Borsa, M. Photocatalytic activity of TiO2/SiO2 systems. J. Hazard. Mater. 2009, 174, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, F.; Jiang, Y.; Xia, M.; Xue, B.; Li, Y. Interface actions between TiO2 and porous diatomite on the structure and photocatalytic activity of TiO2-diatomite. Appl. Surf. Sci. 2014, 303, 290–296. [Google Scholar] [CrossRef]
- Li, X.; Simon, U.; Bekheet, M.F.; Gurlo, A. Mineral-Supported Photocatalysts: A Review of Materials, Mechanisms and Environmental Applications. Energies 2022, 15, 5607. [Google Scholar] [CrossRef]
- Figmig, R.; Estokova, A.; Luptak, M. Concept of Evaluation of Mineral Additives’ Effect on Cement Pastes’ Durability and Environmental Suitability. Materials 2021, 14, 1448. [Google Scholar] [CrossRef]
- Yang, H.; Yang, B.; Chen, W.; Yang, J. Preparation and Photocatalytic Activities of TiO2-Based Composite Catalysts. Catalysts 2022, 12, 1263. [Google Scholar] [CrossRef]
- Mendoza, C.; Valle, A.; Castellote, M.; Bahamonde, A.; Faraldos, M. TiO2 and TiO2–SiO2 coated cement: Comparison of mechanic and photocatalytic properties. Appl. Catal. B Environ. 2015, 178, 155–164. [Google Scholar] [CrossRef]
- Akono, A.-T. Effect of nano-TiO2 on C–S–H phase distribution within Portland cement paste. J. Mater. Sci. 2020, 55, 11106–11119. [Google Scholar] [CrossRef]
- Shchelokova, E.A.; Tyukavkina, V.V.; Tsyryatyeva, A.V.; Kasikov, A.G. Synthesis and characterization of SiO2-TiO2 nanoparticles and their effect on the strength of self-cleaning cement composites. Constr. Build. Mater. 2021, 283, 122769. [Google Scholar] [CrossRef]
- Cardellicchio, L. Self-cleaning and colour-preserving efficiency of photocatalytic concrete: Case study of the Jubilee Church in Rome. Build. Res. Inf. 2020, 48, 160–179. [Google Scholar] [CrossRef]
- Şahin, O.; Bay, S.; İlcan, H.; Yıldırım, G.; Şahmaran, M. Influence of mixing methods on the NOx reduction capability and electrical properties of photocatalytic cementitious systems. Cem. Concr. Compos. 2021, 115, 103840. [Google Scholar] [CrossRef]
- Florean, C.T.; Vermesan, H.; Thalmaier, G.; Neamtu, B.V.; Gabor, T.; Campian, C.; Hegyi, A.; Csapai, A. The Influence of TiO2 Nanoparticles on the Physico–Mechanical and Structural Characteristics of Cementitious Materials. Coatings 2024, 14, 218. [Google Scholar] [CrossRef]
- Hamdany, A.H.; Satyanaga, A.; Zhang, D.; Kim, Y.; Kim, J.R. Photocatalytic Cementitious Material for Eco-Efficient Construction—A Systematic Literature Review. Appl. Sci. 2022, 12, 8741. [Google Scholar] [CrossRef]
- Kaja, A.M.; Brouwers, H.J.H.; Yu, Q.L. NOx degradation by photocatalytic mortars: The underlying role of the CH and C-S-H carbonation. Cem. Concr. Res. 2019, 125, 105805. [Google Scholar] [CrossRef]
- Strokova, V.; Gubareva, E.; Ogurtsova, Y.; Fediuk, R.; Zhao, P.; Vatin, N.; Vasilev, Y. Obtaining and properties of a photocatalytic composite material of the “SiO2–TiO2” system based on various types of silica raw materials. Nanomaterials 2021, 11, 866. [Google Scholar] [CrossRef]
- Antonenko, M.V.; Ogurtsova, Y.N.; Strokova, V.V.; Gubareva, E.N. The effect of titanium dioxide sol stabilizer on the properties of photocatalytic composite material. Lect. Notes Civ. Eng. 2021, 95, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, Y.N.; Strokova, V.V.; Zhao, P.; Antonenko, M.V.; Gubareva, E.N. Properties of cement with photocatalytic composite material. Mat. Sci. Forum 2021, 1040, 153–158. [Google Scholar] [CrossRef]
- Zhang, H.; He, B.; Zhao, B.; Jm Monteiro, P. Using diatomite as a partial replacement of cement for improving the performance of recycled aggregate concrete (RAC)-Effects and mechanism. Constr. Build. Mater. 2023, 385, 131518. [Google Scholar] [CrossRef]
- EN 197-1; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Standards: Plzen, Czech Republic, 2011.
- GOST 8736; Sand for Construction Works. Interstandard (Russia): Voronezh, Russia, 2014.
- EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. European Standards: Plzen, Czech Republic, 2016.
- Ogurtsova, Y.; Antonenko, M.; Gubareva, E.; Nerovnaya, S.; Strokova, V. Composition and properties of fine-grained concrete for self-cleaning coatings. E3S Web Conf. 2023, 410, 01011. [Google Scholar] [CrossRef]
- Black, L. Sustainability of Construction Materials, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 415–457. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. Comparison of test methods to assess pozzolanic activity. Cem. Concr. Comp. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- Nechiporenko, A.P. Donor-Acceptor Properties of the Surface of Solid-Phase Systems. Indicator Method, 1st ed.; Lan: Saint-Petersburg, Russia, 2017; pp. 32–61. [Google Scholar]
- Nelyubova, V.V.; Sivalneva, M.N.; Bondarenko, D.O.; Baskakov, P.S. Study of activity of polydisperse mineral modifiers via unstandardized techniques. J. Phys. Conf. Ser. 2018, 118, 12029. [Google Scholar] [CrossRef]
- UNI 11259; Photocatalysis—Determination of the Photocatalytic Activity of Hidraulic Binders—Rodammina Test Method. Ente Nazionale Italiano di Unificazione (UNI): Roma, Italy, 2008.
- State Standard of Russia (GOST R) 57255-2016; Photo Catalytic Self-Cleaning Concrete. Specifications. Standartinform: Moscow, Russia, 2016.
- Kozlov, D.; Bavykin, D.; Savinov, E. Effect of the acidity of TiO2 surface on its photocatalytic activity in acetone gas-phase oxidation. Catal. Lett. 2003, 86, 169–172. [Google Scholar] [CrossRef]
- Nikolaenko, E.A. Portland-pozzolan cement with increased strength properties. Bull. ESSTUM 2014, 3, 63–69. [Google Scholar]
- Ermolovich, E.A. Effect of grinding on the donor-acceptor properties of surfaces of backfill mix components. J. Min. Sci. 2013, 49, 839–846. [Google Scholar] [CrossRef]
- Zakharova, N.V.; Sychev, M.M.; Korsakov, V.G.; Myakin, S.V. Evolution of donor-acceptor centers on the surface of BaTiO3—CaSnO3 ferroelectric materials in the course of their dispersion. Condens. Matter Interphases 2011, 13, 56–62. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Publishing: London, UK, 1997; pp. 212–214. [Google Scholar]
- Lawrence, P.; Cyr, M.; Ringot, E. Mineral admixtures in mortars: Effect of inert materials on short-term hydration. Cem. Concr. Res. 2003, 33, 1939–1947. [Google Scholar] [CrossRef]
- Ivanov, I.M.; Matveev, D.V.; Orlov, A.A.; Kramar, L.I. Influence of water-cement ratio and superplasticizers on the heat release, cement hydration and hardening processes. Bull. South Ural. State Univ. Ser. Constr. Eng. Archit. 2017, 17, 42–49. [Google Scholar]

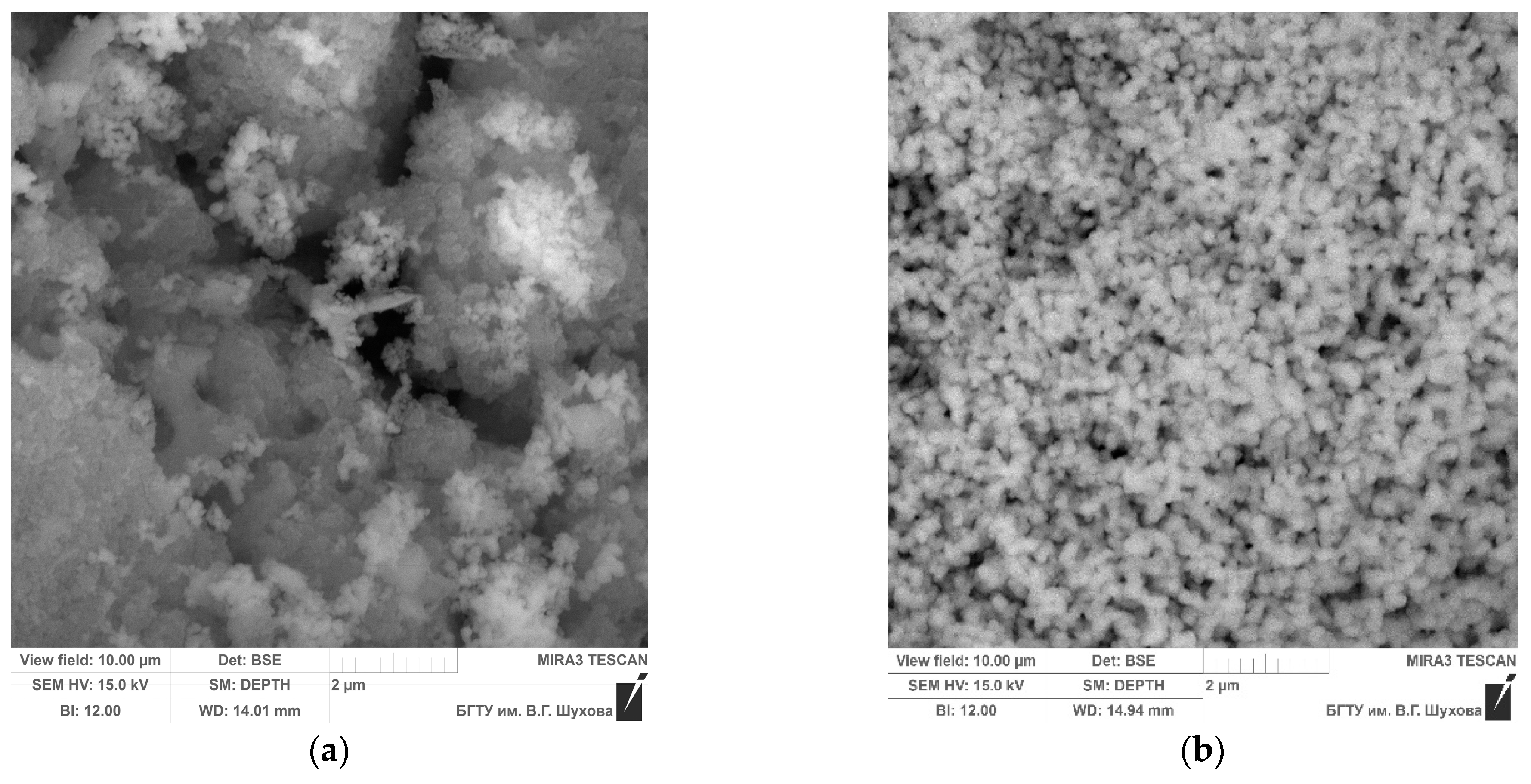
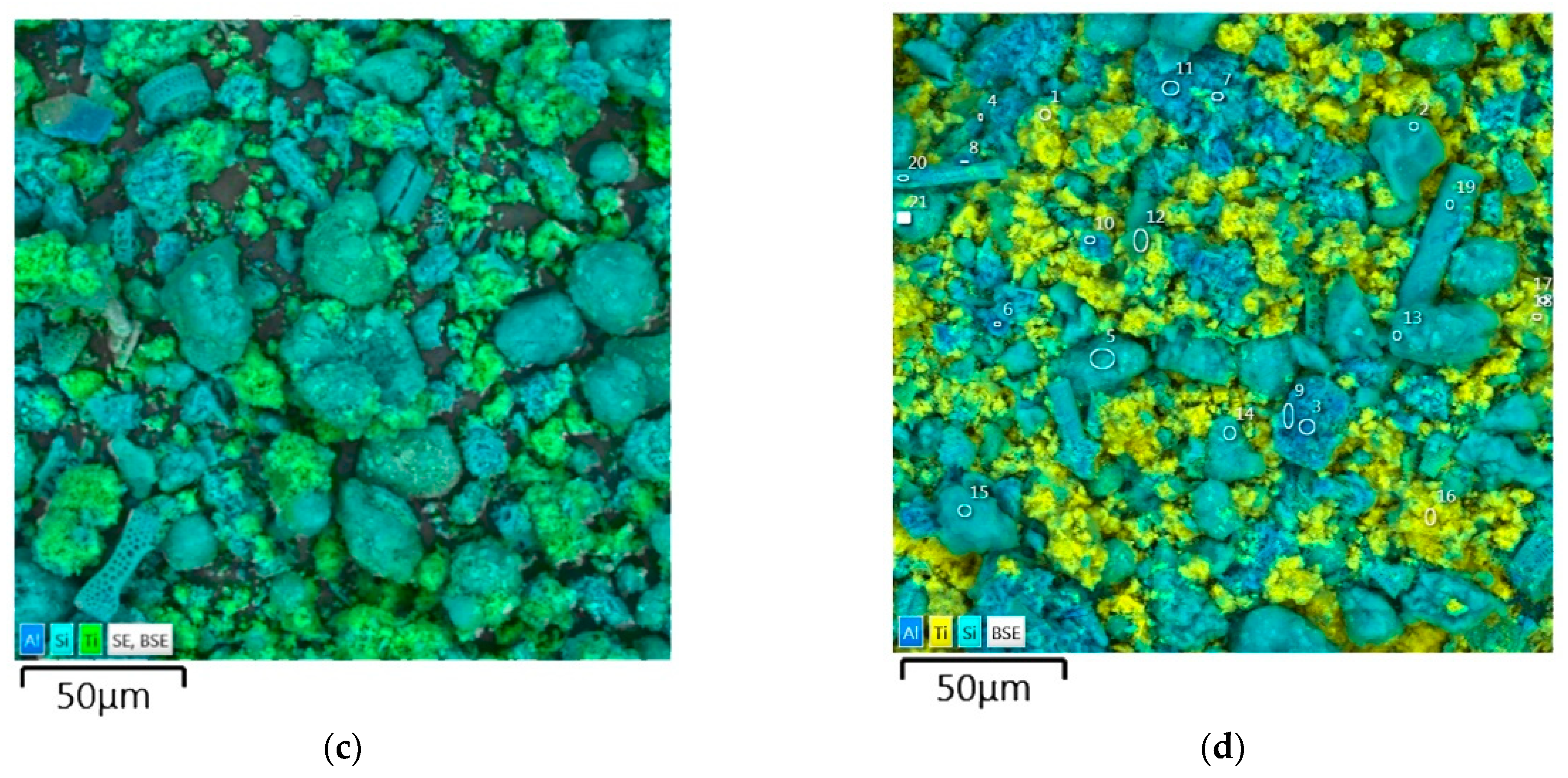
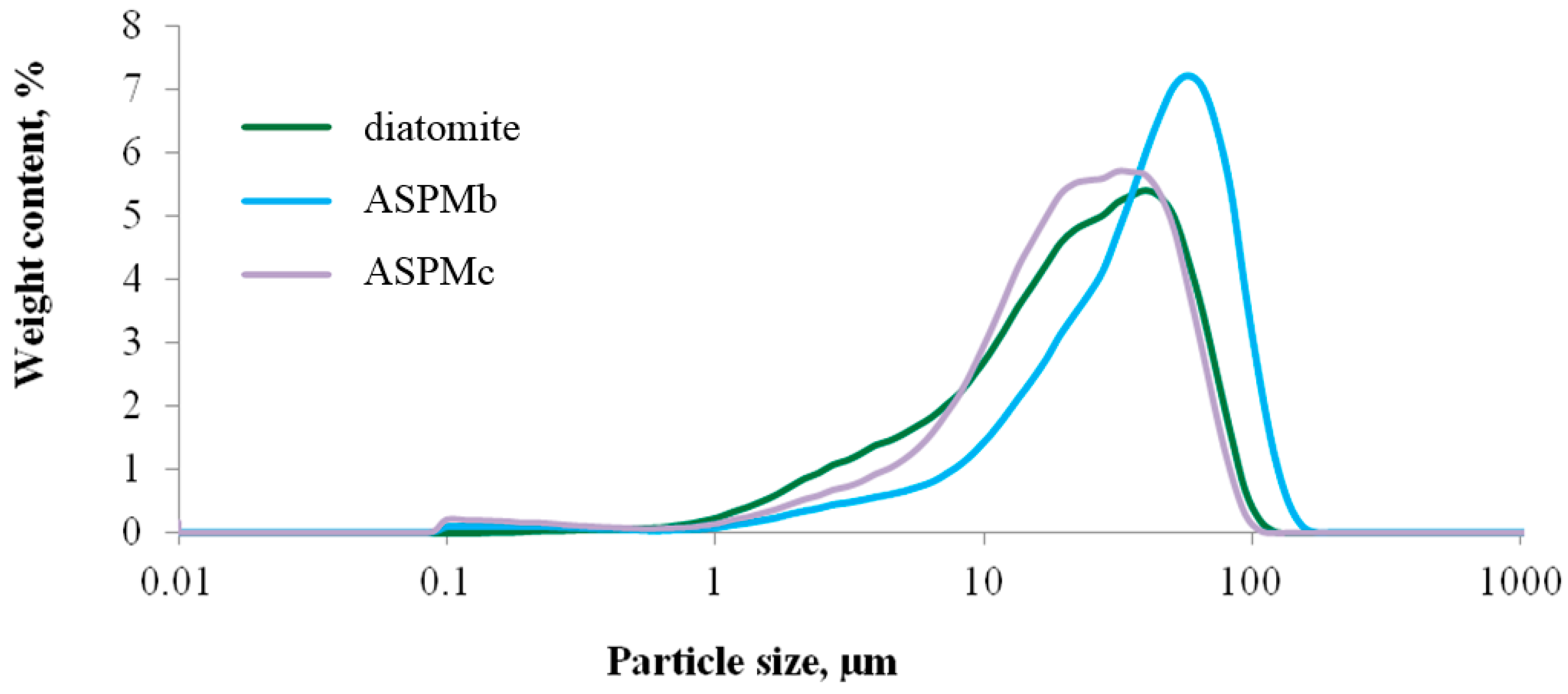
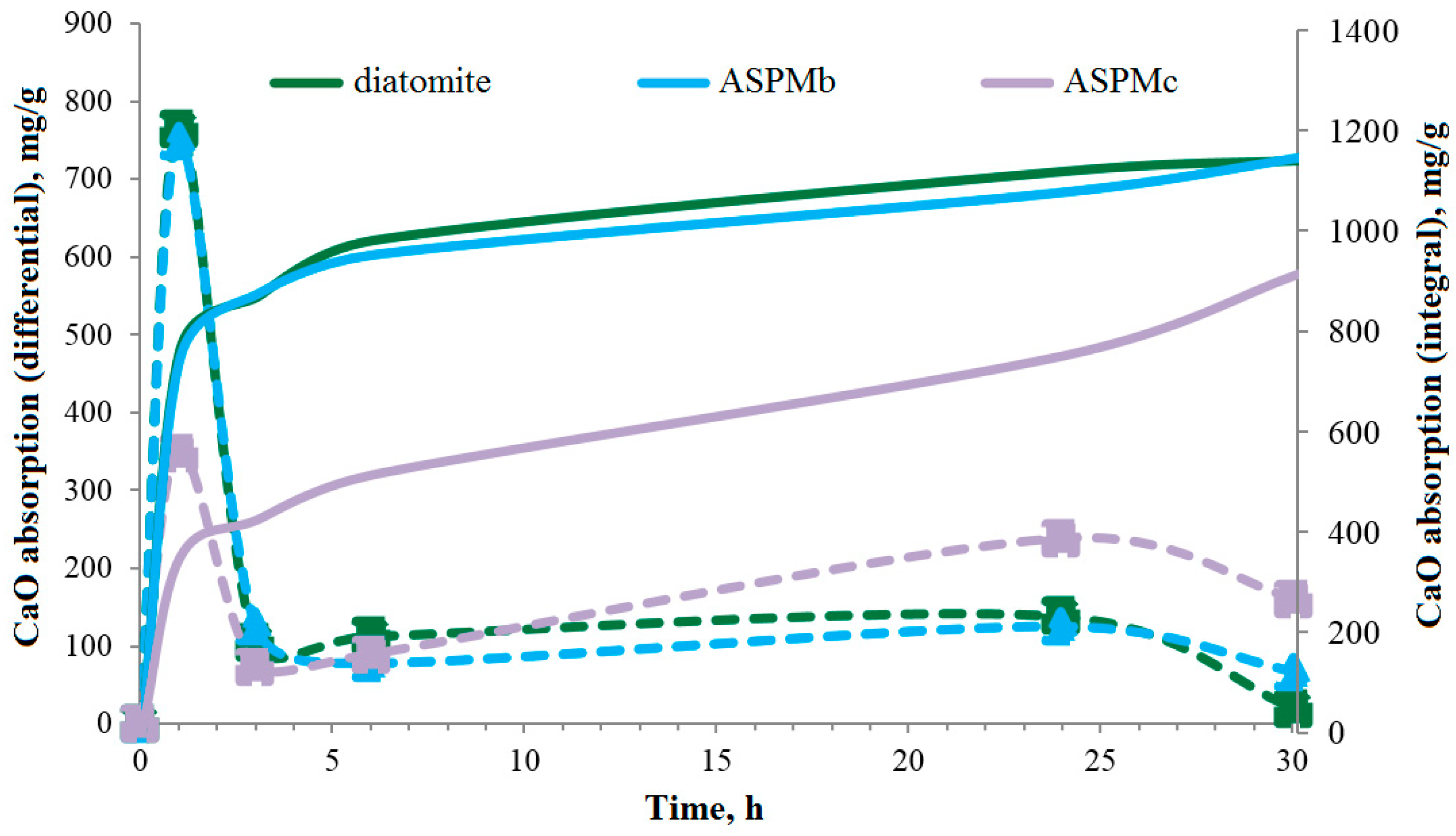




| Parameter Name | Value |
|---|---|
| Appearance | Fine dry powder from light gray to white |
| Particle size, µm: | |
| —average (D50) | 10.2 |
| —maximal (D90) | 36.9 |
| —minimal (D10) | 2.4 |
| Pour density, kg/m3 | 350.0 |
| Mass fraction of moisture, % | 3.4 |
| Specific surface, m2/g | 11.2 |
| pH of the aqueous extract | 7.42 |
| The Content of Oxides, Mass. % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | TiO2 | Others |
| 15.33 | 3.84 | 0.23 | 74.13 | 1.73 | 0.52 | 0.84 | 3.01 | 0.15 | 0.22 |
| Sieve Size, mm | 1.25 | 0.63 | 0.315 | 0.16 | Less than 0.16 | Fineness Modulus |
|---|---|---|---|---|---|---|
| Partial residuals, % | 2.5 | 23.0 | 52.0 | 17.0 | 5.5 | 2.0 |
| Total residuals, % | 2.5 | 25.5 | 77.5 | 94.5 | 100 |
| Material | Tetrabutoxytitanium | Ethyl Alcohol | Diatomite |
|---|---|---|---|
| ASPMb | 1 | 3 | 0.4 |
| ASPMc | 1 | 7 | 0.4 |
| Mass Ratio of Raw Materials | |||||||
|---|---|---|---|---|---|---|---|
| Raw Material | Cement Paste | Cement–Sand Mortar | Concrete | ||||
| Control | With Additive | Control | With Additive | Control | With Diatomite + AEROXIDE® TiO2 P 25 | With ASPMs | |
| WOPC | 1 | 0.85 | 1 | 0.85 | 1 | 0.9 | 0.9 |
| Additive (diatomite or ASPMs) | 0.15 | 0.15 | 0.06 | 0.1 | |||
| Sand | 3 | 3 | 6 | 6 | 6 | ||
| Plasticizing additive | 0.02 | 0.02 | 0.02 | ||||
| AEROXIDE® TiO2 P 25 | 0.04 | ||||||
| Water | 0.5 | 0.5 | 0.5 | 0.5 | 0.35 | 0.35 | 0.35 |
| Material | The Content of Oxides, Mass. % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | Na2O | K2O | CaO | SO3 | Others | |
| Diatomite | 86.81 | 0.28 | 5.91 | 2.67 | 2.35 | 0.34 | 1.12 | 0.41 | 0.01 | 0.01 |
| ASPMb | 56.24 | 37.31 | 2.89 | 1.13 | 0.52 | 0.47 | 0.50 | 0.21 | 0.26 | 0.47 |
| ASPMc | 55.53 | 38.13 | 2.62 | 1.18 | 0.61 | 0.59 | 0.51 | 0.21 | 0.28 | 0.34 |
| Properties | Materials | ||
|---|---|---|---|
| Diatomite | ASPMb | ASPMc | |
| Specific surface according to BET, m2/kg | 62,100 | 63,900 | 70,400 |
| Sum of Bronsted acid sites, 103 mole/g | 105.47 | 62.3 | 31.47 |
| Photocatalytic activity, % | |||
| —4 h later | 12 | 51 | 60 |
| —26 h later | 14 1 | 86 | 89 |
| Binding | Compressive Strength, MPa |
|---|---|
| White ordinary portland cement (WOPC) | 59.8 |
| 85% of WOPC + 15% of ASPMb | 54.7 |
| 85% of WOPC + 15% of ASPMc | 51.0 |
| After Steam Curing (SC) | After SC and Carbonization | ||
|---|---|---|---|
| Before Radiation | After Radiation | Before Radiation | After Radiation |
| Fine-grained concrete without additives | |||
 |  |  |  |
| Fine-grained concrete with AEROXIDE® TiO2 P 25 | |||
 |  |  |  |
| Fine-grained concrete with ASPMb | |||
 |  |  |  |
| Fine-grained concrete with ASPMc | |||
 |  |  |  |
| Sample Type | Removal of Rhodamine B after 26 h of UV Irradiation, % | Time to Reach the Final Contact Angle of wetting (GOST R 57255), h 1 | ||
|---|---|---|---|---|
| After SC | After SC and Carbonization | After SC | After SC and Carbonization | |
| Without additives | 15.88 | 15.81 | contact angle of wetting is not reduced | |
| With AEROXIDE® TiO2 P 25 | 70.18 | 58.62 | 48 | 60 |
| With ASPMb | 57.92 | 57.09 | 56 | 56 |
| With ASPMc | 64.17 | 61.94 | 52 | 56 |
| Type of Sample, Contact Angle of Wetting before Applying Oleic Acid | Initial Contact Angle of Wetting (after Applying Oleic Acid) | Final Contact Angle of Wetting |
|---|---|---|
| Without additives, 31° |  |  |
| With AEROXIDE® TiO2 P 25, 24° |  |  |
| With ASPMb, 32° |  |  |
| With ASPMc, 30° |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strokova, V.; Ogurtsova, Y.; Gubareva, E.; Nerovnaya, S.; Antonenko, M. Multifunctional Anatase–Silica Photocatalytic Material for Cements and Concretes. J. Compos. Sci. 2024, 8, 207. https://doi.org/10.3390/jcs8060207
Strokova V, Ogurtsova Y, Gubareva E, Nerovnaya S, Antonenko M. Multifunctional Anatase–Silica Photocatalytic Material for Cements and Concretes. Journal of Composites Science. 2024; 8(6):207. https://doi.org/10.3390/jcs8060207
Chicago/Turabian StyleStrokova, Valeria, Yulia Ogurtsova, Ekaterina Gubareva, Sofya Nerovnaya, and Marina Antonenko. 2024. "Multifunctional Anatase–Silica Photocatalytic Material for Cements and Concretes" Journal of Composites Science 8, no. 6: 207. https://doi.org/10.3390/jcs8060207
APA StyleStrokova, V., Ogurtsova, Y., Gubareva, E., Nerovnaya, S., & Antonenko, M. (2024). Multifunctional Anatase–Silica Photocatalytic Material for Cements and Concretes. Journal of Composites Science, 8(6), 207. https://doi.org/10.3390/jcs8060207






