Mechanical Properties and Thermal Conductivity of Y-Si and Gd-Si Silicides: First-Principles Calculations
Abstract
:1. Introduction
2. Computation Methods
3. Results and Discussion
3.1. Structural Properties
3.2. Elastic and Mechanical Properties
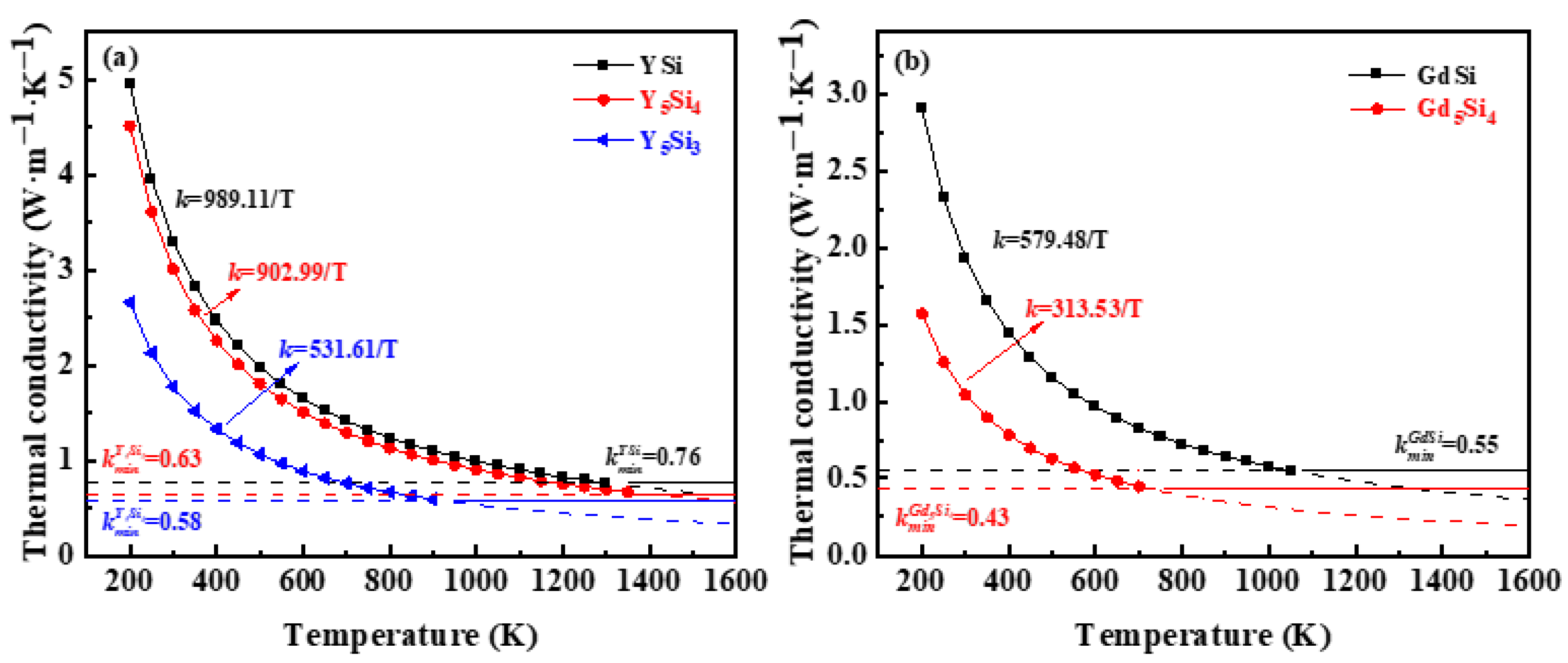
3.3. Thermal Conductivity
4. Conclusions
- (1)
- The results for elastic properties indicated that Y5Si4 is a ductile material, and its G/B value is lower than that of the other materials in this study. This characteristic helps to minimize the thermal stress and enhances the thermal shock resistance when used as coating materials. In addition, Young’s moduli of all the calculated materials are anisotropic.
- (2)
- The calculated thermal conductivity sequence for YxSiy and GdxSiy is as follows: Gd5Si4 < Y5Si3 < GdSi < Y5Si4 < YSi, with Gd5Si4 exhibiting the lowest thermal conductivity at 0.43 W m−1 K−1. This study ascertains that they are promising materials for environmental barrier coatings.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Przyborowski, M.; Hibiya, T.; Eguchi, M.; Egry, I. Surface tension measurement of molten silicon by the oscillating drop method using electromagnetic levitation. J. Cryst. Growth 1995, 151, 60–65. [Google Scholar] [CrossRef]
- Sullivan, R.M. Reformulation of oxide growth equations for oxidation of silicon bond coat in environmental barrier coating systems. J. Eur. Ceram. Soc. 2019, 39, 5403–5409. [Google Scholar] [CrossRef]
- Damby, D.E.; Llewellin, E.W.; Horwell, C.J.; Williamson, B.J.; Najorka, J.; Cressey, G.; Carpenter, M. The α–β phase transition in volcanic cristobalite. J. Appl. Crystallogr. 2014, 47, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Park, Y.; Xue, Z.; Zhang, S.; Byon, E.; Koo, B. Research status of bond coats in environmental barrier coatings. Int. J. Appl. Ceram. Technol. 2022, 19, 1841–1859. [Google Scholar] [CrossRef]
- Tejero-Martin, D.; Bennett, C.; Hussain, T. A review on environmental barrier coatings: History, current state of the art and future developments. J. Eur. Ceram. Soc. 2021, 41, 1747–1768. [Google Scholar] [CrossRef]
- Deijkers, J.A.; Wadley, H.N.G. Hafnium silicate formation during oxidation of a permeable silicon + HfO2 powder composite system. Acta Mater. 2020, 201, 448–461. [Google Scholar] [CrossRef]
- Suzuki, A.; Ashida, H.; Furui, N.; Mameno, K.; Matsunami, H. Thermal Oxidation of SiC and Electrical Properties of Al-SiO2-SiC MOS Structure. Jpn. J. Appl. Phys. 1982, 36, 3770–3778. [Google Scholar] [CrossRef]
- Anton, R.; Leisner, V.; Watermeyer, P.; Engstler, M.; Schulz, U. Hafnia-doped silicon bond coats manufactured by PVD for SiC/SiC CMCs. Acta Mater. 2020, 183, 471–483. [Google Scholar] [CrossRef]
- Xiao, S.; Li, J.; Liu, X.; Chang, Z.; Huang, P.; Zhang, A.; Tian, Y.; Zhang, X.; Zhang, J.; Han, G. Exploration of the oxidation behavior and doping ratio of the Si–HfO2 bond layer used in environmental barrier coatings. Int. J. Appl. Ceram. Technol. 2023, 20, 1753–1763. [Google Scholar] [CrossRef]
- Chen, W.; Han, Q.; He, J.; He, W.; Wang, W.; Guo, H. Effect of HfO2 framework on steam oxidation behavior of HfO2 doped Si coating at high temperatures. Ceram. Int. 2022, 48, 22209–22210. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Zhou, Y. Thermal properties of single-phase Y2SiO5. J. Eur. Ceram. Soc. 2009, 29, 551–557. [Google Scholar] [CrossRef]
- Ogura, Y.; Kondo, M.; Morimoto, T.; Notomi, A.; Sekigawa, T. Oxygen permeability of Y2SiO5. Mater. Trans. 2001, 42, 1124–1130. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, J.; Zhang, T.; Ren, X.; Hu, W.; Zheng, L.; Wang, J. Towards thermal barrier coating application for rare earth silicates RE2SiO5 (RE= La, Nd, Sm, Eu, and Gd). J. Eur. Ceram. Soc. 2019, 39, 1463–1476. [Google Scholar] [CrossRef]
- Dong, Y.; Ren, K.; Wang, Q.; Shao, G.; Wang, Y. Interaction of multicomponent disilicate (Yb0.2Y0.2Lu0.2Sc0.2Gd0.2)2Si2O7 with molten calcia-magnesia-aluminosilicate. J. Adv. Ceram. 2022, 11, 66–74. [Google Scholar] [CrossRef]
- Ramasamy, S.; Tewari, S.N.; Lee, K.N.; Bhatt, R.T.; Fox, D.S. Environmental durability of slurry based mullite–gadolinium silicate EBCs on silicon carbide. J. Eur. Ceram. Soc. 2011, 31, 1123–1130. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, B.-N.; Nagashima, N.; Matsushita, Y.; Jang, B.-K. High-temperature corrosion of spark plasma sintered Gd2SiO5 with volcanic ash for environmental barrier coatings. J. Eur. Ceram. Soc. 2021, 41, 3161–3166. [Google Scholar] [CrossRef]
- Lee, K.N.; Eldridge, J.I.; Robinson, R.C. Residual stresses and their effects on the durability of environmental barrier coatings for SiC ceramics. J. Am. Ceram. Soc. 2005, 88, 3483–3488. [Google Scholar] [CrossRef]
- Olson, D.H.; Deijkers, J.A.; Quiambao-Tomko, K.; Gaskins, J.T.; Richards, B.T.; Opila, E.J.; Hopkins, P.E.; Wadley, H.N. Evolution of microstructure and thermal conductivity of multifunctional environmental barrier coating systems. Mater. Today Phys. 2021, 17, 100304. [Google Scholar] [CrossRef]
- Shukla, A.; Kang, Y.B.; Pelton, A.D. Thermodynamic assessment of the Ce–Si, Y–Si, Mg–Ce–Si and Mg–Y–Si systems. Int. J. Mater. Res. 2009, 100, 208–217. [Google Scholar] [CrossRef]
- Huang, M.; Schlagel, D.L.; Schmidt, F.A.; Lograsso, T.A. Experimental Investigation and Thermodynamic Modeling of the Gd—Si System. J. Alloys Compd. 2007, 441, 94–100. [Google Scholar] [CrossRef]
- Clarke, D.R. Materials selection guidelines for low thermal conductivity thermal barrier coatings. Surf. Coat. Technol. 2003, 163, 67–74. [Google Scholar] [CrossRef]
- Slack, G.A. Nonmetallic crystals with high thermal conductivity. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Hill, R. The elastic behavior of crystalline aggregate. Proc. Phys. Society. Sect. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Voigt, W. Lehrbuch der Kristallphysik; Teubner: Leipzig, Germany, 1928. [Google Scholar]
- Reuss, A. Calculation of the flow limits of mixed crystals on the basis of the plasticity of monocrystals. Z. Angew. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals: Their Representation by Tensors and Matrices; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Green, D.J. An Introduction to the Mechanical Properties of Ceramics; Cambridge University Press: Cambridge, UK, 1998; pp. 1–12. [Google Scholar]
- Nazipov, D.V. First-Principles Study of Elastic Properties of Rare-Earth Oxyorthosilicates R2SiO5. Phys. Status Solidi (B) 2021, 258, 2100181. [Google Scholar] [CrossRef]
- Anderson, O.L. A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Sanditov, B.D.; Tsydypov, S.B.; Sanditov, D.S. Relation between the Grüneisen constant and Poisson’s ratio of vitreous systems. Acoust. Phys. 2007, 53, 594–597. [Google Scholar] [CrossRef]
- Beckstein, O.; Klepeis, J.E.; Hart, G.L.; Pankratov, O. First-principles elastic constants and electronic structure of α-Pt2Si and PtSi. Phys. Rev. B 2001, 13, 134112. [Google Scholar] [CrossRef]
- Wu, Z.J.; Zhao, E.J.; Xiang, H.P.; Hao, X.F.; Liu, X.J.; Meng, J. Publisher’s Note: Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles [Phys. Rev. B 76, 054115 (2007)]. Phys. Rev. B 2007, 76, 059904. [Google Scholar] [CrossRef]
- Schfer, K. Duane C. Wallace: Thermodynamics of Crystals. In Berichte der Bunsengesellschaft für Physikalische Chemie; Verlag Chemie: Weinheim, Germany, 1972; Volume 76. [Google Scholar]
- Makishima, A.; Mackenzie, J.D. Calculation of bulk modulus, shear modulus and Poisson’s ratio of glass. J. Non-Cryst. Solids 1975, 17, 147–157. [Google Scholar] [CrossRef]
- Kulikovsky, V.; Vorlíček, V.; Boháč, P.; Stranyánek, M.; Čtvrtlík, R.; Kurdyumov, A.; Jastrabik, L. Hardness and elastic modulus of amorphous and nanocrystalline SiC and Si films. Surf. Coat. Technol. 2008, 202, 1738–1745. [Google Scholar] [CrossRef]
- Mattesini, M.; Magnuson, M.; Tasnádi, F.; Höglund, C.; Abrikosov, I.A.; Hultman, L. Elastic properties and electrostructural correlations in ternary scandium-based cubic inverse perovskites: A first-principles study. Phys. Rev. B Condens. Matter 2009, 79, 125122. [Google Scholar] [CrossRef]
- Robertson, J.H. Physical properties of crystals: Their representation by tensors and matrices by J. F. Nye. Acta Crystallogr. 1985, 41, 624. [Google Scholar] [CrossRef]
- Brantley, W.A. Calculated Elastic Constants for Stress Problems Associated with Semiconductor Devices. J. Appl. Phys. 1973, 44, 534–535. [Google Scholar] [CrossRef]
- Mohapatra, H.; Eckhardt, C.J. Elastic constants and related mechanical properties of the monoclinic polymorph of the carbamazepine molecular crystal. J. Phys. Chem. B 2008, 112, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- Pecharsky, A.O.; Pecharsky, V.K.; Gschneidner, K.A. Phase relationships and low temperature heat capacities of alloys in the Y5Si4–Y5Ge4 pseudo binary system. J. Alloys Compd. 2004, 379, 127–134. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Fang, Z.; Cao, X.; Li, Y.; Sun, C.; Chen, Z.; Yin, F. First-principles calculations to investigate electronic, elastic, and optical properties of one dimensional electride Y5Si3. Results Phys. 2021, 28, 104615. [Google Scholar] [CrossRef]
- Levin, E.M.; Pecharsky, V.K.; Gschneidner, K.A., Jr.; Miller, G.J. Electrical resistivity, electronic heat capacity, and electronic structure of Gd5Ge4. Phys. Rev. B 2001, 64, 235103. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Y.; Schmitz, B.; Persson, J.; Schmidt, W.; Meuffels, P.; Roth, G.; Brückel, T. Possible magnetic-polaron-switched positive and negative magnetoresistance in the GdSi single crystals. Sci. Rep. 2012, 2, 750. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Sasmal, S.; Kulkarni, R.; Thamizhavel, A. Linear unsaturated magnetoresistance in YSi single crystal. Appl. Phys. Lett. 2021, 119, 017904. [Google Scholar] [CrossRef]

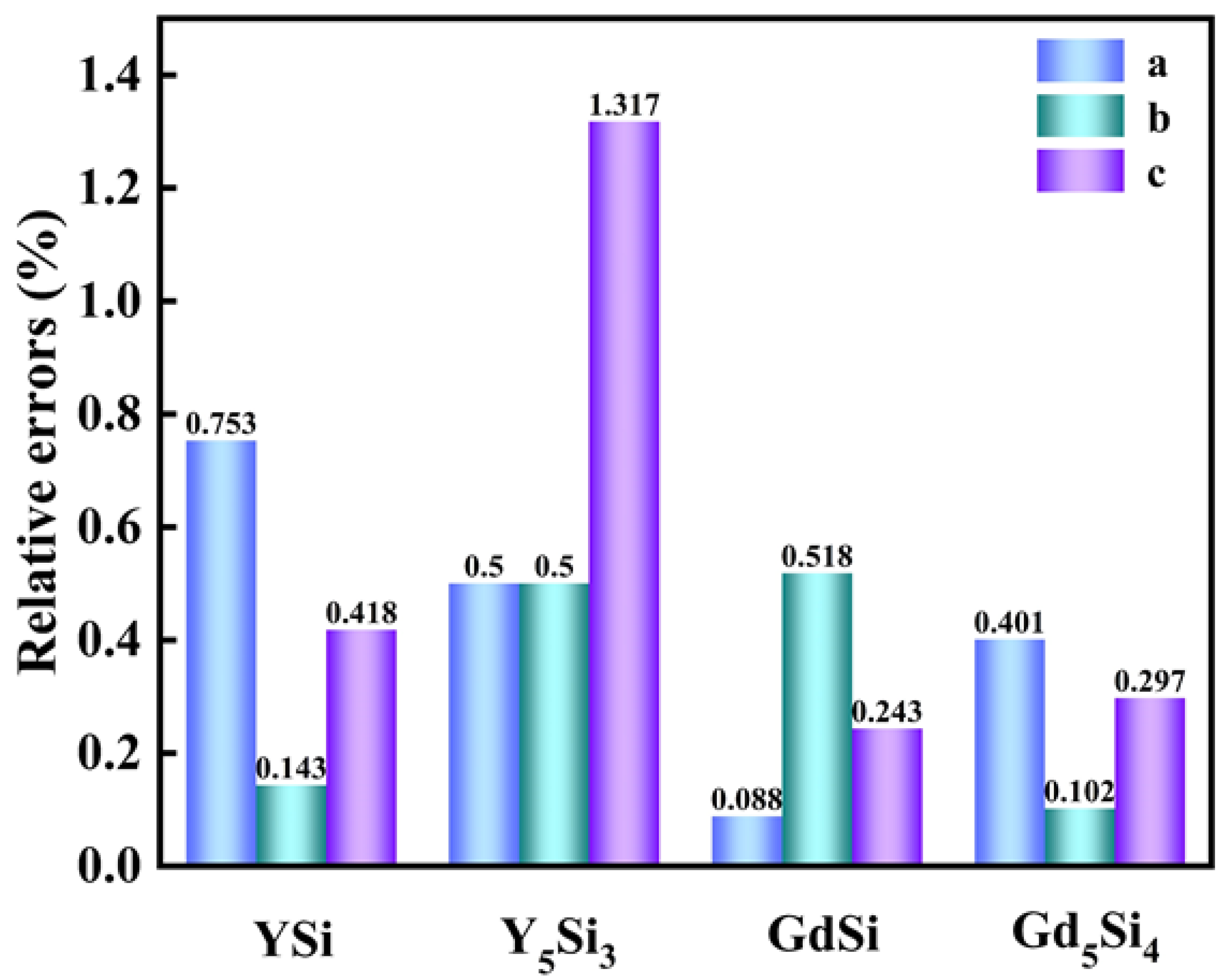


| Materials | () | () | () |
|---|---|---|---|
| YSi | 4.283 | 10.541 | 3.842 |
| YSi (89-2305) | 4.251 | 10.526 | 3.826 |
| Y5Si4 | 7.443 | 14.585 | 7.701 |
| Y5Si3 | 8.445 | 8.445 | 6.386 |
| Y5Si3 (89-3037) | 8.403 | 8.403 | 6.303 |
| GdSi | 7.980 | 3.878 | 5.767 |
| GdSi (80-0705) | 7.973 | 3.858 | 5.753 |
| Gd5Si4 | 7.516 | 14.735 | 7.774 |
| Gd5Si4 (87-2319) | 7.486 | 14.750 | 7.751 |
| Materials | C11 | C12 | C13 | C22 | C23 | C33 | C44 | C55 | C66 |
|---|---|---|---|---|---|---|---|---|---|
| YSi | 161 | 43 | 67 | 203 | 25 | 182 | 48 | 101 | 62 |
| Y5Si4 | 119 | 37 | 51 | 139 | 50 | 139 | 44 | 31 | 52 |
| Y5Si3 | 167 | 40 | 32 | 119 | 51 | 62 | |||
| GdSi | 161 | 43 | 67 | 203 | 25 | 182 | 48 | 101 | 62 |
| Gd5Si4 | 107 | 36 | 48 | 134 | 50 | 130 | 38 | 31 | 51 |
| Materials | B (GPa) | G (GPa) | E (GPa) | μ | H (HV) | G/B |
|---|---|---|---|---|---|---|
| YSi | 91 | 67 | 161 | 0.205 | 13 | 0.736 |
| Y5Si4 | 75 | 42 | 106 | 0.265 | 7 | 0.560 |
| Y5Si3 | 71 | 56 | 133 | 0.194 | 12 | 0.789 |
| GdSi | 74 | 57 | 136 | 0.193 | 12 | 0.730 |
| Gd5Si4 | 62 | 38 | 94 | 0.247 | 7 | 0.613 |
| Materials | vL (km/s) | vT (km/s) | vm (km/s) | ΘD·(K) | kmin(w/(m·K)) |
|---|---|---|---|---|---|
| YSi | 6.34 | 3.86 | 4.27 | 455 | 0.76 |
| Y5Si4 | 5.44 | 3.08 | 3.42 | 356 | 0.58 |
| Y5Si3 | 5.75 | 3.54 | 3.91 | 398 | 0.63 |
| GdSi | 4.65 | 2.87 | 3.17 | 335 | 0.55 |
| Gd5Si4 | 4.03 | 2.34 | 2.59 | 267 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, K.; Huang, P.; Han, G.; Liu, H.; Zhang, W.; Wang, W.; Zhang, J. Mechanical Properties and Thermal Conductivity of Y-Si and Gd-Si Silicides: First-Principles Calculations. J. Compos. Sci. 2024, 8, 221. https://doi.org/10.3390/jcs8060221
Peng K, Huang P, Han G, Liu H, Zhang W, Wang W, Zhang J. Mechanical Properties and Thermal Conductivity of Y-Si and Gd-Si Silicides: First-Principles Calculations. Journal of Composites Science. 2024; 8(6):221. https://doi.org/10.3390/jcs8060221
Chicago/Turabian StylePeng, Kexue, Panxin Huang, Guifang Han, Huan Liu, Weibin Zhang, Weili Wang, and Jingde Zhang. 2024. "Mechanical Properties and Thermal Conductivity of Y-Si and Gd-Si Silicides: First-Principles Calculations" Journal of Composites Science 8, no. 6: 221. https://doi.org/10.3390/jcs8060221





