The Effect of Nanoclay Type on the Mechanical Properties and Antibacterial Activity of Chitosan/PVA Nanocomposite Films
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Nanocomposite Film Preparation
2.3. Characterization
2.3.1. SEM
2.3.2. AFM
2.3.3. XRD
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.5. Tensile Strength (TS)
2.4. Antibacterial Activity
2.5. Statistical Analysis
3. Results
3.1. XRD
3.2. FTIR Results
3.3. SEM/EDX
3.4. AFM
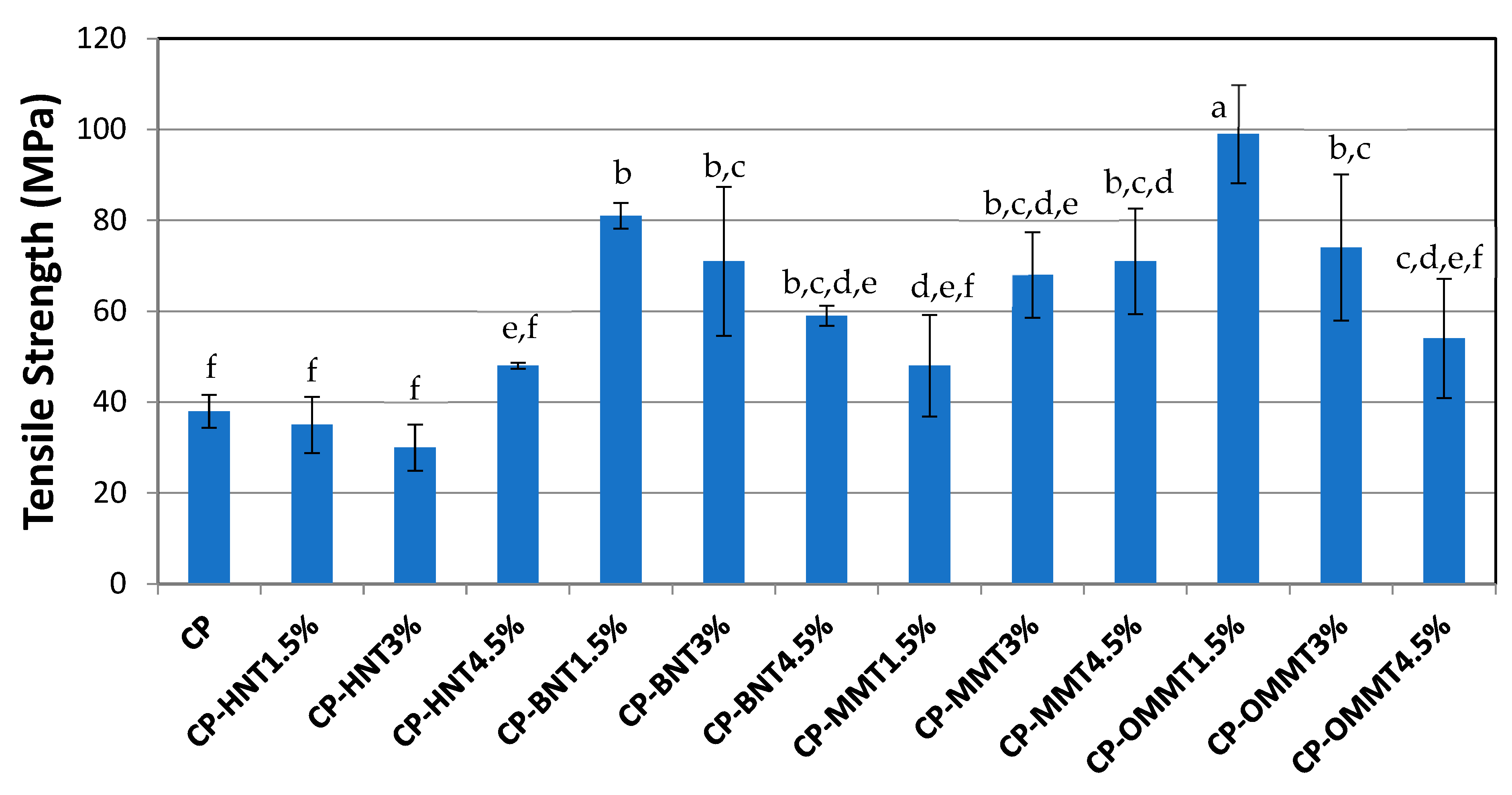
3.5. Tensile Strength
3.6. Antibacterial Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sengwa, R.J.; Dhatarwal, P. PVA/MMT and (PVA/PVP)/MMT hybrid nanocomposites for broad-range radio frequency tunable nanodielectric applications. Mater. Lett. 2021, 299, 130081. [Google Scholar] [CrossRef]
- Borah, D.; Brahma, D.; Roy, S.; Basak, D.; Agarwal, S.; Saikia, H. Bentonite clay as a novel base heterogeneous catalyst in Knoevenagel Condensation of aldehydes with ethyl cyanoacetate in water. Results Chem. 2024, 7, 101238. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Q.; Cheng, J.; Pan, Y.; Yang, G.; Liu, Y.; Wang, L.; Leng, Y.; Tuo, X. Synthesis and characterization of CTAB-modified bentonite composites for the removal of Cs+. J. Radioanal. Nucl. Chem. 2021, 329, 451–461. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J.; Theng, B.; Righi, D.; Delvaux, B. Halloysite clay minerals–a review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Abdouss, M.; Radgoudarzi, N.; Mohebali, A.; Kowsari, E.; Koosha, M.; Li, T. Fabrication of Bio-Nanocomposite Based on HNT-Methionine for Controlled Release of Phenytoin. Polymers 2021, 13, 2576. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, S.; Koosha, M. Designing a pH-responsive drug delivery system for the release of black-carrot anthocyanins loaded in halloysite nanotubes for cancer treatment. Appl. Clay Sci. 2020, 197, 105770. [Google Scholar] [CrossRef]
- Koosha, M.; Raoufi, M.; Moravvej, H. One-pot reactive electrospinning of chitosan/PVA hydrogel nanofibers reinforced by halloysite nanotubes with enhanced fibroblast cell attachment for skin tissue regeneration. Colloids Surf. B Biointerfaces 2019, 179, 270–279. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203. [Google Scholar] [CrossRef]
- De Silva, R.T.; Mantilaka, M.M.M.G.P.G.; Ratnayake, S.P.; Amaratunga, G.A.J.; de Silva, K.M.N. Nano-MgO reinforced chitosan nanocomposites for high performance packaging applications with improved mechanical, thermal and barrier properties. Carbohydr. Polym. 2017, 157, 739–747. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-h.; Wang, M.-C.; Lin, J.-J. Biocompatibility and antimicrobial evaluation of montmorillonite/chitosan nanocomposites. Appl. Clay Sci. 2012, 56, 53–62. [Google Scholar] [CrossRef]
- Taştan, Ö.; Baysal, T. Chitosan as a novel clarifying agent on clear apple juice production: Optimization of process conditions and changes on quality characteristics. Food Chem. 2017, 237, 818–824. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Sila, A.; Haddar, A.; Bougatef, A.; Ayadi, M.A. β-Chitin and chitosan from squid gladius: Biological activities of chitosan and its application as clarifying agent for apple juice. Int. J. Biol. Macromol. 2017, 104, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Racine, L.; Costa, G.; Bayma-Pecit, E.; Texier, I.; Auzély-Velty, R. Design of interpenetrating chitosan and poly(ethylene glycol) sponges for potential drug delivery applications. Carbohydr. Polym. 2017, 170, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Arya, N.; Chakraborty, S.; Dube, N.; Katti, D.S. Electrospraying: A facile technique for synthesis of chitosan-based micro/nanospheres for drug delivery applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88B, 17–31. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S. 6-The use of chitin and chitosan for food packaging applications A2-Chiellini, Emo. In Environmentally Compatible Food Packaging; Woodhead Publishing: Abington, UK, 2008; pp. 137–158. [Google Scholar] [CrossRef]
- van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, F.; Oderinde, O.; Li, K.; Wang, H.; Zhang, Z.; Fu, G. Zinc ions enhanced nacre-like chitosan/graphene oxide composite film with superior mechanical and shape memory properties. Chem. Eng. J. 2017, 321, 502–509. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Arfat, Y.A.; Thai, T.L.A. Mechanical, thermal, structural and barrier properties of crab shell chitosan/graphene oxide composite films. Food Hydrocoll. 2017, 71, 141–148. [Google Scholar] [CrossRef]
- Abraham, A.; Soloman, P.A.; Rejini, V.O. Preparation of Chitosan-Polyvinyl Alcohol Blends and Studies on Thermal and Mechanical Properties. Procedia Technol. 2016, 24, 741–748. [Google Scholar] [CrossRef]
- Kabiri, K.; Mirzadeh, H.; Zohuriaan-Mehr, M.J.; Daliri, M. Chitosan-modified nanoclay–poly (AMPS) nanocomposite hydrogels with improved gel strength. Polym. Int. 2009, 58, 1252–1259. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Dash, M.; Chiellini, F.; Ottenbrite, R.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Nour, M. Polymer/clay nanocomposites. Polimery 2002, 47, 326–331. [Google Scholar] [CrossRef]
- Utracki, L.A. Clay-Containing Polymeric Nanocomposites; iSmithers Rapra Publishing: Shewsbury, UK, 2004; Volume 1. [Google Scholar]
- Koosha, M.; Mirzadeh, H.; Shokrgozar, M.A.; Farokhi, M. Nanoclay-reinforced electrospun chitosan/PVA nanocomposite nanofibers for biomedical applications. RSC Adv. 2015, 5, 10479–10487. [Google Scholar] [CrossRef]
- Yao, J.; Mao, L.; Wang, C.; Liu, X.; Liu, Y. Development of chitosan/poly (vinyl alcohol) active films reinforced with curcumin functionalized layered clay towards food packaging. Prog. Org. Coat. 2023, 182, 107674. [Google Scholar] [CrossRef]
- Sathish Kumar, R.K.; Dhilipkumar, T.; Anita Jessie, J.; Gaayathri, K.K.; Arumugam, S. Advancements in bio-polymeric composite materials for active and intelligent food packaging: A comprehensive review. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; Nair, I.C.; Siengchin, S.; Parameswaranpillai, J.; Radhakrishnan, E.K. Polyvinyl alcohol-nanocomposite films incorporated with clay nanoparticles and lipopeptides as active food wraps against food spoilage microbes. Food Packag. Shelf Life 2021, 30, 100727. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Zhao, Q.; Cheng, X.; Kang, J.; Kong, L.; Zhao, X.; He, X.; Li, J. Polyvinyl alcohol flame retardant film based on halloysite nanotubes, chitosan and phytic acid with strong mechanical and anti-ultraviolet properties. Int. J. Biol. Macromol. 2023, 246, 125682. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Hosseini, R.; Darvishi, F.; Sabzi, M. The release of cefazolin from chitosan/polyvinyl alcohol/sepiolite nanocomposite hydrogel films. Iran. Polym. J. 2016, 25, 933–943. [Google Scholar] [CrossRef]
- Zhai, R.; Zhang, B.; Wan, Y.; Li, C.; Wang, J.; Liu, J. Chitosan–halloysite hybrid-nanotubes: Horseradish peroxidase immobilization and applications in phenol removal. Chem. Eng. J. 2013, 214, 304–309. [Google Scholar] [CrossRef]
- Partovinia, A.; Koosha, M. Fabrication of novel nanocomposite nanofibrous matrices retaining high concentration of microbial cells for heavy crude oil biodegradation. Express Polym. Lett. 2019, 13, 484–499. [Google Scholar] [CrossRef]
- Benucci, I.; Liburdi, K.; Cacciotti, I.; Lombardelli, C.; Zappino, M.; Nanni, F.; Esti, M. Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocoll. 2018, 74, 124–131. [Google Scholar] [CrossRef]
- Liu, M.; Shen, Y.; Ao, P.; Dai, L.; Liu, Z.; Zhou, C. The improvement of hemostatic and wound healing property of chitosan by halloysite nanotubes. RSC Adv. 2014, 4, 23540–23553. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Janghu, P.; Pasrija, R.; Umesh, M.; Chakraborty, P.; Sarojini, S.; Thomas, J. Synthesis and characterization of chitosan nanofibers for wound healing and drug delivery application. J. Drug Deliv. Sci. Technol. 2023, 87, 104858. [Google Scholar] [CrossRef]
- Biswal, A.; Purohit, S.S.; Swain, S.K. Chitosan based composite scaffolds in skin wound repair: A review. J. Drug Deliv. Sci. Technol. 2023, 84, 104549. [Google Scholar] [CrossRef]
- Liu, M.; Wu, C.; Jiao, Y.; Xiong, S.; Zhou, C. Chitosan-halloysite nanotubes nanocomposite scaffolds for tissue engineering. J. Mater. Chem. B 2013, 1, 2078–2089. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Wu, C.; Xiong, S.; Zhou, C. Chitosan/halloysite nanotubes bionanocomposites: Structure, mechanical properties and biocompatibility. Int. J. Biol. Macromol. 2012, 51, 566–575. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Nikolakis, S.-P.; Pamvouxoglou, A.; Koutsopoulou, E. Physicochemical properties of electrostatically crosslinked carrageenan/chitosan hydrogels and carrageenan/chitosan/Laponite nanocomposite hydrogels. Int. J. Biol. Macromol. 2023, 225, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Liu, M.; Zheng, J.; Zhou, C. Adsorption of dyes in aqueous solutions by chitosan–halloysite nanotubes composite hydrogel beads. Microporous Mesoporous Mater. 2015, 201, 190–201. [Google Scholar] [CrossRef]
- Levis, S.R.; Deasy, P.B. Use of coated microtubular halloysite for the sustained release of diltiazem hydrochloride and propranolol hydrochloride. Int. J. Pharm. 2003, 253, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Viseras, C.; Cerezo, P.; Sanchez, R.; Salcedo, I.; Aguzzi, C. Current challenges in clay minerals for drug delivery. Appl. Clay Sci. 2010, 48, 291–295. [Google Scholar] [CrossRef]
- Yang, K.; Shen, L.; Zhang, L.; Sun, W.; Zou, Y.; Ren, Y.; Zeng, R. Antibacterial Activity and Biocompatibility of Ag-Montmorillonite/Chitosan Colloidal Dressing in a Skin Infection Rat Model: An In Vitro and In Vivo Study. J. Funct. Biomater. 2023, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Abdullayev, E. Functional polymer–clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Lvov, Y.M.; Shchukin, D.G.; Möhwald, H.; Price, R.R. Halloysite Clay Nanotubes for Controlled Release of Protective Agents. ACS Nano 2008, 2, 814–820. [Google Scholar] [CrossRef] [PubMed]
- El Kaim Billah, R.; Islam, M.A.; Nazal, M.K.; Bahsis, L.; Soufiane, A.; Abdellaoui, Y.; Achak, M. A novel glutaraldehyde cross-linked chitosan@acid-activated bentonite composite for effectivePb (II) and Cr (VI) adsorption: Experimental and theoretical studies. Sep. Purif. Technol. 2024, 334, 126094. [Google Scholar] [CrossRef]
- Elmahdy, M.M.; Yassin, M.A. Linear and nonlinear optical parameters of biodegradable chitosan/polyvinyl alcohol/sodium montmorillonite nanocomposite films for potential optoelectronic applications. Int. J. Biol. Macromol. 2023, 258, 128914. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Mohebali, A.; Abdouss, M. Layered biocompatible pH-responsive antibacterial composite film based on HNT/PLGA/chitosan for controlled release of minocycline as burn wound dressing. Int. J. Biol. Macromol. 2020, 164, 4193–4204. [Google Scholar] [CrossRef] [PubMed]
- Kusmono; Abdurrahim, I. Water sorption, antimicrobial activity, and thermal and mechanical properties of chitosan/clay/glycerol nanocomposite films. Heliyon 2019, 5, e02342. [Google Scholar] [CrossRef] [PubMed]
- Erklig, A.; Çakır, M.; Bozkurt, Ö. Nano Clay Additive Effect on Shear Strength of GFRP Joints. Sak. Univ. J. Sci. 2019, 23, 1115–1122. [Google Scholar] [CrossRef][Green Version]
- Jiang, W.-T.; Chang, P.-H.; Tsai, Y.; Li, Z. Halloysite nanotubes as a carrier for the uptake of selected pharmaceuticals. Microporous Mesoporous Mater. 2016, 220, 298–307. [Google Scholar] [CrossRef]
- Hayati-Ashtiani, M. Use of FTIR spectroscopy in the characterization of natural and treated nanostructured bentonites (montmorillonites). Part. Sci. Technol. 2012, 30, 553–564. [Google Scholar] [CrossRef]
- Koosha, M.; Mirzadeh, H. Electrospinning, mechanical properties, and cell behavior study of chitosan/PVA nanofibers. J. Biomed. Mater. Res. Part A 2015, 103, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Theng, B.K.G. Chapter 7-Polymer–Clay Nanocomposites. In Developments in Clay Science; Theng, B.K.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4, pp. 201–241. [Google Scholar]
- Pinnavaia, T.J. Intercalated Clay Catalysts. Science 1983, 220, 365–371. [Google Scholar] [CrossRef]
- Daraie, M.; Bagheri, D.; Malmir, M.; Heravi, M.M. Investigation of halloysite nanotubes and Schiff base combination with deposited copper iodide nanoparticles as a novel heterogeneous catalytic system. Sci. Rep. 2021, 11, 23658. [Google Scholar] [CrossRef]
- Atkovska, K.; Bliznakovska, B.; Ruseska, G.; Bogoevski, S.; Boskovski, B.; Grozdanov, A. Adsorption of Fe(II) and Zn(II) ions from landfill leachate by natural bentonite. J. Chem. Technol. Metall. 2016, 51, 215–222. [Google Scholar]
- Qian, Y.; Huang, Z.; Zhou, G.; Chen, C.; Sang, Y.; Yu, Z.; Jiang, L.; Mei, Y.; Wei, Y. Preparation and Properties of Organically Modified Na-Montmorillonite. Materials 2023, 16, 3184. [Google Scholar] [CrossRef]
- Nagarpita, M.V.; Roy, P.; Shruthi, S.B.; Sailaja, R.R.N. Synthesis and swelling characteristics of chitosan and CMC grafted sodium acrylate-co-acrylamide using modified nanoclay and examining its efficacy for removal of dyes. Int. J. Biol. Macromol. 2017, 102, 1226–1240. [Google Scholar] [CrossRef]
- Yang, S.; Ji, Y.; Deng, F.; Sun, X.; Ning, C. Co-exchanged montmorillonite: A potential antibacterial agent with good antibacterial activity and cytocompatibility. J. Mater. Chem. B 2022, 10, 3705–3715. [Google Scholar] [CrossRef]
- Tong, G.; Yulong, M.; Peng, G.; Zirong, X. Antibacterial effects of the Cu(II)-exchanged montmorillonite on Escherichia coli K88 and Salmonella choleraesuis. Vet. Microbiol. 2005, 105, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Cometa, S.; Bonifacio, M.A.; Bellissimo, A.; Pinto, L.; Petrella, A.; De Vietro, N.; Iannaccone, G.; Baruzzi, F.; De Giglio, E. A green approach to develop zeolite-thymol antimicrobial composites: Analytical characterization and antimicrobial activity evaluation. Heliyon 2022, 8, e09551. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, A.; Banerjee, S.L.; Pramanik, N.; Jana, P.; Mitra, T.; Gnanamani, A.; Das, M.; Kundu, P.P. Organically modified clay supported chitosan/hydroxyapatite-zinc oxide nanocomposites with enhanced mechanical and biological properties for the application in bone tissue engineering. Int. J. Biol. Macromol. 2018, 106, 11–19. [Google Scholar] [CrossRef]
- Bhowmick, A.; Jana, P.; Pramanik, N.; Mitra, D.T.; Banerjee, S.; Gnanamani, A.; Das, M.; Kundu, P. Multifunctional zirconium oxide doped chitosan based hybrid nanocomposites as bone tissue engineering materials. Carbohydr. Polym. 2016, 151, 879–888. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res. Int. 2015, 2015, 759348. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, A.B.; Carrara, J.A.; Barroso, C.D.N.; Tuon, F.F.; Faoro, H. Role of Efflux Pumps on Antimicrobial Resistance in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2022, 23, 5779. [Google Scholar] [CrossRef]






| Sample Code | Nanoclay Type | Nanoclay (wt%) |
|---|---|---|
| CP | - | 0 |
| CP/HNT1.5 | HNT | 1.5 |
| CP/HNT3 | HNT | 3 |
| CP/HNT4.5 | HNT | 4.5 |
| CP/BNT1.5 | BNT | 1.5 |
| CP/BNT3 | BNT | 3 |
| CP/BNT4.5 | BNT | 4.5 |
| CP/MMT1.5 | MMT | 1.5 |
| CP/MMT3 | MMT | 3 |
| CP/MMT4.5 | MMT | 4.5 |
| CP/OMMT1.5 | O-MMT | 1.5 |
| CP/OMMT3 | O-MMT | 3 |
| CP/OMMT4.5 | O-MMT | 4.5 |
| Nano-Clay Type | HNT | BNT | MMT | OMMT |
|---|---|---|---|---|
| Nanoclay content with the highest tensile strength (%) | 4.5 | 1.5 | 4.5 | 1.5 |
| Highest tensile strength (MPa) | 48 ± 2.5 | 81 ± 7.9 | 71 ± 6.6 | 99 ± 3.7 |
| Sample | E. coli | P. aeroginosa | S. aureus |
|---|---|---|---|
| Control (CP) | 7 mm | 7 mm | 7 mm |
| CP-MMT1.5 | 7 mm | 7 mm | 10 mm |
| CP-MMT3 | 7 mm | 7 mm | 8 mm |
| CP-MMT4.5 | 11 mm | 7 mm | 7 mm |
| CP-OMMT1.5 | 12 mm | 7 mm | 11 mm |
| CP-OMMT3 | 15 mm | 7 mm | 19 mm |
| CP-OMMT4.5 | 12 mm | 8 mm | 8 mm |
| CP-HNT1.5 | 7 mm | 8 mm | 9 mm |
| CP-HNT3 | 8 mm | 7 mm | 10 mm |
| CP-HNT4.5 | 12 mm | 8 mm | 7 mm |
| CP-BNT1.5 | 14 mm | 12 mm | 7 mm |
| CP-BNT3 | 12 mm | 11 mm | 11 mm |
| CP-BNT4.5 | 7 mm | 7 mm | 7 mm |
| E. coli | P. aeroginosa | S. aureus | |
|---|---|---|---|
| Nano-clay type with the highest antibacterial activity | OMMT | BNT | OMMT |
| Nano-clay content (%) | 3 | 1.5 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrokhi, H.; Koosha, M.; Nasirizadeh, N.; Salari, M.; Abdouss, M.; Li, T.; Gong, Y. The Effect of Nanoclay Type on the Mechanical Properties and Antibacterial Activity of Chitosan/PVA Nanocomposite Films. J. Compos. Sci. 2024, 8, 255. https://doi.org/10.3390/jcs8070255
Farrokhi H, Koosha M, Nasirizadeh N, Salari M, Abdouss M, Li T, Gong Y. The Effect of Nanoclay Type on the Mechanical Properties and Antibacterial Activity of Chitosan/PVA Nanocomposite Films. Journal of Composites Science. 2024; 8(7):255. https://doi.org/10.3390/jcs8070255
Chicago/Turabian StyleFarrokhi, Hadisehsadat, Mojtaba Koosha, Navid Nasirizadeh, Mahshid Salari, Majid Abdouss, Tianduo Li, and Yinghua Gong. 2024. "The Effect of Nanoclay Type on the Mechanical Properties and Antibacterial Activity of Chitosan/PVA Nanocomposite Films" Journal of Composites Science 8, no. 7: 255. https://doi.org/10.3390/jcs8070255
APA StyleFarrokhi, H., Koosha, M., Nasirizadeh, N., Salari, M., Abdouss, M., Li, T., & Gong, Y. (2024). The Effect of Nanoclay Type on the Mechanical Properties and Antibacterial Activity of Chitosan/PVA Nanocomposite Films. Journal of Composites Science, 8(7), 255. https://doi.org/10.3390/jcs8070255






