Development of Mineral Fillers for Acid-Resistant Filling Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
4. Conclusions
- -
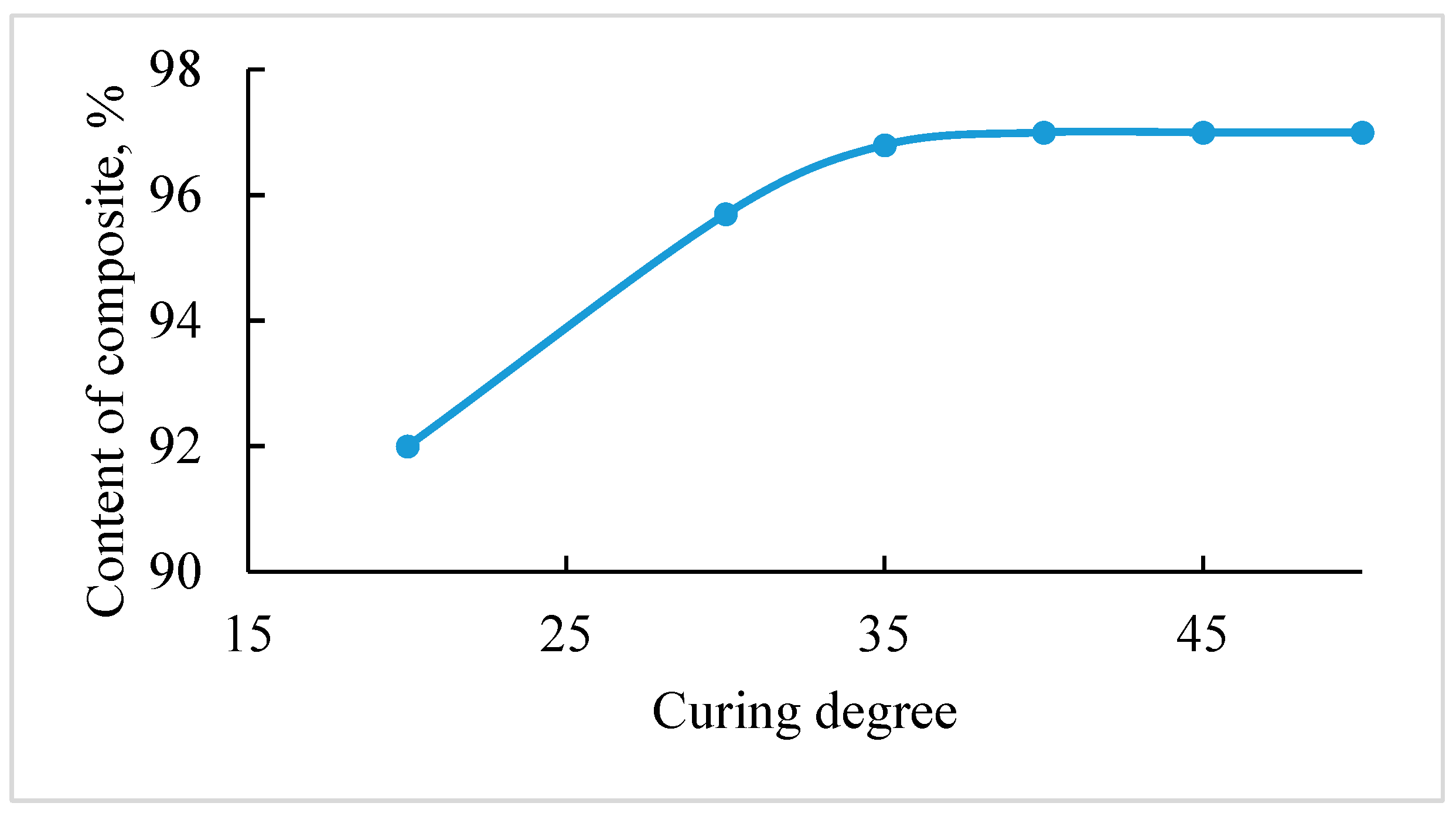
- For the first time, the effect of hydrated calcium compounds on the curing process of filled phenol formaldehyde pouring compositions was established, which consists in binding of polycondensation water and increases the degree of binder curing.
- -
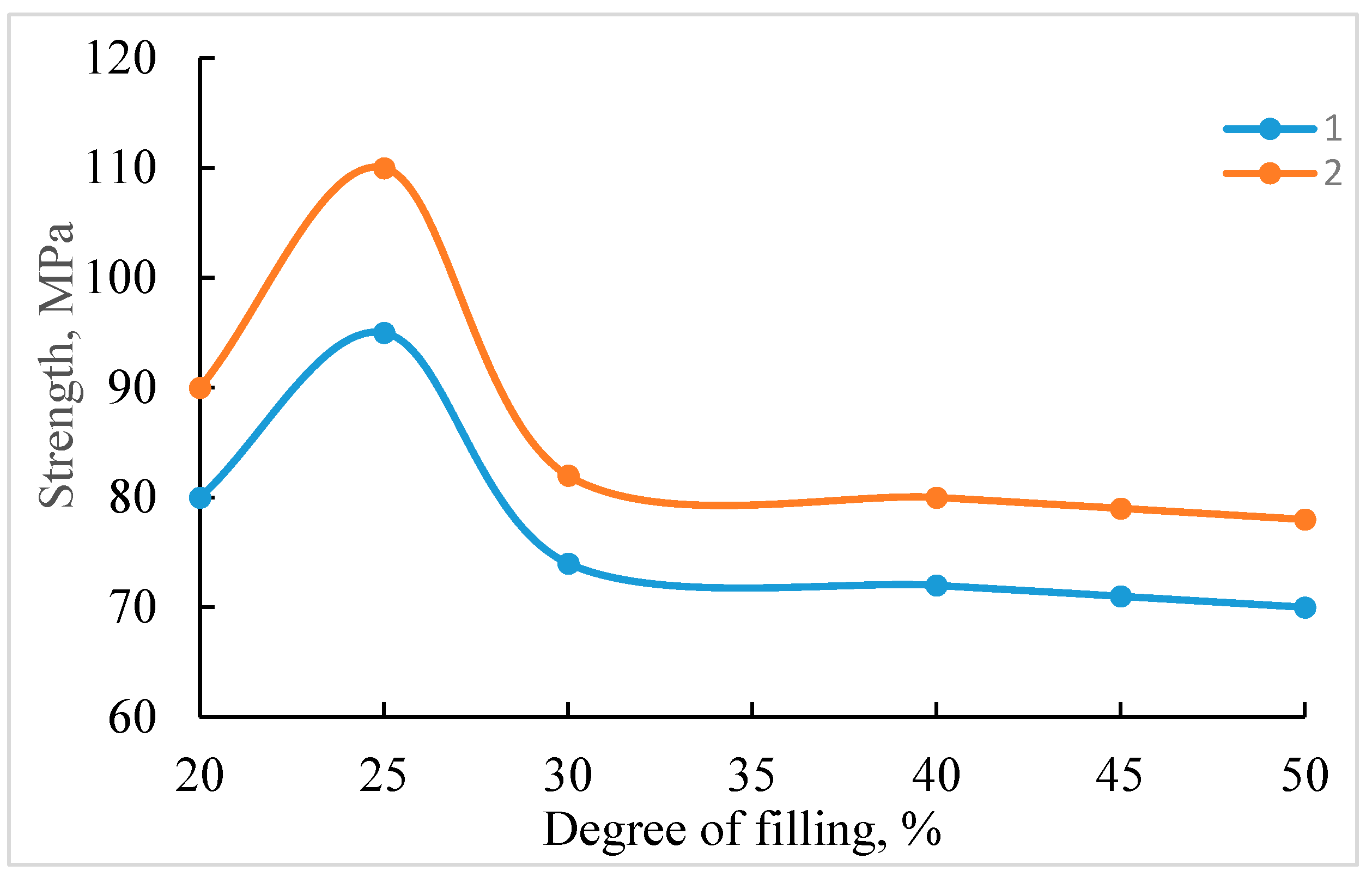
- Using mathematical planning methods, the optimal content of active fillers (10–20 wt.%) was experimentally determined for compositions. These fillers form crystalline hydrates during curing, organizing the structure of the sample and enhancing its chemical resistance and strength properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mazitova, A.; Zaripov, I.; Aminova, G.; Ovod, M.; Suntsova, N. Fillers for polymer composite materials. Nanotechnol. Constr. Sci. Internet-J. 2022, 14, 294–299. [Google Scholar] [CrossRef]
- Taurino, R.; Bondioli, F.; Messori, M. Use of different kinds of waste in the construction of new polymer composites: Review. Mater. Today Sustain. 2023, 21, 100298, ISSN 2589-2347. [Google Scholar] [CrossRef]
- Wiener, J.; Kaineder, H.; Kolednik, O.; Arbeiter, F. Optimization of Mechanical Properties and Damage Tolerance in Polymer-Mineral Multilayer Composites. Materials 2021, 14, 725. [Google Scholar] [CrossRef] [PubMed]
- Savotchenko, S.; Kovaleva, E.; Cherniakov, A. The improvement of mechanical properties of repair and construction compositions based on epoxy resin with mineral fillers. J. Polym. Res. 2022, 29, 280. [Google Scholar] [CrossRef]
- Ismail, H.; Sapuan, S.M.; Ilyas, R.A. (Eds.) Mineral-Filled Polymer Composites Handboo, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; Volume 2. [Google Scholar]
- Atiqah, A.; Maleque, M.A.; Jawaid, M.; Iqbal, M. Development of kenaf-glass reinforced unsaturated polyester hybrid composite for structural applications. Compos. Part B Eng. 2014, 56, 68–73, ISSN 1359-8368. [Google Scholar] [CrossRef]
- Shubhra, Q.T.; Alam, A.; Quaiyyum, M. Mechanical properties of polypropylene composites: A review. J. Thermoplast. Compos. Mater. 2013, 26, 362–391. [Google Scholar] [CrossRef]
- Cabrera, N.; Alcock, B.; Loos, J.; Peijs, T. Processing of all-polypropylene composites for ultimate recyclability. Proceedings of the Institution of Mechanical Engineers. Part L J. Mater. Des. Appl. 2004, 218, 145–155. [Google Scholar] [CrossRef]
- Jancar, J.; Fekete, E.; Hornsby, P.R.; Jancar, J.; Pukánszky, B.; Rothon, R.N. (Eds.) Mineral Fillers in Thermoplastics: Filler Manufacture and Characterisation. In Mineral Fillers in Thermoplastics I. Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1999; Volume 139. [Google Scholar] [CrossRef]
- Ray, S.; Easteal, A.J. Advances in Polymer-Filler Composites: Macro to Nano. Mater. Manuf. Process. 2007, 22, 741–749. [Google Scholar] [CrossRef]
- Roff, W.J.; Scott, J.R. Fibres, Films, Plastics and Rubbers: A Handbook of Common Polymers; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Thomas, S.; Ponnamma, D.; Zachariah, A.K. (Eds.) Polymer Processing and Characterization; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Smejda-Krzewicka, A.; Irzmańska, E.; Mrozowski, K.; Adamus-Włodarczyk, A.; Litwicka, N.; Strzelec, K.; Szynkowska-Jóźwik, M.I. The New Elastomeric Compounds Made of Butyl Rubber Filled with Phyllosilicates, Characterized by Increased Barrier Properties and Hydrophobicity and Reduced Chemical Degradation. Molecules 2024, 29, 1306. [Google Scholar] [CrossRef]
- Negmatov, S.S.; Abed, N.S.; Sultanov, S.U.; Kochkarov, U.K. Machine-building anti-corrosion composite polymeric materials and coatings based on local raw materials and production waste. Int. Sci. Conf. Constr. Mech. Hydraul. Water Resour. Eng. 2023, 401, 05042. [Google Scholar] [CrossRef]
- Kolosova, A.S.; Sokolskaya, M.K.; Vitkalova, I.A.; Torlova, A.S.; Pikalov, E.S. Fillers for modification of modern polymer composite materials. J. Fundam. Res. 2017, 10, 459–465. (In Russian) [Google Scholar]
- Melnichenko, M.A.; Ershova, O.V.; Chuprova, L.V. Influence of filler composition on the properties of polymer composite materials-Text: Direct. Young Sci. 2015, 16, 199–202. (In Russian) [Google Scholar]
- Gizem, A.; Cagla, G.; Kaan, B.; Ceren, Y.; Yusuf, Z.; Senem, A. Seven, Hybrid green composites of PLA incorporated with upcycled waste cellulose and vermiculite. Eur. Polym. J. 2024, 203, 112667, ISSN 0014-3057. [Google Scholar] [CrossRef]
- Hendrik, K.; Paul, S.; Marco, G.; Hans-Jörg, B. Development and thermal performance of a thermoplastic-graphite-composite based plate heat exchanger for use in corrosive media. Appl. Therm. Eng. 2024, 236 (Pt B), 121581, ISSN 1359-4311. [Google Scholar] [CrossRef]
- Ahmad Fauzi, A.A.; Osman, A.F.; Alrashdi, A.A.; Mustafa, Z.; Abdul Halim, K.A. On the Use of Dolomite as a Mineral Filler and Co-Filler in the Field of Polymer Composites: A Review. Polymers 2022, 14, 2843. [Google Scholar] [CrossRef] [PubMed]
- Martikka, O.; Huuhilo, T.; Butylina, S.; Kärki, T. The effect of mineral fillers on the thermal properties of wood-plastic composites. Wood Mater. Sci. Eng. 2012, 7, 107–114. [Google Scholar] [CrossRef]
- Dagdag, O.; El Harfi, A.; Essamri, A.; El Gouri, M.; Chraibi, S.; Assouag, M.; Jodeh, S. Phosphorous-based epoxy resin composition as an effective anticorrosive coating for steel. Int. J. Ind. Chem. 2018, 9, 231–240. [Google Scholar] [CrossRef]
- Rahman, M.M.; Akhtarul, I.M. Application of epoxy resins in building materials: Progress and prospects. Polym. Bull. 2022, 79, 1949–1975. [Google Scholar] [CrossRef]
- Rudawska, A.; Worzakowska, M.; Bociąga, E.; Olewnik-Kruszkowska, E. Investigation of selected properties of adhesive compositions based on epoxy resins. Int. J. Adhes. Adhes. 2019, 92, 23–36. [Google Scholar] [CrossRef]
- Sienkiewicz, N.; Dominic, M.; Parameswaranpillai, J. Natural Fillers as Potential Modifying Agents for Epoxy Composition: A Review. Polymers 2022, 14, 265. [Google Scholar] [CrossRef]
- Rudawska, A.; Czarnota, M. Selected aspects of epoxy adhesive compositions curing process. J. Adhes. Sci. Technol. 2013, 27, 1933–1950. [Google Scholar] [CrossRef]
- Fuda, N.; Weilong, C.; Jingjing, Q.; Junhua, W.; Shiren, W. Additive manufacturing of carbon fiber reinforced thermoplastic composites using fused deposition modeling. Compos. Part B Eng. 2015, 80, 369–378, ISSN 1359-8368. [Google Scholar] [CrossRef]
- Burmistr, M.V.; Boiko, V.S.; Lipko, E.O.; Gerasimenko, K.O.; Gomza, Y.P.; Vesnin, R.L.; Kovalenko, V.L. Antifriction and Construction Materials Based on Modified Phenol-Formaldehyde Resins Reinforced with Mineral and Synthetic Fibrous Fillers. Mech. Compos. Mater. 2014, 50, 213–222. [Google Scholar] [CrossRef]
- Dong, Y.; Xiaofeng, L.; Yue, J.; Hao-Bin, Z.; Bing-Bing, J.; Hui-Ling, M.; Zhong-Zhen, Y. Thermally conductive phenol formaldehyde composites filled with carbon fillers. Mater. Lett. 2014, 118, 212–216, ISSN 0167-577X. [Google Scholar] [CrossRef]
- Xing, Y.; Charles, E. Frazier, Influence of organic fillers on rheological behavior in phenol-formaldehyde adhesives. Int. J. Adhes. Adhes. 2016, 66, 93–98, ISSN 0143-7496. [Google Scholar] [CrossRef]
- Ravindran, L.; Anil Kumar, S.; Thomas, S. Comprehensive review on phenol-formaldehyde resin-based composites and foams. Polym. Compos. 2022, 43, 8602. [Google Scholar] [CrossRef]
- Pattanashetty, B.; Suresha, B.; Hemanth, R. Effect of Filler-Filler Interactions on Mechanical Properties of Phenol Formaldehyde Based Hybrid Composites. Int. J. Eng. Technol. 2017, 13, 24–38. [Google Scholar] [CrossRef]
- Pizzi, A.; Ibeh, C.C. Chapter 2. Phenol-formaldehyde resins. In Handbook of Thermoset Plastics, 4th ed.; William Andrew Publishing: Norwich, NY, USA, 2022; pp. 13–40. [Google Scholar] [CrossRef]
- Turebekova, G.Z.; Akhmetova, S.O.; Bagova, Z.I. Ways of the lead-bearing slag waste utilization. E3S Web Conf. 2021, 262, 04003. [Google Scholar] [CrossRef]
- Krivenko, P.V.; Guziy, S.G. Aluminosilicate coatings with enhanced heat- and corrosion resistance. Appl. Clay Sci. 2013, 73, 65–70, ISSN 0169-1317. [Google Scholar] [CrossRef]
- Wang, M.; Leitch, M.; Xu, C. Synthesis of phenol–formaldehyde resol resins using organosolv pine lignins. Eur. Polym. J. 2009, 45, 3380–3388, ISSN 0014-3057. [Google Scholar] [CrossRef]
- Mirzoev, F.; Ibrohim, A.; Mirzoev, B.; Obidov, Z. Kinetics of hydrochloric acid decomposition of muscovite monomineral//Universum. Tech. Sci. 2024, 4, 21. [Google Scholar]
- Wenxiang, C.; Wei, Y.; Jiahui, P.; Guogang, L.; Suhong, Y. Preparation of anhydrite from phosphogypsum: Influence of phosphorus and fluorine impurities on the performances. Constr. Build. Mater. 2022, 318, 126021, ISSN 0950-0618. [Google Scholar] [CrossRef]
- Abouelleil, H.; Pradelle, N.; Villat, C.; Attik, N.; Colon, P.; Grosgogeat, B. Comparison of mechanical properties of a new fiber reinforced composite and bulk filling composites. Restor. Dent. Endod. 2015, 40, 262–270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Yang, H.; Fang, X.; Wang, X.; Su, Y. Performance study and population structure analysis of hydrolytic acidification immobilized fillers using municipal wastewater. Process Saf. Environ. Prot. 2021, 146, 126–135, ISSN 0957-5820. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, K.M. A Study on Cure Behavior of an Epoxy/Anhydride System and Silica Filler Effects. J. Adhes. Interface 2009, 10, 117–126. [Google Scholar]
- Parameswaran, P.S.; Eby Thomas, T. Modification of Phenol Formaldehyde Resin for Improved Mechanical Properties. Ph.D. Thesis, Cochin University of Science and Technology, Kalamassery, India, 2009. [Google Scholar]
- Yang, Q.; Li, C.; Ren, Q.; Jiang, Z. Properties and microstructure of CO2 activated binder produced by recycling phosphorous slag. Constr. Build. Mater. 2021, 282, 122698. [Google Scholar] [CrossRef]

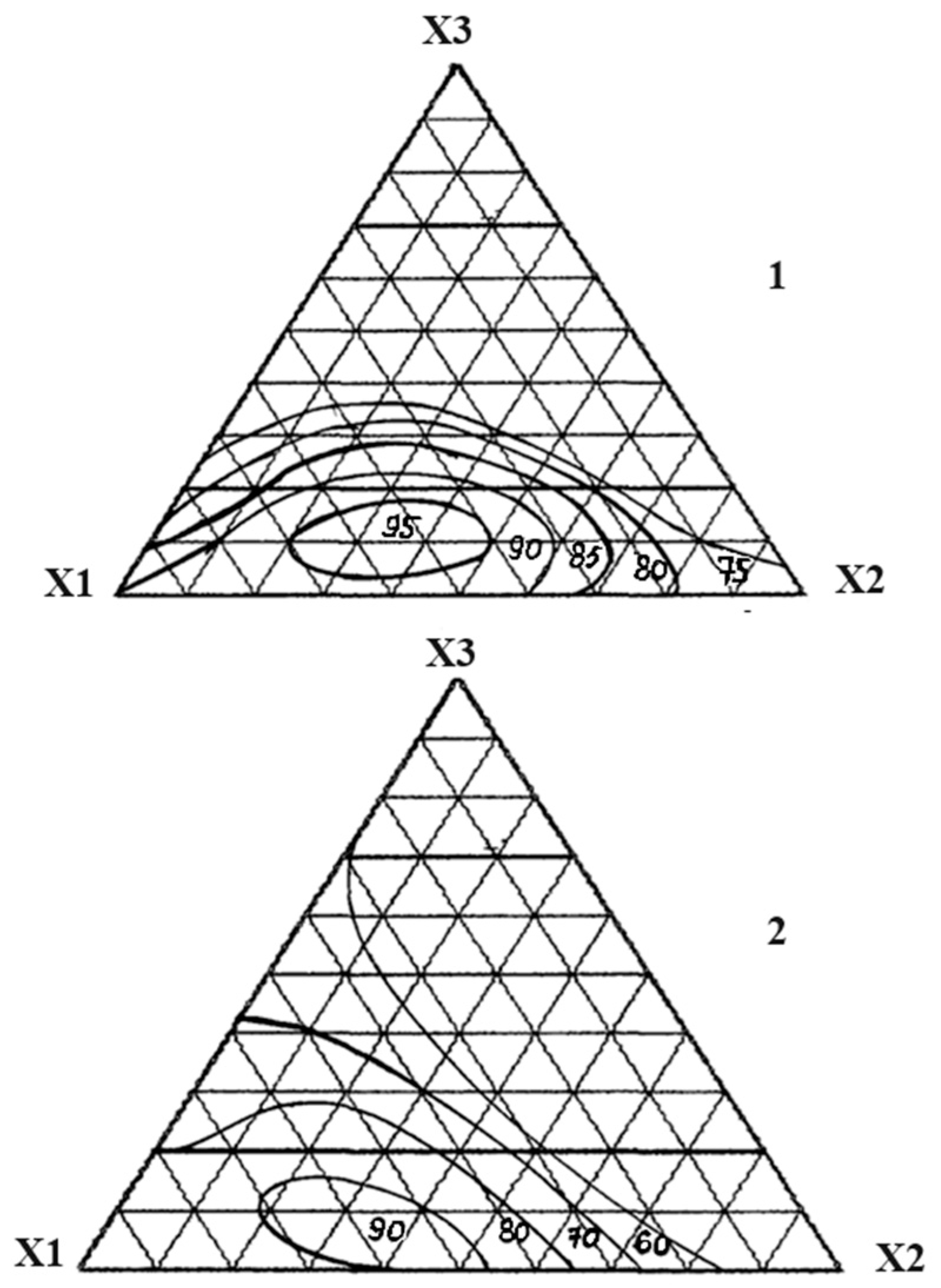
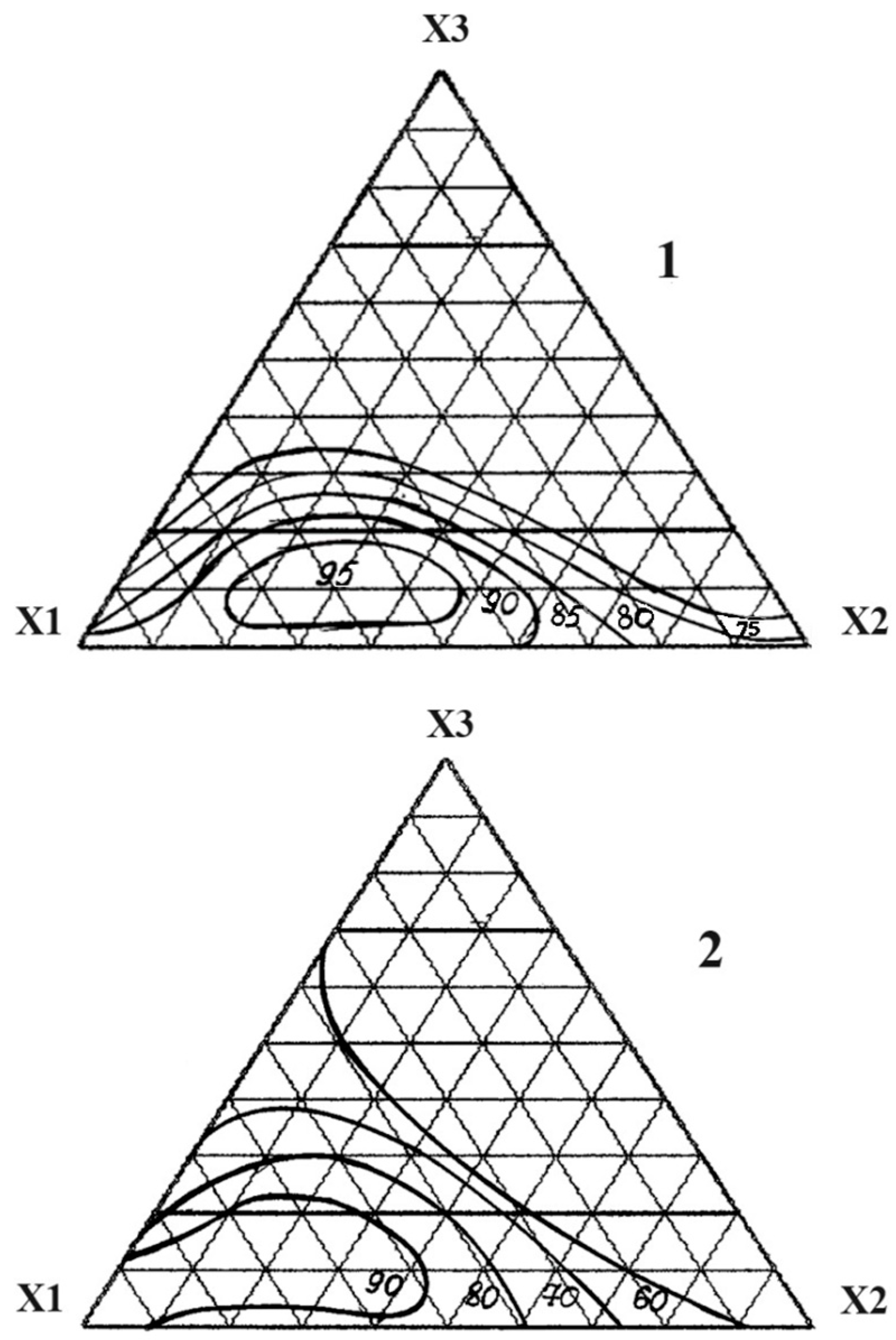



| The Name of Material | Composition, Mass% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | Fetotal | SO3 | P2O5 | Corg. | ∑ | |
| Phosphogypsum | 11.0 | 7.5 | 32.4 | - | - | 42.5 | 6.0 | - | 99.4 |
| Phosphoric slag | 43.4 | 1.2 | 47.6 | 3.7 | 0.3 | 0.6 | 1.5 | - | 98.3 |
| Anhydrite | - | - | 41.2 | - | - | 58.0 | - | - | 99.2 |
| TPP ash | 61.3 | 29.3 | 0.9 | 0.7 | 3.2 | - | - | 3.6 | 99.0 |
| No. | Component, Fraction of Units | Chemical Persistence in 30% HCl, % | Compressive Strength under Compression, MPa | ||
|---|---|---|---|---|---|
| X1 | X2 | X3 | |||
| 1 | 1 | 0 | 0 | 98.0 | 95.0 |
| 2 | 0 | 1 | 0 | 85.4 | 50.2 |
| 3 | 0 | 0 | 1 | 52.2 | 42.4 |
| 4 | ½ | 1/2 | 0 | 90.6 | 75.1 |
| 5 | ½ | 0 | 1/2 | 71.0 | 54.8 |
| 6 | 0 | 1/2 | 1/2 | 48.8 | 40.5 |
| 7 | ¾ | 1/4 | 0 | 93.2 | 68.8 |
| 8 | ¼ | 3/4 | 0 | 80.3 | 60.1 |
| 9 | ¾ | 0 | 1/4 | 75.1 | 75.4 |
| 10 | ¼ | 0 | 3/4 | 52.4 | 54.9 |
| 11 | 0 | 3/4 | 1/4 | 60.7 | 45.1 |
| 12 | 0 | 1/4 | 3/4 | 50.1 | 50.5 |
| 13 | ½ | 1/4 | 1/4 | 95.3 | 70.6 |
| 14 | ¼ | 1/2 | 1/4 | 84.6 | 55.4 |
| 15 | ¼ | 1/4 | 1/2 | 65.0 | 50.2 |
| Components | Contents of the Composites, Mass% | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Phosphogypsum | 15.0 | ||||
| Phosphorus slag | 15.0 | ||||
| Anhydrite | 15.0 | 15.0 | 15.0 | ||
| TPP ash | 20.0 | 20.0 | 20.0 | 25.0 | 30.0 |
| PFR + BSA | 65.0 | 65.0 | 65.0 | 60.0 | 55.0 |
| Cure conditions | |||||
| Temperature, °C | 120 | 120 | 100 | 110 | 120 |
| Time, min | 240 | 200 | 120 | 160 | 180 |
| Chemical resistance, % | |||||
| 30% H2SO4 | 96.6 | 98.8 | 97.0 | 98.5 | 99.3 |
| 30% HCl | 96.2 | 97.4 | 96.0 | 97.5 | 98.0 |
| 20% NaOH | 96.0 | 96.7 | 96.0 | 97.1 | 97.5 |
| Tensile strength, MPa | |||||
| At compression | 45.3 | 53.0 | 50.2 | 57.0 | 60.2 |
| At bending | 18.7 | 23.4 | 21.1 | 25.3 | 28.0 |
| No. | Indicator Name | Number of Composites | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 1 | Density, g/cm3 | 1.37 | 1.38 | 1.38 | 1.37 | 1.39 |
| 2 | Heat resistance according to Martens, °C | 159 | 142 | 156 | 151 | 164 |
| 3 | Linear expansion coefficient 107 | 40.0 | 44.1 | 48.0 | 50.7 | 48.0 |
| 4 | Temperature of destruction, °C | 250 | 255 | 250 | 260 | 250 |
| 5 | Water absorption, mg | 37 | - | 34 | 39 | 38 |
| 6 | Shrinkage, % | 0.47 | 0.46 | 0.41 | 0.47 | 0.42 |
| 7 | Beats volumetric electron resistance, Om | 3.6 | 7.4 | 8.8 | 9.1 | 1.3 |
| 8 | Beats on top Electron resist, Om/cm | 7.4 | 9.5 | 6.2 | 8.7 | 7.6 |
| 9 | Electric strength, MV/m | 20 | 17.7 | 17.8 | 18.3 | 18.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalimoldina, L.M.; Abilkasova, S.O.; Akhmetova, S.O.; Suleimenova, M.S.; Shaikhova, Z.E. Development of Mineral Fillers for Acid-Resistant Filling Composites. J. Compos. Sci. 2024, 8, 284. https://doi.org/10.3390/jcs8070284
Kalimoldina LM, Abilkasova SO, Akhmetova SO, Suleimenova MS, Shaikhova ZE. Development of Mineral Fillers for Acid-Resistant Filling Composites. Journal of Composites Science. 2024; 8(7):284. https://doi.org/10.3390/jcs8070284
Chicago/Turabian StyleKalimoldina, Laila M., Sandugash O. Abilkasova, Saule O. Akhmetova, Mariya Sh. Suleimenova, and Zhanat E. Shaikhova. 2024. "Development of Mineral Fillers for Acid-Resistant Filling Composites" Journal of Composites Science 8, no. 7: 284. https://doi.org/10.3390/jcs8070284






