Transformation of Biomass Power Plant Ash into Composite Fertilizers: A Perspective to Prepare a Rain-Controlled Ammonium Ion–Releasing Composite Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The Reaction between Sulfuric Acid and Biomass Power Plant Ash
3.2. The Phase Composition of the Biomass Power Plant Ash and Sulfuric Acid Reaction Product and Its Transformations with Concentrated Ammonium Nitrate Solutions
4. Conclusions
- The biomass power plant ash, which contained mainly carbonate components, was mixed with concentrated sulfuric acid without any chemical reaction. The protonation-driven carbonic acid evolution was available by controlled adding of water, optionally under pressure, to form a paste-like consistency material containing carbon dioxide bubbles without foam formation. The dwelled paste-like material releases carbon dioxide during drying or depressurizing when a strongly porous solid material is formed.
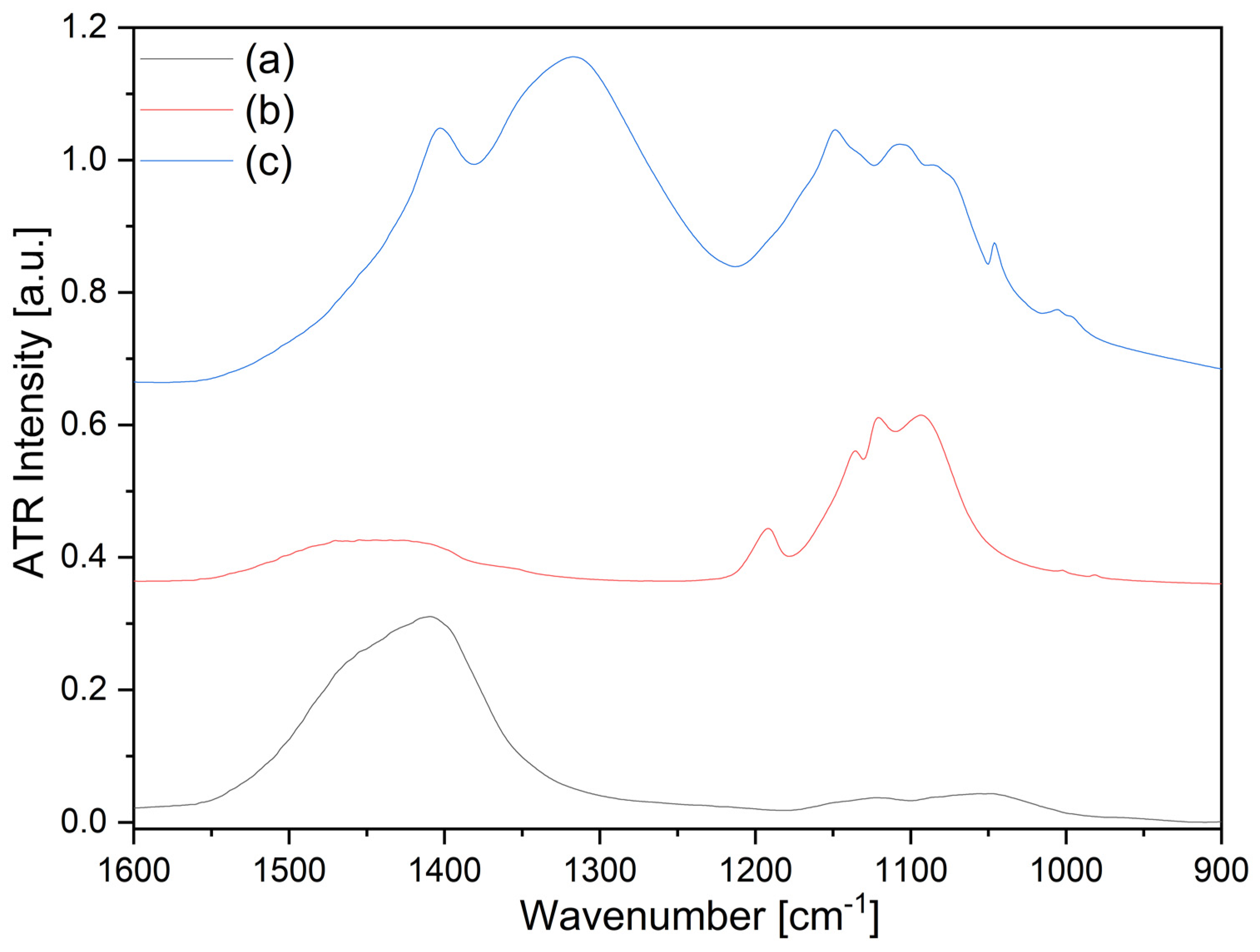
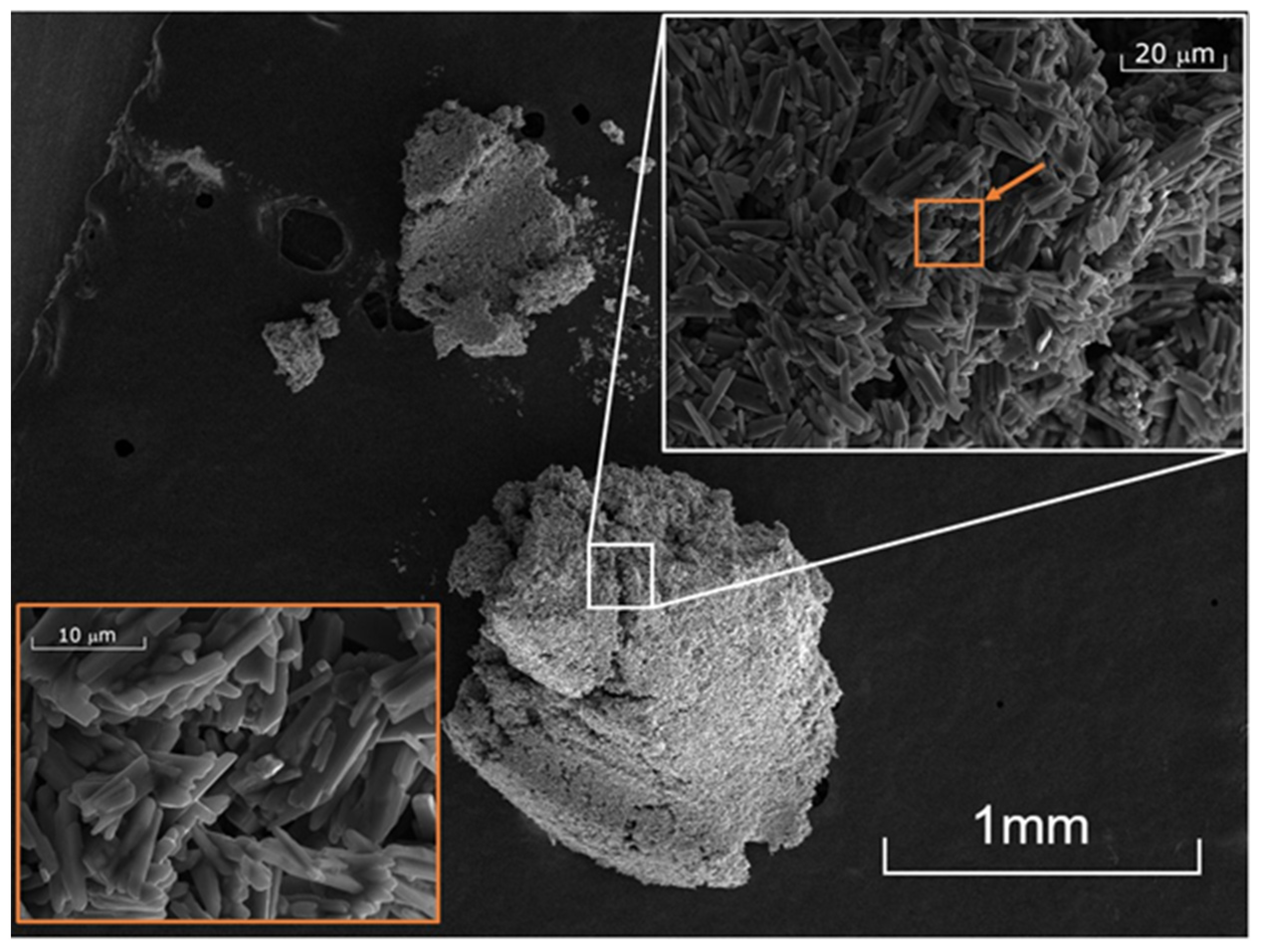
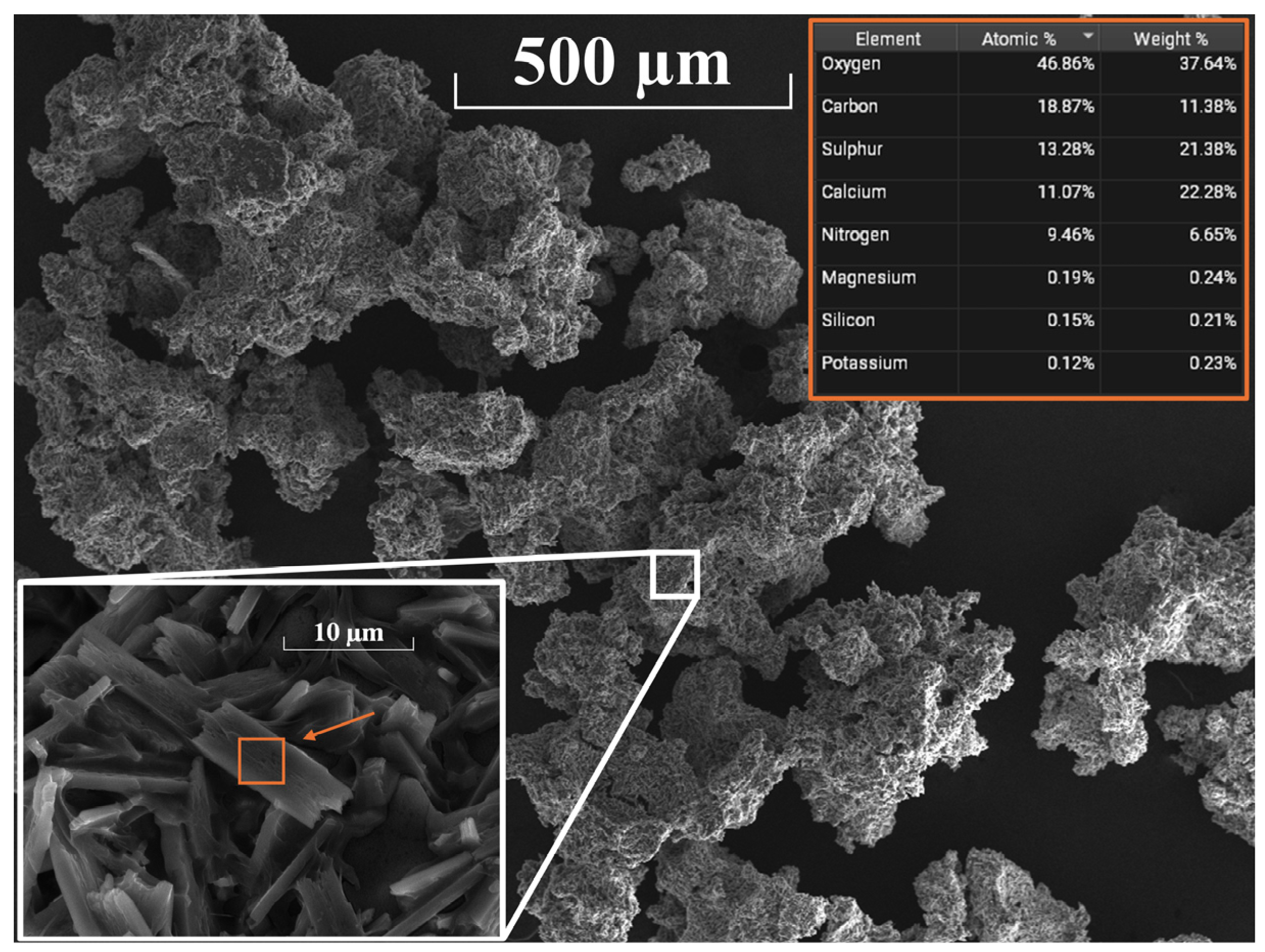
- The reaction products of ash and sulfuric acid in the reaction route described in point 1 were mainly syngenite and polyhalite. These are valuable, slow-releasing, slightly soluble fertilizer materials. The phase identities were assigned by IR and PXRD, and SEM results of the product were also given.
- The sponge-like solid composites formed in the reaction route described in point 1 may be reacted with concentrated ammonium nitrate solution (a cheap intermediate of crystalline ammonium nitrate fertilizer production), which ensures a safe possibility of adding various metal-containing additives, which cannot be performed in the usual ammonium nitrate melt technologies.
- The ammonium nitrate solution reacts with the syngenite content of the composite prepared in the reaction route described in point 1 when ammonium calcium sulfate was formed, which is an excellent slow-releasing ammonium-ion source for soils. It is slightly soluble, so the water content (irrigation, rain) controls its dissolution. The reaction is probably a solid–liquid interaction when the solid syngenite acts as an ion exchanger, and a surface ammonium ion-enriched koktaite formation was found. The phase identities were also assigned by IR and PXRD, and SEM results of the product were also given.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, A.; Arif, S.M.; Aslam, M. Emerging Renewable and Sustainable Energy Technologies: State of the Art. Renew. Sustain. Energy Rev. 2017, 71, 12–28. [Google Scholar] [CrossRef]
- Kotai, L.; Szepvolgyi, J.; Bozi, J.; Gacs, I.; Balint, S.; Gomory, A.; Angyal, A.; Balogh, J.; Li, Z.; Chen, M.; et al. An Integrated Waste-Free Biomass Utilization System for an Increased Productivity of Biofuel and Bioenergy. In Biodiesel—Feedstocks and Processing Technologies; Stoycheva, M., Ed.; InTech: London, UK, 2011; ISBN 978-953-307-713-0. [Google Scholar]
- Manzano-Agugliaro, F.; Alcayde, A.; Montoya, F.G.; Zapata-Sierra, A.; Gil, C. Scientific Production of Renewable Energies Worldwide: An Overview. Renew. Sustain. Energy Rev. 2013, 18, 134–143. [Google Scholar] [CrossRef]
- Munawar, M.A.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Hassan, M.; Liaquat, R.; Dawood, U.F. Challenges and Opportunities in Biomass Ash Management and Its Utilization in Novel Applications. Renew. Sustain. Energy Rev. 2021, 150, 111451. [Google Scholar] [CrossRef]
- Nesterov, D.; Barrera-Martínez, I.; Martínez-Sánchez, C.; Sandoval-González, A.; Bustos, E. Approaching the Circular Economy: Biological, Physicochemical, and Electrochemical Methods to Valorize Agro-Industrial Residues, Wastewater, and Industrial Wastes. J. Environ. Chem. Eng. 2024, 12, 113335. [Google Scholar] [CrossRef]
- Angheluta, S.P.; Burlacu, S.; Diaconu, A.; Curea, C.S. The Energy from Renewable Sources in the European Union: Achieving the Goals. EJSD 2019, 8, 57. [Google Scholar] [CrossRef]
- Mudryk, K.; Frączek, J.; Wróbel, M.; Jewiarz, M.; Dziedzic, K. Agglomeration of Ash-Based Fertilizer Mixtures from Biomass Combustion and Digestate. In Renewable Energy Sources: Engineering, Technology, Innovation; Mudryk, K., Werle, S., Eds.; Springer Proceedings in Energy; Springer International Publishing: Cham, Switzerland, 2018; pp. 823–834. ISBN 978-3-319-72370-9. [Google Scholar]
- Angyal, A.; Hujber, O.; Kotai, L.; Legeza, L.; Sajo, I. Eco Dung Composition and Ecologically Friendly Method for Biomass Ash Transformation into Eco Dung. HU Patent Application P0600390, 10 May 2006. [Google Scholar]
- Kótai, L.; Kazinczy, B.; Gács, I.; Szentmihályi, K.; Keszler, Á.; Szász, K. Utilization of Nitric Acid Wastes from Bleaching Earth Production. Ind. Eng. Chem. Res. 2000, 39, 3920–3925. [Google Scholar] [CrossRef]
- Scherer, H.W. Sulphur in Crop Production—Invited Paper. Eur. J. Agron. 2001, 14, 81–111. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, H.; Ling, G.; Hu, X.; Wang, W. Response of Emitter Clogging Characteristics to Fertilizer Type and Concentration Based on Fertigation. Irrig. Sci. 2023, 41, 769–782. [Google Scholar] [CrossRef]
- Caillat, S.; Vakkilainen, E. Large-Scale Biomass Combustion Plants: An Overview. In Biomass Combustion Science, Technology and Engineering; Elsevier: Amsterdam, The Netherlands, 2013; pp. 189–224. ISBN 978-0-85709-131-4. [Google Scholar]
- Demirbas, A. Combustion of Biomass. Energy Sources A Recovery Util. Environ. Eff. 2007, 29, 549–561. [Google Scholar] [CrossRef]
- Gebreegziabher, T.; Oyedun, A.O.; Luk, H.T.; Lam, T.Y.G.; Zhang, Y.; Hui, C.W. Design and Optimization of Biomass Power Plant. Chem. Eng. Res. Des. 2014, 92, 1412–1427. [Google Scholar] [CrossRef]
- James, A.; Thring, R.; Helle, S.; Ghuman, H. Ash Management Review—Applications of Biomass Bottom Ash. Energies 2012, 5, 3856–3873. [Google Scholar] [CrossRef]
- Rokicki, T.; Koszela, G.; Ochnio, L.; Perkowska, A.; Bórawski, P.; Bełdycka-Bórawska, A.; Gradziuk, B.; Gradziuk, P.; Siedlecka, A.; Szeberényi, A.; et al. Changes in the Production of Energy from Renewable Sources in the Countries of Central and Eastern Europe. Front. Energy Res. 2022, 10, 993547. [Google Scholar] [CrossRef]
- Rosendahl, L. Biomass Combustion Science, Technology and Engineering; Woodhead Publishing Series in Energy; Woodhead Publishing: Oxford, UK, 2013; ISBN 978-0-85709-743-9. [Google Scholar]
- Shi, R.; Li, J.; Jiang, J.; Mehmood, K.; Liu, Y.; Xu, R.; Qian, W. Characteristics of Biomass Ashes from Different Materials and Their Ameliorative Effects on Acid Soils. J. Environ. Sci. 2017, 55, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.C.; Cruz, N.C.; Tarelho, L.A.C.; Rodrigues, S.M. Use of Biomass Ash-Based Materials as Soil Fertilisers: Critical Review of the Existing Regulatory Framework. J. Clean. Prod. 2019, 214, 112–124. [Google Scholar] [CrossRef]
- Touš, M.; Pavlas, M.; Stehlík, P.; Popela, P. Effective Biomass Integration into Existing Combustion Plant. Energy 2011, 36, 4654–4662. [Google Scholar] [CrossRef]
- Vandenbroek, R. Biomass Combustion for Power Generation. Biomass Bioenergy 1996, 11, 271–281. [Google Scholar] [CrossRef]
- Voshell, S.; Mäkelä, M.; Dahl, O. A Review of Biomass Ash Properties towards Treatment and Recycling. Renew. Sustain. Energy Rev. 2018, 96, 479–486. [Google Scholar] [CrossRef]
- Uliasz-Bocheńczyk, A.; Pawluk, A.; Pyzalski, M. Characteristics of Ash from the Combustion of Biomass in Fluidized Bed Boilers. Gospod. Surowcami. Min. 2016, 32, 149–162. [Google Scholar] [CrossRef]
- Albuquerque, A.R.L.; Angélica, R.S.; Merino, A.; Paz, S.P.A. Chemical and Mineralogical Characterization and Potential Use of Ash from Amazonian Biomasses as an Agricultural Fertilizer and for Soil Amendment. J. Clean. Prod. 2021, 295, 126472. [Google Scholar] [CrossRef]
- Chojnacka, K.; Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Gorazda, K.; Kulczycka, J.; Kominko, H.; Moustakas, K.; Witek-Krowiak, A. Practical Aspects of Biowastes Conversion to Fertilizers. Biomass. Conv. Bioref. 2024, 14, 1515–1533. [Google Scholar] [CrossRef]
- Cole, J.C.; Smith, M.W.; Penn, C.J.; Cheary, B.S.; Conaghan, K.J. Nitrogen, Phosphorus, Calcium, and Magnesium Applied Individually or as a Slow Release or Controlled Release Fertilizer Increase Growth and Yield and Affect Macronutrient and Micronutrient Concentration and Content of Field-Grown Tomato Plants. Sci. Hortic. 2016, 211, 420–430. [Google Scholar] [CrossRef]
- Cruz-Paredes, C.; López-García, Á.; Rubæk, G.H.; Hovmand, M.F.; Sørensen, P.; Kjøller, R. Risk Assessment of Replacing Conventional P Fertilizers with Biomass Ash: Residual Effects on Plant Yield, Nutrition, Cadmium Accumulation and Mycorrhizal Status. Sci. Total Environ. 2017, 575, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, L.; Sousa Correia, D.S.; Van Caneghem, J. Linking Phosphorus Extraction from Different Types of Biomass Incineration Ash to Ash Mineralogy, Ash Composition and Chemical Characteristics of Various Types of Extraction Liquids. Waste Biomass Valor. 2021, 12, 5235–5248. [Google Scholar] [CrossRef]
- Pasquali, M.; Zanoletti, A.; Benassi, L.; Federici, S.; Depero, L.E.; Bontempi, E. Stabilized Biomass Ash as a Sustainable Substitute for Commercial P-fertilizers. Land Degrad. Dev. 2018, 29, 2199–2207. [Google Scholar] [CrossRef]
- Zhang, Z.; He, F.; Zhang, Y.; Yu, R.; Li, Y.; Zheng, Z.; Gao, Z. Experiments and Modelling of Potassium Release Behavior from Tablet Biomass Ash for Better Recycling of Ash as Eco-Friendly Fertilizer. J. Clean. Prod. 2018, 170, 379–387. [Google Scholar] [CrossRef]
- Mladenov, M.; Pelovski, Y. Granulation Study of Composite Mixtures on the Basis of Ash from Burned Wooden Biomass, for Production of Soil Conditioners. J. Univ. Chem. Technol. Metall. 2021, 46, 131–136. [Google Scholar]
- Wierzbowska, J.; Sienkiewicz, S.; Żarczyński, P.; Krzebietke, S. Environmental Application of Ash from Incinerated Biomass. Agronomy 2020, 10, 482. [Google Scholar] [CrossRef]
- Dormeshkin, O. Interactions between Components of Complex Fertilizers Chemical and Physico-Chemical Interactions at the Stages of Mixing, Granulating and Drying during Their Production; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2019; ISBN 978-613-9-45198-2. [Google Scholar]
- Moure Abelenda, A.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Impact of Sulphuric, Hydrochloric, Nitric, and Lactic Acids in the Preparation of a Blend of Agro-Industrial Digestate and Wood Ash to Produce a Novel Fertiliser. J. Environ. Chem. Eng. 2021, 9, 105021. [Google Scholar] [CrossRef]
- Richaud, R.; Herod, A.A.; Kandiyoti, R. Comparison of Trace Element Contents in Low-Temperature and High-Temperature Ash from Coals and Biomass. Fuel 2004, 83, 2001–2012. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Ding, Z.; Martens, W.N.; Schuiling, R.D.; Duong, L.V.; Frost, R.L. Thermal Decomposition of Syngenite, K2Ca(SO4)2·H2O. Thermochim. Acta 2004, 417, 143–155. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Schuiling, R.D.; Ding, Z.; Hickey, L.; Wharton, D.; Frost, R.L. Vibrational Spectroscopic Study of Syngenite Formed during the Treatment of Liquid Manure with Sulphuric Acid. Vib. Spectrosc. 2002, 28, 209–221. [Google Scholar] [CrossRef]
- Gorbounov, M.; Petrovic, B.; Ozmen, S.; Clough, P.; Masoudi Soltani, S. Activated Carbon Derived from Biomass Combustion Bottom Ash as Solid Sorbent for CO2 Adsorption. Chem. Eng. Res. Des. 2023, 194, 325–343. [Google Scholar] [CrossRef]
- Gorbounov, M.; Hecquet-Perrot, L.; Ignatova, S.; Hewitson, P.; Soltani, S.M. Acidic Surface Chemical Modification of Biomass Combustion Ash-Derived Activated Carbon for CO2 Adsorption. Next Mater. 2025, 6, 100321. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, 1st ed.; Wiley: Hoboken, NJ, USA, 2008; ISBN 978-0-471-74339-2. [Google Scholar]
- Grau, F.; Choo, H.; Hu, J.; Jung, J. Engineering Behavior and Characteristics of Wood Ash and Sugarcane Bagasse Ash. Materials 2015, 8, 6962–6977. [Google Scholar] [CrossRef]
- Gezerman, A.O. Mathematical Modeling for Prilling Processes in Ammonium Nitrate Production. Eng. Rep. 2020, 2, e12173. [Google Scholar] [CrossRef]
- Kirova-Yordanova, Z. Exergy-Based Estimation and Comparison of Urea and Ammonium Nitrate Production Efficiency and Environmental Impact. Energy 2017, 140, 158–169. [Google Scholar] [CrossRef]
- Maxwell, G.R. Ammonium Nitrate. In Synthetic Nitrogen Products; Kluwer Academic Publishers: Boston, MA, USA, 2005; pp. 251–265. ISBN 978-0-306-48225-0. [Google Scholar]
- Bell, J.M.; Taber, W.C. The Solubility of Gypsum in Solutions of Ammonium Sulphate. J. Phys. Chem. 1906, 10, 119–122. [Google Scholar] [CrossRef]
- Bell, J.M.; Taber, W.C. A Supposed Ammonium Syngenite. J. Phys. Chem. 1907, 11, 492–494. [Google Scholar] [CrossRef]
- Coates, R.V.; Woodard, G.D. X-ray Powder Diffraction Data for Solid Solutions and Double Salts Occurring in Granular Compound Fertilisers. J. Sci. Food. Agric. 1963, 14, 398–404. [Google Scholar] [CrossRef]
- Lloyd, K.R.; Baker, J.; Lund, J.J. Methods of Microbially Producing Acids and Minerals and Uses Thereof. PCT International Patent Application WO2017041028A1, 30 August 2018. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Part 1. Phase–Mineral and Chemical Composition and Classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Walter, K.H.; Baird, R.J. A Coating Process for Fertilizers. Patent Application AU749213B2, 16 September 1999. [Google Scholar]
- Kótai, L.; Argay, G.; Holly, S.; Keszler, Á.; Pukánszky, B.; Banerji, K.K. Study on the Existence of Hydrogen Bonds in Ammonium Permanganate. Z. Anorg. Allg. Chem. 2001, 627, 114. [Google Scholar] [CrossRef]




| CaCO3 (g) | CaSO4·5H2O (g) | H2SO4 (g) | Bulk Density, g/mL | Mechanical Strength | Water Absorption Capacity, % |
|---|---|---|---|---|---|
| 0 | 2 | 4 | 0.46 | weak | 118 |
| 0 | 2 | 8 | 0.87 | weak | 55 |
| 2 | 0 | 4 | 0.60 | weak | 99 |
| 2 | 0 | 8 | 0.68 | weak | 83 |
| 4 | 0 | 4 | 0.57 | weak | 93 |
| 4 | 0 | 8 | 0.78 | Very weak | 74 |
| CaCO3 (g) | CaSO4·5H2O (g) | H2SO4 (g) | Density, g/mL | Mechanical Strength | Water Absorption Capacity, % |
|---|---|---|---|---|---|
| 0 | 2 | 8 | 0.87 | medium | 56 |
| 0 | 2 | 16 | 1.56 | medium | 27 |
| 2 | 0 | 16 | 0.60 | strong | 73 |
| 4 | 0 | 16 | 0.77 | Very strong | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kótai, L.; Windisch, M.; Béres, K.A. Transformation of Biomass Power Plant Ash into Composite Fertilizers: A Perspective to Prepare a Rain-Controlled Ammonium Ion–Releasing Composite Fertilizer. J. Compos. Sci. 2024, 8, 336. https://doi.org/10.3390/jcs8090336
Kótai L, Windisch M, Béres KA. Transformation of Biomass Power Plant Ash into Composite Fertilizers: A Perspective to Prepare a Rain-Controlled Ammonium Ion–Releasing Composite Fertilizer. Journal of Composites Science. 2024; 8(9):336. https://doi.org/10.3390/jcs8090336
Chicago/Turabian StyleKótai, László, Márk Windisch, and Kende Attila Béres. 2024. "Transformation of Biomass Power Plant Ash into Composite Fertilizers: A Perspective to Prepare a Rain-Controlled Ammonium Ion–Releasing Composite Fertilizer" Journal of Composites Science 8, no. 9: 336. https://doi.org/10.3390/jcs8090336






