Enhancing Phase Change Characteristics of Hybrid Nanocomposites for Latent Heat Thermal Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nano-Enhanced PCM
2.2. Experimental Test Facility
2.3. Experimental Procedure
2.4. Performance Calculations
2.5. Measurement and Uncertainty
3. Results and Discussion
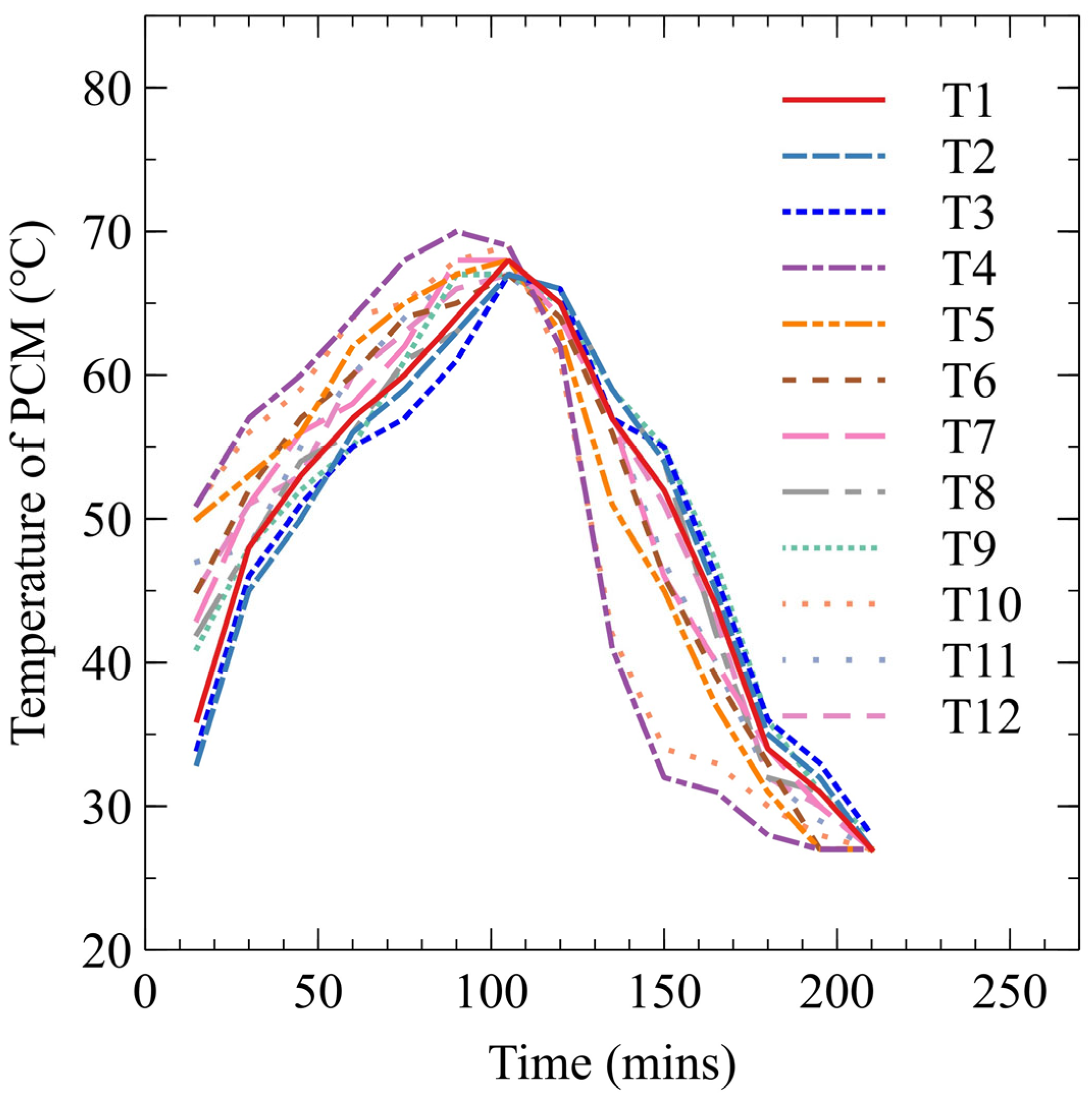
3.1. PCM Fusion in a TES Container
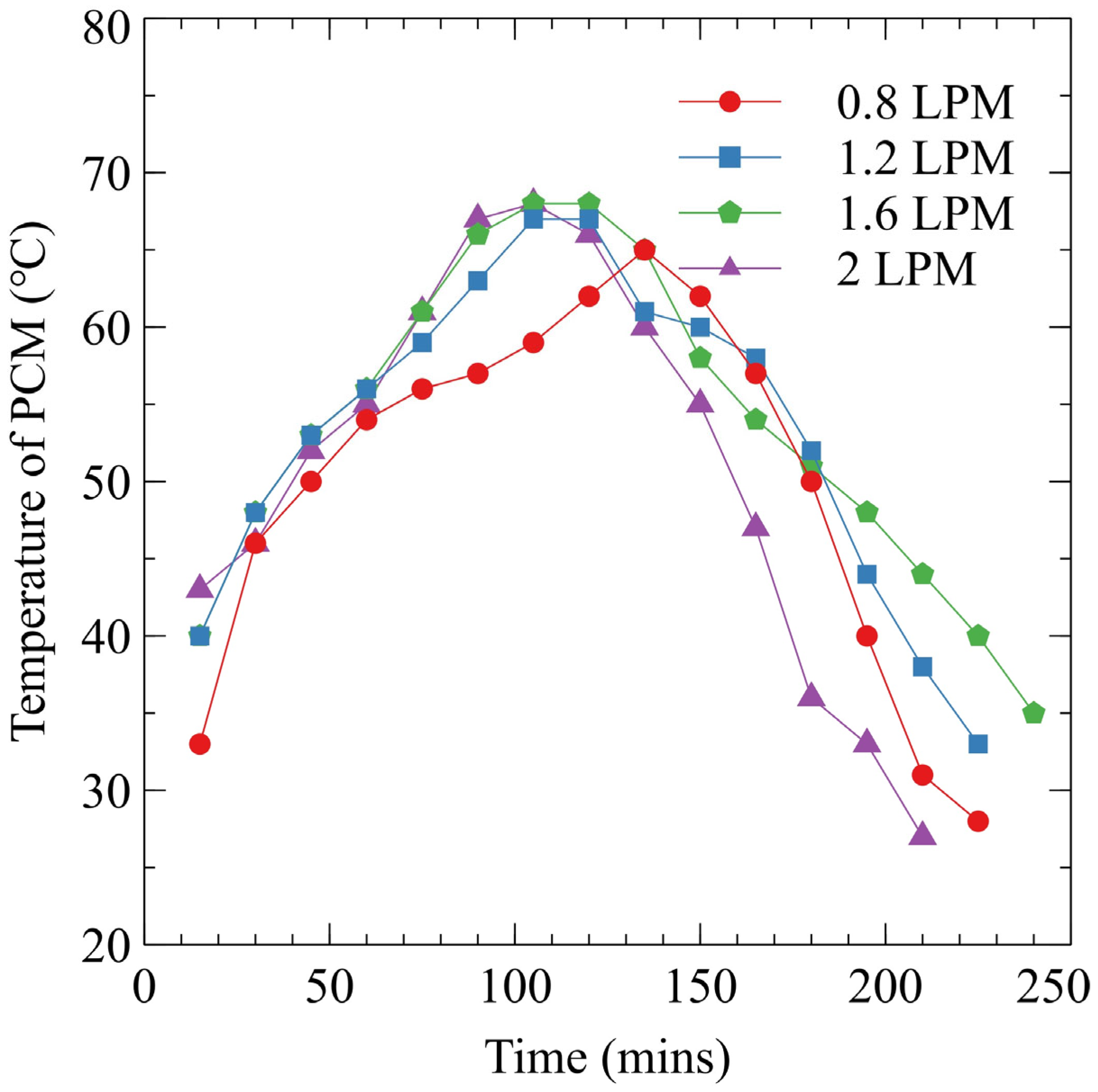
3.2. Effect of Flow Rate on PCM Fusion
3.3. Effect of HTF Temperature
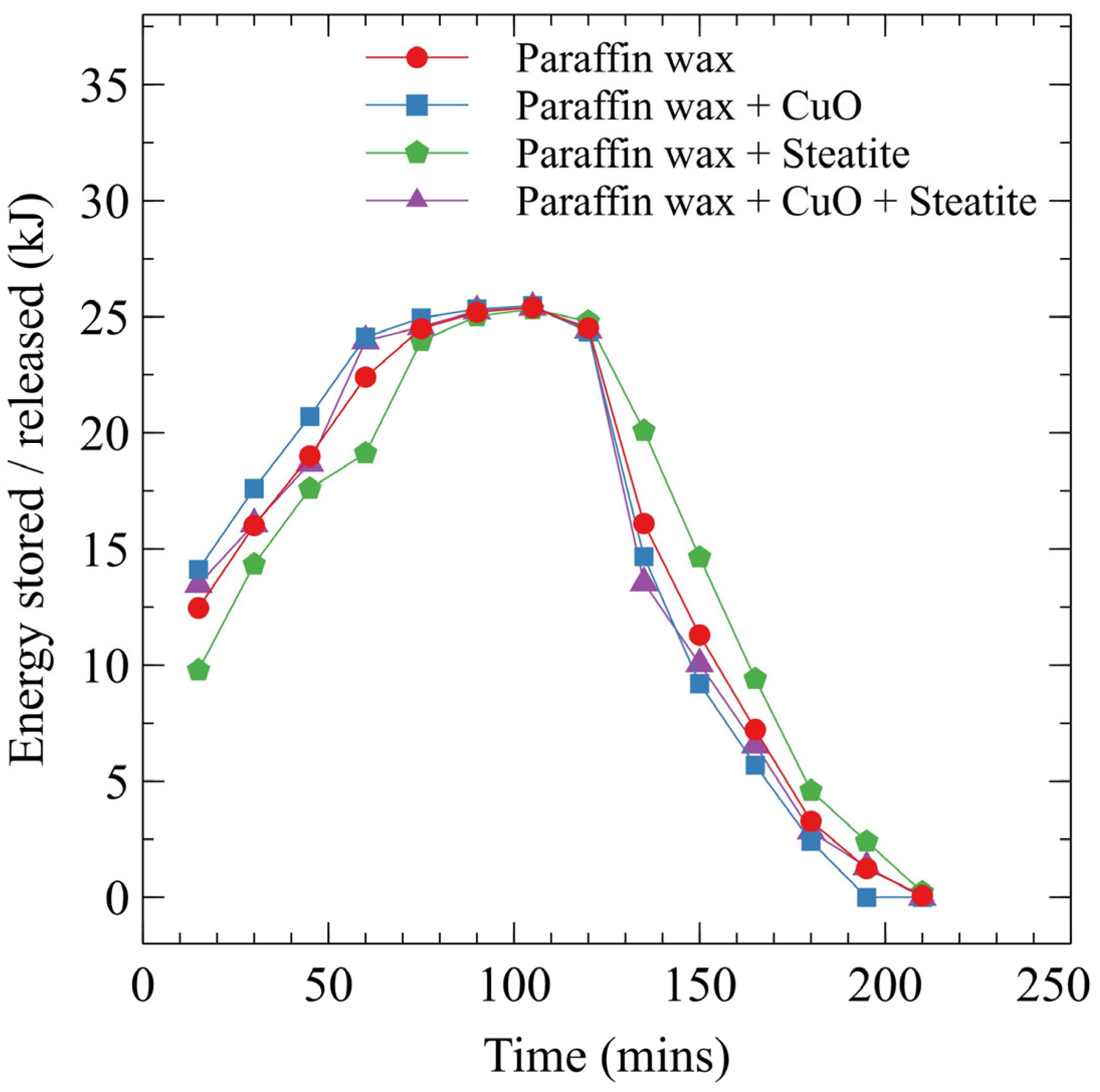
3.4. Energy Stored and Released
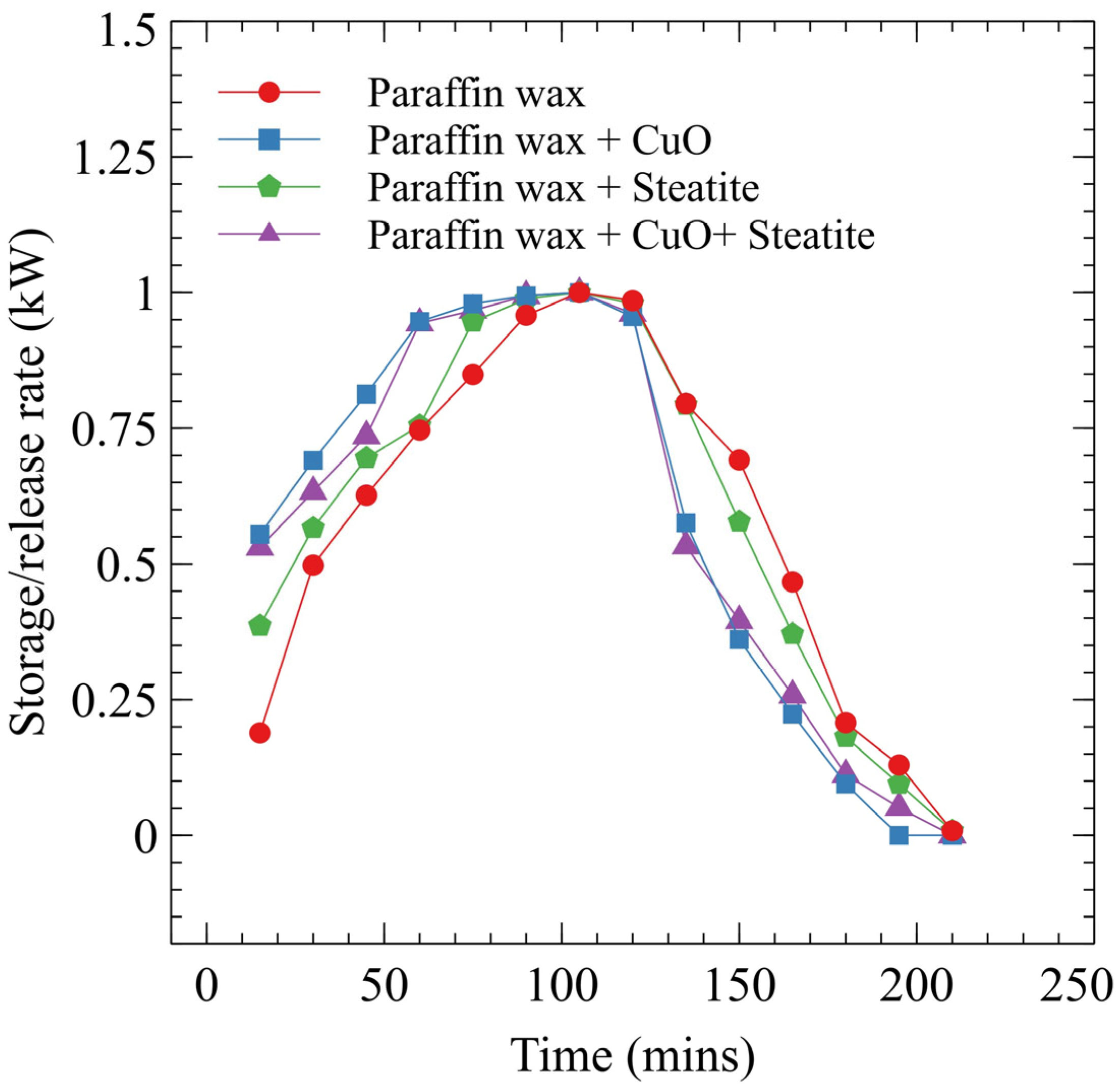
3.5. Energy Storage/Release Rate
3.6. Heat Storage Effectiveness
4. Conclusions
- PCM melts around an HTT, facilitated by heat exchange with a high-temperature HTF. The less dense melted PCM rises to the container’s top, exchanging heat with solid PCM and further promoting melting through natural convection.
- Nanocomposites with steatite and CuO enhanced thermal conductivity and expedited PCM fusion. Steatite increased PCM melting by reducing solid PCM settlement, while CuO facilitated faster heat exchange and melting despite having minor impacts on natural convection.
- During solidification, the PCM temperature drops as HTF at 27 °C flows through the HTT. This creates a significant temperature difference, aiding in solidification as per the second law of thermodynamics. The presence of heat transfer surfaces at both the top and bottom accelerates solidification despite PCM’s low thermal conductivity.
- Case 3 utilizes a blend of paraffin wax and CuO, demonstrates superior heat transfer capabilities, and achieves the fastest overall fusion time of 90 min, highlighting its potential for efficient TES applications.
- The flow rate of the HTF significantly influences PCM fusion in the horizontal TES system. Optimal fusion was observed at 2 LPM, where increased turbulence enhanced heat transport efficiency despite challenges at higher flow rates that reduced heat exchange efficiency.
- The HTF inlet temperature of 70 °C proved effective in melting PCM, with steatite and CuO additives further enhancing heat transfer. Their inclusion facilitated faster PCM melting and solidification, improving overall system efficiency.
- The energy stored and released for case 3 is 20.71 and 9.2 kJ. There is an increase of 30.5% in heat storage and a decrease of 47.4% in heat release in case 3 compared to case 1. This indicates case 3 is effective in expediting fusion.
- The storage and release rate for case 3 is 0.506 and 0.667 kW. A 14.6 and 4.3% decrease in the storage and release rate is seen in case 3 compared to case 1.
- The energy effectiveness for case 3 is 0.812 and 0.360. There is an increase of 29.7% and a decrease of 47.9% in effectiveness found in case 3. It displayed enhanced effectiveness due to improved heat transfer.
- Cases with nanocomposite additives showed varied energy storage capacities, with enhanced thermal conductivity leading to quicker heat storage and release cycles.
- Cases incorporating nanocomposites exhibited accelerated rates due to improved heat transfer efficiency, influencing the overall effectiveness of PCM fusion.
- Configurations with nanocomposites demonstrated higher effectiveness, particularly in facilitating rapid PCM fusion and efficient heat transfer during storage and release cycles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GO | Graphene oxide |
| CuO | Copper oxide |
| HTF | Heat transfer fluid |
| HTT | Heat transfer tube |
| LHS | Latent heat storage |
| MWCNT | Multiwall carbon nanotube |
| NePCM | Nano-enhanced phase change material |
| PCM | Phase change material |
| PEG | Polyethylene glycol |
| TES | Thermal energy storage |
References
- Martins, T.; Barreto, A.C.; Souza, F.M.; Souza, A.M. Fossil fuels consumption and carbon dioxide emissions in G7 countries: Empirical evidence from ARDL bounds testing approach. Environ. Pollut. 2021, 291, 118093. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Shukla, S.K.; Rathore, P.K.S. A comprehensive review on solar to thermal energy conversion and storage using phase change materials. J. Energy Storage 2023, 72, 108280. [Google Scholar] [CrossRef]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent TES technologies and applications: A review. Int. J. Thermofluids 2020, 5–6, 100039. [Google Scholar] [CrossRef]
- Jiang, R.; Qian, G.; Li, Z.; Yu, X.; Lu, Y. Progress and challenges of latent thermal energy storage through external field-dependent heat transfer enhancement methods. Energy 2024, 304, 132101. [Google Scholar] [CrossRef]
- Tofani, K.; Tiari, S. Nano-enhanced phase change materials in latent heat thermal energy storage systems: A review. Energies 2021, 14, 3821. [Google Scholar] [CrossRef]
- Reji Kumar, R.; Samykano, M.; Pandey, A.K.; Kadirgama, K.; Tyagi, V.V. Phase change materials and nano-enhanced phase change materials for thermal energy storage in photovoltaic thermal systems: A futuristic approach and its technical challenges. Renew. Sustain. Energy Rev. 2020, 133, 110341. [Google Scholar] [CrossRef]
- Said, Z.; Pandey, A.K.; Tiwari, A.K.; Kalidasan, B.; Jamil, F.; Thakur, A.K.; Tyagi, V.V.; Sarı, A.; Ali, H.M. Nano-enhanced phase change materials: Fundamentals and applications. Prog. Energy Combust. Sci. 2024, 104, 101162. [Google Scholar] [CrossRef]
- Mukhesh, R.; Sarath, K.P.; Feroz Osman, M.; Deepu, M.; Manu, K.V. Asymmetric PCM melting and thermal convection in a rectangular enclosure with straight and wavy heat transfer passages. Int. J. Heat Mass Transfer 2023, 217, 124625. [Google Scholar] [CrossRef]
- Punniakodi, B.M.S.; Senthil, R. Recent developments in nano-enhanced phase change materials for solar thermal storage. Sol. Energy Mater. Sol. Cells 2022, 238, 111629. [Google Scholar] [CrossRef]
- Thangapandian, E.; Palanisamy, P.; Selvaraj, S.K.; Chadha, U.; Khanna, M. Detailed experimentation and prediction of thermophysical properties in lauric acid-based nanocomposite phase change material using artificial neural network. J. Energy Storage 2023, 74, 109345. [Google Scholar] [CrossRef]
- Mishra, G.; Memon, A.; Gupta, A.K.; Nirmalkar, N. Computational study on effect of enclosure shapes on melting characteristics of phase change material around a heated cylinder. Case Stud. Therm. Eng. 2022, 34, 102032. [Google Scholar] [CrossRef]
- Talele, V.; Zhao, P. Effect of nano-enhanced phase change material on the thermal management of a 18650 NMC battery pack. J. Energy Storage 2023, 64, 107068. [Google Scholar] [CrossRef]
- Patel, P.; Sharma, A.; Monde, A.D.; Sharma, M.; Mondal, B.; Kothadia, H.B. Performance analysis of melting phenomena in an ice-freezing type direct-contact heat exchanger. J. Energy Storage 2022, 50, 104575. [Google Scholar] [CrossRef]
- Athawale, V.; Jakhar, A.; Jegatheesan, M.; Rath, P.; Bhattacharya, A. A 3D resolved-geometry model for unstructured and structured packed bed encapsulated phase change material system. J. Energy Storage 2022, 51, 104430. [Google Scholar] [CrossRef]
- Teja, P.N.S.; Gugulothu, S.K.; Reddy, P.D.S.; Barmavatu, P. Effect of orientation and nanoparticle addition of an encapsulated phase change material on heat transfer in a packed bed thermal energy storage system—A numerical analysis. J. Energy Storage 2024, 78, 110023. [Google Scholar] [CrossRef]
- Das, S.; Athawale, V.; Joy, J.M.; Jegatheesan, M.; Rath, P.; Bhattacharya, A. A 3D pore-scale model for macro-encapsulated phase change material-metal foam hybrid energy storage system. J. Energy Storage 2023, 63, 106995. [Google Scholar] [CrossRef]
- Nahak, P.K.; Athawale, V.; Jegatheesan, M.; Rath, P.; Bhattacharya, A. Effect of variable capsule size distribution for unstructured packed bed encapsulated phase change material system. Int. J. Heat Mass Transfer 2022, 197, 123354. [Google Scholar] [CrossRef]
- Bharathiraja, R.; Ramkumar, T.; Selvakumar, M. Studies on the thermal characteristics of nano-enhanced paraffin wax phase change material (PCM) for thermal storage applications. J. Energy Storage 2023, 73, 109216. [Google Scholar] [CrossRef]
- Kumar, R.; Samykano, M.; Pandey, A.K.; Kadirgama, K.; Tyagi, V.V. A comparative study on thermophysical properties of functionalized and non-functionalized Multi-Walled Carbon Nano Tubes (MWCNTs) enhanced salt hydrate phase change material. Sol. Energy Mater. Sol. Cells 2022, 240, 111697. [Google Scholar] [CrossRef]
- Duan, P.; Abed, A.M.; Chaturvedi, R.; Ahmad, S.F.; Absalamov, T.; Dahari, M.; Fouad, Y. Heat absorption/release efficiency betterment of phase change material inside a shell-and-tube latent heat storage system under six different conditions of tube and fins. J. Energy Storage 2024, 90, 111880. [Google Scholar] [CrossRef]
- Punniakodi, B.M.S.; Senthil, R. Enhanced heat transfer in a phase change energy storage with helical tubes. J. Energy Storage 2023, 58, 106352. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhanja, D.; Nath, S. Numerical investigation of heat transfer and melting process of phase change material in trapezoidal cavities with different shapes and different heated tube positions. J. Energy Storage 2023, 72, 108285. [Google Scholar] [CrossRef]
- Senthil, R.; Cheralathan, M. Simultaneous testing of a parabolic dish concentrated PCM and non-PCM solar receiver. Int. J. Mech. Prod. Eng. Res. Dev. 2017, 7, 79–85. [Google Scholar]
- Sutradhar, J.; Kothari, R.; Sahu, S.K. Melting and solidification analysis of phase change material-metal foam composite with expansion/shrinkage void in rectangular system. J. Energy Storage 2022, 47, 103596. [Google Scholar] [CrossRef]
- Jeyaseelan, T.R.; Vigneshwaran, P.; Saboor, S.; Kumar, D.S.; Saxena, K.K.; Arıcı, M. Experimental study on effect of mild steel scrap vertical insert in charging and discharging process of NaNO3:KNO3 phase change material. J. Energy Storage 2024, 85, 111074. [Google Scholar] [CrossRef]
- Alqaed, S.; Almehmadi, F.A.; Mustafa, J.; Husain, S.; Cheraghian, G. Effect of nano phase change materials on the cooling process of a triangular lithium battery pack. J. Energy Storage 2022, 51, 104326. [Google Scholar] [CrossRef]
- Rajeswari, K.; Hari Suthan, V.; Suganthi, K.S.; Thiruvenkatam, S.; Devaraj, S.; Rajan, K.S. Graphene oxide–adipic acid nanocomposites for thermal energy storage: Assessment of thermophysical properties and energy storage performance. J. Energy Storage 2024, 77, 109949. [Google Scholar] [CrossRef]
- Bharathiraja, R.; Ramkumar, T.; Karthick, L.; Mohanraj, M. Performance investigation on flat plate solar water collector using a hybrid nano-enhanced phase change material (PCM). J. Energy Storage 2024, 86, 111163. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Premnath, D.; Cheralathan, M.; Senthil, R. Experimental thermal performance of deionized water and iron oxide nanofluid for cold thermal storage. Environ. Sci. Pollut. Res. 2024, 31, 26330–26339. [Google Scholar] [CrossRef]
- Kumar, A.; Saha, S.K.; Kumar, K.R.; Rakshit, D. Study of melting of paraffin dispersed with copper nanoparticles in square cavity subjected to external magnetic field. J. Energy Storage 2022, 50, 104338. [Google Scholar] [CrossRef]
- Selvaraj, V.; Krishnan, H. Acidic functionalized graphene dispersed polyethylene glycol nano-phase change material for the active cooling of a simulated heat-generating electronic system. J. Energy Storage 2022, 45, 103774. [Google Scholar] [CrossRef]
- Saranprabhu, M.K.; Rajan, K.S. Thermal energy storage system with a high-temperature nanoparticle enhanced phase change material: Charging and discharging characteristics upon integration with process preheating. J. Energy Storage 2022, 55, 105295. [Google Scholar] [CrossRef]
- Bharathiraja, R.; Ramkumar, T.; Selvakumar, M.; Radhika, N. Thermal characteristics enhancement of paraffin wax phase change material (PCM) for thermal storage applications. Renew. Energy 2024, 222, 119986. [Google Scholar] [CrossRef]
- Kushwaha, P.K.; Sharma, N.K.; Kumar, A.; Meena, C.S. Recent advancements in augmentation of solar water heaters using nanocomposites with PCM: Past, present, and future. Buildings 2023, 13, 79. [Google Scholar] [CrossRef]
- Paul, J.; Samykano, M.; Pandey, A.K.; Kadirgama, K.; Tyagi, V.V. Nano-engineered paraffin-based phase change material for building thermal management. Buildings 2023, 13, 900. [Google Scholar] [CrossRef]
- Sivashankar, M.; Selvam, C.; Suresh, S. Experimental study on the performance of low concentrated solar photovoltaic system with nano-enhanced phase change material encapsulated heat sink. Appl. Therm. Eng. 2025, 262, 125254. [Google Scholar] [CrossRef]
- Venkatraman, S.; Jidhesh, P.; Rathnaraj, J.D.; Selvam, C. Experimental studies on the enhancement in discharging characteristics of phase change material with steatite nanoparticles. J. Energy Storage 2023, 73, 109103. [Google Scholar] [CrossRef]
- Brychka, S.; Brychka, A.; Hedin, N.; Mondeshki, M. Sustainable composite materials based on carnauba wax and montmorillonite nanoclay for energy storage. Materials 2024, 17, 1978. [Google Scholar] [CrossRef]
- Vijay Krishna, N.; Manikandan, S.; Selvam, C. Enhanced performance of thermoelectric cooler with phase change materials: An experimental study. Appl. Therm. Eng. 2022, 212, 118612. [Google Scholar] [CrossRef]
- Tselepi, M.; Prouskas, C.; Papageorgiou, D.G.; Lagaris, I.E.; Evangelakis, G.A. Graphene-based phase change composite nano-materials for thermal storage applications. Energies 2022, 15, 1192. [Google Scholar] [CrossRef]
- Pereira, J.; Moita, A.; Moreira, A. An overview of the nano-enhanced phase change materials for energy harvesting and conversion. Molecules 2023, 28, 5763. [Google Scholar] [CrossRef] [PubMed]
- Khlissa, F.; Mhadhbi, M.; Aich, W.; Hussein, A.K.; Alhadri, M.; Selimefendigil, F.; Öztop, H.F.; Kolsi, L. Recent advances in nanoencapsulated and nano-enhanced phase-change materials for thermal energy storage: A review. Processes 2023, 11, 3219. [Google Scholar] [CrossRef]
- Calotă, R.; Pop, O.; Bode, F.; Croitoru, C.; Serafim, A.; Bărbulescu, A.; Damian, C.; Tefas, L. A novel concept of nano-enhanced phase change material. Materials 2024, 17, 4268. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.K.; Rahman, S.; Yadav, A.; Samykano, M.; Tyagi, V.V. Graphene–silver hybrid nanoparticle-based organic phase change materials for enhanced thermal energy storage. Sustainability 2022, 14, 13240. [Google Scholar] [CrossRef]
- Fayyaz, H.; Hussain, A.; Ali, I.; Shahid, H.; Ali, H.M. Experimental analysis of nano-enhanced phase-change material with different configurations of heat sinks. Materials 2022, 15, 8244. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Velraj, R.; Senthil, R. Overview of numerical, experimental, and parametric studies on the spherical container-based packed bed latent heat storage. J. Energy Storage 2024, 102, 114089. [Google Scholar] [CrossRef]
- Chen, C.; Sheng, T.; Du, X.; Yang, L.; Romagnoli, A. Performance of packed bed thermal energy storage with cascaded multiple capsuled phase change materials. Int. J. Heat Mass Transfer 2024, 235, 126146. [Google Scholar] [CrossRef]
- Yadav, A.; Samykano, M.; Kalidasan, B.; Sharma, K.; Pandey, A.K. Optimizing thermal energy storage using multi-walled carbon nano tube infused polyethylene glycol composites: An experimental and simulation study. Process Saf. Environ. Prot. 2025, 194, 246–262. [Google Scholar] [CrossRef]
- Paul, J.; Samykano, M.; Pandey, A.K.; Kadirgama, K.; Jacob, J.; Saidur, R. A comprehensive assessment of thermophysical properties of MXene doped Polyethylene glycol 400 for cold chain logistics. J. Mol. Liq. 2025, 419, 126799. [Google Scholar] [CrossRef]
- Apmann, K.; Fulmer, R.; Soto, A.; Vafaei, S. Thermal Conductivity and Viscosity: Review and Optimization of Effects of Nanoparticles. Materials 2021, 14, 1291. [Google Scholar] [CrossRef] [PubMed]
- Shchegolkov, A.V.; Shchegolkov, A.V.; Galunin, E.V.; Popova, A.A.; Krivosheev, R.M.; Memetov, N.R.; Tkachev, A.G. Graphene-Modified Heat-Accumulating Materials and Aspects of their Application in Thermotherapy and Biotechnologies. Nano Hybrids and Composites 2017, 13, 21–25. [Google Scholar] [CrossRef]
- Amidu, M.A.; Ali, M.; Alkaabi, A.K.; Addad, Y. A critical assessment of nanoparticles enhanced phase change materials (NePCMs) for latent heat energy storage applications. Sci. Rep. 2023, 13, 7829. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, S.; Huang, S.-J.; Rathnaraj, D.; Srinivasan, S.A. Effect of Ball-Milled Steatite Powder on the Latent Heat Energy Storage Properties and Heat Charging–Discharging Periods of Paraffin Wax as Phase Change Material. Micromachines 2022, 13, 1456. [Google Scholar] [CrossRef]
- Takale, B.B.; Patil, R.S. Preparation and Characterization of CuO Nanoparticles Dopped Paraffin Wax Composite for Solar Thermal Energy Storage in the Solar Drying Application. In Advances in Clean Energy and Sustainability, Green Energy and Technology; Tatiparti, S.S.V., Seethamraju, S., Eds.; Springer: Singapore, 2024; pp. 201–211. [Google Scholar] [CrossRef]
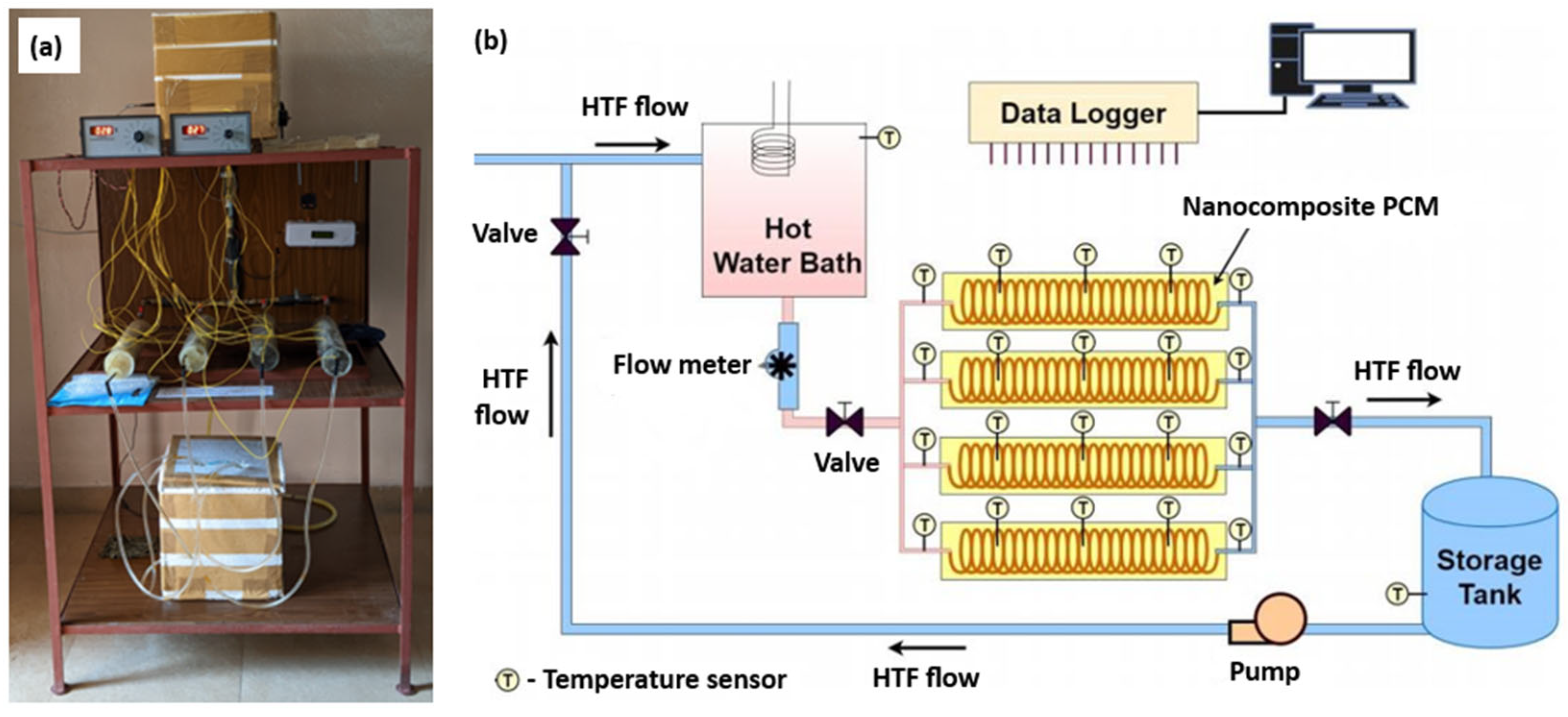

| TES Material | Characteristics | Advantages | Disadvantages | Applications | Ref. |
|---|---|---|---|---|---|
| Adipic acid with 0–2 wt% graphene oxide (GO) | 1 wt% addition of GO with adipic acid enhanced the thermal conductivity by 16% | 44% enhancement in the initial solidification rate | The latent heat was reduced by 3.4% and specific heat by 3.6% | TES systems | [28] |
| Paraffin wax with different proportions of MWCNT and SiO2 | Thermal conductivity increased from 0.24 to 0.451 W/mK | Thermal efficiency improved using hybrid PCM from 64.7% to 71.7% | There was less hot water production during the morning due to the PCM charging | Flat-plate solar water heater | [29] |
| Deionized water with suspended iron oxide nanoparticles | Surface heat flux increased by 200% when NePCM was used | The subcooling effect of deionized water was eliminated without nucleating agents | The scalability of NePCM needs to be explored | Large-scale air conditioning system | [30] |
| Paraffin wax with copper nanoparticles (0.5, 1, and 2%) | PCM melting rate and energy storage capacity of NePCM increased at 2 and 0.5% concentrations of nanoparticles | The highest energy storage capacity was found at a 0.5% concentration of Cu nanoparticles | Higher external magnetic field decreased the melting rate and energy storage capacity | Electronic cooling and battery thermal management | [31] |
| PEG with functionalized graphene | 72% enhanced thermal conductivity for 0.3 vol.% NePCM compared to pure PCM | 24% enhanced latent heat for 0.2 vol.% NePCM compared to pure PEG | Latent heat enhancement decreased with nanoparticle vol.% increase | Electronic cooling system | [32] |
| Solar salt with copper and graphene oxide | Solar salt with copper (0.5 wt%) and GO (0.5 wt%) | The overall heat transfer coefficient was enhanced using nanoparticles | Higher concentration decreases the LHS | Preheating applications | [33] |
| Paraffin wax with graphene nanoplatelets and nano-SiO2 particles | Mixing of nanoparticles (0.5 and 1 wt%) enhanced the thermal conductivity | The thermal conductivity enhancement was highest when hybrid nanoparticles were present at 1 wt% | Nanoparticles decreased the melting temperature and increased the solidification temperature of hybrid PCMs | TES systems | [34] |
| Paraffin wax with Al and CuO | Paraffin wax with 0.05 to 0.5% concentrations of Al and CuO improved thermal conductivity | Thermal conductivity was increased with Al and CuO addition of up to 0.5 wt% | An increased percentage of nanoparticles reduced the latent heat | Solar water heater | [35] |
| Graphene–silver nanofillers dispersed in paraffin | 6.7% enhanced latent heat was found for 0.3 wt% nanocomposite | Great potential in shielding ultraviolet rays | PCM decomposition limit could be considered | Building thermal management | [36] |
| Property | CuO | Steatite | PCM (RT60) |
|---|---|---|---|
| Specific heat (kJ/kg. °C) | 0.385 | 0.921 | 2.1 (solid at 15 °C), 2.14 (liquid at 60 °C) |
| Density (kg/m3) | 8960 | 2700 | 882 (solid at 15 °C), 774 (liquid at 60 °C) |
| Thermal conductivity (W/m. °C) | 3.94 | 2.9 | 0.22 (solid), 0.2 (liquid) |
| Melting point (°C) | 1058 | 1600 | 60 |
| Heat of fusion (kJ/kg) | - | - | 180 |
| Volumetric expansion (%) | 0.13 | 0.115 | 12.5 |
| Maximum operating temperature (°C) | 1000 | 1200 | 80 |
| Parameter | Instrument | Range | Uncertainty |
|---|---|---|---|
| Mass (kg) | Mass balance | 0–0.6 | ±0.00001 kg |
| Temperature (°C) | K-type thermocouple | 73–1533 | ±0.5 °C |
| Flow rate (kg/h) | Flow meter | 0–200 | ±1.5 kg/h |
| Heat stored (kJ) | - | - | ±0.105 kJ |
| Heat storage rate (kW) | - | - | ±0.015 kW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumalsamy, J.; Punniakodi, S.B.M.; Selvam, C.; Senthil, R. Enhancing Phase Change Characteristics of Hybrid Nanocomposites for Latent Heat Thermal Energy Storage. J. Compos. Sci. 2025, 9, 120. https://doi.org/10.3390/jcs9030120
Perumalsamy J, Punniakodi SBM, Selvam C, Senthil R. Enhancing Phase Change Characteristics of Hybrid Nanocomposites for Latent Heat Thermal Energy Storage. Journal of Composites Science. 2025; 9(3):120. https://doi.org/10.3390/jcs9030120
Chicago/Turabian StylePerumalsamy, Jidhesh, Swami B. M. Punniakodi, Chandrasekaran Selvam, and Ramalingam Senthil. 2025. "Enhancing Phase Change Characteristics of Hybrid Nanocomposites for Latent Heat Thermal Energy Storage" Journal of Composites Science 9, no. 3: 120. https://doi.org/10.3390/jcs9030120
APA StylePerumalsamy, J., Punniakodi, S. B. M., Selvam, C., & Senthil, R. (2025). Enhancing Phase Change Characteristics of Hybrid Nanocomposites for Latent Heat Thermal Energy Storage. Journal of Composites Science, 9(3), 120. https://doi.org/10.3390/jcs9030120









