Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care
Abstract
:1. Introduction
2. Hydrogels in Wound Care Management
2.1. Hydrogels Properties
2.2. Hydrogels’ Mechanism of Action in Wound Healing
3. Applications in Burn Treatment
| Product | Hydrogel Composition | Purpose | Side Effects | Ref. |
|---|---|---|---|---|
| Aquacel Hydrogel | Sodium carboxymethylcellulose | Balances moisture, preventing fluid spread and maceration Micro-contours to the wound bed, leaving no gaps Immobilizes bacteria and MMPs to aid in tissue regeneration Draws fluid away from the wound bed through vertical wicking De-sloughs the wound bed, reducing infection and inflammation Aids autolytic debridement, creating a cleaner wound bed | Burning on initial application Possible allergic contact dermatitis | [119,120] |
| Derma-Gel Hydrogel | Polyethylene glycol (PEG) | Provides a moist environment to the injured skin site (wound, burn, etc.), through a water-based gel polymer that does not dry out. Forms a homogenous protective barrier on the surface of the damaged skin site Easy wound management through hydrophilic properties and a long-term moist environment. Bacterial control, anti-inflammatory activity, protective film effect, absorbing wound exudates Ideal for uncovered wounds or to allow for the removal of secondary dressings without adherence | n.r. | [121] |
| HydroTac | Polyacrylate, Polyurethane | Hydroactive polymer foam dressing with a hydrogel layer Accelerates the healing process Stimulate epithelialization process due to growth factor accumulation Optimizes pH values for epithelialization Reduces the potential for sticking to wounds and supports painless dressing changes High retention under pressure, including when used in combination with compression Protects wound edges against exudate, reducing the potential for maceration | Local skin reactions (irritation, redness, swelling) In rare cases, causes allergic reactions | [122] |
| Intrasite Gel | Carboxymethylcellulose (CMC) Propylene glycol | Applipak system provides simple, directable, and controlled applications even in awkward wound sites Absorbs slough and exudate without damaging fragile granulation tissue Range of sizes available for different wound sizes Creates a moist wound management environment Rehydrates necrotic tissue and aids debridement Non-adherent | Reddening of the skin may occur with lengthy exposure May cause mild irritation to eyes If it is accidentally ingested No significant signs or symptoms. Large amounts may case gastric disturbances | [123] |
| Purilon Gel | Alginate Carboxymethylcellulose (CMC) | Purilon® gel is indicated for dry and sloughy necrotic wounds as well as wounds with a mix of necrotic and granulated tissue such as leg ulcers, pressure ulcers, and non-infected diabetic foot ulcers, and it may be used on 1st- and 2nd-degree burns. May be used to provide a moist healing environment throughout the healing process. Purilon® gel should be used in conjunction with a secondary dressing. | n.r. | [124] |
| Solosite Gel | Sodium Carboxymethylcellulose Polyvinylpyrrolidone | Provides a moist environment for optimal wound healing. Protects the user against dehydration and bacterial contamination while absorbing exudates from the wound. | n.r. | [125] |
| Article Title | Treatment | Aim of the Study | Testing Stage | Results | Ref. |
|---|---|---|---|---|---|
| 3D-Printed Gelatin-Alginate Hydrogel Dressings for Burn Wound Healing: A Comprehensive Study | 3D-printed gelatin and alginate-based hydrogel | Evaluation of gelatin and alginate hydrogels | In vitro In vivo | The hydrogel dressing based on 75% gelatin and 25% alginate showed the best balance between mechanical, hydration, and biological properties. 3D-printed dressing in vivo tests, on Sprague Dawley rats, showed faster wound closure, follicular regeneration, and atraumatic removal. | [126] |
| In Vitro and In Vivo Evaluation of Metformin Hydrochloride Hydrogels Developed with Experimental Design in the Treatment of Burns | Hydrogel-based Poloxamer 407®, Carbopol 934®, and sodium carboxymethyl cellulose (Na-CMC) Metformin | Testing of different concentrations of Metformin (4 mg/g, 6 mg/g, and 8 mg/g) with optimization of the formulation on loading efficiency and release of the active substance. | In vitro In vivo | In vitro tests analyzed Metformin’s physicochemical properties, pH, viscosity, and release profiles. For the in vivo evaluation, female Wistar rats were used to assess burn wound healing activity. Poloxamer® hydrogel with 8 mg/g metformin demonstrated the greatest reduction in burn size and the best results in tissue regeneration. Histopathologically, significant re-epithelialization and an increase in neovascularization were observed in the metformin hydrogel-treated groups. The beneficial effects were more pronounced in the acute phase of healing. | [127] |
| Hyaluronic acid hydrogel loaded by adipose stem cells enhances wound healing by modulating IL-1β, TGF-β1, and bFGF in burn wound models in rat | Hyaluronic acid-based hydrogel (HA) Adipose stem cells (ASCs) Acellular dermal matrix (ADM) | Efficacity investigation of hyaluronic acid (HA) hydrogel loaded with adipose tissue-derived stem cells (ASCs) in accelerating the healing of burn wounds. | In vitro In vivo | In terms of cell viability, HA significantly increased cell viability in the culture medium. ADM-HA/ASCs accelerated wound closure compared to ADM and ADM-HA groups, reducing inflammation and stimulating angiogenesis. ADM-HA/ASCs had a significant impact on TGF-β1 (proliferation) and bFGF (maturation and angiogenesis) expression, as well as on reducing inflammation (decreased IL-1β). Wounds treated with ADM-HA/ASCs showed complete epithelialization at 14 days, with faster granulation tissue and blood vessels’ formation. | [128] |
| Hydrogel nanocomposite based on alginate/zeolite for burn wound healing: In vitro and in vivo study | Nanocomposite hydrogels based on alginate (Alg) and natural zeolite (clinoptilolite) | Evaluation of the antibacterial and regenerative characteristics of Alg/Zeolite (Alg/Zeo) composite hydrogel. Comparison of wound healing efficacy between plain hydrogel (Alg)- and composite hydrogel (Alg/Zeo)-treated groups versus the control group. | In vitro In vivo | The hydrogels were biocompatible without inducing significant cytotoxicity at moderate concentrations. Alg and Alg/Zeo accelerated wound contraction and re-epithelialization process compared to the control group. There were no significant differences between the Alg and Alg/Zeo groups in terms of granulation tissue thickness, angiogenesis, and inflammatory cell recruitment. The Alg/Zeo group showed almost complete epithelialization (86%) and better collagen organization compared to the control group after 14 days | [129] |
| Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury | Injectable hydrogel based on hyaluronic acid (HA) and polyethylene glycol (PEG) Adipose-derived stem cells (ASCs) RGD short peptide | Developing a conformable hydrogel to deliver and protect stem cells in an inflammatory environment. Evaluating the hydrogel’s ability to accelerate burn healing and reduce scar formation. | In vitro In vivo | The presence of RGD peptide promotes significative cell proliferation, and viability, and promotes secretion of factors such as PDGF, HGF, and MMP-9. Healing was accelerated and the hydrogel- and cell-treated group demonstrated a significantly higher healing rate than the control group. The average healing time was 11.8 days. The hydrogel + cells group showed increased vascularization and a significant reduction in fibroblasts involved in scar formation (α-SMA positive). The type III/I collagen type III/I ratio was higher, indicating reduced scarring. | [130] |
| Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing | Hydrogel-based carboxymethyl chitosan (CMC) and carbomer 940 (CBM) loaded with Eucalyptus (EEO), Ginger (GEO), and Cumin (CEO) essential oils | This study aimed to develop an antibacterial polysaccharide-based hydrogel with essential oil for burn skin repair. | In vitro In vivo | The hydrogels did not exhibit obvious cytotoxicity on L929 cells up to a low extraction concentration (1000 mg/mL), while, at a low concentration, the cell viability decreased for all hydrogels. CBM/CMC/EEO hydrogel also induced significant migration of L929 cells, which was attributed to the presence of the essential oil. CBM/CMC/EEO hydrogel exhibited a minimal effect on apoptosis. Essential oils enhanced the antimicrobial effect of hydrogels. CBM/CMC/EEO hydrogel was observed to possess the highest antibacterial activity toward S. aureus and E. coli. The in vivo results illustrated that the CBM/CMC/EEO hydrogel treatment induced the regeneration of collagen fibers, epidermis, and appendages The histology staining, and analysis of IL6, TNF-α, TGF-β, VEGF, and EGF levels showed that the CBM/CMC/EEO hydrogel treatment significantly accelerated burn wound repair in a mouse model. | [119] |
| Thermo-responsive chitosan hydrogel for healing of full-thickness wounds infected with XDR bacteria isolated from burn patients: In vitro and in vivo animal model | Chitosan-based hydrogel cross-linked with different concentrations of β-glycerolphosphate disodium salt pentahydrate (β-GP) | Evaluation of thermo-responsive chitosan (TCTS) hydrogel potential for protection against full-thickness wounds containing extensively drug-resistant (XDR) bacteria isolated from burn patients. The hydrogel was evaluated both in vitro and in vivo in a rat model. | In vitro In vivo | Cell viability assay showed no significant cytotoxicity of the TCTS hydrogel for Hu02 fibroblast cells. In vitro, antibacterial assay showed no antibacterial activity of TCTS hydrogel against standard strain bacteria. In hydrogel-treated wounds, a decrease in the number of bacterial colonies was observed, which was explained by the accelerated healing induced by the hydrogel. The wounds were completely re-epithelialized and closed on postoperative day 14. | [131] |
| Oxygen-releasing hydrogels promote burn healing under hypoxic conditions | Oxygen delivery system made of self-healing hydrogel (QGO) (gallic acid-grafted quaternized chitosan and oxidized hyaluronic acid) Oxygen-releasing microspheres (GC) (calcium peroxide (CaO2) and gelatin) | Using oxygen-releasing hydrogels to accelerate burn healing under hypoxic conditions | In vitro In vivo | QGO/GC has stable mechanical properties and is self-healing Consistently releases oxygen over 10 days Promotes the migration and proliferation of endothelial cells and the capillary formation Stimulates angiogenesis by increasing VEGF expression and other GF Wound healing was significantly accelerated compared to control groups Reduced inflammation (decreased TNF-α expression) and increased collagen deposition and granulation formation The hydrogel showed no cytotoxicity and good tissue compatibility | [132] |
| Silk fibroin hydrogel promotes burn wound healing through regulating TLN1 expression and affecting cell adhesion and migration | Silk fibroin hydrogel | Silk fibroin hydrogel for grade II burns healing by regulating TLN1 (Talin1) expression and influencing cell adhesion and migration | In vitro In vivo | Porous structure favors nutrient transport and cell proliferation In the hydrogel-treated group, accelerated healing was observed compared to the other groups, with a complete regeneration of the epidermis in 12 days. Histologically, the hydrogel-treated burns showed faster re-epithelialization and a considerable reduction in inflammation Hydrogel promoted cell proliferation and reduced cell apoptosis Hacat and HDF cells had a considerably higher migration rate TLN1 expression was significantly upregulated, promoting cell dehydration and migration | [133] |
4. Applications in Chronic Wound Care
5. Innovations and Advanced Technologies
6. Limitations, Challenges, and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.d.P.d.L.; Passos, M.F. Skin wounds, the healing process, and hydrogel-based wound dressings: A short review. J. Biomater. Sci. Polym. Ed. 2021, 32, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Dai, J.; Zhang, J.; Li, Z. Accelerated Skin Wound Healing by Electrical Stimulation. Adv. Healthc. Mater. 2021, 10, 2100557. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef]
- WHO. Burns. Available online: https://who.int/news-room/fact-sheets/detail/burns (accessed on 9 January 2025).
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Verdolino, D.V.; Thomason, H.A.; Fotticchia, A.; Cartmell, S. Wound dressings: Curbing inflammation in chronic wound healing. Emerg. Top. Life Sci. 2021, 5, 523–537. [Google Scholar] [CrossRef]
- Su, L.; Jia, Y.; Fu, L.; Guo, K.; Xie, S. The emerging progress on wound dressings and their application in clinic wound management. Heliyon 2023, 9, e22520. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Ngoc Le, T.T.; Nguyen, A.T.; Thien Le, H.N.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Weir, D. Wound Dressings. In Local Wound Care for Dermatologists; Alavi, A., Maibach, H.I., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 25–34. [Google Scholar]
- Kaya, S.; Acar, S. Properties of ideal wound dressingideal yara örtüsünün özellikleri. Ank. Univ. Eczaci. Fak. Derg. 2023, 47, 5. [Google Scholar] [CrossRef]
- Nazarko, L. Wound healing and moisture balance: Selecting dressings. Nurs. Resid. Care 2009, 11, 286–291. [Google Scholar] [CrossRef]
- Obagi, Z.; Damiani, G.; Grada, A.; Falanga, V. Principles of Wound Dressings: A Review. Surg. Technol. Int. 2019, 35, 1–57. [Google Scholar]
- Niculescu, A.-G.; Grumezescu, A.M. An up-to-date review of biomaterials application in wound management. Polymers 2022, 14, 421. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.A.; Rastegarpanah, H.; Ansari, N.; Dortaj, H.; Shafaghi, S.; Ghorbani, F.; Shafaghi, M. Preparation and in vivo Evaluation of an Astragalus Gummifer Hydrogel-Based Dressing for Excisional Wound. Biointerface Res. Appl. Chem. 2024, 14, 95. [Google Scholar]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and Hydrogel-Derived Materials for Energy and Water Sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef]
- Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Hydrogels in Cutaneous Wound Healing: Insights into Characterization, Properties, Formulation and Therapeutic Potential. Gels 2024, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Singh, M. Hydrogels and Wound Healing: Current and Future Prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Wang, W.; Ummartyotin, S.; Narain, R. Advances and challenges on hydrogels for wound dressing. Curr. Opin. Biomed. Eng. 2023, 26, 100443. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Ranote, S.; Kumar, K.; Chauhan, S.; Chauhan, G.S. New Nicotinic Acid-Based Hydrogel: Swelling and Insulin Uptake Studies. Biointerface Res. Appl. Chem. 2023, 13, 102. [Google Scholar]
- Soetaredjo, F.E.; Ismadji, S.; Santoso, S.P.; Putro, J.N.; Foe, K.; Waworuntu, G.L. pH-Sensitive CNC-Chitosan Hydrogel for Drug Delivery. Lett. Appl. NanoBioSci. 2024, 13, 168. [Google Scholar]
- Zhang, W.; Liu, L.; Cheng, H.; Zhu, J.; Li, X.; Ye, S.; Li, X. Hydrogel-based dressings designed to facilitate wound healing. Mater. Adv. 2024, 5, 1364–1394. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Yu, P.; Wei, L.; Yang, Z.; Liu, X.; Ma, H.; Zhao, J.; Liu, L.; Wang, L.; Chen, R.; Cheng, Y. Hydrogel Wound Dressings Accelerating Healing Process of Wounds in Movable Parts. Int. J. Mol. Sci. 2024, 25, 6610. [Google Scholar] [CrossRef]
- Al-Mamari, A.; Shahitha, F.; Al-Sibani, M.; Al Saadi, A.; Al Harrasi, A.; Ahmad, A. Novel antibacterial wound healing hydrogels based on HEC/SA/HA using gree n chemistry approach. Lett. Appl. NanoBioSci. 2023, 12, 69. [Google Scholar]
- He, H.; Xiao, Z.; Zhou, Y.; Chen, A.; Xuan, X.; Li, Y.; Guo, X.; Zheng, J.; Xiao, J.; Wu, J. Zwitterionic poly(sulfobetaine methacrylate) hydrogels with optimal mechanical properties for improving wound healing in vivo. J. Mater. Chem. B 2019, 7, 1697–1707. [Google Scholar] [CrossRef]
- Ho, T.T.-P.; Tran, H.A.; Doan, V.K.; Maitz, J.; Li, Z.; Wise, S.G.; Lim, K.S.; Rnjak-Kovacina, J. Natural Polymer-Based Materials for Wound Healing Applications. Adv. NanoBiomed Res. 2024, 4, 2300131. [Google Scholar] [CrossRef]
- Pan, Z.; Ye, H.; Wu, D. Recent advances on polymeric hydrogels as wound dressings. APL Bioeng. 2021, 5, 011504. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Tang, P.; Zheng, T.; Zhang, X.; Zhang, Y.; Li, G. Collagen-based hydrogels cross-linked via laccase-mediated system incorporated with Fe3+ for wound dressing. Colloids Surf. B Biointerfaces 2022, 219, 112825. [Google Scholar] [CrossRef]
- Ye, R.; Liu, S.; Zhu, W.; Li, Y.; Huang, L.; Zhang, G.; Zhang, Y. Synthesis, Characterization, Properties, and Biomedical Application of Chitosan-Based Hydrogels. Polymers 2023, 15, 2482. [Google Scholar] [CrossRef]
- Lin, G.-S.; Peng, W.; Gao, J.; Wahlen, A.; Tong, Z. Chapter Two—Functional naturally derived materials to improve the environment: Chemical structures, modifications, applications, and future perspectives. In Advances in Bioenergy; Li, Y., Chang, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 8, pp. 93–144. [Google Scholar]
- Zhang, Y.; Kumar, P.; Lv, S.; Xiong, D.; Zhao, H.; Cai, Z.; Zhao, X. Recent advances in 3D bioprinting of vascularized tissues. Mater. Des. 2021, 199, 109398. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Claudio-Rizo, J.; Espíndola-Serna, L.; Becerra, J.; Cano-Salazar, L.; Flores Guia, T. Recent Advances in the Synthesis and Applications of Collagen Based Hydrogels: A Review. Mediterr. J. Basic Appl. Sci. 2019, 3, 54–98. [Google Scholar]
- Devernois, E.; Coradin, T. Synthesis, Characterization and Biological Properties of Type I Collagen–Chitosan Mixed Hydrogels: A Review. Gels 2023, 9, 518. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Minculescu, M.A.; Niculescu, A.-G.; Hudiță, A.; Holban, A.M.; Alberts, A.; Grumezescu, A.M. Nanoparticle-Enhanced Collagen Hydrogels for Chronic Wound Management. J. Funct. Biomater. 2025, 16, 91. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar]
- Hong, F.; Qiu, P.; Wang, Y.; Ren, P.; Liu, J.; Zhao, J.; Gou, D. Chitosan-based hydrogels: From preparation to applications, a review. Food Chem. X 2024, 21, 101095. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Singha, I.; Basu, A. Chitosan based injectable hydrogels for smart drug delivery applications. Sens. Int. 2022, 3, 100168. [Google Scholar] [CrossRef]
- Che, X.; Zhao, T.; Hu, J.; Yang, K.; Ma, N.; Li, A.; Sun, Q.; Ding, C.; Ding, Q. Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers 2024, 16, 344. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Feng, Y. Chitosan Hydrogel as Tissue Engineering Scaffolds for Vascular Regeneration Applications. Gels 2023, 9, 373. [Google Scholar] [CrossRef]
- Yadav, M.; Kaushik, B.; Rao, G.K.; Srivastava, C.M.; Vaya, D. Advances and challenges in the use of chitosan and its derivatives in biomedical fields: A review. Carbohydr. Polym. Technol. Appl. 2023, 5, 100323. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Y.; Luo, D.; Li, P.; Chen, Y.; Fu, X.; Yue, Y.; Hou, R.; Liu, J.; Wang, X. Advantages and disadvantages of various hydrogel scaffold types: A research to improve the clinical conversion rate of loaded MSCs-Exos hydrogel scaffolds. Biomed. Pharmacother. 2024, 179, 117386. [Google Scholar] [CrossRef]
- Farshidfar, N.; Iravani, S.; Varma, R.S. Alginate-Based Biomaterials in Tissue Engineering and Regenerative Medicine. Mar. Drugs 2023, 21, 189. [Google Scholar] [CrossRef]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar] [CrossRef]
- Kibungu, C.; Kondiah, P.P.D.; Kumar, P.; Choonara, Y.E. This Review Recent Advances in Chitosan and Alginate-Based Hydrogels for Wound Healing Application. Front. Mater. 2021, 8, 681960. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef]
- Luo, Y.; Tan, J.; Zhou, Y.; Guo, Y.; Liao, X.; He, L.; Li, D.; Li, X.; Liu, Y. From crosslinking strategies to biomedical applications of hyaluronic acid-based hydrogels: A review. Int. J. Biol. Macromol. 2023, 231, 123308. [Google Scholar] [CrossRef]
- Chen, X.; Wu, T.; Bu, Y.; Yan, H.; Lin, Q. Fabrication and Biomedical Application of Alginate Composite Hydrogels in Bone Tissue Engineering: A Review. Int. J. Mol. Sci. 2024, 25, 7810. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef]
- Hwang, H.S.; Lee, C.-S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Ngece, K.; Alven, S.; Aderibigbe, B.A. Gelatin-Based Hybrid Scaffolds: Promising Wound Dressings. Polymers 2021, 13, 2959. [Google Scholar] [CrossRef]
- Kang, J.I.; Park, K.M. Advances in gelatin-based hydrogels for wound management. J. Mater. Chem. B 2021, 9, 1503–1520. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft. Matter. 2012, 8, 3280–3294. [Google Scholar] [CrossRef]
- Li, H.; Qi, Z.; Zheng, S.; Chang, Y.; Kong, W.; Fu, C.; Yu, Z.; Yang, X.; Pan, S. The Application of Hyaluronic Acid-Based Hydrogels in Bone and Cartilage Tissue Engineering. Adv. Mater. Sci. Eng. 2019, 2019, 3027303. [Google Scholar] [CrossRef]
- Wang, M.; Deng, Z.; Guo, Y.; Xu, P. Designing functional hyaluronic acid-based hydrogels for cartilage tissue engineering. Mater. Today Bio 2022, 17, 100495. [Google Scholar] [CrossRef]
- Onofrei, M.; Filimon, A. Cellulose-Based Hydrogels: Designing Concepts, Properties, and Perspectives for Biomedical and Environmental Applications. In Polymer Science: Research Advances, Practical Applications and Educational Aspects, 1st ed.; Méndez Vilas Antonio, S.M.A., Ed.; Formatex Research Center S.L.: Norristown, PA, USA, 2016. [Google Scholar]
- Kundu, R.; Mahada, P.; Chhirang, B.; Das, B. Cellulose hydrogels: Green and sustainable soft biomaterials. Curr. Res. Green Sustain. Chem. 2022, 5, 100252. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef]
- Lyu, Y.; Liu, Y.; He, H.; Wang, H. Application of Silk-Fibroin-Based Hydrogels in Tissue Engineering. Gels 2023, 9, 431. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A. How to Improve Physico-Chemical Properties of Silk Fibroin Materials for Biomedical Applications?—Blending and Cross-Linking of Silk Fibroin—A Review. Materials 2021, 14, 1510. [Google Scholar] [CrossRef]
- Liu, J.; Ge, X.; Liu, L.; Xu, W.; Shao, R. Challenges and Opportunities of Silk Protein Hydrogels in Biomedical Applications. Mater. Adv. 2022, 3, 2291–2308. [Google Scholar] [CrossRef]
- Madappura, A.P.; Madduri, S. A comprehensive review of silk-fibroin hydrogels for cell and drug delivery applications in tissue engineering and regenerative medicine. Comput. Struct. Biotechnol. J. 2023, 21, 4868–4886. [Google Scholar] [CrossRef]
- Akdag, Z.; Ulag, S.; Kalaskar, D.M.; Duta, L.; Gunduz, O. Advanced Applications of Silk-Based Hydrogels for Tissue Engineering: A Short Review. Biomimetics 2023, 8, 612. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, D.; Zhang, Y.; Li, M.; Chai, R. Silk fibroin hydrogels for biomedical applications. Smart Med. 2022, 1, e20220011. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, J.; Hu, J.; Cheng, Y.; Chen, X.; Gu, Z.; Li, Y. Bioinspired polydopamine hydrogels: Strategies and applications. Prog. Polym. Sci. 2023, 146, 101740. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Syed, A.; Wong, M.; Hicks, J.; Nunez, G.; Jitianu, A.; Siler, Z.; Peterson, M. Polydopamine Antioxidant Hydrogels for Wound Healing Applications. Gels 2020, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Fu, J.; Liu, E.; Gao, J.; Lv, Y.; Li, Z. Injectable hydrogels doped with PDA nanoparticles for photothermal bacterial inhibition and rapid wound healing in vitro. RSC Adv. 2024, 14, 2778–2791. [Google Scholar] [CrossRef]
- Kadlec, M.; Pekař, M.; Smilek, J. Mechanical properties of agarose hydrogels tuned by amphiphilic structures. Colloids Surf. A Physicochem. Eng. Asp. 2024, 700, 134791. [Google Scholar] [CrossRef]
- Zhao, J.; Marczynski, M.; Henkel, M.; Lieleg, O. Agarose-based hydrogels with tunable, charge-selective permeability properties. J. Appl. Polym. Sci. 2023, 140, e54303. [Google Scholar] [CrossRef]
- Jarosz, A.; Kapusta, O.; Gugała-Fekner, D.; Barczak, M. Synthesis and Characterization of Agarose Hydrogels for Release of Diclofenac Sodium. Materials 2023, 16, 6042. [Google Scholar] [CrossRef]
- Jiang, F.; Xu, X.-W.; Chen, F.-Q.; Weng, H.-F.; Chen, J.; Ru, Y.; Xiao, Q.; Xiao, A.-F. Extraction, Modification and Biomedical Application of Agarose Hydrogels: A Review. Mar. Drugs 2023, 21, 299. [Google Scholar] [CrossRef]
- Akhtar, A.; Farzam Rad, V.; Moradi, A.-R.; Yar, M.; Bazzar, M. Emerging polymeric biomaterials and manufacturing-based tissue engineering approaches for neuro regeneration-A critical review on recent effective approaches. Smart Mater. Med. 2023, 4, 337–355. [Google Scholar] [CrossRef]
- Lee, S.; Tong, X.; Yang, F. Effects of the poly(ethylene glycol) hydrogel crosslinking mechanism on protein release. Biomater. Sci. 2016, 4, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Keys, K.B.; Torres-Lugo, M.; Lowman, A.M. Poly(ethylene glycol)-containing hydrogels in drug delivery. J. Control. Release 1999, 62, 81–87. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jayathilaka, P.B.; Islam, M.S.; Tanaka, C.B.; Silberstein, M.N.; Kilian, K.A.; Kruzic, J.J. Structural aspects controlling the mechanical and biological properties of tough, double network hydrogels. Acta Biomater. 2022, 138, 301–312. [Google Scholar] [CrossRef]
- Lima, T.D.; Canelas, C.A.; Dutra, J.D.; Rodrigues, A.P.; Brígida, R.T.; Concha, V.O.; da Costa, F.A.; Passos, M.F. Poly (ε-caprolactone)-Based Scaffolds with Multizonal Architecture: Synthesis, Characterization, and In Vitro Tests. Polymers 2023, 15, 4403. [Google Scholar] [CrossRef]
- Borkar, T.; Goenka, V.; Jaiswal, A.K. Application of poly-ε-caprolactone in extrusion-based bioprinting. Bioprinting 2021, 21, e00111. [Google Scholar] [CrossRef]
- Idumah, C.I. Poly (α-caprolactone) (PCL) biopolymeric bionanoarchitectures for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2024, 74, 1–30. [Google Scholar] [CrossRef]
- Wang, M.; Bai, J.; Shao, K.; Tang, W.; Zhao, X.; Lin, D.; Huang, S.; Chen, C.; Ding, Z.; Ye, J. Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials. Int. J. Polym. Sci. 2021, 2021, 2225426. [Google Scholar] [CrossRef]
- Liang, X.; Zhong, H.-J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.-M.; He, J. Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef]
- Bercea, M. Recent Advances in Poly(vinyl alcohol)-Based Hydrogels. Polymers 2024, 16, 2021. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and properties of Poly(vinyl alcohol) hydrogels with high strength and toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Zhong, Y.; Lin, Q.; Yu, H.; Shao, L.; Cui, X.; Pang, Q.; Zhu, Y.; Hou, R. Construction methods and biomedical applications of PVA-based hydrogels. Front. Chem. 2024, 12, 1376799. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tang, Z.; Zhang, X.; Li, Z.; Xiao, H.; Zhang, M.; Liu, K.; Ni, Y.; Huang, L.; Chen, L.; et al. A self-healing, stretchable, and conductive Poly(N-vinylpyrrolidone)/gallic acid composite hydrogel formed via hydrogen bonding for wearable electronic sensors. Compos. Sci. Technol. 2020, 198, 108294. [Google Scholar] [CrossRef]
- Podaru, I.A.; Stănescu, P.O.; Ginghină, R.; Stoleriu, Ş.; Trică, B.; Şomoghi, R.; Teodorescu, M. Poly(N-vinylpyrrolidone)–Laponite XLG Nanocomposite Hydrogels: Characterization, Properties and Comparison with Divinyl Monomer-Crosslinked Hydrogels. Polymers 2022, 14, 4216. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, Y.; Pan, Y.; Yu, M.; Lin, X.; Mondal, A.K. Biocompatible, Injectable, and Self-Healing Poly(N-vinylpyrrolidone)/Carboxymethyl Cellulose Hydrogel for Drug Release. ACS Omega 2024, 9, 5854–5861. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2020, 10, 685–698. [Google Scholar] [CrossRef]
- Liu, B.; Chen, K. Advances in Hydrogel-Based Drug Delivery Systems. Gels 2024, 10, 262. [Google Scholar] [CrossRef]
- Kamini; Puri, D. Hydrogel-based drug delivery systems—A review. Polym. Plast. Technol. Mater. 2024, 63, 2213–2236. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar] [CrossRef]
- Wanis, N. Hydrogel Biomaterials for Drug Delivery: Mechanisms, Design, and Drugs. In Hydrogels; Lăcrămioara, P., Mihaela Violeta, G., Cristina-Elena, D.-P., Eds.; IntechOpen: Rijeka, Croatia, 2022; p. Ch. 3. [Google Scholar]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Arbab, S.; Ullah, H.; Muhammad, N.; Wang, W.; Zhang, J. Latest advance anti-inflammatory hydrogel wound dressings and traditional Lignosus rhinoceros used for wound healing agents. Front. Bioeng. Biotechnol. 2024, 12, 1488748. [Google Scholar] [CrossRef]
- Olteanu, G.; Neacșu, S.M.; Joița, F.A.; Musuc, A.M.; Lupu, E.C.; Ioniță-Mîndrican, C.B.; Lupuliasa, D.; Mititelu, M. Advancements in Regenerative Hydrogels in Skin Wound Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 3849. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhou, J.; Lu, Y.; Zhang, H.; Chen, B.; Dong, S.; Xue, Y.; Zhan, K.; Chen, C.; Sun, Y.; et al. Advancements in Wound Management: Microenvironment-Sensitive Bioactive Dressings with On-Demand Regulations for Diabetic Wounds. Engineering 2025. [Google Scholar] [CrossRef]
- Legrand, J.M.D.; Martino, M.M. Growth Factor and Cytokine Delivery Systems for Wound Healing. Cold Spring Harbor Perspect. Biol. 2022, 14, a041234. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kilgour, C.; Cabral, J.; Wise, L. Advancements in the Delivery of Growth Factors and Cytokines for the Treatment of Cutaneous Wound Indications. Adv. Wound Care 2020, 10, 596–622. [Google Scholar] [CrossRef]
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef]
- Cook, K.A.; Martinez-Lozano, E.; Sheridan, R.; Rodriguez, E.K.; Nazarian, A.; Grinstaff, M.W. Hydrogels for the management of second-degree burns: Currently available options and future promise. Burn. Trauma 2022, 10, tkac047. [Google Scholar] [CrossRef]
- Radzikowska-Büchner, E.; Łopuszyńska, I.; Flieger, W.; Tobiasz, M.; Maciejewski, R.; Flieger, J. An Overview of Recent Developments in the Management of Burn Injuries. Int. J. Mol. Sci. 2023, 24, 6357. [Google Scholar] [CrossRef]
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122. [Google Scholar] [CrossRef]
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef]
- Goh, M.; Du, M.; Peng, W.R.; Saw, P.E.; Chen, Z. Advancing burn wound treatment: Exploring hydrogel as a transdermal drug delivery system. Drug Deliv. 2024, 31, 2300945. [Google Scholar] [CrossRef]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burn. Trauma 2021, 9, tkab041. [Google Scholar] [CrossRef] [PubMed]
- Williams, C. An investigation of the benefits of Aquacel Hydrofibre wound dressing. Br. J. Nurs. 1999, 8, 676–680. [Google Scholar] [CrossRef]
- Derma-Gel. Available online: https://www.derma-gel.com/human/index.html (accessed on 21 January 2025).
- Hartman. HydroTac. Available online: https://www.hartmann.info/en-us/search?page=1&searchTerm=HydroTac (accessed on 21 January 2025).
- Nephew, S.A. Intrasite Gel. Available online: https://www.smith-nephew.com/en/health-care-professionals/products/advanced-wound-management/intrasite-gel-ppl#reference-materials (accessed on 21 January 2025).
- Coloplast. Purilon® Gel. Available online: https://products.coloplast.co.uk/coloplast/wound-care/purilon-gel-/ (accessed on 21 January 2025).
- Nephew, S.A. Solosite Gel. Available online: https://hsastore.com/smith-and-nephew-solosite-hydrogel-wound-gel---3-oz./9207.html?srsltid=AfmBOopgWYjeGnF1kZ8A9HsGfBH7_G7k0Q5b9gzHclYCqQ-WjhvZ_uxa (accessed on 21 January 2025).
- Fayyazbakhsh, F.; Khayat, M.J.; Leu, M.C. 3D-Printed Gelatin-Alginate Hydrogel Dressings for Burn Wound Healing: A Comprehensive Study. Int. J. Bioprint. 2022, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Ozyilmaz, E.D.; Celikkaya, R.; Comoglu, T.; Ozakpinar, H.R.; Behzatoglu, K. In Vitro and In Vivo Evaluation of Metformin Hydrochloride Hydrogels Developed with Experimental Design in the Treatment of Burns. AAPS PharmSciTech 2023, 24, 248. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Oryan, A.; Mohammadi, A.A. Hyaluronic acid hydrogel loaded by adipose stem cells enhances wound healing by modulating IL-1β, TGF-β1, and bFGF in burn wound model in rat. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 555–567. [Google Scholar] [CrossRef]
- Samadian, H.; Vahidi, R.; Salehi, M.; Hosseini-Nave, H.; Shahabi, A.; Zanganeh, S.; Lashkari, M.; Kouhbananinejad, S.M.; Rezaei Kolarijani, N.; Amini, S.M.; et al. Hydrogel nanocomposite based on alginate/zeolite for burn wound healing: In vitro and in vivo study. Iran J. Basic Med. Sci. 2023, 26, 708–716. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Aliakbar Ahovan, Z.; Khosravimelal, S.; Eftekhari, B.S.; Mehrabi, S.; Hashemi, A.; Eftekhari, S.; Brouki Milan, P.; Mobaraki, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Thermo-responsive chitosan hydrogel for healing of full-thickness wounds infected with XDR bacteria isolated from burn patients: In vitro and in vivo animal model. Int. J. Biol. Macromol. 2020, 164, 4475–4486. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Zheng, C.; Sun, N.; Chen, W.; Gao, Q.; Liu, J.; Hu, F.; Zhou, T.; Zhang, Y.; Lu, T. Oxygen-releasing hydrogels promote burn healing under hypoxic conditions. Acta Biomater. 2022, 154, 231–243. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, F.; Zhang, X.; Peng, Z.; Jiang, B.; Liang, M.; Wang, Y. Silk fibroin hydrogel promote burn wound healing through regulating TLN1 expression and affecting cell adhesion and migration. J. Mater. Sci. Mater. Med. 2020, 31, 48. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Hasson, J.; Reed, M. Clinical approach to chronic wound management in older adults. J. Am. Geriatr. Soc. 2021, 69, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Dalla Paola, L. Challenges in the management of chronic wound infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef]
- Bettle, G.; Bell, D.P.; Bakewell, S.J. A Novel Comprehensive Therapeutic Approach to the Challenges of Chronic Wounds: A Brief Review and Clinical Experience Report. Adv. Ther. 2024, 41, 492–508. [Google Scholar] [CrossRef]
- Wang, P.; Cai, F.; Li, Y.; Yang, X.; Feng, R.; Lu, H.; Bai, X.; Han, J. Emerging trends in the application of hydrogel-based biomaterials for enhanced wound healing: A literature review. Int. J. Biol. Macromol. 2024, 261, 129300. [Google Scholar] [CrossRef]
- Mani, M.P.; Mohd Faudzi, A.A.; Ramakrishna, S.; Ismail, A.F.; Jaganathan, S.K.; Tucker, N.; Rathanasamy, R. Sustainable electrospun materials with enhanced blood compatibility for wound healing applications—A mini review. Curr. Opin. Biomed. Eng. 2023, 27, 100457. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Activheal. ACTIVHEAL® HYDROGEL. Available online: https://activheal.com/wound-care-dressing-range/hydrogel-dressing/ (accessed on 27 January 2025).
- Macgowan, H.; McKay, M.; Hilley, P.; Wilson, E.; Warnock, I.; Dobson, L. Wound Management Guidance and Formulary; NHS Forth Valley: Larbert, UK, 2022. [Google Scholar]
- DermaRite. AquaDerm. Available online: https://dermarite.com/product/aquaderm/ (accessed on 27 January 2025).
- DermaRite. AquaDermTM Hydrogel Sheet Wound Dressing; DermaRite Industries LLC: North Berge, NJ, USA, 2017. [Google Scholar]
- Weller, C.D.; Team, V.; Sussman, G. First-line interactive wound dressing update: A comprehensive review of the evidence. Front. Pharmacol. 2020, 11, 155. [Google Scholar] [CrossRef]
- Corp, I.L. MEDIHONEY. Available online: https://www.woundsource.com/product/medihoney-hydrogel-sheet-dressing-adhesive (accessed on 27 January 2025).
- Kikgel. Neoheal Hydrogel. Available online: https://kikgel.com.pl/en/products/neoheal/#-1 (accessed on 27 January 2025).
- 3M™. Nu-Gel™ Hydrogel with Alginate. Available online: https://www.3m.co.uk/3M/en_GB/p/d/b5005265144/ (accessed on 27 January 2025).
- Hollister LTD Restore Hydrogel. Available online: https://www.hollister.com/-/media/files/pdfs-for-download/wound-care/restore-hydrogel-techsheet-911140-1110.ashx (accessed on 27 January 2025).
- Rauscher, L. Suprasorb® G Gel Dressing. Available online: https://www.lohmann-rauscher.com/en/products/wound-care/modern-wound-care/suprasorb-g/ (accessed on 27 January 2025).
- Shen, Z.; Zhang, C.; Wang, T.; Xu, J. Advances in Functional Hydrogel Wound Dressings: A Review. Polymers 2023, 15, 2000. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, C.X.; Xu, B.; Yu, Z. Diabetic foot ulcers: Classification, risk factors and management. World J. Diabetes 2022, 13, 1049–1065. [Google Scholar] [CrossRef] [PubMed]
- Güiza-Argüello, V.R.; Solarte-David, V.A.; Pinzón-Mora, A.V.; Ávila-Quiroga, J.E.; Becerra-Bayona, S.M. Current Advances in the Development of Hydrogel-Based Wound Dressings for Diabetic Foot Ulcer Treatment. Polymers 2022, 14, 2764. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef]
- Krizanova, O.; Penesova, A.; Hokynkova, A.; Pokorna, A.; Samadian, A.; Babula, P. Chronic venous insufficiency and venous leg ulcers: Aetiology, on the pathophysiology-based treatment. Int. Wound J. 2024, 21, e14405. [Google Scholar] [CrossRef]
- Bernatchez, S.F.; Eysaman-Walker, J.; Weir, D. Venous Leg Ulcers: A Review of Published Assessment and Treatment Algorithms. Adv. Wound Care 2021, 11, 28–41. [Google Scholar] [CrossRef]
- Mayrovitz, H.N.; Wong, S.; Mancuso, C. Venous, Arterial, and Neuropathic Leg Ulcers with Emphasis on the Geriatric Population. Cureus 2023, 15, e38123. [Google Scholar] [CrossRef] [PubMed]
- Roussou, E.; Fasoi, G.; Stavropoulou, A.; Kelesi, M.; Vasilopoulos, G.; Gerogianni, G.; Alikari, V. Quality of life of patients with pressure ulcers: A systematic review. Med. Pharm. Rep. 2023, 96, 123–130. [Google Scholar] [CrossRef]
- Luo, J.; Carter, G.C.; Agarwal, J.P.; Kwok, A.C. The 5-Factor Modified Frailty Index as a Predictor of 30-day Complications in Pressure Ulcer Repair. J. Surg. Res. 2021, 265, 21–26. [Google Scholar] [CrossRef]
- Källman, U.; Hommel, A.; Borgstedt Risberg, M.; Gunningberg, L.; Sving, E.; Bååth, C. Pressure ulcer prevalence and prevention interventions—A ten-year nationwide survey in Sweden. Int. Wound J. 2022, 19, 1736–1747. [Google Scholar] [CrossRef]
- Gomes, F.; Furtado, G.E.; Henriques, M.; Sousa, L.B.; Santos-Costa, P.; Bernardes, R.; Apóstolo, J.; Parreira, P.; Salgueiro-Oliveira, A. The skin microbiome of infected pressure ulcers: A review and implications for health professionals. Eur. J. Clin. Investig. 2022, 52, e13688. [Google Scholar] [CrossRef]
- Boyko, T.V.; Longaker, M.T.; Yang, G.P. Review of the Current Management of Pressure Ulcers. Adv. Wound Care 2016, 7, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Dou, Z.; Zhang, Y.; Li, F.; Xing, B.; Hu, Z.; Li, X.; Liu, Z.; Yang, W.; Liu, Z. Smart Responsive and Controlled-Release Hydrogels for Chronic Wound Treatment. Pharmaceutics 2023, 15, 2735. [Google Scholar] [CrossRef]
- Shang, S.; Zhuang, K.; Chen, J.; Zhang, M.; Jiang, S.; Li, W. A bioactive composite hydrogel dressing that promotes healing of both acute and chronic diabetic skin wounds. Bioact. Mater. 2024, 34, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, R.; Guan, Y.; Zhang, Z.; Yang, F.; Xiao, C.; Wang, Z.; Yu, P.; Hu, L.; Zhou, Z.; et al. Piezoelectric Hydrogel for Prophylaxis and Early Treatment of Pressure Injuries/Pressure Ulcers. ACS Biomater. Sci. Eng. 2022, 8, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cai, E.; Qi, X.; Ge, X.; Xiang, Y.; Wang, J.; Li, Y.; Lv, L.; Zheng, H.; Qian, Y.; et al. Immunomodulatory gallium/glycyrrhizic acid hydrogels for treating multidrug-resistant Pseudomonas aeruginosa-infected pressure ulcers. Chem. Eng. J. 2024, 487, 150756. [Google Scholar] [CrossRef]
- Nazemoroaia, M.; Bagheri, F.; Mirahmadi-Zare, S.Z.; Eslami-kaliji, F.; Derakhshan, A. Asymmetric natural wound dressing based on porous chitosan-alginate hydrogel/electrospun PCL-silk sericin loaded by 10-HDA for skin wound healing: In vitro and in vivo studies. Int. J. Pharm. 2025, 668, 124976. [Google Scholar] [CrossRef]
- Khaliq, T.; Sohail, M.; Minhas, M.U.; Mahmood, A.; Munir, A.; Qalawlus, A.H.M.; Jabeen, N.; Kousar, M.; Anwar, Z. Hyaluronic acid/alginate-based biomimetic hydrogel membranes for accelerated diabetic wound repair. Int. J. Pharm. 2023, 643, 123244. [Google Scholar] [CrossRef]
- Rahman, M.M.; Garcia, N.; Loh, Y.S.; Marks, D.C.; Banakh, I.; Jagadeesan, P.; Cameron, N.R.; Yung-Chih, C.; Costa, M.; Peter, K.; et al. A platelet-derived hydrogel improves neovascularisation in full thickness wounds. Acta Biomater. 2021, 136, 199–209. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.; Dai, W.-Q.; Cui, N.; Liu, J.-Q.; Wang, M.-G.; Zhou, Y.-F.; Fang, L.-X.; Sun, J.; Jiang, G.-B.; et al. Lutein-loaded multifunctional hydrogel dressing based on carboxymethyl chitosan for chronic wound healing. Int. J. Biol. Macromol. 2025, 300, 140219. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, J.; Chen, W.; Lu, C.; Zeng, Z.; Jiang, T.; Liu, W. Gelatin/Dopamine/Zinc-Doped Ceria/Curcumin nanocomposite hydrogels for repair of chronic refractory wounds. Int. J. Pharm. 2024, 663, 124575. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.; Vini, F. Hydrogel Dressings in Wound Management: Advances, Applications, and Future Directions. Int. J. Med. Sci. Clin. Res. Stud. 2023, 3, 2674–2680. [Google Scholar] [CrossRef]
- Teoh, J.H.; Mozhi, A.; Sunil, V.; Tay, S.M.; Fuh, J.; Wang, C.-H. 3D Printing Personalized, Photocrosslinkable Hydrogel Wound Dressings for the Treatment of Thermal Burns. Adv. Funct. Mater. 2021, 31, 2105932. [Google Scholar] [CrossRef]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3665. [Google Scholar] [CrossRef]
- Wang, S.; Lee, J.M.; Yeong, W.Y. Smart hydrogels for 3D bioprinting. Int. J. Bioprint. 2015, 1, 3–14. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Huang, Y.; Li, J.; Dong, R.; Yun, X.; Ren, Y.; Liu, X.; Hui, H.; Wu, L.; et al. Thermal-responsive microgels incorporated PVA composite hydrogels: Integration of two-stage drug release and enhanced self-healing ability for chronic wound treatment. Chem. Eng. J. 2025, 506, 159813. [Google Scholar] [CrossRef]
- Bei, Z.; Ye, L.; Tong, Q.; Ming, Y.; Yang, T.; Zhu, Y.; Zhang, L.; Li, X.; Deng, H.; Liu, J.; et al. Thermostimulated shrinking and adhesive hydrogel dressing for treating chronic diabetic wounds. Cell Rep. Phys. Sci. 2024, 5, 102289. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Liu, H.; Ren, M.; Wang, Z.; Wang, X.; Liu, H.; Feng, Y.; Lin, Q.; Wang, C.; et al. pH-responsive hydrogel loaded with insulin as a bioactive dressing for enhancing diabetic wound healing. Mater. Des. 2021, 210, 110104. [Google Scholar] [CrossRef]
- Li, N.; Liu, W.; Zheng, X.; Wang, Q.; Shen, L.; Hui, J.; Fan, D. Antimicrobial hydrogel with multiple pH-responsiveness for infected burn wound healing. Nano Res. 2023, 16, 11139–11148. [Google Scholar] [CrossRef]
- Irmukhametova, G.S.; Mun, G.A.; Khutoryanskiy, V.V. Hydrogel Dressings. In Therapeutic Dressings and Wound Healing Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 185–207. [Google Scholar]
- Zeng, Z.; Zhu, M.; Chen, L.; Zhang, Y.; Lu, T.; Deng, Y.; Ma, W.; Xu, J.; Huang, C.; Xiong, R. Design the molecule structures to achieve functional advantages of hydrogel wound dressings: Advances and strategies. Compos. Part B Eng. 2022, 247, 110313. [Google Scholar] [CrossRef]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Vizetto-Duarte, C.; Moay, Z.K.; Setyawati, M.I.; Rakshit, M.; Kathawala, M.H.; Ng, K.W. Composite Hydrogels in Three-Dimensional in vitro Models. Front. Bioeng. Biotechnol. 2020, 8, 611. [Google Scholar] [CrossRef]
- Tian, L.; Liu, T.; Jiang, Y.; He, B.; Hao, H. Multifunctional hydrogel sensor with Tough, self-healing capabilities and highly sensitive for motion monitoring and wound healing. Chem. Eng. J. 2024, 497, 154890. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. New Perspectives of Hydrogels in Chronic Wound Management. Molecules 2025, 30, 686. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammadi, S.; Sedghi Aminabad, N.; Sabzi, A.; Zarebkohan, A.; Razavi, M.; Vosough, M.; Bodaghi, M.; Maleki, H. Smart and Biomimetic 3D and 4D Printed Composite Hydrogels: Opportunities for Different Biomedical Applications. Biomedicines 2021, 9, 1537. [Google Scholar] [CrossRef]
- Kim, S.H.; Seo, Y.B.; Yeon, Y.K.; Lee, Y.J.; Park, H.S.; Sultan, M.T.; Lee, J.M.; Lee, J.S.; Lee, O.J.; Hong, H.; et al. 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials 2020, 260, 120281. [Google Scholar] [CrossRef]
- Damiati, L.A.; Alsudir, S.A.; Mohammed, R.Y.; Majrashi, M.A.; Albrahim, S.H.; Algethami, A.; Alghamdi, F.O.; Alamari, H.A.; Alzaydi, M.M. 4D printing in skin tissue engineering: A revolutionary approach to enhance wound healing and combat infections. Bioprinting 2025, 45, e00386. [Google Scholar] [CrossRef]



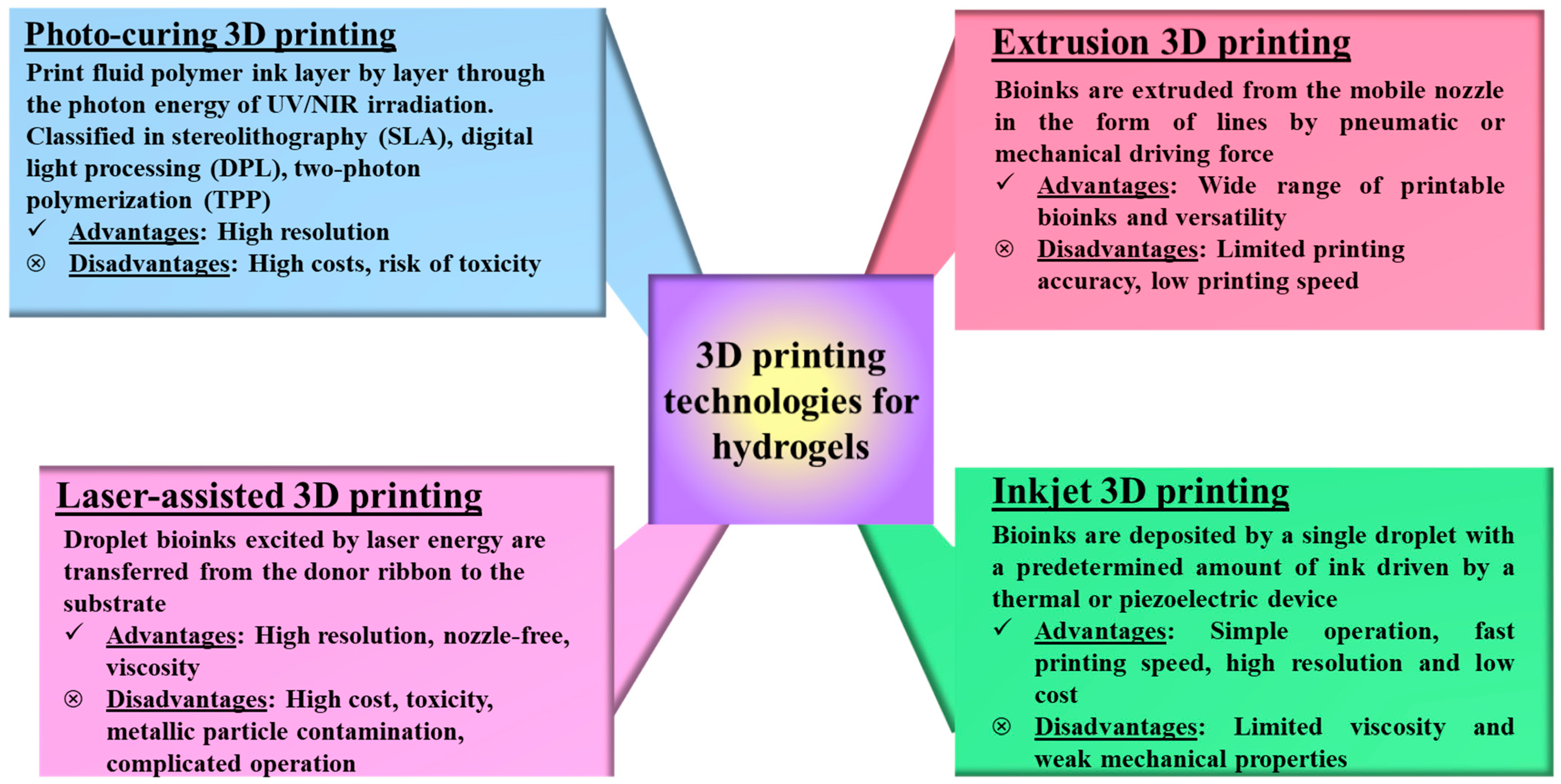
| Polymer Type | Polymer Name | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| Natural | Collagen (Col) | most abundant structural protein in animals main component of ECM biocompatible and non-toxic polymer stable good capacity to retain water can be loaded with therapeutic agents enhances the activity of therapeutic agents allows cell adhesion, proliferation, and differentiation represents an ideal microenvironment for angiogenesis low-cost and versatile polymer | its properties depend on fabrication parameters (e.g., Col source, pH, gelation) poor long-term stability exhibits poor mechanical properties (e.g., low stiffness) not easy to functionalize has a high rate of proteolytic degradation that limits its applicability insoluble in water | [36,37,38,39,40,41,42,43,44] |
| Chitosan (CS) | biocompatible and biodegradable polymer stimuli-responsive polymer can be modified by both physical and chemical crosslinking does not induce toxic or immunological effects presents bioadhesive and antimicrobial effects can act as a chelating, hemostatic, antioxidant, and pain-relief agent can accelerate homeostasis and epidermal cell growth can be used as a drug delivery system can maintain the moisture of injuries that is necessary for the wound-healing process | low blood compatibility low angiogenic activity poor mechanical properties high rate of degradability poor solubility in aqueous solutions | [38,45,46,47,48,49,50,51] | |
| Alginate (ALG) | has a great biocompatibility and biodegradability soluble and stable has multiple obtaining sources, with easy gelation stimuli-responsive polymer does not produce toxic or immunological effects can form gels in the presence of divalent cations can be easily functionalized with drugs and cells, and the degradation rate can be modified | poor cell adhesion properties has weak mechanical strength and needs to be combined with other polymers | [52,53,54,55,56,57,58,59] | |
| Gelatin (Gel) | high biocompatibility and biodegradability, and low toxicity cost-effective good water absorption properties can mimic the natural dermal EMC can promote erythropoiesis and increase the quantity of platelets and white blood cells to prevent bleeding | not thermostable must be coated with another material to improve its low mechanical strength has a high degradation rate | [60,61,62,63,64] | |
| Hyaluronic Acid (HA) | biocompatible polymer with great biodegradability promotes cellular adhesion and can provide sufficient biological activity that stimulates the microenvironment destined for cell survival can act as a space-filler and lubricant has high hydrophilicity, containing a high amount of water | insufficient mechanical strength susceptibility to degradation by hyaluronidase has poor degradation rates | [57,65,66,67] | |
| Cellulose | biocompatible, biodegradable low-cost material non-toxic polymer excellent hydrophilicity good mechanical properties excellent capacity to absorb fluids | cannot be used in its natural form because of its high number of hydroxyl groups high costs and energy consumption for fabricating dressings | [68,69,70] | |
| Silk Fibroin (SF) | biocompatible, non-toxic, and safe to use low immunogenicity degradation rate can be easily controllable can be combined with other polymers or materials similar structure to ECM great viscoelastic, swelling, and morphological properties excellent tissue repair functions enhances cell adhesion, adaptation, and proliferation cost-effective polymer that has a stable source to provide | lacks mechanical strength very brittle, which makes it difficult to use in scaffold fabrication needs purification because of the residual sericin that can create problems with biocompatibility unstable in an aqueous environment degrades at a slower rate, inhibiting tissue renewal, which can be a disadvantage when considering resorbable hydrogels insoluble in most organic solvents and water | [71,72,73,74,75,76] | |
| Polydopamine (PDA) | polymerized form of dopamine can be easily integrated into tissues and promotes healing can provide a reliable and suitable tissue adhesion has antioxidant properties due to its numerous catechols biocompatible can confer stability to drugs and biomolecules has great photothermal properties | still has biosecurity issues requires further studies to determine its effectiveness in hydrogel manufacturing | [77,78,79] | |
| Agarose | biocompatible and non-toxic low-cost polysaccharide ease of controllable gelation capable of forming physically cross-linked hydrogels in aqueous medium high water-absorption capacity can mimic the ECM has favorable and tunable mechanical properties can be used as drug-delivery systems can be mixed with other polysaccharides, peptides, and magnetic nanoparticles | exhibits brittleness and contractility does not support cell adhesion and must be combined with other polymers | [80,81,82,83,84] | |
| Synthetic | Polyethylene glycol (PEG) | biocompatible and non-toxic resistant to protein adsorption can be easily modified with other polymers has a high drug encapsulation rate has high hydrophilicity, tunable physicochemical properties, and anti-fouling properties can undergo rapid clearance from the body | because of its bioinert properties, it cannot provide an adhesive effect to cells does not promote tissue formation should be combined with other polymers to enhance its properties | [85,86,87,88] |
| Poly-ε-caprolactone (PCL) | biocompatible and biodegradable has high hydrophobicity and a slow degradation rate soluble in chlorinated solvents can be easily combined with other polymers | has low hydrophilicity; this disadvantage can be altered through combination with hydrophilic materials has a slow rate of resorption | [89,90,91] | |
| Polyvinyl alcohol (PVA) | has a low toxicity, high biocompatibility, and biodegradable properties has a great absorption capacity and tunable mechanical properties chemically stable in the presence of body fluids, stimuli-responsive, and resistant to aging | provides a weak network with low endurance at high temperatures and is incompatible with the human body network unable to support cell attachment | [92,93,94,95,96] | |
| Poly-N-vinylpyrrolidone (PVP) | biocompatible and non-toxic hydrophilic and soluble in water and organic solvents can be combined with both hydrophobic and hydrophilic polymers improves bioavailability of poor water-soluble incorporated drugs | has poor mechanical properties and bioactivity has poor self-healing performance | [97,98,99,100] |
| Product | Hydrogel Composition | Applications | Side Effects | Refs. |
|---|---|---|---|---|
| ActivHeal® | Calcium sodium alginate | Used as a primary dressing on dry and sloughy wounds with nil to low exudate: Pressure ulcers Cavity wounds Leg ulcers Graft and donor sites Diabetic ulcers Post-op surgical wounds Lacerations and abrasions | Potential issues for patients with sensitivity to calcium alginate or other known allergic skin conditions | [22,24,107,140,141] |
| AquaDermTM | 2-Acrylamido-2-methyl-1-propanesulfonic acid sodium Propylene Glycol Poly(ethylene glycol) dimethacrylate 2-Hydroxy-2-methylpropiophenone Purified water | For the management of partial and full-thickness wounds that are dry or have minimal exudate, including: Pressure ulcers Minor burns Radiation tissue damage | Potential allergic reactions in patients with sensitivity to dressing components Propylene glycol component may cause allergic reactions in older people | [22,24,107,142,143,144] |
| MEDIHONEY | Active Leptospermum honey in combination with a hydrogel sheet dressing | Indicated for non-draining to lightly exuding wounds such as: Diabetic foot ulcers Leg ulcers (venous insufficiency ulcers Arterial ulcers Leg ulcers of mixed etiology) Pressure ulcers (partial- and full-thickness) | Slight transient stinging Increase in exudate Potential issues for patients with sensitivity to honey May raise blood glucose in diabetic patients | [22,24,107,141,145] |
| Neoheal® Hydrogel | Polyvinylopyrrolidone Polyethylene glycol Agar | Recommended for treatment of: Ulcerations Bedsores All kinds of skin damage in which a humid medium is favorable | n.r. | [22,24,107,146] |
| NU-GEL | Sodium Alginate | Indicated for the autolytic debridement of necrotic and sloughy wounds. | n.r. | [22,24,107,147] |
| Restore Hydrogel | Deionized water, glycerin USP 99.7%, sodium polyacrylate, propylene glycol USP, hyaluronic acid, sodium metabisulfite FCC, methylparaben NF, propylparaben NF | It promotes a moist environment in a variety of wounds: Stages I-IV pressure ulcers Diabetic skin ulcers Venous ulcers Skin tears Cuts Abrasions Conditions associated with peristomal care | Propylene glycol component may cause allergic reactions in older people | [22,24,107,144,148] |
| Suprasorb G | Hydrogel: water, acrylic polymers based on a taurate derivative, polyethylene, phenoxyethanol Carrier film: polyethylene White application aid: polyethylene | Indicated for the management of dry to moderately exuding chronic and acute wounds, including but not limited to: Venous leg ulcers Diabetic foot ulcers Arterial ulcers Moderate skin tears Malignant wounds and palliative care Extravasation injury | n.r. | [22,24,107,149] |
| Article Title | Treatment | Aim of the Study | Testing Stage | Results | Ref. |
|---|---|---|---|---|---|
| A bioactive composite hydrogel dressing that promotes the healing of both acute and chronic diabetic skin wounds | Carboxymethyl chitosan (CMCS) hydrogel loaded with chitosan nanoparticles, Mesenchymal Stem Cell (MSC)-derived exosomes, bioglass (BG) and TiO2 | Development of a bioactive composite hydrogel bioactive wound dressing for the treatment of acute and chronic wounds, including diabetic lesions and burns. | In vitro In vivo | Hydrogel loaded with exosomes, chitosan, BG, and TiO2 provides a sustained release of bioactive substances, stimulating healing The hydrogel promotes cell proliferation via the migration of endothelial cells and fibroblasts due to its optimal porosity Promotes angiogenesis by increasing VEGFA and VEGFR2 expression Reduces inflammation by decreasing TNF-α, IL-1β and IL-6, and increasing IL-10 In vivo tests demonstrated accelerated healing in rats In diabetic wounds, the hydrogel promoted granulation formation and collagen synthesis Showed significant antimicrobial activity against E. coli and S. aureus strains The hydrogel prevented complications associated with burns and promoted epidermal regeneration | [163] |
| Piezoelectric hydrogel for prophylaxis and early treatment of pressure injuries/pressure ulcers | Electroactive hydrogel of polyacrylonitrile-acrylamide-styrene sulfate-poly (vinylidene fluoride) (PAAN-PVDF) | Obtaining a piezoelectric hydrogel for the prevention and early treatment of PrU | In vitro Ex vivo | Hydrogel promotes L929 cell proliferation The hydrogel is biocompatible and also shows high blood compatibility In vitro tests demonstrate that the hydrogel stimulated angiogenesis via piezoelectric stimulation Fresh pig skin was used to simulate the effect of the hydrogel on pressure distribution in bony protrusive areas. Results showed that the hydrogel reduced the pressure applied to these areas | [164] |
| Immunomodulatory gallium/glycyrrhizic acid hydrogels for treating multidrug-resistant Pseudomonas aeruginosa-infected pressure ulcers | Gallium and glycyrrhizic acid (Ga/GA)-based immunomodulatory hydrogel (Ga/GA) | Development of (Ga/GA)-based immunomodulatory hydrogel for the treatment of pressure ulcers infected with antibiotic-resistant P. aeruginosa (MRPA) | In vitro In vivo | The Ga/GA hydrogel has demonstrated a water-holding capacity that can facilitate the maintenance of a humid environment The hydrogel has a great antimicrobial effect, preventing bacterial biofilm formation and eliminating pre-existing biofilms Stimulates fibroblast and macrophage proliferation Facilitates the transition of macrophages from M1 to M2 phenotype, thereby reducing inflammation and accelerating tissue regeneration Neutralizes reactive oxygen species (ROS), protecting cells against oxidative stress In MRPA-infected PrU models, the hydrogel promoted healing by reducing inflammation and wound contraction, facilitating the synthesis of collagen and angiogenesis | [165] |
| Asymmetric natural wound dressing based on porous chitosan-alginate hydrogel/electrospun PCL-silk sericin loaded by 10-HDA for skin wound healing: In vitro and in vivo studies | Asimetric hydrogel dressing based on chitosan-alginate (CS-Alg) with PCL-silk sericin (PCL-SS) membrane loaded with 10-hydroxy-2-decenoic acid (10-HAD) | The development of an asymmetric natural wound dressing based on a porous CS-Alg hydrogel and an electrospun PCL-SS membrane loaded with 10-HAD with enhanced antimicrobial, anti-inflammatory, wound healing, and regenerative properties | In vitro In vivo | CS-Alg-based hydrogel demonstrated excellent biocompatibility The hydrogel promoted fibroblast proliferation and metabolic cellular activity In Wistar rats, the dressing significantly accelerated wound healing by preventing the formation of granulation tissue 10-HDA is released gradually, followed by a sustained release The hydrogel has great antimicrobial efficiency against S. aureus and E. coli strains | [166] |
| Hyaluronic acid/alginate-based biomimetic hydrogel membranes for accelerated diabetic wound repair | Biomimetic hyaluronic acid (HA) and alginate (Alg), Polyvinyl alcohol (PVA)-based hydrogel loaded with cefotaxime (CTX) | Development of a biomimetic HA-Alg-PVA hydrogel biomimetic membrane to accelerate diabetic wound healing through the controlled release of CTX. | In vitro In vivo | The hydrogel allows oxygen to pass through and regulates humidity Prevents the build-up of exudate It has a great antimicrobial effect on the S. aureus and P. aeruginousa strains because of the CTX It does not have a toxic effect on fibroblasts The in vitro cytotoxicity assay demonstrates a cell viability of over 80% In a diabetic rat model, the membrane significantly accelerated the healing process The group treated with CTX-loaded membrane showed complete epidermal and dermal formation without signs of necrosis | [167] |
| A platelet-derived hydrogel improves neovascularization in full thickness wounds. | Paletels, fibrin, and thrombin-based hydrogel | Development of a hydrogel derived from platelets and fibrin using expired platelets to stimulate wound repair. | In vivo | The hydrogel promotes angiogenesis and collagen synthesis The healing was accelerated by collagen fibers deposition, with a faster re-epithelization | [168] |
| Lutein-loaded multifunctional hydrogel dressing based on carboxymethyl chitosan for chronic wound healing | Hydrogel based on carboxymethylated chitosan (CMC), polyvinylpyrrolidone (PVP) loaded with lutein and tannic acid (TA) | Development of a multifunctional lutein/CMC/PVP/TA-based hydrogel wound dressing for chronic wounds, especially diabetic wounds. | In vitro In vivo | Hydrogels with moderate lutein concentration (5 mg/mL) supported cell proliferation The hydrogel has demonstrated effective removal ROS The hydrogel prevented the development of biofilm biofilms and showed a good antimicrobial effect Wound sizes were significantly reduced in the group treated with 5 mg/mL lutein concentration, with faster wound closure and formation of well-organized epithelial tissue. Hydrogels with moderate lutein concentration (5 mg/mL) promoted collagen synthesis and its uniform deposition Hydrogels with moderate lutein concentration (5 mg/mL) promoted angiogenesis Hydrogels with moderate lutein concentration (5 mg/mL) reduced the inflammation | [169] |
| Gelatin/dopamine/zinc-doped ceria/curcumin nanocomposite hydrogels for repair of chronic refractory wounds | Methylacrylated gelatin modified with dopamine (GelMD) with zinc-doped hollow mesoporous cerium oxide nanoparticles loaded with curcumin-based nanocomposite hydrogels (gelmd-Cur@zhmce) | Development of biocompatible multifunctional nanocomposite (GelMD-Cur@ZHMCe) for chronic wounds treatment | In vitro In vivo | Curcumin is effectively released in an acidic environment such as infected wounds The hydrogel supported free radical scavenging GelMD-Cur@ZHMCe significantly inhibited the growth of bacteria such as E. coli and S. aureus, preventing biofilm formation The hydrogel stimulated endothelial cell migration and angiogenesis The healing time was 14 days GelMD-Cur@ZHMCe con led to epithelial regeneration, increased collagen deposits, and reduced inflammation | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberts, A.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. J. Compos. Sci. 2025, 9, 133. https://doi.org/10.3390/jcs9030133
Alberts A, Moldoveanu E-T, Niculescu A-G, Grumezescu AM. Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. Journal of Composites Science. 2025; 9(3):133. https://doi.org/10.3390/jcs9030133
Chicago/Turabian StyleAlberts, Adina, Elena-Theodora Moldoveanu, Adelina-Gabriela Niculescu, and Alexandru Mihai Grumezescu. 2025. "Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care" Journal of Composites Science 9, no. 3: 133. https://doi.org/10.3390/jcs9030133
APA StyleAlberts, A., Moldoveanu, E.-T., Niculescu, A.-G., & Grumezescu, A. M. (2025). Hydrogels for Wound Dressings: Applications in Burn Treatment and Chronic Wound Care. Journal of Composites Science, 9(3), 133. https://doi.org/10.3390/jcs9030133










