Photocatalytic Degradation of Acetaminophen by g-C3N4/CQD/Ag Nanocomposites from Aqueous Media
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Materials
2.2. Fabrication of Nanocomposite
2.2.1. Synthesis of g-C3N4
2.2.2. Synthesis of Ag/g-C3N4 Nanocomposites
2.2.3. Synthesis of CQDs
2.2.4. Synthesis of g-C3N4/CQD
2.2.5. Synthesis of g-C3N4/CQDs/Ag
2.3. Characterization
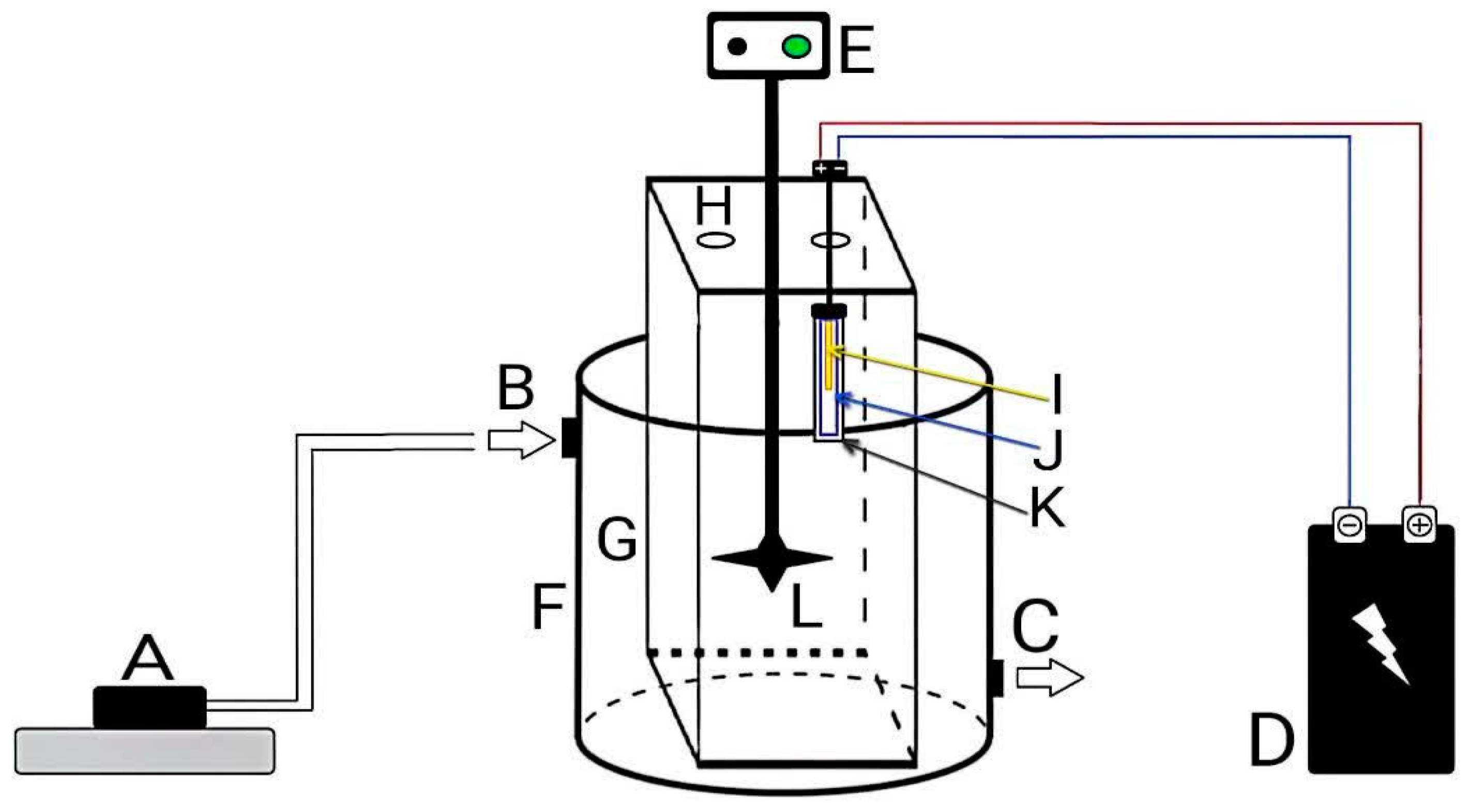
2.4. Photocatalytic Effectiveness Experiments
2.5. Scavenger-Quenching Experiments
2.6. Statistical Analysis
3. Results and Discussion
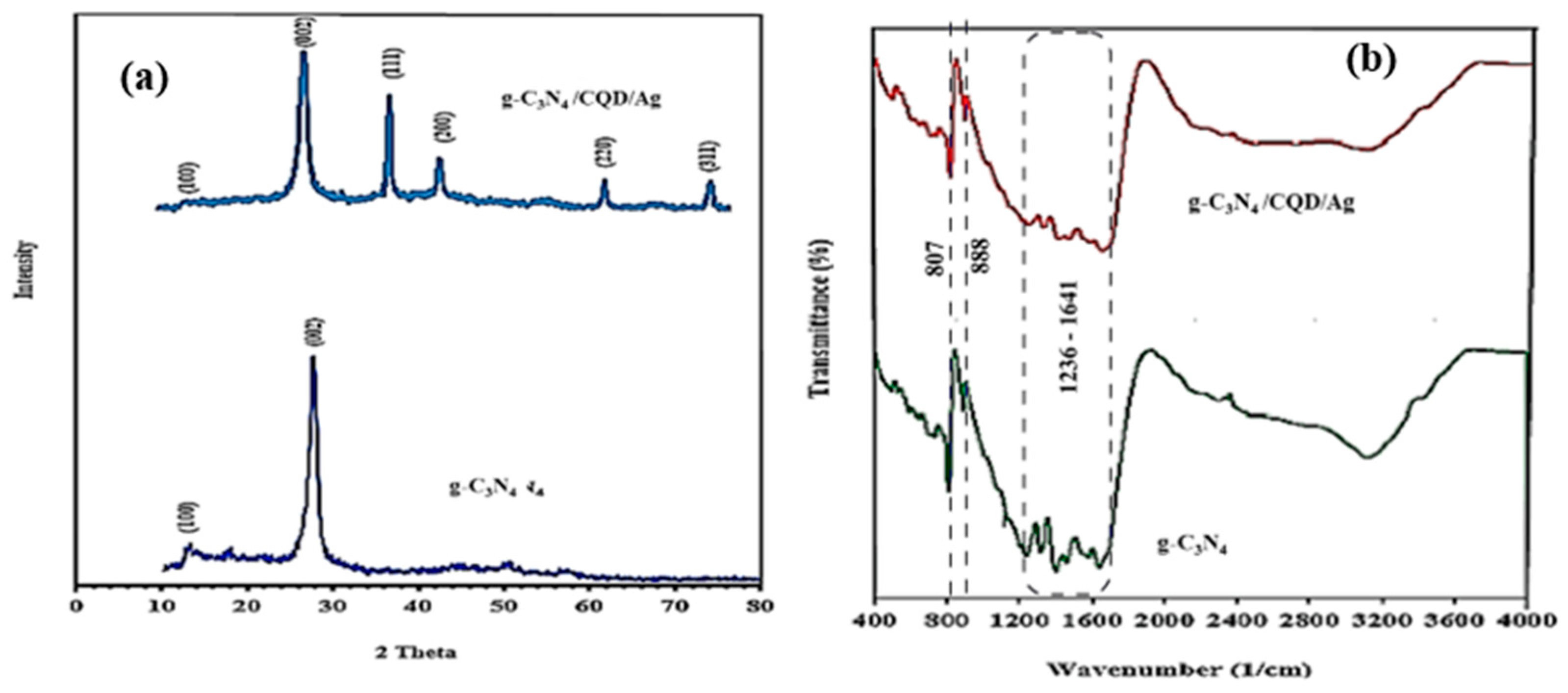
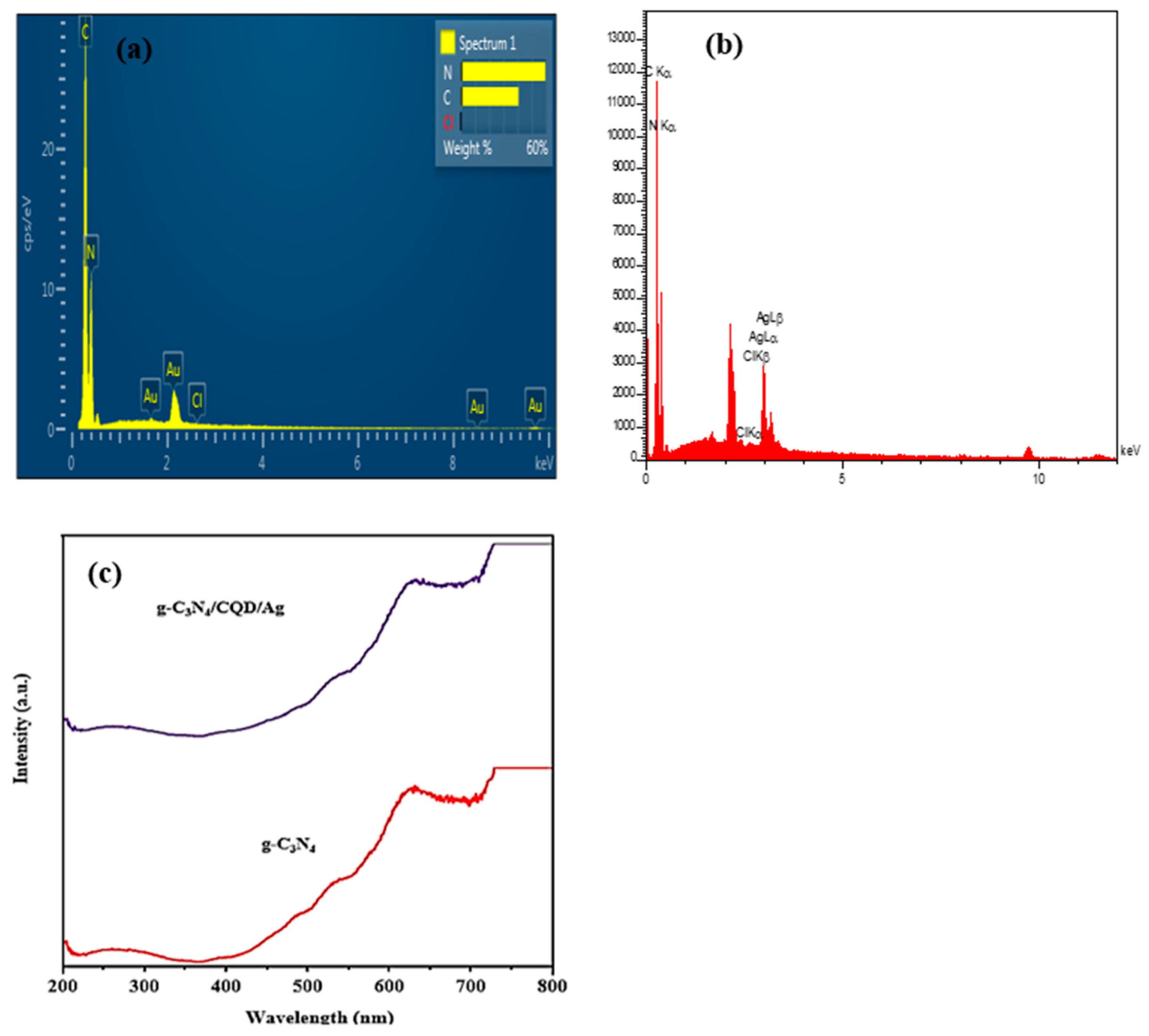
3.1. Structure and Composition Photocatalysts
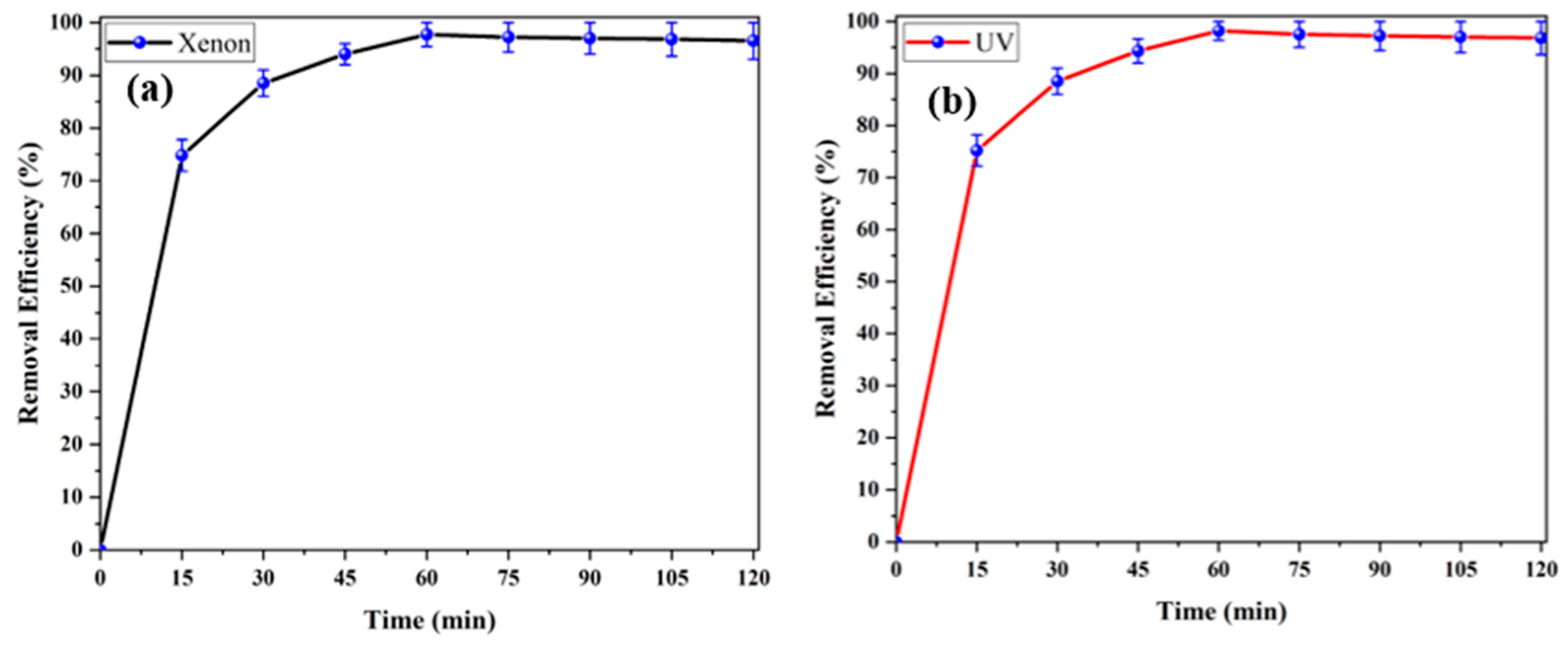
3.2. Photocatalytic Decomposition of ACT Under Ultraviolet and XenonXenon-Light Illumination
3.2.1. Impact of Media pH
3.2.2. Impact of Oxidation Time
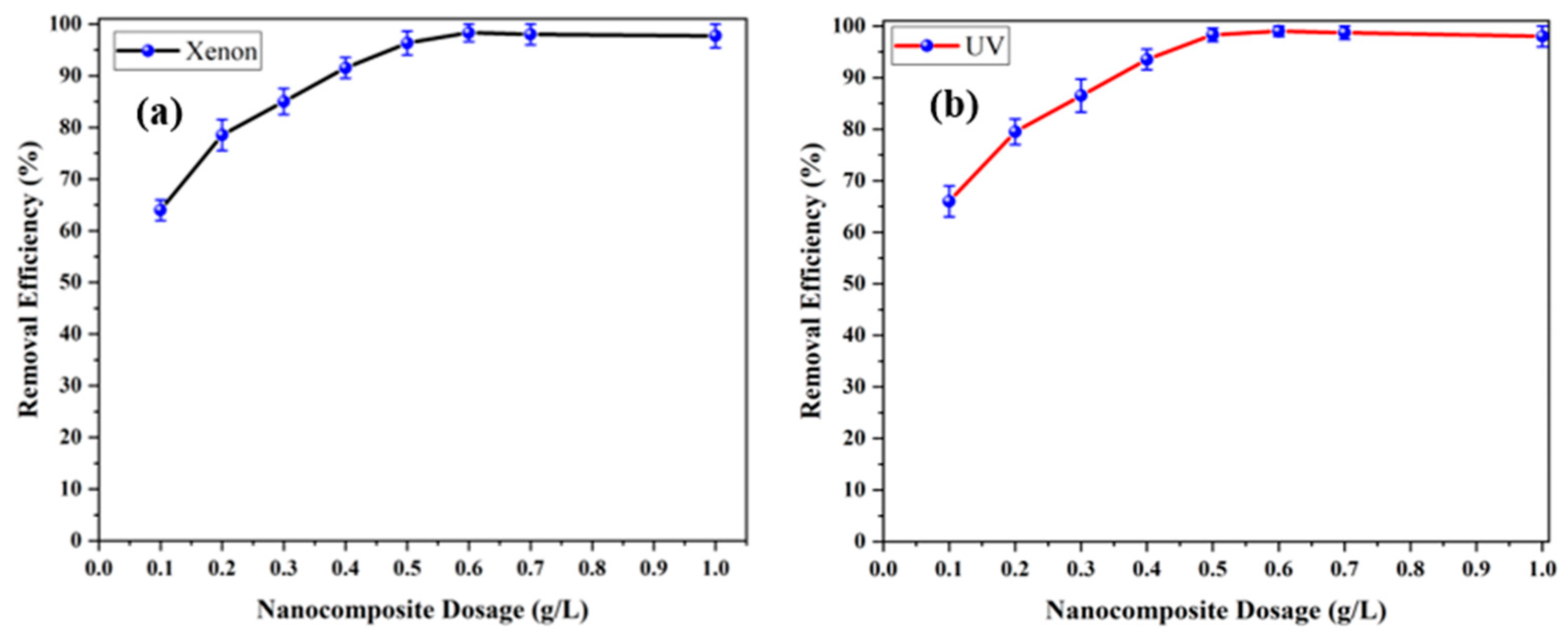
3.2.3. Impact of Photocatalyst Content
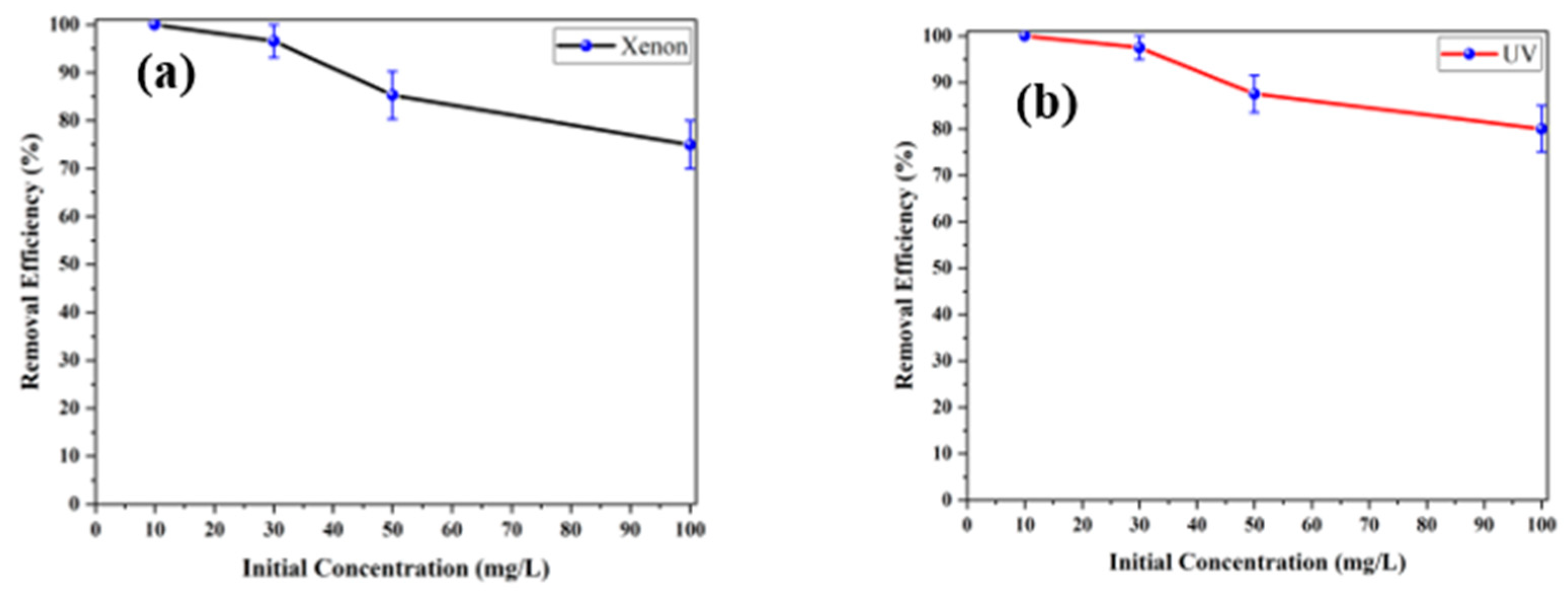
3.2.4. Impact of Initial Acetaminophen Content
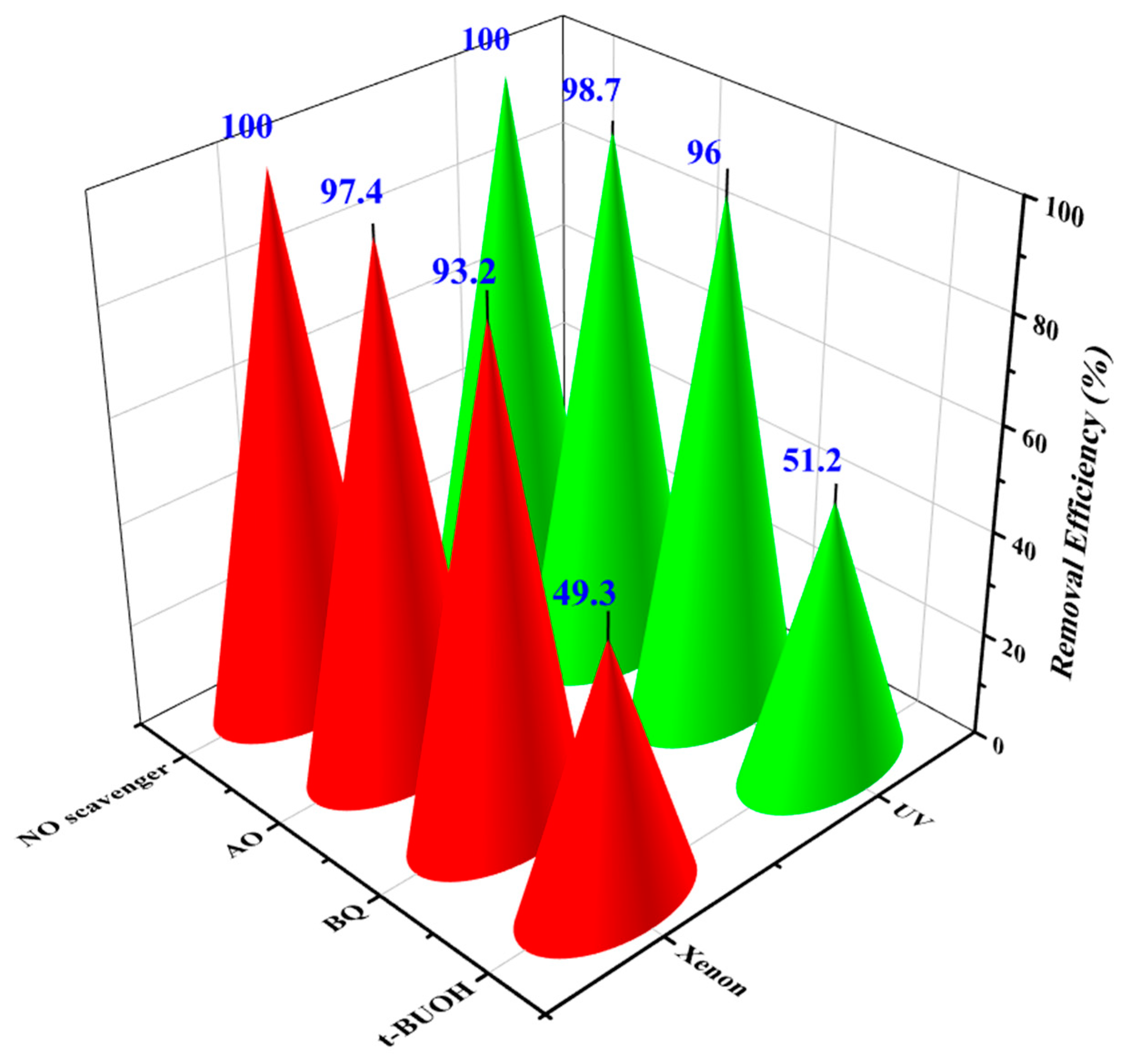
3.2.5. Trapping Experiments
3.2.6. Photocatalytic Decomposition Rate of Previously Published Research on g-C3N4 and Its Materials in Comparison with the Current g-C3N4/CQD/Ag Material
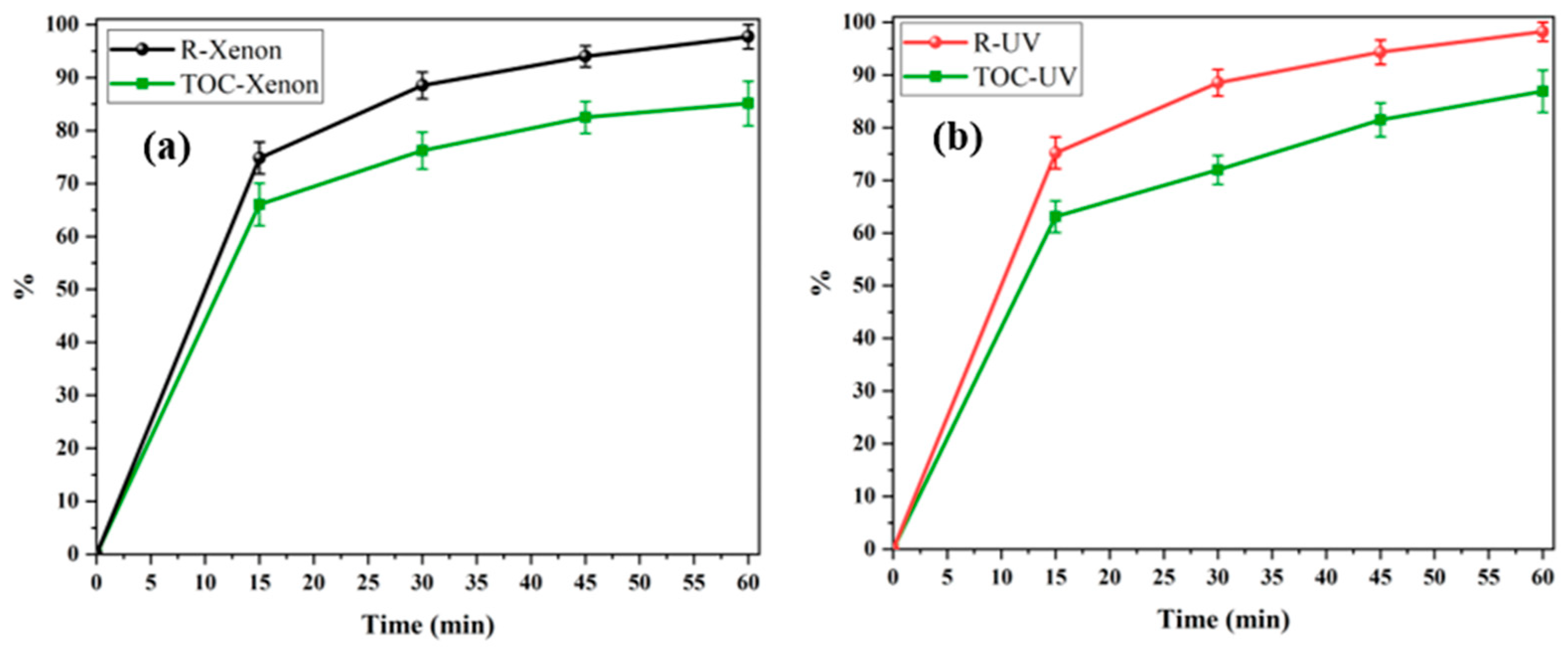
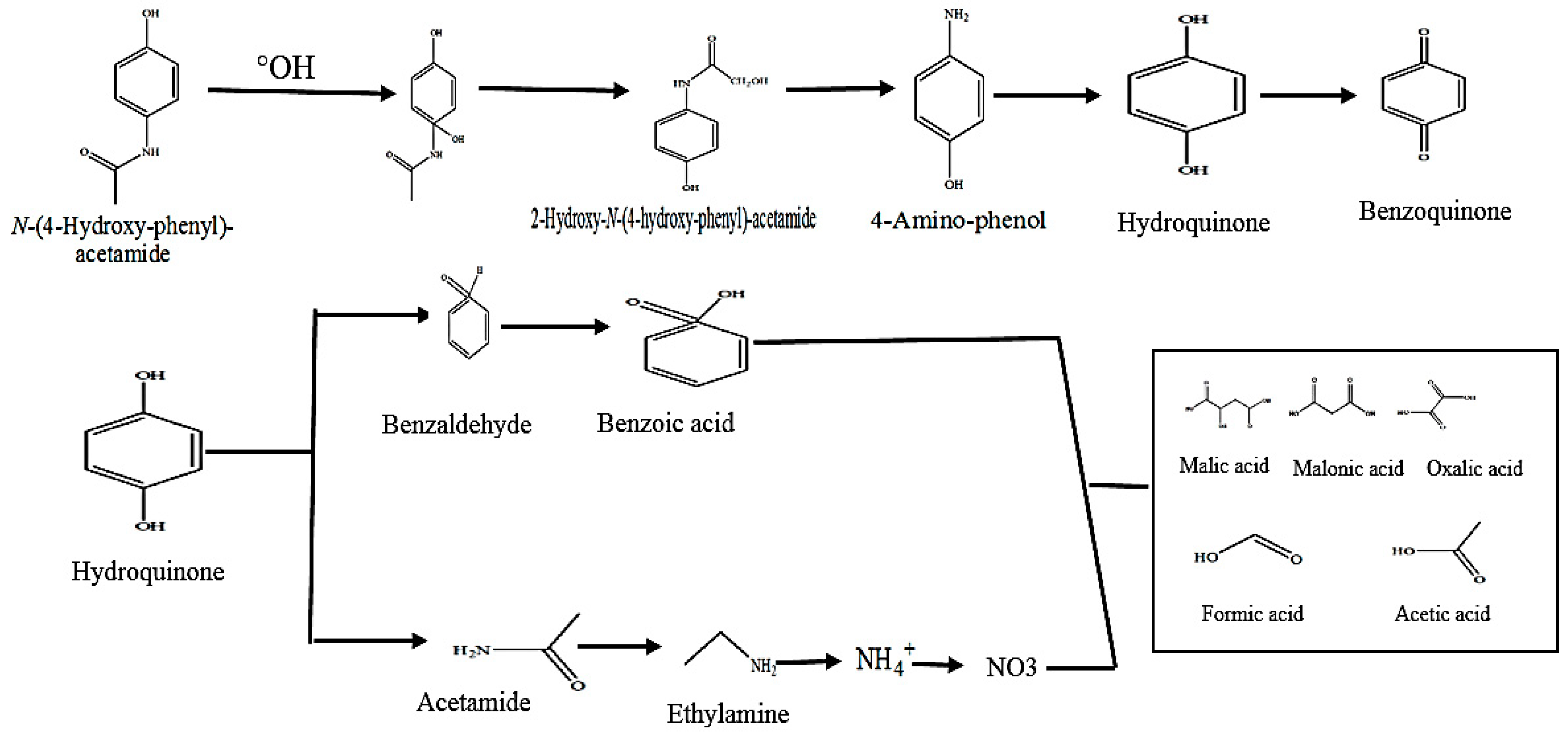
3.2.7. Determination of By-Products and Mineralization
3.2.8. Acetaminophen Degradation Pathway
3.3. Effect of Dark Conditions on ACT Adsorption by g-C₃N₄/CQD/Ag
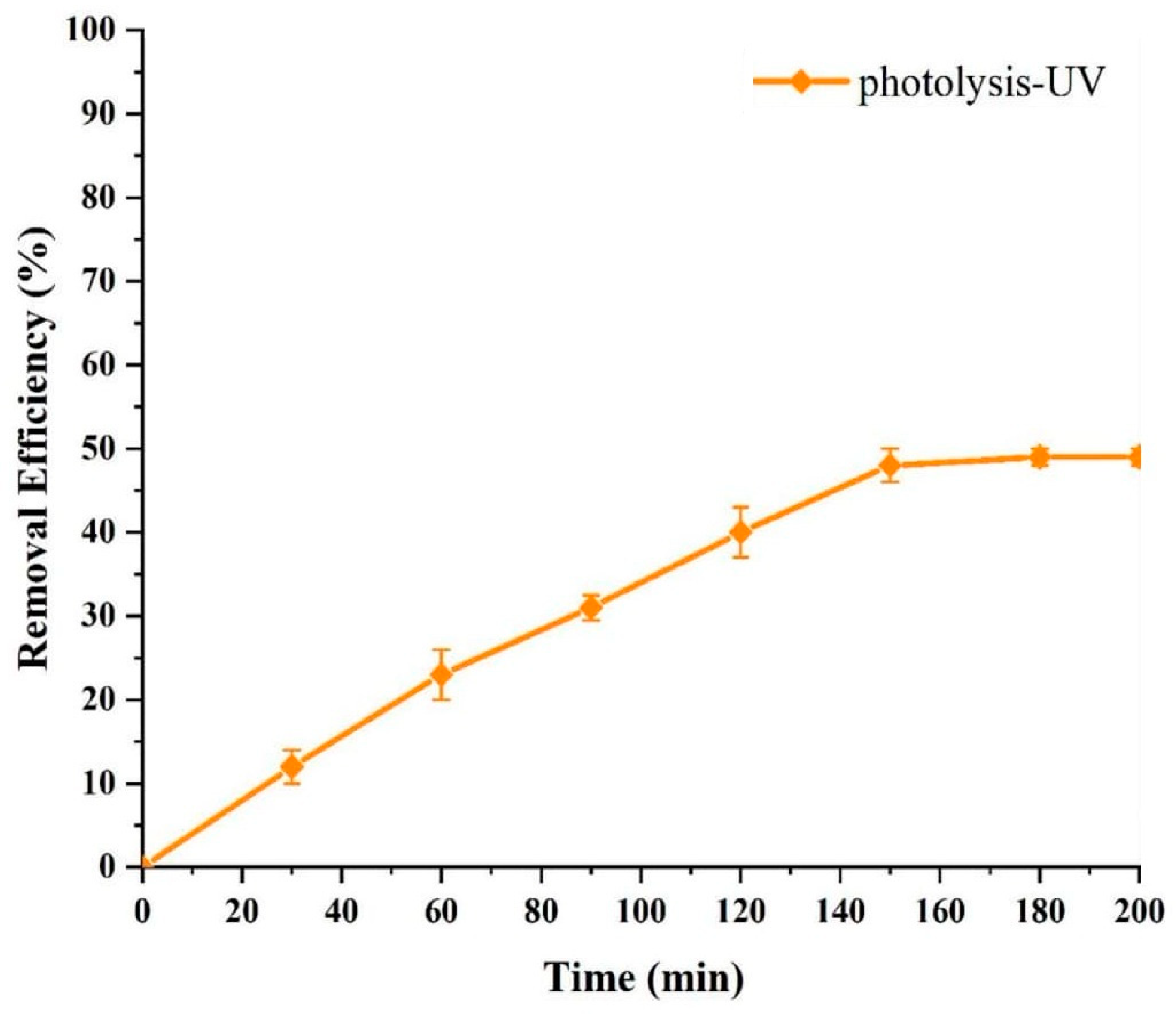
3.4. The Effect of UV Radiation on Photolysis of ACT
3.5. Comparison of the Findings of This Study with Those of Other Studies
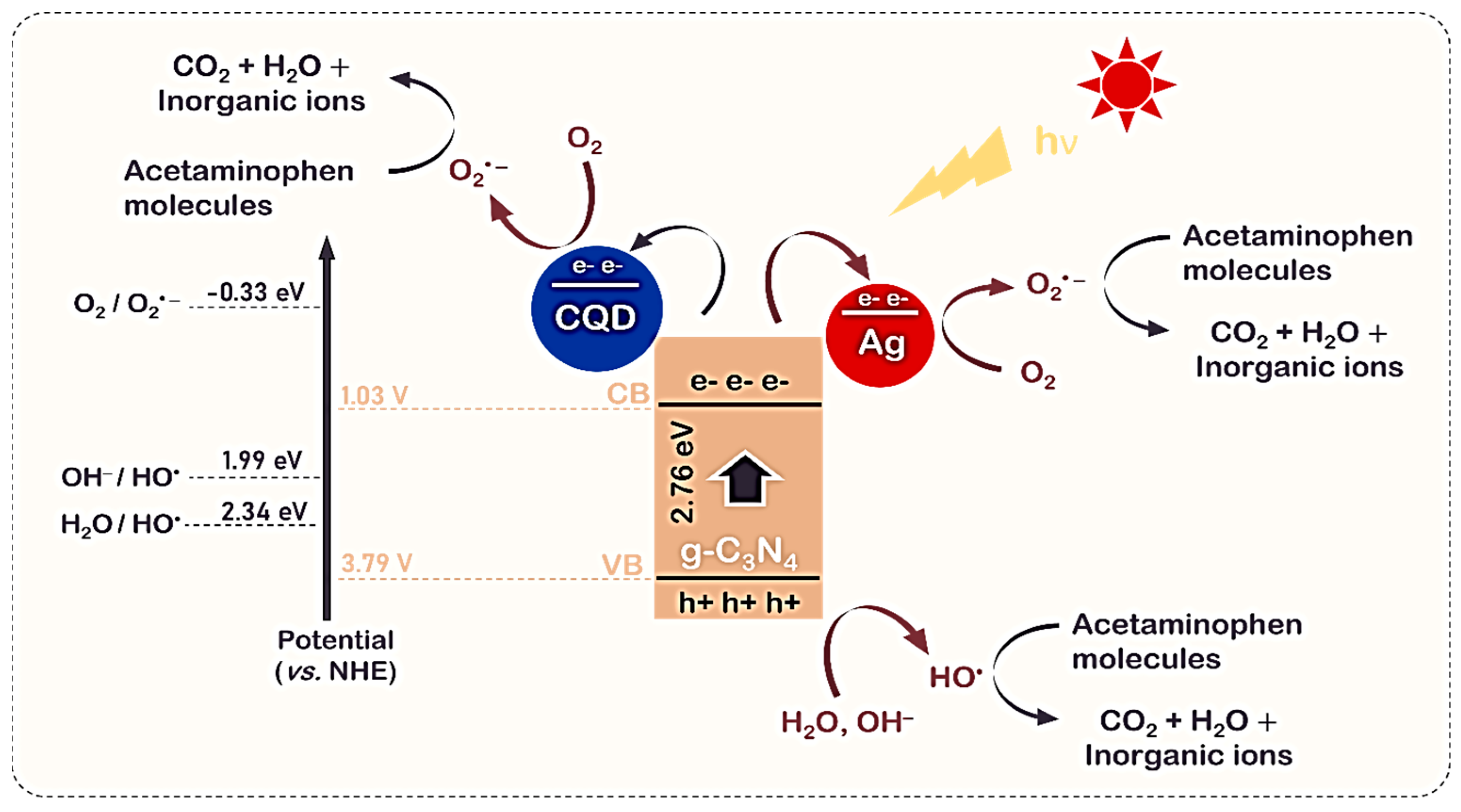
3.6. Mechanism Elucidation of g-C3N4/CQD/Ag Photocatalyst
3.6.1. Band Structure Analysis of g-C3N4
3.6.2. Charge Transfer Mechanism
- CQDs act as electron acceptors due to their lower CB potential. Electrons transfer from C3N4’s CB to CQDs’ CB, thermodynamically favoring O2 reduction [97].
3.6.3. Redox Reactions and Radical Formation
3.7. Comparison of the Photocatalytic Performance of g-C3N4, CQD, Ag, and Their Composites, in the Photocatalytic Removal Efficiency of Acetaminophen Under UV Light
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.E.; Han, T.H.; Khan, M.M.; Karim, M.R.; Cho, M.H. Environmentally sustainable fabrication of Ag@ g-C3N4 nanostructures and their multifunctional efficacy as antibacterial agents and photocatalysts. ACS Appl. Nano Mater. 2018, 1, 2912–2922. [Google Scholar] [CrossRef]
- Mafa, P.J.; Malefane, M.E.; Idris, A.O.; Liu, D.; Gui, J.; Mamba, B.B.; Kuvarega, A.T. Multi-elemental doped g-C3N4 with enhanced visible light photocatalytic activity: Insight into naproxen degradation, kinetics, effect of electrolytes, and mechanism. Sep. Purif. Technol. 2022, 282, 120089. [Google Scholar] [CrossRef]
- Tri, N.L.M.; Kim, J.; Giang, B.L.; Al Tahtamouni, T.; Huong, P.T.; Lee, C.; Viet, N.M. Ag-doped graphitic carbon nitride photocatalyst with remarkably enhanced photocatalytic activity towards antibiotic in hospital wastewater under solar light. J. Ind. Eng. Chem. 2019, 80, 597–605. [Google Scholar] [CrossRef]
- Wang, X.; Tan, F.; Wang, W.; Qiao, X.; Qiu, X.; Chen, J. Anchoring of silver nanoparticles on graphitic carbon nitride sheets for the synergistic catalytic reduction of 4-nitrophenol. Chemosphere 2017, 172, 147–154. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Yao, K.; Lv, W. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Dadigala, R.; Bandi, R.; Gangapuram, B.R.; Guttena, V. Carbon dots and Ag nanoparticles decorated g-C3N4 nanosheets for enhanced organic pollutants degradation under sunlight irradiation. J. Photochem. Photobiol. A Chem. 2017, 342, 42–52. [Google Scholar] [CrossRef]
- González-González, R.B.; Sharma, A.; Parra-Saldívar, R.; Ramirez-Mendoza, R.A.; Bilal, M.; Iqbal, H.M. Decontamination of emerging pharmaceutical pollutants using carbon-dots as robust materials. J. Hazard. Mater. 2022, 423, 127145. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.; Brown, R.; Hashib, M. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef]
- Azizi-Toupkanloo, H.; Karimi-Nazarabad, M.; Shakeri, M.; Eftekhari, M. Photocatalytic mineralization of hard-degradable morphine by visible light-driven Ag@ gC 3 N 4 nanostructures. Environ. Sci. Pollut. Res. 2019, 26, 30941–30953. [Google Scholar] [CrossRef]
- Thang, N.Q.; Sabbah, A.; Chen, L.-C.; Chen, K.-H.; Thi, C.M.; Van Viet, P. High-efficient photocatalytic degradation of commercial drugs for pharmaceutical wastewater treatment prospects: A case study of Ag/g-C3N4/ZnO nanocomposite materials. Chemosphere 2021, 282, 130971. [Google Scholar] [CrossRef]
- Liu, Z.-y.; Zhang, M.-y.; Wu, J.-l. Enhanced Visible-Light Photocatalytic and Antibacterial Activities of Ag-Doped g-C3N4 Nanocomposites. ChemistrySelect 2018, 3, 10630–10636. [Google Scholar] [CrossRef]
- Shamilov, R.R.; Muzipov, Z.M.; Sagdeev, D.O.; Kholin, K.V.; Saifina, A.F.; Gubaidullin, A.T.; Galyametdinov, Y.G. Photocatalytic materials based on g-C3N4 obtained by the one-Pot calcination method. C 2023, 9, 85. [Google Scholar] [CrossRef]
- Chebanenko, M.; Omarov, S.O.; Dmitriev, D.; Martinson, K.; Tomkovich, M.; Popkov, V. Visible Light–Induced Photocatalytic Degradation of Tetracycline over Exfoliated Graphitic C3N4 Doped with Cubic Co3O4. Kinet. Catal. 2024, 65, 548–555. [Google Scholar] [CrossRef]
- Hong, Y.; Meng, Y.; Zhang, G.; Yin, B.; Zhao, Y.; Shi, W.; Li, C. Facile fabrication of stable metal-free CQDs/g-C3N4 heterojunctions with efficiently enhanced visible-light photocatalytic activity. Sep. Purif. Technol. 2016, 171, 229–237. [Google Scholar] [CrossRef]
- Faisal, M.; Ismail, A.A.; Harraz, F.A.; Al-Sayari, S.; El-Toni, A.M.; Al-Assiri, M. Synthesis of highly dispersed silver doped g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity. Mater. Des. 2016, 98, 223–230. [Google Scholar] [CrossRef]
- Ling, Y.; Liao, G.; Xu, P.; Li, L. Fast mineralization of acetaminophen by highly dispersed Ag-g-C3N4 hybrid assisted photocatalytic ozonation. Sep. Purif. Technol. 2019, 216, 1–8. [Google Scholar] [CrossRef]
- Song, Y.; Qi, J.; Tian, J.; Gao, S.; Cui, F. Construction of Ag/g-C3N4 photocatalysts with visible-light photocatalytic activity for sulfamethoxazole degradation. Chem. Eng. J. 2018, 341, 547–555. [Google Scholar] [CrossRef]
- Patial, S.; Sudhaik, A.; Chandel, N.; Ahamad, T.; Raizada, P.; Singh, P.; Chaukura, N.; Selvasembian, R. A review on carbon quantum dots modified g-C3N4-based photocatalysts and potential application in wastewater treatment. Appl. Sci. 2022, 12, 11286. [Google Scholar] [CrossRef]
- Wu, K.; Chen, D.; Fang, J.; Wu, S.; Yang, F.; Zhu, X.; Fang, Z. One-step synthesis of sulfur and tungstate co-doped porous g-C3N4 microrods with remarkably enhanced visible-light photocatalytic performances. Appl. Surf. Sci. 2018, 462, 991–1001. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Liu, F.; Jiang, W.; Zhang, D.; Liang, J. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 2019, 151, 8–19. [Google Scholar] [CrossRef]
- Gupta, S.; Gandhi, J.; Kokate, S.; Raikar, L.G.; Kopuri, V.G.; Prakash, H. Augmented photocatalytic degradation of Acetaminophen using hydrothermally treated g-C3N4 and persulfate under LED irradiation. Heliyon 2023, 9, e16450. [Google Scholar] [CrossRef]
- Lyth, S.M.; Nabae, Y.; Moriya, S.; Kuroki, S.; Kakimoto, M.-a.; Ozaki, J.-i.; Miyata, S. Carbon nitride as a nonprecious catalyst for electrochemical oxygen reduction. J. Phys. Chem. C 2009, 113, 20148–20151. [Google Scholar] [CrossRef]
- Di, T.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. A direct Z-scheme g-C3N4/SnS2 photocatalyst with superior visible-light CO2 reduction performance. J. Catal. 2017, 352, 532–541. [Google Scholar] [CrossRef]
- Yan, J.; Rodrigues, M.T.F.; Song, Z.; Li, H.; Xu, H.; Liu, H.; Wu, J.; Xu, Y.; Song, Y.; Liu, Y. Reversible formation of g-C3N4 3D hydrogels through ionic liquid activation: Gelation behavior and room-temperature gas-sensing properties. Adv. Funct. Mater. 2017, 27, 1700653. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Feng, Y.; Zeng, Y.; Xie, Z.; Zhang, Q.; Su, Y.; Chen, P.; Liu, Y.; Yao, K. Novel ternary photocatalyst of single atom-dispersed silver and carbon quantum dots co-loaded with ultrathin g-C3N4 for broad spectrum photocatalytic degradation of naproxen. Appl. Catal. B Environ. 2018, 221, 510–520. [Google Scholar] [CrossRef]
- Hao, P.; Wang, G.; Wen, J.; Li, X.; Suo, Y.; Zhan, H.; Bi, S.; Liu, W. Efficient photocatalytic-fenton oxidation performance of Fe3+/g-C3N4/NCDs nanorods: Structure-activity relationship of photocatalytic degradation of water pollutants. J. Environ. Chem. Eng. 2022, 10, 107728. [Google Scholar] [CrossRef]
- Azqandi, M.; Nateq, K.; Golrizkhatami, F.; Nasseh, N.; Seyedi, N.; Moghaddam, N.S.M.; Fanaei, F. Innovative RGO-bridged S-scheme CuFe2O4@ Ag2S heterojunction for efficient Sun-light-driven photocatalytic disintegration of Ciprofloxacin. Carbon 2025, 231, 119725. [Google Scholar] [CrossRef]
- Munoz-Batista, M.J.; Fontelles-Carceller, O.; Ferrer, M.; Fernández-García, M.; Kubacka, A. Disinfection capability of Ag/g-C3N4 composite photocatalysts under UV and visible light illumination. Appl. Catal. B Environ. 2016, 183, 86–95. [Google Scholar] [CrossRef]
- Xu, W.; Lai, S.; Pillai, S.C.; Chu, W.; Hu, Y.; Jiang, X.; Fu, M.; Wu, X.; Li, F.; Wang, H. Visible light photocatalytic degradation of tetracycline with porous Ag/graphite carbon nitride plasmonic composite: Degradation pathways and mechanism. J. Colloid Interface Sci. 2020, 574, 110–121. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Yu, C.; Wang, P.; Wang, X.; Chen, F.; Yu, H. Silver-melamine nanowire-assisted synthesis of net-like AgCl-Ag/g-C3N4 for highly efficient photocatalytic degradation ability. J. Alloys Compd. 2019, 806, 263–271. [Google Scholar] [CrossRef]
- Noroozi, R.; Gholami, M.; Farzadkia, M.; Jonidi Jafari, A. Degradation of ciprofloxacin by CuFe2O4/GO activated PMS process in aqueous solution: Performance, mechanism and degradation pathway. Int. J. Environ. Anal. Chem. 2022, 102, 174–195. [Google Scholar] [CrossRef]
- Cheng, Q.; He, Y.; Ge, Y.; Zhou, J.; Song, G. Ultrasensitive detection of heparin by exploiting the silver nanoparticle-enhanced fluorescence of graphitic carbon nitride (gC3N4) quantum dots. Mikrochim. Acta 2018, 185, 332. [Google Scholar] [CrossRef]
- Cako, E.; Dudziak, S.; Głuchowski, P.; Trykowski, G.; Pisarek, M.; Borzyszkowska, A.F.; Sikora, K.; Zielińska-Jurek, A. Heterojunction of (P, S) co-doped g-C3N4 and 2D TiO2 for improved carbamazepine and acetaminophen photocatalytic degradation. Sep. Purif. Technol. 2023, 311, 123320. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Shi, J.; Deng, H. Fabrication of novel visible-light-driven AgI/g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J. Colloid Interface Sci. 2017, 496, 167–176. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Jin, X.; Zheng, X.; Wang, Y.; Wei, D.; Zhang, Q.; Feng, Y.; Xie, Z.; Chen, P. Highly active metal-free carbon dots/g-C3N4 hollow porous nanospheres for solar-light-driven PPCPs remediation: Mechanism insights, kinetics and effects of natural water matrices. Water Res. 2020, 172, 115492. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Xu, Y.; Feng, Z.; Huang, J. Defect-assisted surface modification enhances the visible light photocatalytic performance of g-C3N4@ C-TiO2 direct Z-scheme heterojunctions. Chin. J. Catal. 2019, 40, 424–433. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Pal, D.; Mishra, J.; Thakur, C. Noble metal–free doped graphitic carbon nitride (g-C3N4) for efficient photodegradation of antibiotics: Progress, limitations, and future directions. Environ. Sci. Pollut. Res. 2023, 30, 25546–25558. [Google Scholar] [CrossRef]
- Gotostos, M.J.N.; Su, C.C.; De Luna, M.D.G.; Lu, M.c. Kinetic study of acetaminophen degradation by visible light photocatalysis. J. Environ. Sci. Health Part A 2014, 49, 892–899. [Google Scholar] [CrossRef]
- Huang, W.; Huang, Y.; Yang, L.; Li, J.; Liu, C.; Li, N.; Lai, B. Novel ternary catalyst Ag2O/NCDs@ porous tubular g-C3N4 as efficient visible-light-driven peroxymonosulfate activator. Chem. Eng. J. 2023, 465, 142911. [Google Scholar] [CrossRef]
- Li, C.; Sun, T.; Yi, G.; Zhang, D.; Zhang, Y.; Lin, X.; Liu, J.; Shi, Z.; Lin, Q. Microwave-assisted method synthesis of Ag/CNQDs/g-C3N4 with excellent photocatalytic activity for the degradation of norfloxacin. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 131001. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, Z.; Zeng, H.; Tang, R.; Li, L.; Wang, J.; Feng, C.; Gong, D.; Tang, L.; Huang, Y. Phosphorus and kalium co-doped g-C3N4 with multiple-locus synergies to degrade atrazine: Insights into the depth analysis of the generation and role of singlet oxygen. Appl. Catal. B Environ. 2023, 320, 121942. [Google Scholar] [CrossRef]
- Zhang, W.; Bian, Z.; Xin, X.; Wang, L.; Geng, X.; Wang, H. Comparison of visible light driven H2O2 and peroxymonosulfate degradation of norfloxacin using Co/g-C3N4. Chemosphere 2021, 262, 127955. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhou, H.; Xiong, Z.; Liu, Y.; Yao, G.; Lai, B. Heterogeneous activation of peroxymonosulfate by CoMgFe-LDO for degradation of carbamazepine: Efficiency, mechanism and degradation pathways. Chem. Eng. J. 2020, 391, 123604. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A review. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Humayun, M.; Fu, Q.; Zheng, Z.; Li, H.; Luo, W. Improved visible-light catalytic activities of novel Au/P-doped g-C3N4 photocatalyst for solar fuel production and mechanism. Appl. Catal. A Gen. 2018, 568, 139–147. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Liu, C.; Huo, P.; Wang, H. Construction of 3D porous g-C3N4/AgBr/rGO composite for excellent visible light photocatalytic activity. Appl. Surf. Sci. 2018, 458, 586–596. [Google Scholar] [CrossRef]
- Wei, F.; Li, J.; Dong, C.; Bi, Y.; Han, X. Plasmonic Ag decorated graphitic carbon nitride sheets with enhanced visible-light response for photocatalytic water disinfection and organic pollutant removal. Chemosphere 2020, 242, 125201. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, X.; Shi, H.; Wang, Y.; Ren, Y.; Liu, E.; Zhang, X.; Fan, J.; Hu, X. Photocatalytic activity enhanced by synergistic effects of nano-silver and ZnSe quantum dots co-loaded with bulk g-C3N4 for Ceftriaxone sodium degradation in aquatic environment. Chem. Eng. J. 2018, 353, 56–68. [Google Scholar] [CrossRef]
- He, Q.; Zhou, F.; Zhan, S.; Yang, Y.; Liu, Y.; Tian, Y.; Huang, N. Enhancement of photocatalytic and photoelectrocatalytic activity of Ag modified Mpg-C3N4 composites. Appl. Surf. Sci. 2017, 391, 423–431. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Q.; Li, Y.; Li, Y.; Gou, J.; Cheng, X. Degradation of sulfamethoxazole by peroxymonosulfate activated by waste eggshell supported Ag2O-Ag nano–particles. Chem. Eng. J. 2021, 405, 126719. [Google Scholar] [CrossRef]
- Feng, C.; Deng, Y.; Tang, L.; Zeng, G.; Wang, J.; Yu, J.; Liu, Y.; Peng, B.; Feng, H.; Wang, J. Core-shell Ag2CrO4/N-GQDs@ g-C3N4 composites with anti-photocorrosion performance for enhanced full-spectrum-light photocatalytic activities. Appl. Catal. B Environ. 2018, 239, 525–536. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Lu, J.; Guo, Z.; Zhang, M.; Li, D.; Zhao, Z.; Song, S.; Liu, Y.; Qin, L. A CQD/CdS/g-C3N4 photocatalyst for dye and antibiotic degradation: Dual carrier driving force and tunable electron transfer pathway. Sep. Purif. Technol. 2023, 305, 122333. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Deng, F.; Li, X.; Luo, X.; Peng, Y.; Zhang, J.; Zou, J.; Liu, X.; Ding, L. Ag Quantum Dots Decorated Ultrathin GC3N4 Nanosheets for Boosting Degradation of Pharmaceutical Contaminants and Antibiosis: Insight from Interfacial Electric Field Induced by Local Surface Plasma Resonance. Chem. Eng. J. 2023, 463, 142313. [Google Scholar] [CrossRef]
- Li, C.; Sun, T.; Yi, G.; Zhang, D.; Zhang, Y.; Lin, X.; Liu, J.; Shi, Z.; Lin, Q. In-depth insight into the Ag/CNQDs/g-C3N4 photocatalytic degradation of typical antibiotics: Influence factor, mechanism and toxicity evaluation of intermediates. Molecules 2023, 28, 1597. [Google Scholar] [CrossRef]
- Lv, S.; Ng, Y.H.; Zhu, R.; Li, S.; Wu, C.; Liu, Y.; Zhang, Y.; Jing, L.; Deng, J.; Dai, H. Phosphorus vapor assisted preparation of P-doped ultrathin hollow g-C3N4 sphere for efficient solar-to-hydrogen conversion. Appl. Catal. B Environ. 2021, 297, 120438. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Rong, C.; Li, A.; Hua, X.; Dong, D.; Liang, D.; Liu, H. Photocatalytic degradation of acetaminophen in aqueous environments: A mini review. Toxics 2023, 11, 604. [Google Scholar] [CrossRef]
- Xin, J.; Li, F.; Li, Z.; Zhao, J.; Wang, Y. Controlling the band structure and photocatalytic performance of single atom Ag/C 3 N 4 catalysts by variation of silver concentration. Inorg. Chem. Front. 2022, 9, 302–309. [Google Scholar] [CrossRef]
- Tian, S.; Yin, Y.; Liu, M.; Shi, L.; Zhang, S.; Asif, A.H.; Li, X.; Liu, M.; Duan, X.; Wang, S. Atomically dispersed Cu-N3 on hollow spherical carbon nitride for acetaminophen degradation: Generation of 1O2 from H2O2. Sep. Purif. Technol. 2023, 318, 124016. [Google Scholar] [CrossRef]
- Balakumar, V.; Ramalingam, M.; Sekar, K.; Chuaicham, C.; Sasaki, K. Fabrication and characterization of carbon quantum dots decorated hollow porous graphitic carbon nitride through polyaniline for photocatalysis. Chem. Eng. J. 2021, 426, 131739. [Google Scholar] [CrossRef]
- Liu, N.; Liu, J.; Kong, W.; Li, H.; Huang, H.; Liu, Y.; Kang, Z. One-step catalase controllable degradation of C 3 N 4 for N-doped carbon dot green fabrication and their bioimaging applications. J. Mater. Chem. B 2014, 2, 5768–5774. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Raizada, P.; Singh, P.; Kumar, A.; Khan, A.A.P.; Asiri, A.M. Exploring recent advances in silver halides and graphitic carbon nitride-based photocatalyst for energy and environmental applications. Arab. J. Chem. 2020, 13, 8271–8300. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Cao, D.; Wang, Y.; Zhu, Y. Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid. Appl. Catal. B Environ. 2017, 211, 79–88. [Google Scholar] [CrossRef]
- Li, B.; Ma, X.; Deng, J.; Li, Q.; Chen, W.; Li, G.; Chen, G.; Wang, J. Comparison of acetaminophen degradation in UV-LED-based advance oxidation processes: Reaction kinetics, radicals contribution, degradation pathways and acute toxicity assessment. Sci. Total Environ. 2020, 723, 137993. [Google Scholar] [CrossRef]
- Hong, J.; Hwang, D.K.; Selvaraj, R.; Kim, Y. Facile synthesis of Br-doped g-C3N4 nanosheets via one-step exfoliation using ammonium bromide for photodegradation of oxytetracycline antibiotics. J. Ind. Eng. Chem. 2019, 79, 473–481. [Google Scholar] [CrossRef]
- John, P.; Johari, K.; Gnanasundaram, N.; Appusamy, A.; Thanabalan, M. Enhanced photocatalytic performance of visible light driven TiO2/g-C3N4 for degradation of diclofenac in aqueous solution. Environ. Technol. Innov. 2021, 22, 101412. [Google Scholar] [CrossRef]
- Quyen, V.T.; Kim, H.J.; Kim, J.; Huong, P.T.; Thanh, D.M.; Viet, N.M.; Thang, P.Q. Synthesizing S-doped graphitic carbon nitride for improvement photodegradation of tetracycline under solar light. Solar Energy 2021, 214, 288–293. [Google Scholar] [CrossRef]
- Pattanayak, D.S.; Pal, D.; Mishra, J.; Thakur, C.; Wasewar, K.L. Doped graphitic carbon nitride (g-C3N4) catalysts for efficient photodegradation of tetracycline antibiotics in aquatic environments. Environ. Sci. Pollut. Res. 2023, 30, 24919–24926. [Google Scholar] [CrossRef]
- Wu, K.; Chen, D.; Lu, S.; Fang, J.; Zhu, X.; Yang, F.; Pan, T.; Fang, Z. Supramolecular self-assembly synthesis of noble-metal-free (C, Ce) co-doped g-C3N4 with porous structure for highly efficient photocatalytic degradation of organic pollutants. J. Hazard. Mater. 2020, 382, 121027. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, C.; Yu, K.; Lu, Z.; Liu, Z.; Huo, P.; Tang, X.; Yan, Y. Synthesis Ce-doped biomass carbon-based g-C3N4 via plant growing guide and temperature-programmed technique for degrading 2-Mercaptobenzothiazole. Appl. Catal. B Environ. 2020, 268, 118432. [Google Scholar] [CrossRef]
- Masunga, N.; Mamba, B.B.; Kefeni, K.K. Trace samarium doped graphitic carbon nitride photocatalytic activity toward metanil yellow dye degradation under visible light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125107. [Google Scholar] [CrossRef]
- Kalisamy, P.; Lallimathi, M.; Suryamathi, M.; Palanivel, B.; Venkatachalam, M. ZnO-embedded S-doped gC3N4 heterojunction: Mediator-free Z-scheme mechanism for enhanced charge separation and photocatalytic degradation. RSC Adv. 2020, 10, 28365–28375. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.R.; Sharma, R.; Panchal, P.; Nehra, S.; Gupta, A.; Sharma, A. Synthesis, characterization and application of silver doped graphitic carbon nitride as photocatalyst towards visible light photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2020, 45, 23937–23946. [Google Scholar] [CrossRef]
- Doufar, N.; Benamira, M.; Lahmar, H.; Trari, M.; Avramova, I.; Caldes, M. Structural and photochemical properties of Fe-doped ZrO2 and their application as photocatalysts with TiO2 for chromate reduction. J. Photochem. Photobiol. A Chem. 2020, 386, 112105. [Google Scholar] [CrossRef]
- Chen, M.; Guo, C.; Hou, S.; Wu, L.; Lv, J.; Hu, C.; Zhang, Y.; Xu, J. In-situ fabrication of Ag/Pg-C3N4 composites with enhanced photocatalytic activity for sulfamethoxazole degradation. J. Hazard. Mater. 2019, 366, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Lakhi, K.S.; Park, D.-H.; Al-Bahily, K.; Cha, W.; Viswanathan, B.; Choy, J.-H.; Vinu, A. Mesoporous carbon nitrides: Synthesis, functionalization, and applications. Chem. Soc. Rev. 2017, 46, 72–101. [Google Scholar] [CrossRef]
- Wang, L.; Hong, Y.; Liu, E.; Duan, X.; Lin, X.; Shi, J. A bottom-up acidification strategy engineered ultrathin g-C3N4 nanosheets towards boosting photocatalytic hydrogen evolution. Carbon 2020, 163, 234–243. [Google Scholar] [CrossRef]
- Di, G.; Zhu, Z.; Dai, Q.; Zhang, H.; Shen, X.; Qiu, Y.; Huang, Y.; Yu, J.; Yin, D.; Küppers, S. Wavelength-dependent effects of carbon quantum dots on the photocatalytic activity of g-C3N4 enabled by LEDs. Chem. Eng. J. 2020, 379, 122296. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Yang, C.; Wang, X. Nanospherical carbon nitride frameworks with sharp edges accelerating charge collection and separation at a soft photocatalytic interface. Adv. Mater. 2014, 26, 4121–4126. [Google Scholar] [CrossRef]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A. g-C3N4/carbon dot-based nanocomposites serve as efficacious photocatalysts for environmental purification and energy generation: A review. J. Clean. Prod. 2020, 276, 124319. [Google Scholar] [CrossRef]
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Liu, Y.; Alharbi, N.S.; Rabah, S.O.; Wang, S.; Wang, X. Synthesis and fabrication of g-C3N4-based materials and their application in elimination of pollutants. Sci. Total. Environ. 2020, 731, 139054. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Sacchi, B.; Pirola, C.; Demartin, F.; Cerrato, G.; Morandi, S.; Capucci, V. Aspirin and paracetamol removal using a commercial micro-sized TiO2 catalyst in deionized and tap water. Environ. Sci. Pollut. Res. 2017, 24, 12646–12654. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liya, E.Y.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- El Najjar, N.H.; Touffet, A.; Deborde, M.; Journel, R.; Leitner, N.K.V. Kinetics of paracetamol oxidation by ozone and hydroxyl radicals, formation of transformation products and toxicity. Sep. Purif. Technol. 2014, 136, 137–143. [Google Scholar] [CrossRef]
- Kim, I.; Yamashita, N.; Tanaka, H. Performance of UV and UV/H2O2 processes for the removal of pharmaceuticals detected in secondary effluent of a sewage treatment plant in Japan. J. Hazard. Mater. 2009, 166, 1134–1140. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Briones, R.M.; Su, C.-C.; Lu, M.-C. Kinetics of acetaminophen degradation by Fenton oxidation in a fluidized-bed reactor. Chemosphere 2013, 90, 1444–1448. [Google Scholar] [CrossRef]
- Cai, H.; Zou, J.; Lin, J.; Li, J.; Huang, Y.; Zhang, S.; Yuan, B.; Ma, J. Sodium hydroxide-enhanced acetaminophen elimination in heat/peroxymonosulfate system: Production of singlet oxygen and hydroxyl radical. Chem. Eng. J. 2022, 429, 132438. [Google Scholar] [CrossRef]
- Fan, J.; Qin, H.; Jiang, S. Mn-doped g-C3N4 composite to activate peroxymonosulfate for acetaminophen degradation: The role of superoxide anion and singlet oxygen. Chem. Eng. J. 2019, 359, 723–732. [Google Scholar] [CrossRef]
- Feng, X.; Wang, P.; Hou, J.; Qian, J.; Wang, C.; Ao, Y. Oxygen vacancies and phosphorus codoped black titania coated carbon nanotube composite photocatalyst with efficient photocatalytic performance for the degradation of acetaminophen under visible light irradiation. Chem. Eng. J. 2018, 352, 947–956. [Google Scholar] [CrossRef]
- Xu, B.; Zhan, G.; Xu, B.; Du, H.; Luo, H.; Wang, T.; Zhan, C.; Yang, Y. Degradation of acetaminophen in aqueous solution by UV and UV-activated sludge processes. Water Sci. Technol. 2018, 78, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, B.; Jeyadharmarajan, J.; Chinnaiyan, P. Novel organic assisted Ag-ZnO photocatalyst for atenolol and acetaminophen photocatalytic degradation under visible radiation: Performance and reaction mechanism. Environ. Sci. Pollut. Res. 2021, 28, 39637–39647. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, O.F.S.; Palaniandy, P.; Ahmadipour, M.; Mohammadi, H.; Bin Hamdan, M.R. Removal of acetaminophen using Fe2O3-TiO2 nanocomposites by photocatalysis under simulated solar irradiation: Optimization study. J. Environ. Chem. Eng. 2021, 9, 104921. [Google Scholar] [CrossRef]
- Yanyan, L.; Kurniawan, T.A.; Ying, Z.; Albadarin, A.B.; Walker, G. Enhanced photocatalytic degradation of acetaminophen from wastewater using WO3/TiO2/SiO2 composite under UV–VIS irradiation. J. Mol. Liq. 2017, 243, 761–770. [Google Scholar] [CrossRef]
- Nateq, K.; Amarzadeh, M.; Shohani Zadeh, M.; Rostami, M.; Danaee, I.; Schwaminger, S.; Khosravi-Nikou, M.R.; Mirzapoor, A.; Gol, G. Construction of engineered heterojunction based on CdS and MgO material co-integrated into a flat plane-like graphene for tetracycline decontamination: Ecological hazard assessment and toxicity alleviation. J. Water Process Eng. 2024, 68, 106361. [Google Scholar] [CrossRef]
- Moradi, S.; Isari, A.A.; Hayati, F.; Rezaei Kalantary, R.; Kakavandi, B. Co-implanting of TiO2 and liquid-phase-delaminated g-C3N4 on multi-functional graphene nanobridges for enhancing photocatalytic degradation of acetaminophen. J. Chem. Eng. 2021, 414, 128618. [Google Scholar] [CrossRef]
- Wen, J.; Zhou, L.; Tang, O.; Xiao, X.; Sun, S.H. Photocatalytic degradation of organic pollutants by carbon quantum dots functionalized g-C3N4: A review. J. Ecotoxicol Environ Saf. 2023, 262, 115133. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Huo, J.; Zhang, P.; Zeng, J.; Wang, T.; Zen, H. Improving the photocatalytic hydrogen production of Ag/gC3N4 nanocomposites by dye-sensitization under visible light irradiation. Nanoscale 2016, 8, 2249–2259. [Google Scholar] [CrossRef]
- Saran, S.; Mohanty, K. Facile Green Synthesis of Ag@g-C3N4 for Enhanced Photocatalytic and Catalytic Degradation of Organic Pollutant. J. Clust. Sci. 2021, 32, 585–592. [Google Scholar]
- Ghodsi, S.; Esrafili, A.; Sobhi, H.R.; Rezaei Kalantary, R.; Gholami, M.; Maleki, R. Synthesis and application of g-C3N4/Fe3O4/Ag nanocomposite for the efficient photocatalytic inactivation of Escherichia coli and Bacillus subtilis bacteria in aqueous solutions. AMB Express 2021, 11, 161. [Google Scholar] [CrossRef]
- Azqandi, M.; Nateq, K.; Amarzadeh, M.; Yoosefian, M.; Yaghoot-Nezhad, A.; Ahmad, A.; Ramavandi, B.; Nasshe, N. Intensified photo-decontamination of tetracycline antibiotic by S-scheme spinel manganese ferrite-grafted ZIF-8 heterojunction photocatalyst: Mechanism conception, degradation pathway and DFT studies. J. Environ. Chem. Eng. 2024, 12, 112875. [Google Scholar] [CrossRef]




| Category | Detail | Category | Detail |
|---|---|---|---|
| Structure |  | IUPAC name | N-(4-hydroxyphenyl) Acetamide |
| Molecular mass | 151.2 g/mol | Trade names | Tylenol/Panadol |
| Density | 1.263 g/cm3 | Melting point | 169–172 °C |
| Log Kow | 2 | Water solubility | 1400–2400 mg/L |
| pKa | 9.38 |
| Components | g-C3N4 | |
|---|---|---|
| Weight % | Atomic % | |
| C | 40.57 | 44.32 |
| N | 59.43 | 55.68 |
| Ag | - | - |
| Total | 100 | 100 |
| Components | g-C3N4/CQD/Ag | |
| Weight % | Atomic % | |
| C | 35 | 42.1 |
| N | 52 | 55.3 |
| Ag | 13 | 2.59 |
| Total | 100 | 100 |
| Photocatalyst | Illumination Source | Implementation | Degradation Efficiency (%) | Ref |
|---|---|---|---|---|
| Ag/g-C3N4 | 200 W tungsten lamps (420 nm cut off filter) | Degradation of MB dye | 96 | [47] |
| SDAg-CQD/UCN | 350 W Xenon lamps (420 nm cut off filter) | Decontamination of naproxen | 87.5 | [48] |
| ZnSe-Ag/CN | 300 W Xenon lamps (420 nm cut off filter) | Decontamination of ceftriaxone sodium | 89.24 | [49] |
| Ag/g-C3N4 | 300 W Xenon lamps (400 nm cut off filter) | Decontamination of sulfamethoxazole | 87.3 | [50] |
| Ag2O/NCDs@PTCN | 300 W Xenon lamps (420 nm cut off filter) | Decontamination of CBZ | 99.9 | [51] |
| Ag/CNQDs/g-C3N4 | Microwave | Degradation of norfloxacin | 100 | [52] |
| CQD/cds/g-C3N4 | 45 W LED lamps (420 nm cut off filter) | Decontamination of Rhodamine B and sulfadiazine | 94.7 & 76 | [53] |
| Ag/UCN | 300 W Xenon lamps | Degradation of ofloxacin | 95.2 | [54] |
| Ag/UTCN | 800 W Xenon lamps (420 nm cut off filter) | Degradation of acetaminophen | 85 | [55] |
| Ag/CNQDs/g-C3N4 | 300 W Xenon lamps (400 nm cut off filter) | Decontamination of norfloxacin, sulfamethoxazole and tetracycline hydrochloride | 100 & 81 | [56] |
| g-C3N4/NCDs/Ag | 300 W Xenon lamps (400 nm cut off filter) | Decontamination of methyl orange, Rhodamine B, tetracycline hydrochloride and chrysin hydrochloride | 100 | [57] |
| Ag/g-C3N4/O3 | High pressure Xenon lamp | Degradation of acetaminophen | 83.1 | [58] |
| HT-g-C3N4/PS system | LED lamps 400 nm | Degradation of acetaminophen | 41 | [59] |
| CQD/CN-PANI | 500 W Xenon lamps (420 nm cut off filter) | Decontamination of ciprofloxacin, imidacloprid, tetracycline, phenol and Rhodamine B | 94.7, 80.1, 93.2, 45.6 & 97.9 | [60] |
| CDs/g-C3N4 | 300 W Xenon lamps (400 nm cut off filter) | Decontamination of phenol | 87 | [61] |
| Ag/g-C3N4 | 5 W LED lamps | Degradation of ciprofloxacin | 84 | [62] |
| GCN-Ag | 200 W Xenon lamps | Degradation of methylene blue, crystal violet and rose bangal | 96, 80 & 78 | [63] |
| Br/g-C3N4 | 38.5W WLEL | Degradation of OTC | 75 | [64] |
| Ag/g-C3N4 | Solar light | Degradation of tetracycline | 96.8 | [65] |
| g-C3N4/TiO2 | 300 W Xenon lamps (400 nm cut off filter) | Degradation of ciprofloxacin | 88.1 | [66] |
| g-C3N4/RGO/WO3 | 500 W Xenon lamps (400 nm cut off filter) | Decontamination of ciprofloxacin | 85 | [67] |
| g-C3N4/TiO2/Kaolinite | Xenon lamps (400 nm cut off filter) | Decontamination of ciprofloxacin | 92 | [68] |
| CN-Ce (Cerium ш) | 300 W Xenon lamps (420 nm cut off filter) | Decontamination of naproxen | 69 | [69] |
| CN-Er (Erbium ш) | 300 W Xenon lamps (420 nm cut off filter) | Decontamination of naproxen | 44 | [70] |
| CN-Sm (samarium ш) | 300 W Xenon lamps (420 nm cut off filter) | Decontamination of naproxen | 40.1 | [71] |
| NCDs/g-C3N4 | 350 W Xenon lamps (420 nm cut off filter) | Decontamination of indomethacin | 91.5 | [72] |
| Ag/g-C3N4 | 300 W Xenon lamps (400 nm cut off filter) | Decontamination of diclofenac | 54 | [73] |
| Ag/CDs/CNNs | Sun light | Degradation of methyl orange and p-nitrophenol | 98.6 & 92 | [74] |
| Ag/g-C3N4 | Sun light | Degradation of morphine | 91 | [75] |
| Ag/g-C3N4/ZnO | 300 W Xenon lamps (400 nm cut off filter) | Decontamination of paracetamol, amoxicillin and cefalexin | 85.3, 41.36 & 71.74 | [76] |
| CQD/g-C3N4 | 250 W Xenon lamps (420 nm cut off filter) | Decontamination of Rhodamine B and tetracycline hydrochloride | 95.2 & 78.6 | [77] |
| Ag/g-C3N4-PNP | 300 W Xenon lamps (400 nm cut off filter) | Decontamination of sulfamethoxazole | 87.3 | [78] |
| CQD/g-C3N4 | 300 W Xenon lamps | Decontamination of diclofenac | 100 | [79] |
| Ag/g-C3N4/PCN | 300 W Xenon lamps (420 nm cut off filter) | Decontamination f tetracycline | 83 | [80] |
| AgI/g-C3N4 | 300 W Xenon lamps (400 nm cut off filter) | Decontamination of diclofenac | 45 | [81] |
| HCNs/CDs | 350 W Xenon lamps (420 nm cut off filter) | Decontamination of indomethacin, naproxen, ciprofloxacin, norfloxacin, diclofenac, bisphenol A and naproxen | 4.8, 98.6, 47.2, 41.7, 10.9 & 20.9 | [82] |
| g-C3N4/CQD/Ag | 500 W Xenon lamps (420 nm cut off filter) and UV lamp 250 W (λUV = 254 nm) | Decontamination of acetaminophen | Under visible light = 85.3 Under UV irradiation = 87.5 | In this study |
| Catalyst | Light Source (Power Values) | pH | Dose of Catalyst (g/L) | Initial Concentration (mg/L) | Irradiation Time (min) | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| Ag-ZnO | Tungsten halogen lamp (300 W) | 8.5 | 1 | 5 | 12 | 90.8 | [92] |
| (P,S)-g-C3N4/TiO2 (5%) | Xenon lamp (30 mW/cm2) | 5 and 7 | 1 | 20 | 60 | 100 | [34] |
| Fe2O3-TiO2 | halogen lamp (500 W) | 11 | 1.25 | 30 | 180 | 95.8 | [93] |
| WO3/TiO2/SiO2 | Xenon lamp (500 W) | 9 | 1.5 | 5 | 240 | 95 | [94] |
| g-C3N4/CQD/Ag | Xenon lamp (500 W) | 7 | 0.6 | 50 | 60 | 85.3 | In this study |
| UV lamp (250 W) | 7 | 0.6 | 50 | 60 | 87.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toolabi, A.; Tahergorabi, M.; Mehralipour, J.; Seyedi, N.; Nasseh, N. Photocatalytic Degradation of Acetaminophen by g-C3N4/CQD/Ag Nanocomposites from Aqueous Media. J. Compos. Sci. 2025, 9, 197. https://doi.org/10.3390/jcs9050197
Toolabi A, Tahergorabi M, Mehralipour J, Seyedi N, Nasseh N. Photocatalytic Degradation of Acetaminophen by g-C3N4/CQD/Ag Nanocomposites from Aqueous Media. Journal of Composites Science. 2025; 9(5):197. https://doi.org/10.3390/jcs9050197
Chicago/Turabian StyleToolabi, Ali, Mahsa Tahergorabi, Jamal Mehralipour, Neda Seyedi, and Negin Nasseh. 2025. "Photocatalytic Degradation of Acetaminophen by g-C3N4/CQD/Ag Nanocomposites from Aqueous Media" Journal of Composites Science 9, no. 5: 197. https://doi.org/10.3390/jcs9050197
APA StyleToolabi, A., Tahergorabi, M., Mehralipour, J., Seyedi, N., & Nasseh, N. (2025). Photocatalytic Degradation of Acetaminophen by g-C3N4/CQD/Ag Nanocomposites from Aqueous Media. Journal of Composites Science, 9(5), 197. https://doi.org/10.3390/jcs9050197





