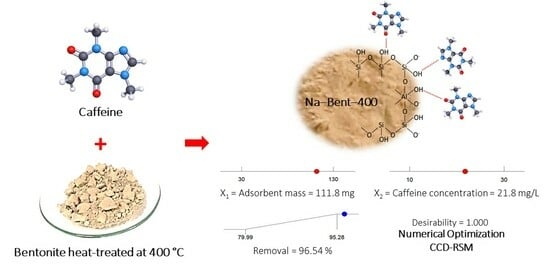
Caffeine Adsorption on a Thermally Modified Bentonite: Adsorbent Characterization, Experimental Design, Equilibrium and Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Clay Modification and Characterization
2.3. Experimental Conditions for Adsorption
2.4. Experimental Design
2.5. Adsorption Isotherm and Reuse Tests
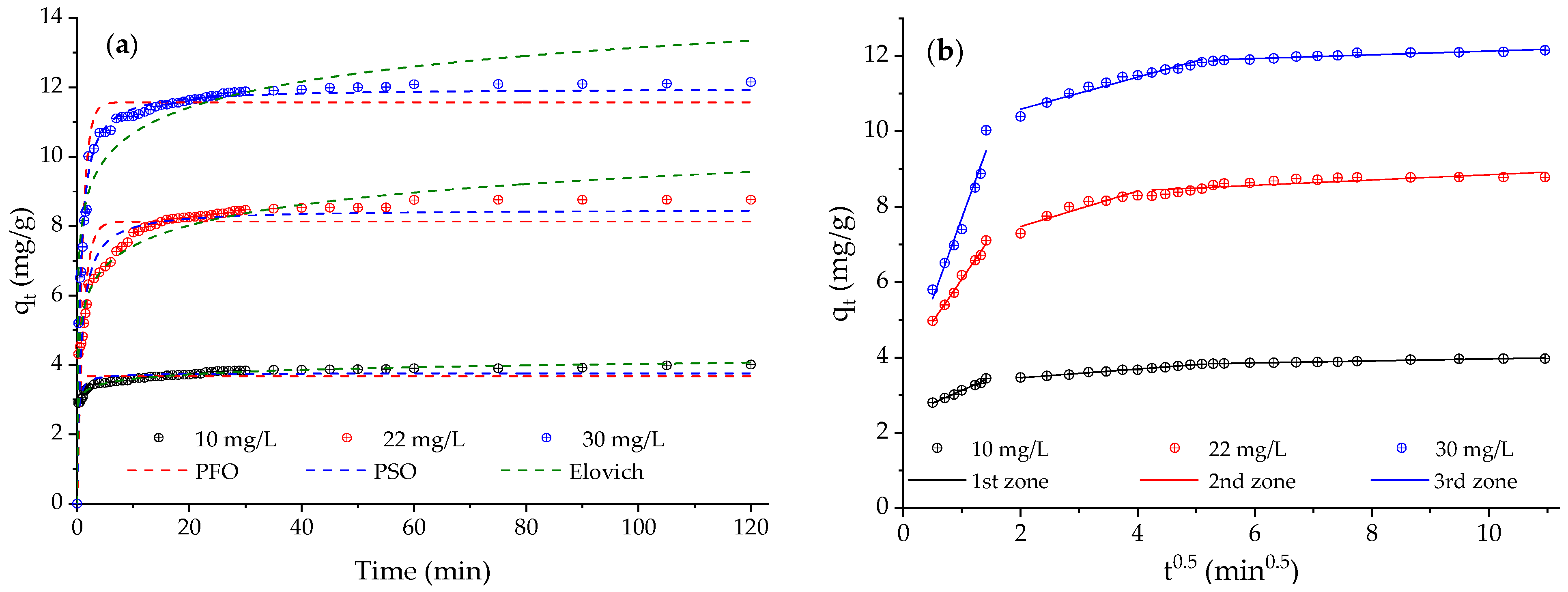
2.6. Adsorption Kinetics and Mechanism
3. Results and Discussion
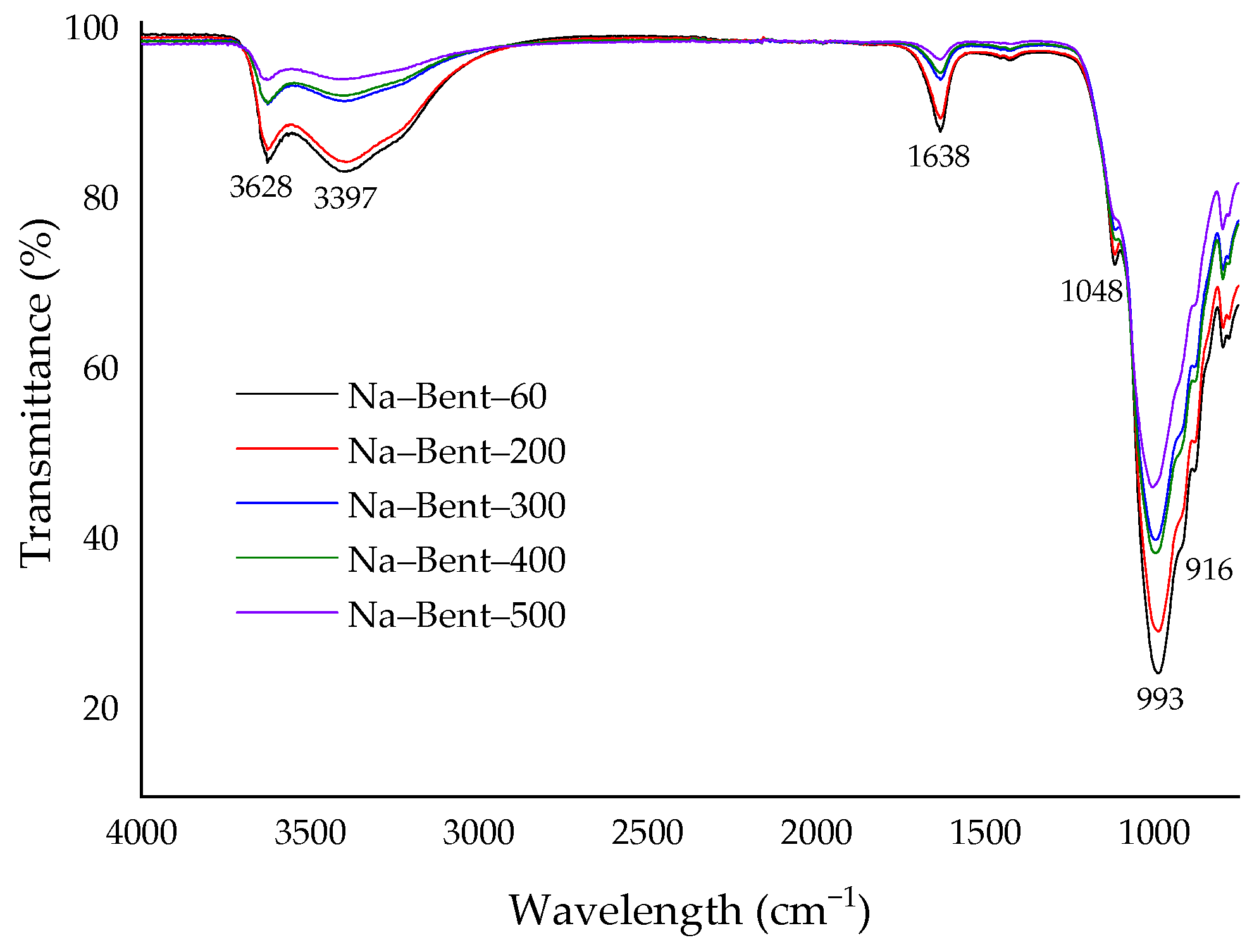
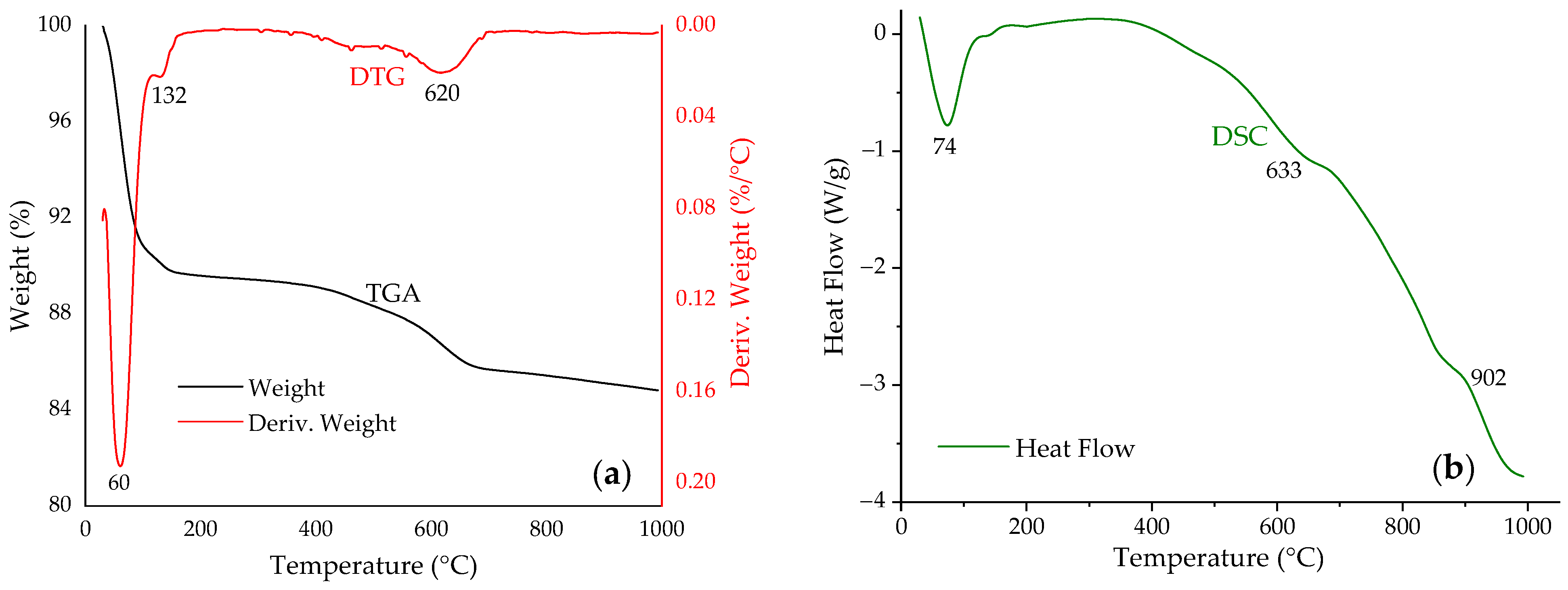
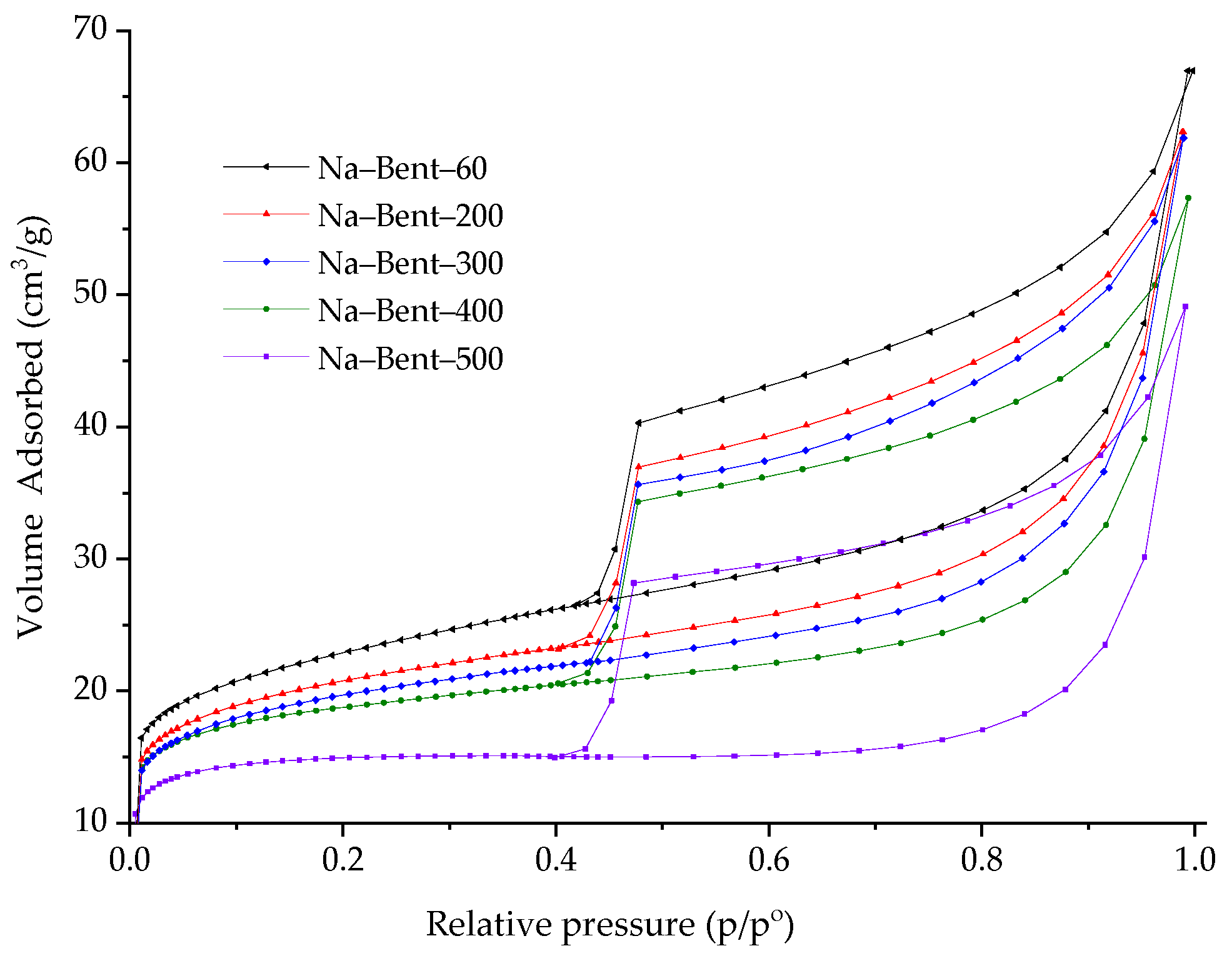
3.1. Adsorbent Characterization
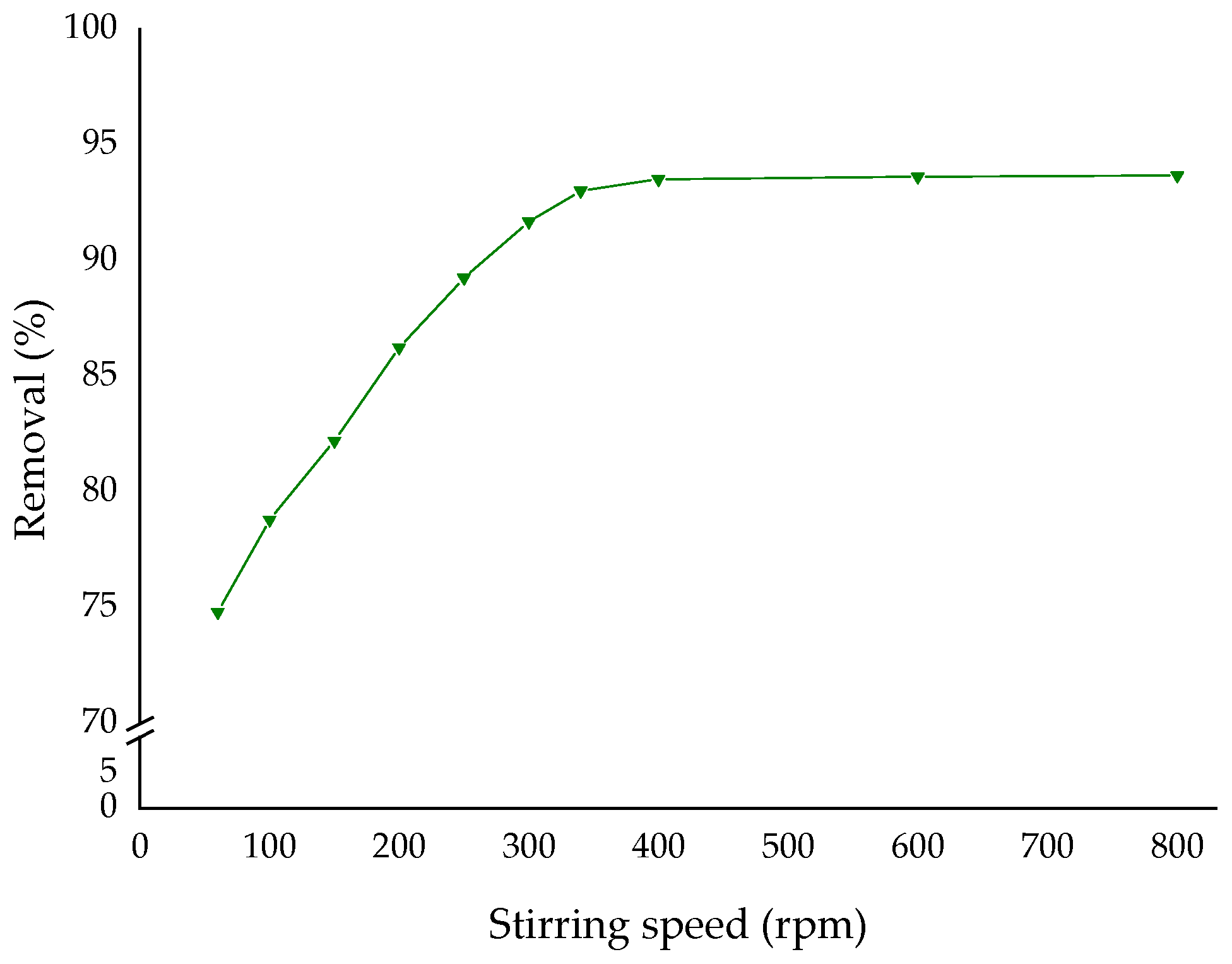
3.2. Experimental Conditions for Adsorption
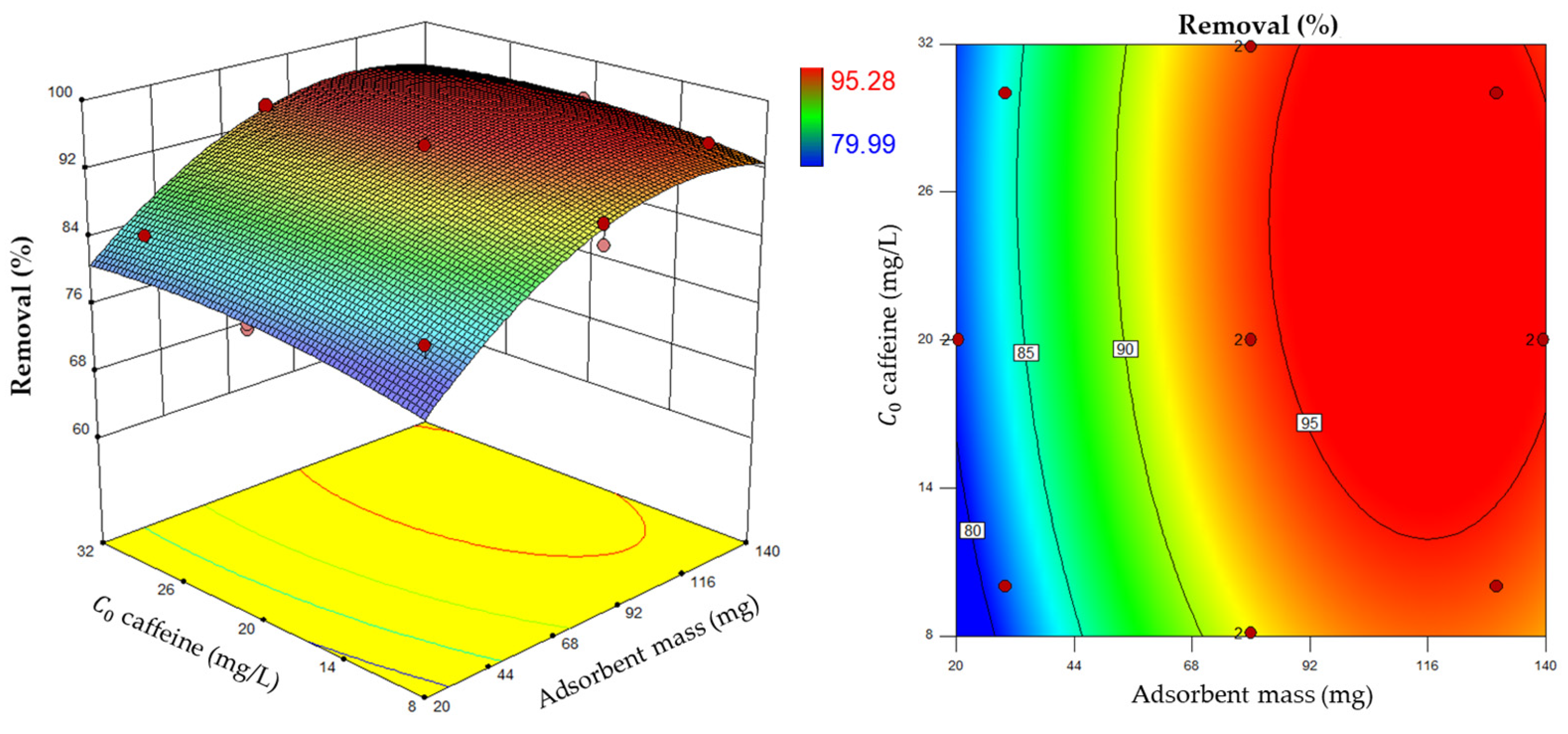
3.3. Experimental Design for Caffeine Adsorption
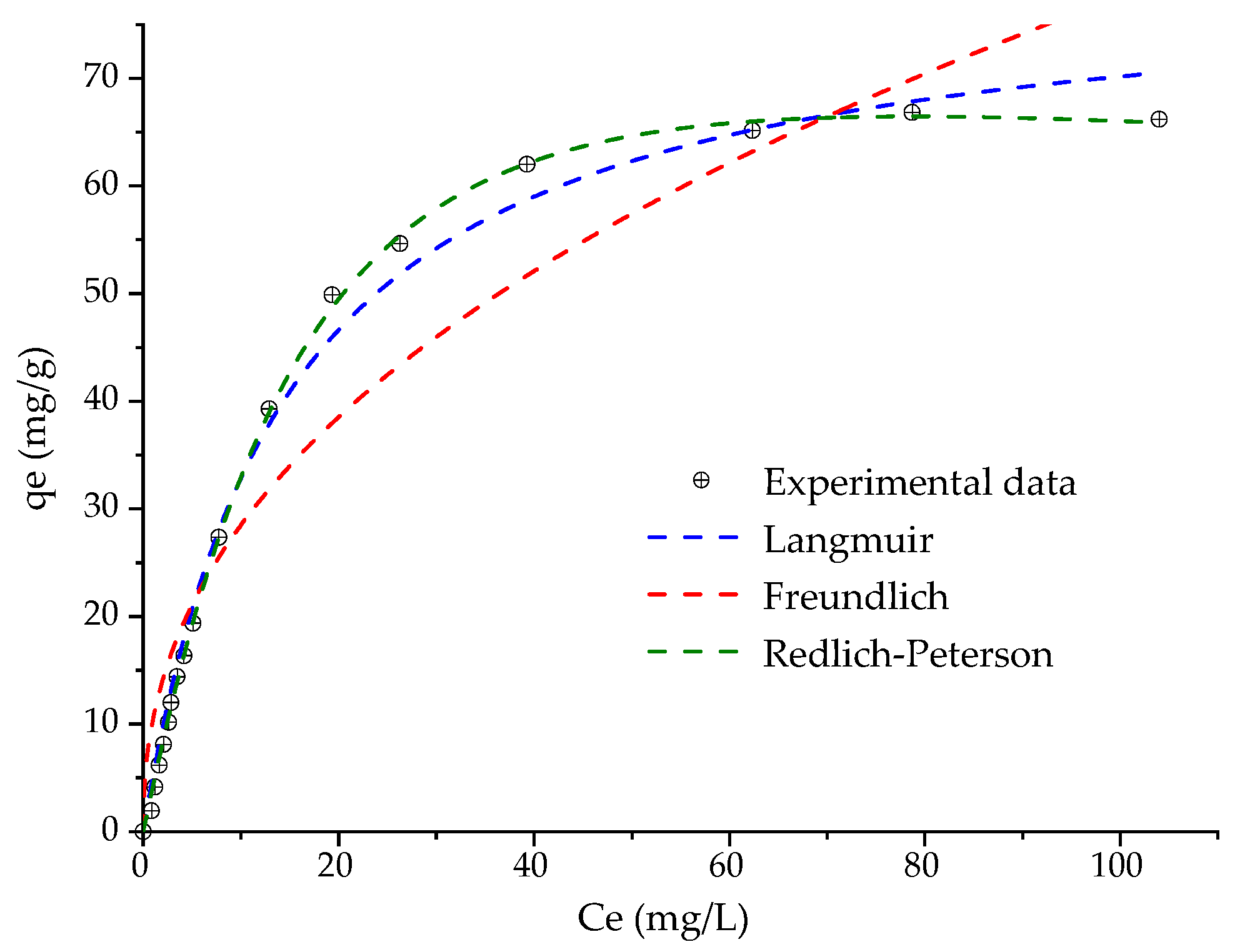
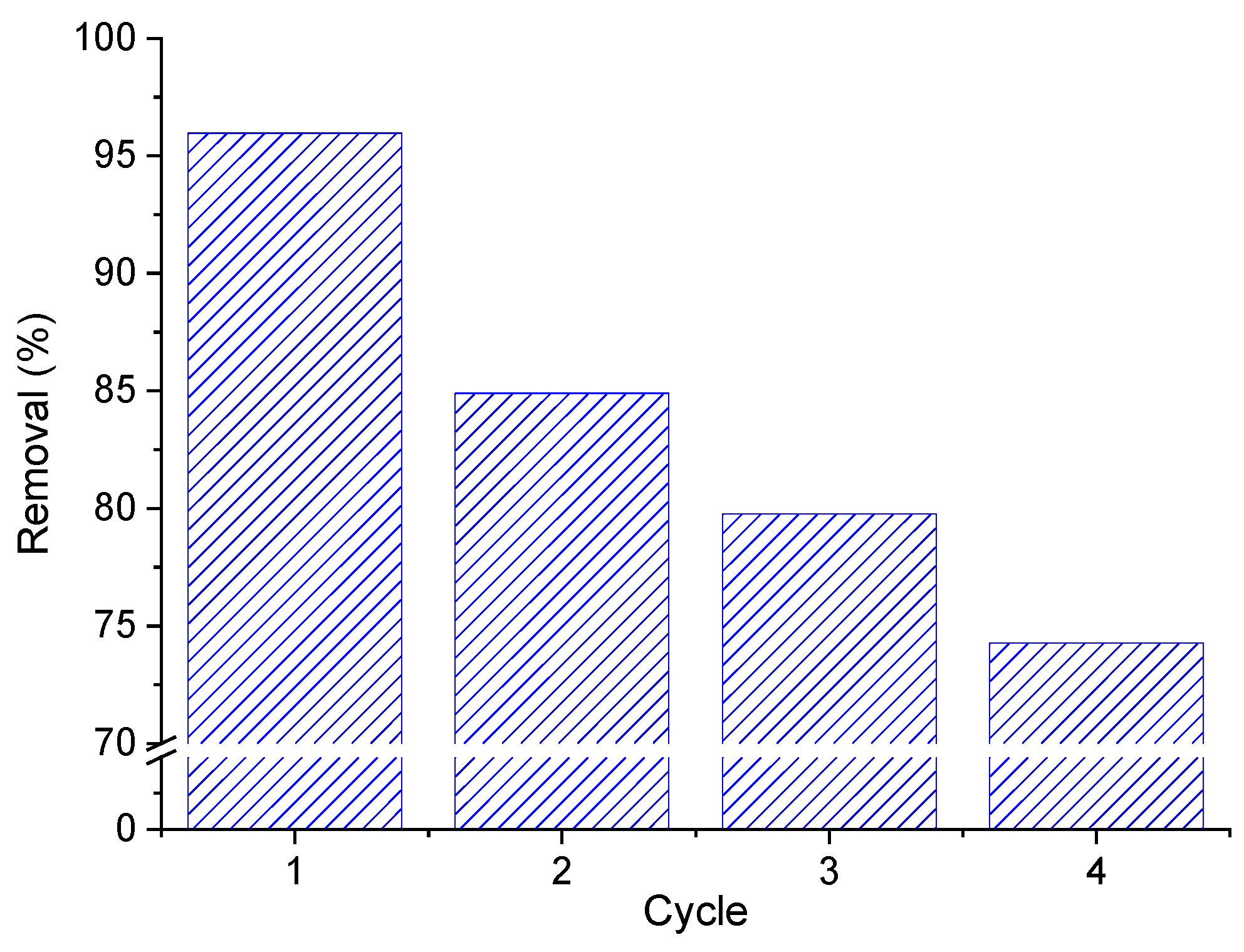
3.4. Adsorption Isotherm and Reuse Tests
3.5. Adsorption Kinetics and Proposed Mechanism for the Adsorption of Caffeine
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naidu, R.; Jit, J.; Kennedy, B.; Arias, V. Emerging contaminant uncertainties and policy: The chicken or the egg conundrum. Chemosphere 2016, 154, 385–390. [Google Scholar] [CrossRef]
- Álvarez, S.; Rodríguez, A.; Ovejero, G.; Gómez, J.M.; García, J. Removal of caffeine from pharmaceutical wastewater by adsorption: Influence of NOM, textural and chemical properties of the adsorbent. Environ. Technol. 2016, 37, 1618–1630. [Google Scholar] [CrossRef] [PubMed]
- Yargeau, V.; Lopata, A.; Metcalfe, C. Pharmaceuticals in the Yamaska River, Quebec, Canada. Water Qual. Res. J. Can. 2007, 42, 231–239. [Google Scholar] [CrossRef]
- Buerge, I.J.; Poiger, T.; Muller, M.; Buser, H.R. Caffeine, an anthropogenic marker for wastewater contamination of surface waters. Environ. Sci. Technol. 2003, 37, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Rigueto, C.; Nazari, M.; De Souza, C.; Cadore, J.; Brião, V.; Piccin, J. Alternative techniques for caffeine removal from wastewater: An overview of opportunities and challenges. J. Water Process Eng. 2020, 35, 101231. [Google Scholar] [CrossRef]
- Lin, T.; Yu, S.; Chen, W. Occurrence, removal and risk assessment of pharmaceutical and personal care products (PPCPs) in an advanced drinking water treatment plant (ADWTP) around Taihu Lake in China. Chemosphere 2016, 152, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Rey, A.; Beltrán, F.J. TiO2 photocatalytic oxidation of a mixture of emerging contaminants: A kinetic study independent of radiation absorption based on the direct-indirect model. Chem. Eng. J. 2018, 339, 369–380. [Google Scholar] [CrossRef]
- Li, L.; Gong, L.; Wang, Y.X.; Liu, Q.; Zhang, J.; Mu, Y.; Yu, H.Q. Removal of halogenated emerging contaminants from water by nitrogen-doped graphene decorated with palladium nanoparticles: Experimental investigation and theoretical analysis. Water Res. 2016, 98, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Dordio, A.; Miranda, S.; Ramalho, J.P.; Carvalho, A.P. Mechanisms of removal of three widespread pharmaceuticals by two clay materials. J. Hazard. Mater. 2017, 323, 575–583. [Google Scholar] [CrossRef]
- Marcal, L.; de Faria, E.H.; Nassar, E.J.; Trujillano, R.; Martin, N.; Vicente, M.A.; Rives, V.; Gil, A.; Korili, S.A.; Ciuffi, K.J. Organically modified saponites: SAXS study of swelling and application in caffeine removal. ACS Appl. Mater. Interfaces 2015, 7, 10853–10862. [Google Scholar] [CrossRef]
- Quintero-Jaramillo, J.A.; Carrero-Mantilla, J.I.; Sanabria-González, N.R. A review of caffeine adsorption studies onto various types of adsorbents. Sci. World J. 2021, 2021, 9998924. [Google Scholar] [CrossRef] [PubMed]
- Bergaya, F.; Lagaly, G. General Introduction: Clays, Clay Minerals, and Clay Science. In Developments in Clay Science. Handbokk of Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 1–18, Chapter 1. [Google Scholar]
- Odom, I.E. Smectite clay minerals: Properties and uses. Phil. Trans. R. Soc. Land. A 1984, 311, 391–409. [Google Scholar]
- Murray, H.H. Bentonite Applications. In Developments in Clay Science. Applied Clay Mineralogy; Murray, H.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 111–130, Chapter 6. [Google Scholar]
- Ewis, D.; Ba-Abbad, M.M.; Benamor, A.; El-Naas, M.H. Adsorption of organic water pollutants by clays and clay minerals composites: A comprehensive review. Appl. Clay Sci. 2022, 229, 106686. [Google Scholar] [CrossRef]
- França, D.B.; Oliveira, L.S.; Filho, F.G.N.; Filho, E.C.S.; Osajima, J.A.; Jaber, M.; Fonseca, M.G. The versatility of montmorillonite in water remediation using adsorption: Current studies and challenges in drug removal. J. Environ. Chem. Eng. 2022, 10, 107341. [Google Scholar] [CrossRef]
- My Linh, N.L.; Hoang Van, D.; Duong, T.; Tinh, M.X.; Quang Khieu, D. Adsorption of arsenate from aqueous solution onto modified Vietnamese bentonite. Adv. Mater. Sci. Eng. 2019, 2019, 2710926. [Google Scholar] [CrossRef]
- Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; Neves, G.d.A. Adsorption of anionic dye on the acid-functionalized bentonite. Materials 2020, 13, 3600. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Li, Q.; Tian, Q.; Chen, X.; Almásy, L.; Liu, Y.; Sun, G.; Tuo, X.; Yan, M.; Henderson, M.J. (Ce-Al)-oxide pillared bentonite: A high affinity sorbent for plutonium. J. Hazard. Mater. 2018, 352, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Shiono, T.; Matsui, Y.; Yoneda, M. Interaction of caffeine with montmorillonite. Part. Sci. Technol. 2019, 37, 325–332. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shiono, T.; Matsui, Y.; Yoneda, M. Changes the structure and caffeine adsorption property of calcined montmorillonite. Int. J. Geomate. 2016, 11, 2301–2306. [Google Scholar] [CrossRef]
- Yamamoto, K.; Shiono, T.; Yoshimura, R.; Matsui, Y.; Yoneda, M. Influence of hydrophilicity on adsorption of caffeine onto montmorillonite. Adsorpt. Sci. Technol. 2018, 36, 967–981. [Google Scholar] [CrossRef]
- Pradas, E.G.; Sánchez, M.V.; Cruz, F.C.; Viciana, M.S.; Pérez, M.F. Adsorption of cadmium and zinc from aqueous solution on natural and activated bentonite. J. Chem. Tech. Biotechnol. 1994, 59, 289–295. [Google Scholar] [CrossRef]
- Padilla, E.; Medellín, N.; Robledo, A. Comparative study of the effect of structural arrangement of clays in the thermal activation: Evaluation of their adsorption capacity to remove Cd(II). J. Environ. Chem. Eng. 2020, 8, 103850. [Google Scholar] [CrossRef]
- Iamsaard, K.; Weng, C.-H.; Tzeng, J.-H.; Anotai, J.; Jacobson, A.R.; Lin, Y.-T. Systematic optimization of biochars derived from corn wastes, pineapple leaf, and sugarcane bagasse for Cu(II) adsorption through response surface methodology. Bioresour. Technol. 2023, 382, 129131. [Google Scholar] [CrossRef] [PubMed]
- Ni’mah, Y.L.; Subandi, A.P.K.; Suprapto, S. The application of silica gel synthesized from chemical bottle waste for zinc (II) adsorption using Response Surface Methodology (RSM). Heliyon 2022, 8, e11997. [Google Scholar] [CrossRef] [PubMed]
- Homem, V.; Alves, A.; Santos, L. Amoxicillin degradation at ppb levels by Fenton’s oxidation using design of experiments. Sci. Total Environ. 2010, 408, 6272–6280. [Google Scholar] [CrossRef] [PubMed]
- Macias-Quiroga, I.F.; Giraldo-Gómez, G.I.; Sanabria-González, N.R. Characterization of colombian clay and its potential use as adsorbent. Sci. World J. 2018, 2018, 5969178. [Google Scholar] [CrossRef] [PubMed]
- Januário, E.F.D.; Vidovix, T.B.; Ribeiro, A.C.; da Costa Neves Fernandes de Almeida Duarte, E.; Bergamasco, R.; Vieira, A.M.S. Evaluation of hydrochar from peach stones for caffeine removal from aqueous medium and treatment of a synthetic mixture. Environ. Technol. 2022, 45, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Couto, O.M., Jr.; Matos, I.; da Fonseca, I.M.; Arroyo, P.A.; da Silva, E.A.; de Barros, M.A.S.D. Effect of solution pH and influence of water hardness on caffeine adsorption onto activated carbons. Can. J. Chem. Eng. 2015, 93, 68–77. [Google Scholar] [CrossRef]
- Álvarez, A.; Moreno, S.; Molina, R.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A. Gold supported on pillared clays for CO oxidation reaction: Effect of the clay aggregate size. Appl. Clay Sci. 2012, 69, 22–29. [Google Scholar] [CrossRef]
- Önal, M.; Sarıkaya, Y. Thermal behavior of a bentonite. J. Therm. Anal. Calorim. 2007, 90, 167–172. [Google Scholar] [CrossRef]
- Damonte, M.; Sánchez, R.; dos Santos Afonso, M. Some aspects of the glyphosate adsorption on montmorillonite and its calcined form. Appl. Clay. Sci. 2007, 36, 86–94. [Google Scholar] [CrossRef]
- Gil, A.; Korili, S.; Vicente, M. Recent advances in the control and characterization of the porous structure of pillared clay catalysts. Catal. Rev. Sci. Eng. 2008, 50, 153–221. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Guechi, E.-K.; Hamdaoui, O. Biosorption of methylene blue from aqueous solution by potato (Solanum tuberosum) peel: Equilibrium modelling, kinetic, and thermodynamic studies. Desalin. Water Treat. 2016, 57, 10270–10285. [Google Scholar] [CrossRef]
- Balistrieri, L.; Murray, J.W. The surface chemistry of goethite (αFeOOH) in major ion seawater. Am. J. Sci. 1981, 281, 788–806. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. E. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Sivrikaya, O.; Uzal, B.; Ozturk, Y. Practical charts to identify the predominant clay mineral based on oxide composition of clayey soils. Appl. Clay Sci. 2017, 135, 532–537. [Google Scholar] [CrossRef]
- Vieira, M.; Neto, A.A.; Gimenes, M.; Da Silva, M. Sorption kinetics and equilibrium for the removal of nickel ions from aqueous phase on calcined Bofe bentonite clay. J. Hazard. Mater. 2010, 177, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Andrini, L.; Toja, R.M.; Gauna, M.R.; Conconi, M.S.; Requejo, F.G.; Rendtorff, N. Extended and local structural characterization of a natural and 800 C fired Na-montmorillonite–Patagonian bentonite by XRD and Al/Si XANES. Appl. Clay Sci. 2017, 137, 233–240. [Google Scholar] [CrossRef]
- Noyan, H.; Önal, M.; Sarikaya, Y. The effect of heating on the surface area, porosity and surface acidity of a bentonite. Clay Miner. 2006, 54, 375–381. [Google Scholar] [CrossRef]
- Patel, H.A.; Somani, R.S.; Bajaj, H.C.; Jasra, R.V.J. Nanoclays for polymer nanocomposites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment. Bull. Mater. Sci. 2006, 29, 133–145. [Google Scholar] [CrossRef]
- Russell, J.D.; Fraser, A.R. Infrared Methods. In Clay Mineralogy: Spectroscopic and Chemical Determinative Methods; Wilson, M.J., Ed.; Springer Dordrecht: London, UK, 1995; Volume 30, pp. 11–67. [Google Scholar]
- Fil, B.A.; Özmetin, C.; Korkmaz, M. Characterization and electrokinetic properties of montmorillonite. Bulg. Chem. Commun. 2014, 46, 258–263. [Google Scholar]
- Heller-Kallai, L. Chapter 7.2 Thermally Modidied Clay Minerals. In Developments in Clay Science; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 289–308. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Orolínová, Z.; Mockovčiaková, A.; Dolinská, S.; Briančin, J. Effect of thermal treatment on the bentonite properties. Arh. Teh. Nauke. 2012, 7, 49–56. [Google Scholar] [CrossRef]
- Karimifard, S.; Alavi Moghaddam, M. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640-641, 772–797. [Google Scholar] [CrossRef] [PubMed]
- Veza, I.; Spraggon, M.; Fattah, I.M.R.; Idris, M. Response surface methodology (RSM) for optimizing engine performance and emissions fueled with biofuel: Review of RSM for sustainability energy transition. Results Eng. 2023, 18, 101213. [Google Scholar] [CrossRef]
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthes, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. J. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Galhetas, M.; Mestre, A.S.; Pinto, M.L.; Gulyurtlu, I.; Lopes, H.; Carvalho, A.P. Chars from gasification of coal and pine activated with K2CO3: Acetaminophen and caffeine adsorption from aqueous solutions. J. Colloid. Interface Sci. 2014, 433, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Luján, M.J.; Iborra, M.I.; Mendoza, J.A.; Alcaina, M.I. Pharmaceutical compounds removal by adsorption with commercial and reused carbon coming from a drinking water treatment plant. J. Clean. Prod. 2019, 238, 117866. [Google Scholar] [CrossRef]
- Portinho, R.; Zanella, O.; Féris, L. Grape stalk application for caffeine removal through adsorption. J. Environ. Manage. 2017, 202, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Da Silva, M.; Vieira, M. Equilibrium and kinetic studies of caffeine adsorption from aqueous solutions on thermally modified verde-lodo bentonite. Appl. Clay Sci. 2019, 168, 366–373. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- do Nascimento, D.C.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of propranolol hydrochloride from aqueous solutions onto thermally treated bentonite clay: A complete batch system evaluation. J. Mol. Liq. 2021, 337, 116442. [Google Scholar] [CrossRef]
- El-Khaiary, M.I.; Malash, G.F. Common data analysis errors in batch adsorption studies. Hydrometallurgy 2011, 105, 314–320. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Keerthanan, S.; Bhatnagar, A.; Mahatantila, K.; Jayasinghe, C.; Ok, Y.S.; Vithanage, M. Engineered tea-waste biochar for the removal of caffeine, a model compound in pharmaceuticals and personal care products (PPCPs), from aqueous media. Environ. Technol. Innov. 2020, 19, 100847. [Google Scholar] [CrossRef]
- Bachmann, S.A.L.; Calvete, T.; Féris, L.A. Caffeine removal from aqueous media by adsorption: An overview of adsorbents evolution and the kinetic, equilibrium and thermodynamic studies. Sci. Total. Environ. 2021, 767, 144229. [Google Scholar] [CrossRef] [PubMed]
- Švorc, Ľ. Determination of caffeine: A comprehensive review on electrochemical methods. Int. J. Electrochem. Sci. 2013, 8, 5755–5773. [Google Scholar] [CrossRef]
- Sakuma, H.; Tamura, K.; Hashi, K.; Kamon, M. Caffeine adsorption on natural and synthetic smectite clays: Adsorption mechanism and effect of interlayer cation valence. J. Phys. Chem. C 2020, 124, 25369–25381. [Google Scholar] [CrossRef]




| Contact Time (min) | pH | Thermal Treatment (°C) | Stirring Speed (rpm) |
|---|---|---|---|
| 2–120 | 7.0 | 60–500 | 400 |
| t selected | 4.0–10.0 | 60–500 | 400 |
| t selected | pH selected | T selected | 400 |
| t selected | pH selected | T selected | 60–800 |
| t selected | pH selected | T selected | Stirring speed selected |
| Variables | Coded and Non-Coded Levels | ||||
|---|---|---|---|---|---|
| −α −1.1892 | −1 | 0 | +1 | +α +1.1892 | |
| Adsorbent mass (mg), X1 | 20.5 | 30 | 80 | 130 | 139.5 |
| Initial caffeine concentration (mg/L), X2 | 8.1 | 10 | 20 | 30 | 31.9 |
| Terms CCD | |||||
| Replicas axial points | 2 | ||||
| Replicas central points | 2 | ||||
| Total tests | 14 | ||||
| Model | Equation | Units |
|---|---|---|
| PFO | (1/min) (mg/g) | |
| PSO | (g/mg min) (mg/g) | |
| Elovich | (g/mg) (mg/g min) | |
| IPD | (mg/g min0.5) I (mg/g) |
| Oxide (wt.%) | Raw Clay | Na–Bent–60 | Na–Bent–400 | Montmorillonite * |
|---|---|---|---|---|
| SiO2 | 62.09 | 61.13 | 61.98 | 48.24–65.07 |
| Al2O3 | 17.73 | 17.23 | 17.89 | 14.73–24.54 |
| Fe2O3 | 9.57 | 8.93 | 8.98 | 0.07–6.87 |
| CaO | 3.67 | 1.80 | 1.99 | 0.06–3.74 |
| MgO | 2.99 | 2.44 | 2.48 | 1.64–7.38 |
| K2O | 1.78 | 1.69 | 1.85 | 0.03–3.33 |
| Na2O | 0.25 | 2.35 | 2.34 | 0.00–3.87 |
| MnO | 0.19 | 0.21 | 0.23 | 0.93–2.15 |
| Sample | SBET m2/g | SExt m2/g | Sμp m2/g | Vμp cm3/g | V0.99 cm3/g | pHPZC |
|---|---|---|---|---|---|---|
| Na–Bent–60 | 83.1 | 52.6 | 30.5 | 0.0121 | 0.0101 | 7.6 |
| Na–Bent–200 | 77.8 | 48.3 | 29.5 | 0.0111 | 0.0967 | 8.3 |
| Na–Bent–300 | 73.6 | 46.1 | 27.5 | 0.0106 | 0.0967 | 8.7 |
| Na–Bent–400 | 66.7 | 40.4 | 26.3 | 0.0093 | 0.0886 | 8.8 |
| Na–Bent–500 | 57.1 | 31.1 | 26.0 | 0.0071 | 0.0650 | 8.9 |
| Run | Values | Result | |||
|---|---|---|---|---|---|
| Coded | Experimental | Removal (%) | |||
| X1 | X2 | X1 | X2 | Y | |
| 1 | −α | 0 | 20.5 | 20.0 | 79.99 |
| 2 | 0 | 0 | 80.0 | 20.0 | 94.79 |
| 3 | +1 | −1 | 130.0 | 10.0 | 95.10 |
| 4 | 0 | +α | 80.0 | 31.9 | 94.38 |
| 5 | 0 | +α | 80.0 | 31.9 | 94.12 |
| 6 | −1 | −1 | 30.0 | 10.0 | 82.98 |
| 7 | −α | 0 | 20.5 | 20.0 | 80.60 |
| 8 | 0 | −α | 80.0 | 8.1 | 89.29 |
| 9 | 0 | −α | 80.0 | 8.1 | 91.70 |
| 10 | +α | 0 | 139.5 | 20.0 | 95.20 |
| 11 | +1 | +1 | 130.0 | 30.0 | 95.28 |
| 12 | −1 | +1 | 30.0 | 30.0 | 84.30 |
| 13 | 0 | 0 | 80.0 | 20.0 | 94.79 |
| 14 | +α | 0 | 139.5 | 20.0 | 95.20 |
| Source | Sum of Squares | df a | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 449.45 | 5 | 89.89 | 71.66 | <0.0001 b |
| X1 | 354.99 | 1 | 354.99 | 283.02 | <0.0001 b |
| X2 | 11.27 | 1 | 11.27 | 8.98 | 0.0171 b |
| X1×2 | 0.32 | 1 | 0.32 | 0.26 | 0.6245 c |
| X12 | 76.66 | 1 | 76.66 | 61.11 | <0.0001 b |
| X22 | 4.86 | 1 | 4.86 | 3.87 | 0.0846 c |
| Residual | 10.03 | 8 | 1.25 | ||
| Lack of fit | 6.91 | 3 | 2.30 | 3.69 | 0.0971 b |
| Pure Error | 459.48 | 5 | 0.62 | ||
| R2 | 0.9782 | ||||
| Adjusted R2 | 0.9645 |
| mads mg | mg/L | Removal (%) | Error % | |
|---|---|---|---|---|
| Experimental | Predicted | |||
| 80.0 | 15.0 | 91.64 | 93.51 | 1.87 |
| 100.0 | 20.0 | 93.48 | 96.05 | 0.42 |
| 111.8 | 21.8 | 94.77 | 96.54 | 1.77 |
| 120.0 | 25.0 | 91.97 | 96.53 | 4.56 |
| Model | Equation | Parameters |
|---|---|---|
| Langmuir | = 0.069 ± 0.005 L/mg = 80.331 ± 2.110 mg/g R2 = 0.991 | |
| Freundlich | = 10.469 ± 1.780 (mg/g) (L/mg)1/n = 2.299 ± 0.234 R2 = 0.912 | |
| Redlich–Peterson | = 4.356 ± 0.104 L/g = 0.018 ± 0.002 (L/mg)βRP = 1.241 ± 0.023 R2 = 0.999 |
| Model | Parameters | |||
|---|---|---|---|---|
| 10 | 22 | 30 | ||
| PFO | (1/min) | 4.01 ± 0.56 | 0.92 ± 0.1 | 1.08 ± 0.07 |
| (mg/g) | 3.68 ± 0.04 | 8.13 ± 0.12 | 11.59 ± 0.10 | |
| R2 | 0.851 | 0.835 | 0.925 | |
| PSO | (g/mg min) | 1.86 ± 0.22 | 0.18 ± 0.02 | 0.16 ± 0.01 |
| (mg/g) | 3.76 ± 0.02 | 8.49 ± 0.08 | 11.97 ± 0.06 | |
| R2 | 0.942 | 0.938 | 0.982 | |
| Elovich | (mg/g min) | 4.13 × 106 ± 1.83 × 106 | 486.54 ± 150.18 | 2271.94 ± 1256.11 |
| (g/mg) | 5.33 ± 0.13 | 1.16 ± 0.04 | 0.93 ± 0.06 | |
| R2 | 0.995 | 0.964 | 0.928 | |
| IPD Linear zone 1 | (mg/g min−0.5) (mg/g) R2 | 0.68 ± 0.03 | 2.27 ± 0.10 | 4.30 ± 0.40 |
| 2.45 ± 0.31 | 3.81 ± 0.10 | 3.41 ± 0.42 | ||
| 0.991 | 0.991 | 0.958 | ||
| IPD Linear zone 2 | 0.17 ± 0.01 | 0.47 ± 0.08 | 0.43 ± 0.03 | |
| 3.23 ± 0.01 | 6.53 ± 0.24 | 9.74 ± 0.11 | ||
| 0.991 | 0.888 | 0.957 | ||
| IPD Linear zone 3 | 0.03 ± 0.01 | 0.071 ± 0,01 | 0.05 ± 0.01 | |
| 3.69 ± 0.01 | 8.15 ± 0.08 | 11.64 ± 0.04 | ||
| 0.965 | 0.705 | 0.997 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Jaramillo, J.A.; Carrero, J.I.; Sanabria-González, N.R. Caffeine Adsorption on a Thermally Modified Bentonite: Adsorbent Characterization, Experimental Design, Equilibrium and Kinetics. Colloids Interfaces 2024, 8, 26. https://doi.org/10.3390/colloids8020026
Quintero-Jaramillo JA, Carrero JI, Sanabria-González NR. Caffeine Adsorption on a Thermally Modified Bentonite: Adsorbent Characterization, Experimental Design, Equilibrium and Kinetics. Colloids and Interfaces. 2024; 8(2):26. https://doi.org/10.3390/colloids8020026
Chicago/Turabian StyleQuintero-Jaramillo, Javier A., Javier Ignacio Carrero, and Nancy R. Sanabria-González. 2024. "Caffeine Adsorption on a Thermally Modified Bentonite: Adsorbent Characterization, Experimental Design, Equilibrium and Kinetics" Colloids and Interfaces 8, no. 2: 26. https://doi.org/10.3390/colloids8020026