Highlights
What are the main findings?
- The predictors included in the final nomogram model were hypoproteinemia, long-term combined antibiotic use, intubation times, mechanical ventilation days, and tracheotomy/intubation.
- The area under the curve (AUC) was 0.937 (95% CI: 0.902–0.972) and 0.925 (0.867–0.982) in the training and validation datasets, respectively, suggesting that the model demonstrated effective discrimination. Our model also demonstrated strong concordance performance and clinical applicability.
What is the implication of the main finding?
- Using this nomogram model, clinicians can assess VAP risk in elderly ICU patients and identify those at high risk.
- External validation of the nomogram model in larger populations is still required.
Abstract
Background: Ventilator-associated pneumonia (VAP) causes heavy losses in terms of finances, hospitalization, and death for elderly patients in the intensive care unit (ICU); however, the risk is difficult to evaluate due to a lack of reliable assessment tools. We aimed to create and validate a nomogram to estimate VAP risk to provide early intervention for high-risk patients. Methods: Between January 2016 and March 2021, 293 patients from a tertiary hospital in China were retrospectively reviewed as a training set. Another 84 patients were enrolled for model validation from April 2021 to February 2022. Least absolute shrinkage and selection operator (LASSO) regression and multivariable logistic regression analysis were employed to select predictors, and a nomogram model was constructed. The calibration, discrimination, and clinical utility of the nomogram were verified. Finally, a web-based online scoring system was created to make the model more practical. Results: The predictors were hypoproteinemia, long-term combined antibiotic use, intubation time, length of mechanical ventilation, and tracheotomy/intubation. The area under the curve (AUC) was 0.937 and 0.925 in the training and validation dataset, respectively, suggesting the model exhibited effective discrimination. The calibration curve demonstrated high consistency with the observed result and the estimated values. Decision curve analysis (DCA) demonstrated that the nomogram was clinically applicable. Conclusions: We have created a novel nomogram model that can be utilized to anticipate VAP risk in elderly ICU patients, which is helpful for healthcare professionals to detect patients at high risk early and adopt protective interventions.
1. Introduction
Ventilator-associated pneumonia (VAP) refers to pneumonia arising more than 48 h after tracheotomy or endotracheal intubation for mechanical ventilation. VAP also includes pneumonia that occurs within 48 h after the removal of mechanical ventilation and extubation. In intensive care units (ICUs), mechanical ventilation is commonly used to save patients’ lives. However, VAP in ICUs is a complicated and multifaceted clinical disorder that has been linked to high morbidity (4–61%), high mortality (40–43%), and substantial therapeutic costs [1,2,3,4]. The occurrence of VAP exerts a financial burden on the healthcare sector and increases the need for medical supplies [5]. The onset of VAP entails extra health costs ranging from $5000 to $40,000 for each patient and extends hospital stays by 6–30 days [6].
Common risk factors for VAP may include sex, Acute Physiology and Chronic Health Evaluation (APACHE) II score, hypoproteinemia, state of consciousness, prior antibiotic therapy, age, intubation times, mechanical ventilation days, tracheotomy, and underlying diseases such as diabetes and chronic obstructive pulmonary disease [7,8,9,10,11]. Furthermore, previous studies have shown that oral care, bed elevation of 30–45°, and continuing aspiration of subglottic secretions might decrease VAP risk [12,13,14]. The number of elderly patients admitted to ICUs is rising with the aging population. In 1992, 2007, and 2022, the average ages of ICU patients in three extensive studies were 51, 60.7, and 64 years old, respectively [15,16,17]. The incidence of VAP elevates with age. VAP risk increased over 1.15-fold with every 1-year increase, according to Liu et al. [10]. Therefore, it is of great significance to identify high-risk groups regarding VAP, especially in elderly patients, because effective measures can be taken early to reduce the occurrence of VAP.
To the best of our knowledge, there are few validated tools to assess the risk of VAP in elderly patients in the ICU. The nomogram is an intuitive and convenient predictive tool that can quantitatively calculate the hazard ratio of individual clinical events [18,19,20]. It has been widely used in various medical fields [21,22,23]. Consequently, the objectives of this study were to evaluate the significant predictor variables associated with VAP among elderly ICU patients and to construct and validate a nomogram for estimating VAP risk.
2. Methods
2.1. Study Design and Study Sample
Between January 2016 and March 2021, the data of 293 elderly ICU mechanical ventilation patients from the Wenzhou Hospital of Integrative Medicine were retrospectively reviewed as a training set, and another dataset of 84 elderly ICU mechanical ventilation patients from the same hospital between April 2021 and February 2022 was collected for model validation. If a patient had multiple admissions, only the initial entry was evaluated.
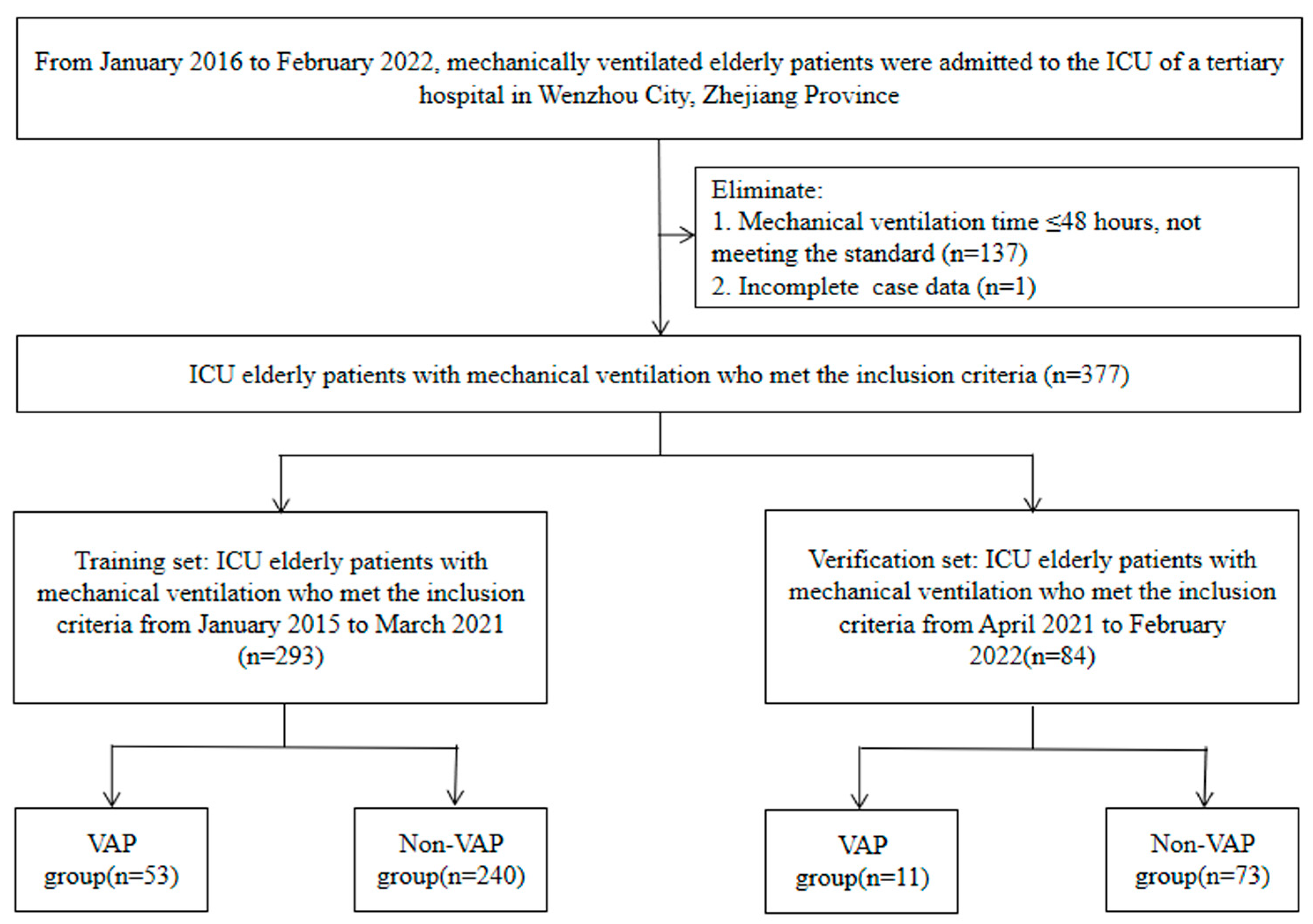
The inclusion criteria included: (1) age 60 years or older; (2) complete clinical data; (3) mechanical ventilation time > 48 h; (4) if multiple VAP infections during hospitalization had occurred, only the first infection was included. We further excluded patients who were admitted to the ICU with a VAP infection. In the end, 137 patients whose mechanical ventilation time did not meet the criteria and 1 patient whose medical record was incomplete were excluded. Finally, 377 patients were included.
Clinical information was collected retrospectively through the real-time hospital infection surveillance system, the software program Zexin (version 3.0), and electronic medical records. This real-time surveillance system could collect data from multiple hospital databases and provide warnings of hospital infection through information such as positive bacterial culture and fever. The cut-off time for the survey was 48 h after extubation in the non-VAP group and the date of infection in the VAP group. The time range for mechanical ventilation days, albumin levels, state of consciousness, intubation times, and tracheotomy/intubation values was from the start of mechanical ventilation to the end of the investigation. The time range for antibiotic use was from admission to the end of the investigation. APACHE II score was taken after the patient was admitted to the ICU.
As a standard rule of thumb, when creating a predictive model for binary or time-to-event outcomes, at minimum, ten events must be collected for each predictor variable (i.e., each β term in the regression equation), then reviewed for inclusion in the predicting model formula; this commonly requires at least ten events per variable (10 EPV) [24,25,26]. In this study, the final model contained five predictors.
The screening flow chart is shown in Figure 1. The ethics committee of the Wenzhou Hospital of Integrative Medicine (2022-L079) authorized this research. Due to the retrospective nature of this investigation, the requirement of informed consent was waived by the ethics committee of the Wenzhou Hospital of Integrative Medicine.
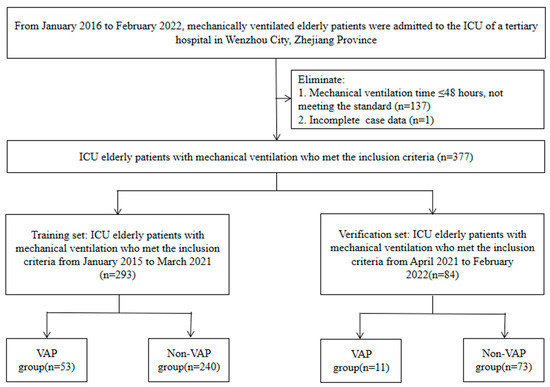
Figure 1.
Case screening flow chart.
2.2. Assessment of Potential Predictors
Clinical information, including gender, APACHE II score, hypoproteinemia (defined as a blood albumin concentration below 30 g per liter), long-term combined use of antibiotics, state of consciousness, advanced age, intubation times, mechanical ventilation days, tracheotomy/intubation, and basic disease status (hypertension, diabetes, cerebral infarction, cardiac insufficiency, malignant tumor, Parkinson’s disease, Alzheimer’s disease, hepatic insufficiency, renal insufficiency, chronic obstructive pulmonary disease, and respiratory failure) were collected.
According to clinical significance, the cut-off points of hypoproteinemia, advanced age, and long-term combined use of antibiotics were 30 g/L, ≥80 years old, and ≥2 kinds and ≥7 days of usage. For mechanical ventilation days, APACHE II, and other continuous variables, a two-piece linear regression model with a smoothing function was adopted to evaluate the nonlinear associations of the variable with VAP. A log likelihood ratio test was employed to contrast the one-line linear regression model with a two-piecewise linear model.
2.3. Outcome Ascertainment
For the diagnosis of VAP, the clinicians made a preliminary diagnosis, and the hospital’s infection control staff further reviewed it. If there were any disagreements, the two departments would discuss them further to reach a consensus. Based on the 2018 Chinese guidelines for the diagnosis and treatment of hospital-acquired pneumonia and VAP in adults [27], VAP refers to pneumonia arising more than 48 h after tracheotomy or endotracheal intubation for mechanical ventilation. VAP also includes pneumonia that occurs within 48 h after the removal of mechanical ventilation and extubation. A clinical diagnosis of pneumonia can be established via chest X-ray or CT showing new or progressive, infiltrative, solid or ground glass shadows, plus two or more of the following three clinical signs: (1) fever with temperature > 38 degrees; (2) purulent airway discharge; and (3) peripheral blood leukocyte count >10 × 109/L or <4 × 109/L. The VAP concept was consistent with the principles obtained from the Infectious Diseases Society of America and the American Thoracic Society [28].
2.4. Statistical Analysis
Continuous variables that were not distributed normally were presented as medians (interquartile range, IQR), and the Mann–Whitney U test was used to compare between-group differences. The categorical variables were presented as n (%), and the between-group comparison was made using the chi-square test. Due to the relatively small percentage of missing data (<5%), this study excluded patients with incomplete data. Internal validation was conducted via the repeated self-sampling method (bootstrap of 1000 times), and further validation was conducted with the data of the validation set.
Feature selection and model development. We used the least absolute shrinkage and selection operator (LASSO) regression approach to select the optimum predictors from the training set’s ten potential predictors. Tenfold cross-validation was applied to determine the optimum value of the penalty parameter λ. The selection criterion for the optimal lambda parameter was lambda.1se. (maximum lambda for error mean within one standard deviation of the minimum). Multivariable logistic regression was used to construct a nomogram model using selected variables.
Model performance evaluation. Calibration and discrimination were used to evaluate the nomogram’s performance in predicting VAP risk. The discriminatory ability of the nomogram was evaluated using the receiver operating characteristic (ROC) curve. Generally, an area under the curve (AUC) between 0.71 and 0.90 indicates moderate discrimination, while above 0.90 indicates high discrimination. The calibration capacity of the nomogram was validated utilizing the calibration curve and the Hosmer–Lemeshow goodness-of-fit test, which measures the level of coincidence between the predictive value and the actual estimated values.
Clinical validity evaluation. We conducted a decision curve analysis (DCA) to assess the clinical validity of the nomogram. DCA is a tool that evaluates the net clinical benefit at various threshold probabilities [29,30]. By subtracting the percentage of false-positive patients from the percentage of true-positive patients and weighing the relative harms of not intervening with the negative consequences of unnecessary interventions, the net benefits were calculated [29]. Moreover, the maximum Youden index was used to determine the optimal clinical cutoff value (sensitivity + specificity-1).
Statistical analysis was conducted using R (version 3.4.3) and EmpowerStats (version 3.0, X&Y solutions, Inc., Boston, MA, USA). All statistical tests were two-sided, and a p-value < 0.05 was considered as statistically significant.
3. Results
3.1. Sample Characteristics
A total of 377 elderly ICU patients with mechanical ventilation were included in the current study, and 64 had VAP. VAP was observed in 53 (18.1%) and 11 (13.1%) patients in the training set and the validation set, respectively (Figure 1). Table 1 displays the clinical features of the participants.

Table 1.
Comparison of general features between the training set and the validation set.
3.2. Variable Selection
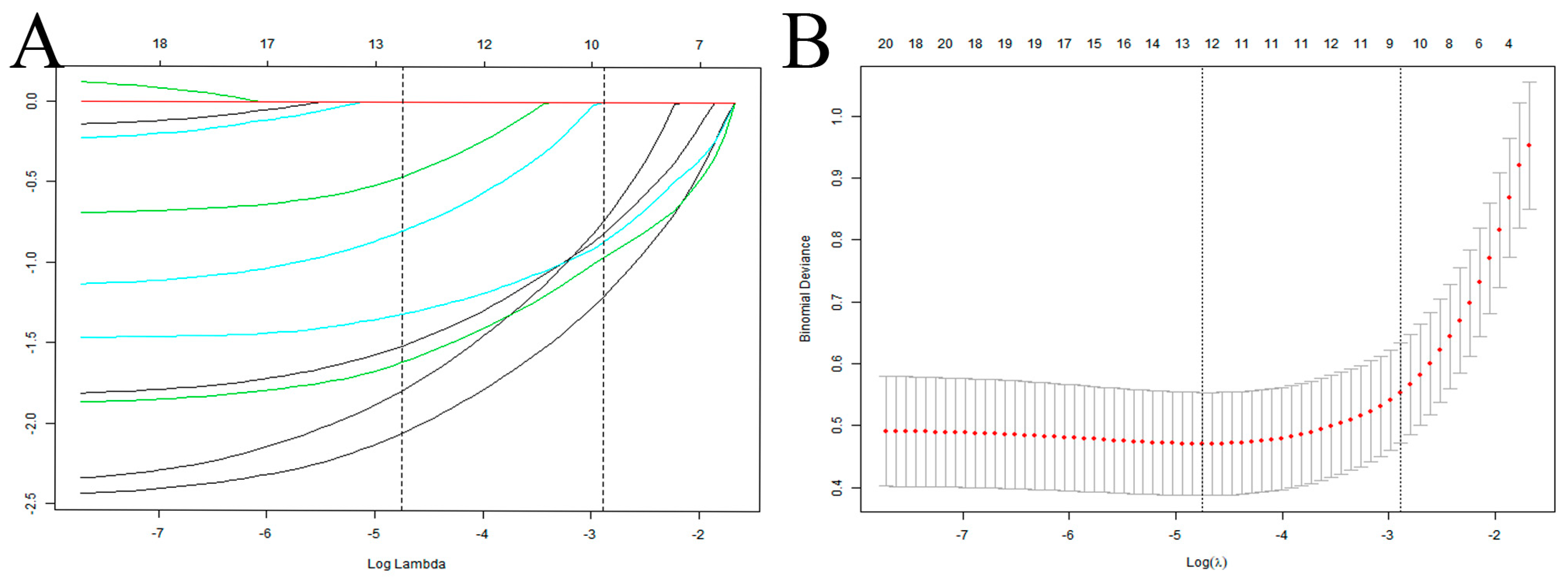
In this study, 10 variables were dimensionally reduced using LASSO regression, and 5 predictors were selected: hypoproteinemia, long-term combined antibiotics, intubation times, mechanical ventilation days, and tracheotomy/intubation (Figure 2).
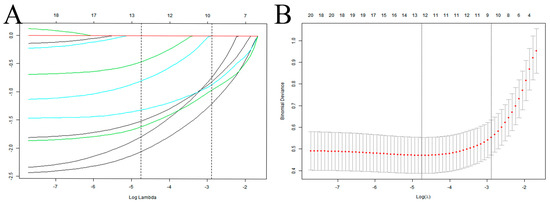
Figure 2.
Selection of predictive variables for VAP infection in elderly ICU patients using LASSO regression. (A) Profiles of the LASSO coefficients for the ten candidate variables. (B) Tenfold cross-validation is used to identify the optimal penalization coefficient (λ) for the LASSO model. Note: The left vertical line indicates the minimum error, while the right vertical line reflects the one standard error of the minimum criteria (1SE criterion).
3.3. Model Development
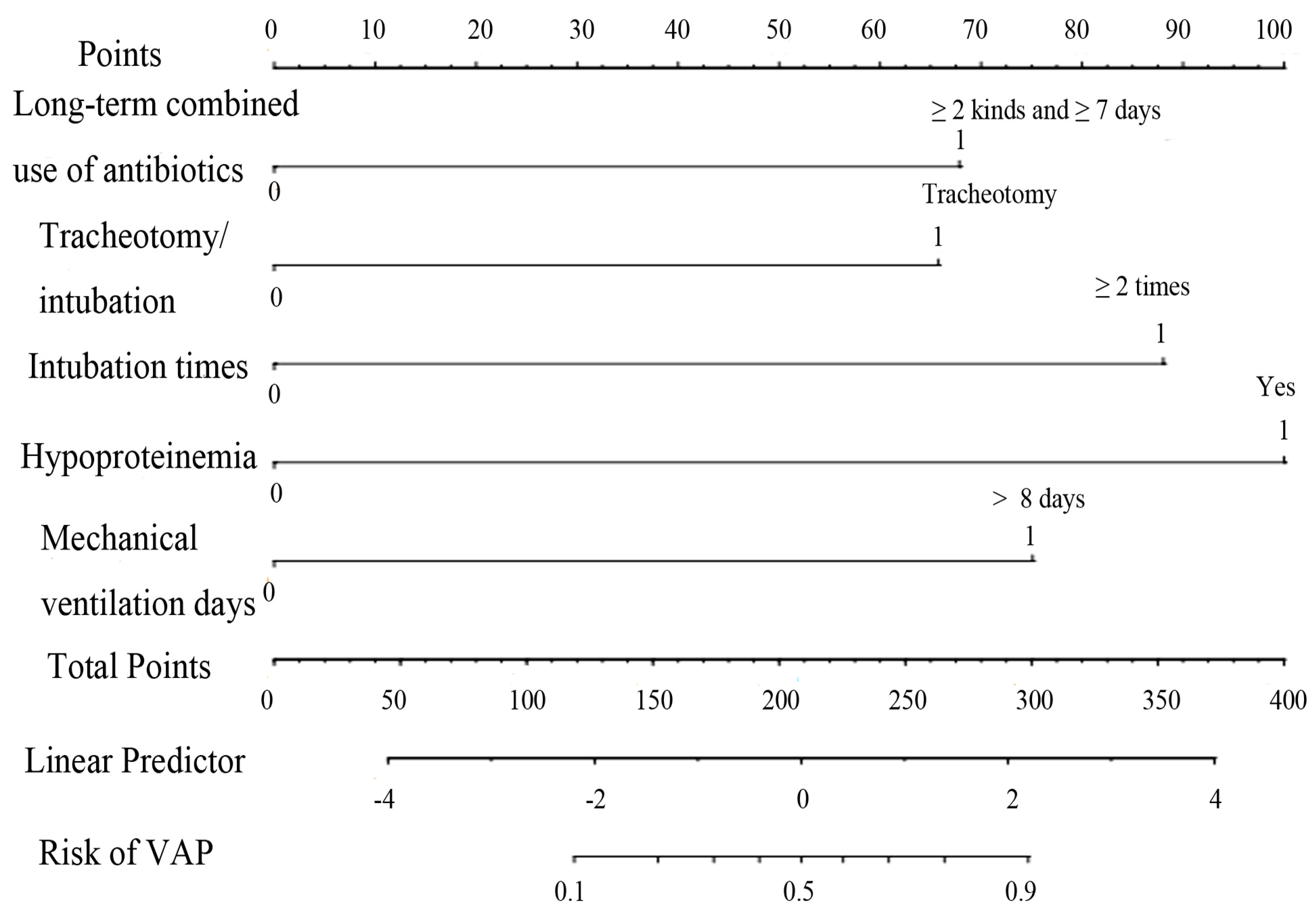
Hypoproteinemia, long-term combined use of antibiotics, intubation times, mechanical ventilation days, and tracheotomy/intubation were reliable predictive variables of VAP in ICU elderly patients independently (p < 0.05) (Table 2), and a nomogram model of VAP in elderly ICU patients was constructed (Figure 3). To make the operation of the nomogram more practical and convenient, we created a web-based online scoring system. Users can open the URL (https://vapmodel.shinyapps.io/dynnomapp/) (accessed on 12 December 2023) when needed and enter variable information or scan a QR code with their cell phone (Figure S1) to obtain the predicted probability of VAP. For example, for an elderly patient who had undergone mechanical ventilation for 12 days, with the combined use of two antibiotics for 10 days, and hypoproteinemia, using the online scoring system indicated that the patient had a VAP risk of approximately 70% (accessed on 6 November 2022) (Figure S2).

Table 2.
Multivariate logistic analysis of VAP infection in ICU elderly patients.
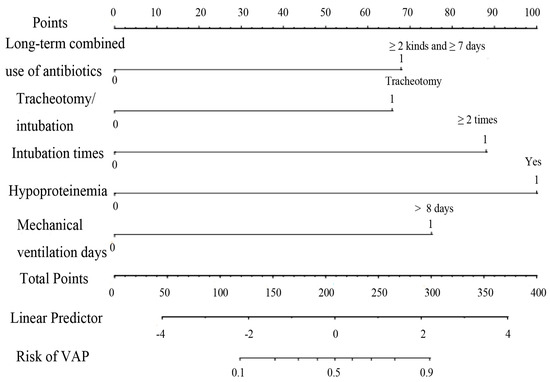
Figure 3.
Nomogram of VAP risk prediction for elderly ICU patients with mechanical ventilation.
3.4. Model Performance
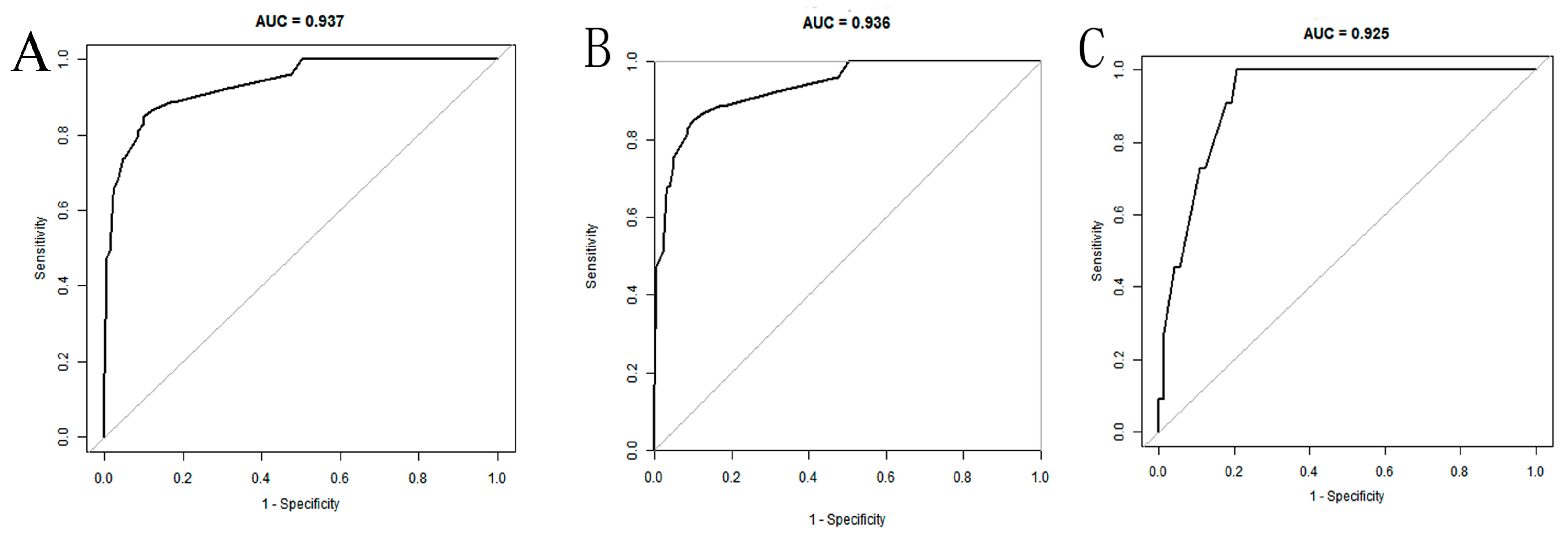
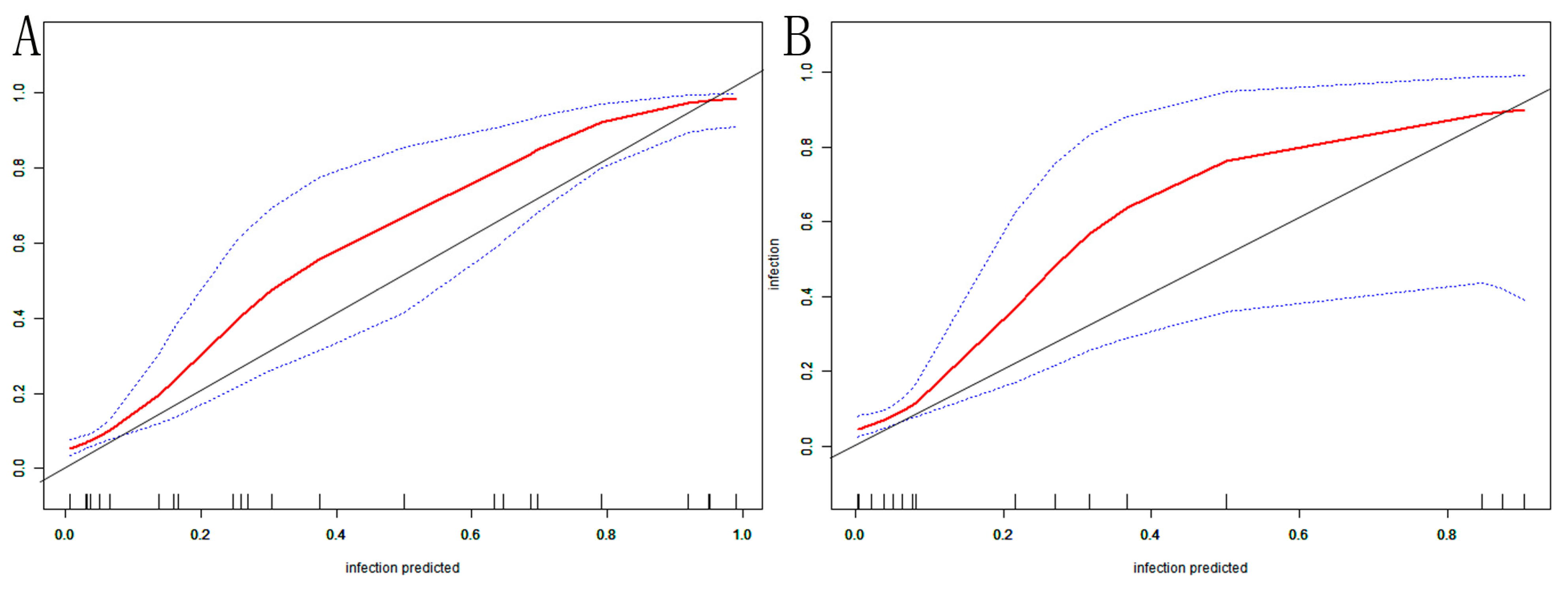
The AUC of the nomogram was 0.937 (95% CI: 0.902–0.972) in the training set (Figure 4A). At the highest Youden index point, the optimal threshold was 0.207; the corresponding specificity was 90.00%, and the sensitivity was 84.91%. The repeated self-sampling method (bootstrap 1000 times) was used for internal validation of the nomogram, and the AUC was 0.936 (95% CI: 0.901–0.972) (Figure 4B). The AUC was 0.925 (95% CI: 0.867–0.982) in the validation set, indicating that the model had good discrimination (Figure 4C). Hosmer–Lemeshow goodness-of-fit test in the training set p = 0.265 > 0.05, validation set p = 0.956 > 0.05. The calibration curve and the Hosmer–Lemeshow test showed high consistency with the observed result and the predicted values in the training set (Figure 5A). This was further verified in the validation set (Figure 5B).
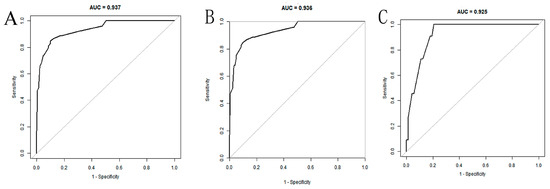
Figure 4.
ROC curves of the nomogram: (A) training set; (B) training set (with bootstrap, 1000 times); (C) validation set.
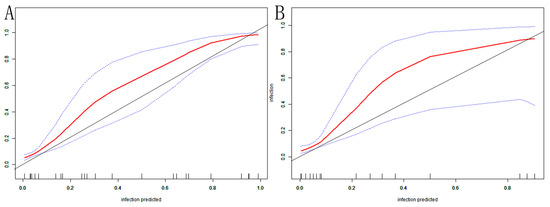
Figure 5.
VAP nomogram calibration curve in elderly ICU patients: (A) training set; (B) validation set. The X-axis depicts the predictive risk of VAP in elderly ICU patients; the Y-axis depicts the actual risk of VAP in elderly ICU patients; the black line depicts the best estimation of the optimal model; the red color is the calibration curve; the blue interval is the 95% CI of the calibration curve.
3.5. Application in Clinical Practice
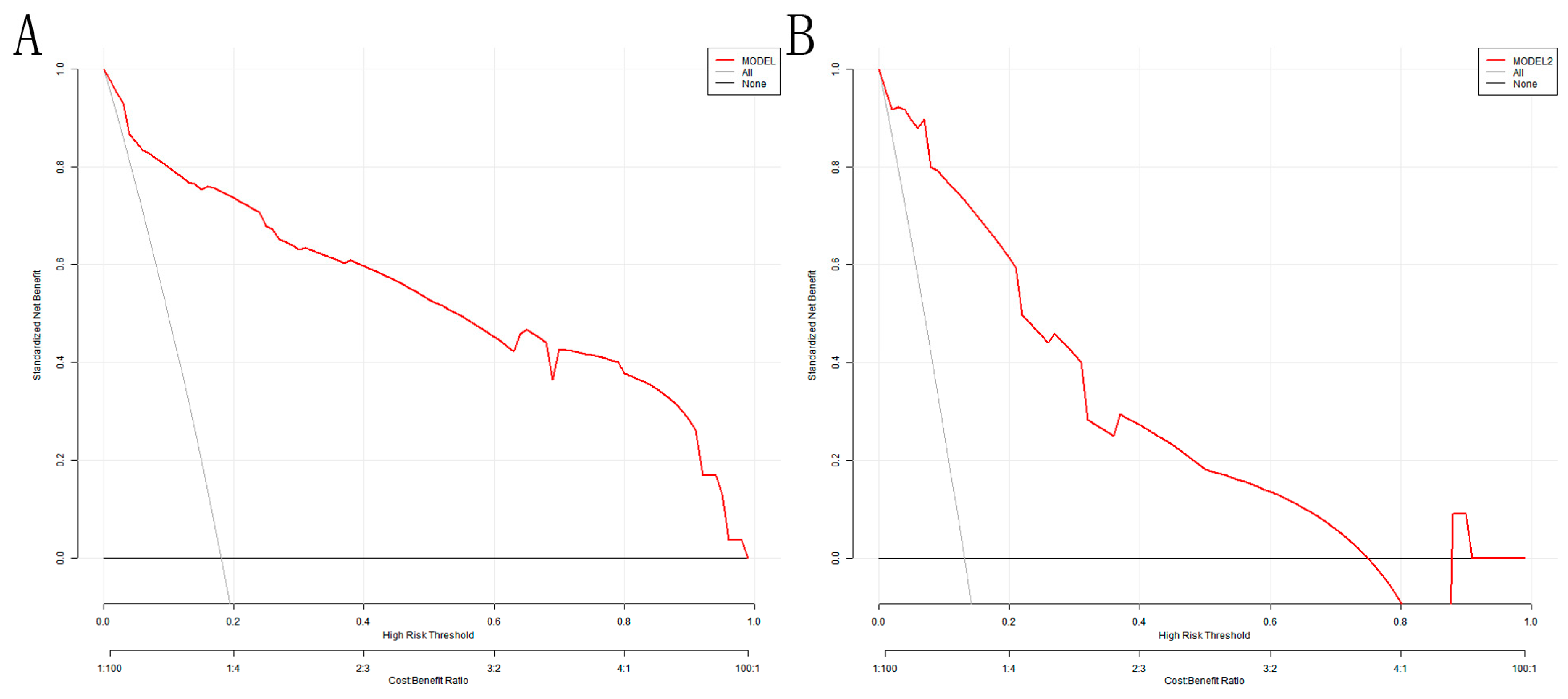
The DCA analysis shows that the model’s threshold probabilities reached 0–0.98 and 0–0.75 in the training and validation sets, respectively, which could produce net clinical benefits (Figure 6A,B). The optimal threshold of 0.207 for the training set was substituted into Figure 6A, and the clinical net benefit was approximately 75%, indicating a good clinical application value.
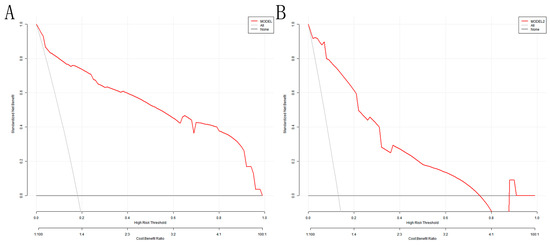
Figure 6.
DCA for the nomogram model: (A) training set; (B) validation set. The Y-axis depicts the net benefit; the gray line in the figure represents the occurrence of VAP in all elderly ICU patients with mechanical ventilation; the black horizontal line indicates that none of the elderly ICU patients developed VAP, and the red represents the decision curve of the risk assessment model.
4. Discussion
In this study, we constructed and validated a nomogram model combining tracheotomy/intubation, intubation times, mechanical ventilation days, long-term combined use of antibiotics, and hypoproteinemia. The area under the curve (AUC) was 0.937 (95% CI: 0.902–0.972) and 0.925 (0.867–0.982) in the training and validation dataset, respectively. The model will help clinicians assess the risk of VAP in elderly ICU patients and identify high-risk patients, which is conducive to taking the necessary interventions to reduce the incidence of VAP early.
Shuhua Li et al. [31] recently established and validated a nomogram model for ventilator-associated pneumonia in elderly ICU patients, but the AUC for the training and validation groups was 0.859 (95% CI: 0.828–0.890) and 0.813 (95% CI: 0.700–0.850), respectively. Additionally, they only included variables with p < 0.05 in the univariate analysis in the logistic regression analysis. Zahar et al. [32] developed and validated a VAP model in ICU patients aged > 16 years, The AUC of their model in the training and validation set was 0.881 and 0.848, respectively, but the study did not show the nomogram and calibration curve, and there was no clinical effectiveness evaluation. Wu et al. [33] investigated a VAP prediction model; however, their focus was acute respiratory distress syndrome (ARDS) patients, and they performed a secondary analysis of the early versus delayed enteral nutrition using ARDSNet. In this model, the area under the curve was 0.744, lacking internal and external validation. In elderly ICU patients, few effective tools exist for assessing VAP risk. This research aimed to develop and validate an operable nomogram model.
The predictors included in the nomogram model were tracheotomy/intubation, intubation times, mechanical ventilation days, long-term combined use of antibiotics, and hypoproteinemia. Based on the calibration curve and the AUC results, the nomogram exhibited strong concordance performance as well as good discrimination. Additionally, DCA results in our study demonstrated a good clinical utility of the prediction model. Several studies have proved the advantages of the most recent DCA approach and support its usage [34,35].
In the ICU, the invasive factors of VAP associated with mechanical ventilation include tracheostomy, reintubation, and duration of mechanical ventilation. Tracheotomy impaired the trachea’s normal anatomical and physiological function, corresponding to a previous systematic review performed by Ding et al. [36]. The respiratory system communicates with the external environment immediately, bypassing typical respiratory defense systems, including the oropharynx and cilia; this weakens the cough and reflex role of the trachea, allowing infectious agents to easily colonize the tracheal tube and form biofilm, leading to an increase in the opportunity for VAP infection [37,38,39]. Reintubation was related to the inhalation of potentially pathogenic organisms in the oropharynx [36], and was a risk variable for VAP [40]. The increased number of intubations would damage the airway mucosa and reduce the cilia function, affecting the incidence of VAP. Furthermore, the prevalence of VAP increased from 5% in patients who experienced one day of mechanical ventilation to 65% in patients who experienced 30 days of mechanical ventilation [41]. Moreover, long-term mechanical ventilation would cause damage to the airway mucosa and local cilia function of patients. As mechanical ventilation duration increased, the frequency of procedures, including sputum suction, was elevated, the airway mucosa of patients was damaged more seriously, the cough reflex of patients would be weakened, the barrier function of the respiratory tract was damaged, and the risk of VAP increased. Therefore, reducing invasive operations related to mechanical ventilation, testing the weaning scheme, and minimizing mechanical ventilation time are essential measures to control VAP.
In addition to invasive operations, prior long-term combined use of antibiotics was also a potential independent risk variable for VAP, similar to some prior investigations. The ICU houses the majority of severely ill patients, and the chance of antibiotic administration is extremely high. Antibiotic overuse may modify the infestation of normal microorganisms, resulting in invasion by opportunistic pathogens or drug resistance formation in numerous bacteria strains, hence raising the prevalence of VAP [42,43]. Therefore, it is suggested that care providers follow the principle of antibacterial drug use, carry out a antimicrobial drug susceptibility test before using antibacterial drugs, use antibacterial drugs rationally according to the antimicrobial drug susceptibility test results, and pay attention to the concentration and dosage of antibacterial drugs.
Among patients with VAP, malnutrition plays a key role in addition to medical factors. Albumins are incorporated into all predicting–nutritional variables, and hypoproteinemia is pointed out as a factor of malnutrition, as well as indicating a serious chance of complications [44]. Elderly patients have more underlying diseases than young patients, often have gastrointestinal dysfunction, and are more prone to albumin reduction. Hypoproteinemia may lead to lymphocytopenia, which reduces the immune system’s defensive capacity [45], and in patients with decreased immune resistance, the promotion of the proliferation of drug-resistant bacteria is easy, inducing infection; in particular, severe hypoproteinemia accompanied by pulmonary infection generally had a poor prognosis [46]. The recommendations of the European society for clinical nutrition and metabolism validate the use of nutritional therapy [47]. The variables finally included in this model had clinical significance and a sufficient theoretical basis.
The current study has several advantages. First, the nomogram model was used to establish a diagnostic model for the occurrence of VAP in elderly ICU patients, and a web-based online scoring system was used, which was more intuitive, practical and operable, and conducive to early intervention. Second, a comprehensive evaluation of the model was carried out in all aspects: in addition to the ROC curves showing strong concordance performance, we also used calibration and DCA curves to reflect the model’s calibration degree and clinical validity. Third, internal verification was performed: repeated self-sampling method (bootstrap 1000 times) and verification data divided by the time were used for verification. Temporal validation is a prospective evaluation of a model independent of the modeling data and the modeling process, so it can sometimes be viewed as an external validation [48]. Fourth, it is easy for clinicians to obtain information on the five predictors in our model through electronic medical records. There is no need to increase the patient’s additional examination items or the doctor’s other recording work; these features made our nomogram a useful clinical tool.
However, this study still had limitations. First, this study was a single-center retrospective investigation that excluded a small number of patients with incomplete records, which might be subject to selection bias, and only temporal validation was performed without external validation. Therefore, multicenter prospective studies and external validation should be carried out later. Second, the count of predictors was limited, and only a few readily available and actionable predictors were selected. Although the sample size of this study meets the minimum sample size requirements, the sample sizes of the training set and the verification set were generally small. Third, although the diagnosis of VAP was made by a senior clinician and infection control practitioner to avoid bias caused by misclassification, misdiagnosis and missed diagnosis might still have occurred.
5. Conclusions
Our nomogram model showed strong concordance performance, good discrimination, and clinical application value. Using this nomogram model, clinicians can assess VAP risk in elderly ICU patients and identify those at high risk. However, external validation of the nomogram model in larger populations is still required.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/arm92010010/s1, Figure S1; Figure S2.
Author Contributions
W.G. conceived and designed the study. W.G. and Z.T. were responsible for data acquisition. W.G. and Z.C. analyzed and interpreted the data. W.G. drafted the first version of the manuscript. W.L., W.G. and Z.C. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Youth Natural Science Foundation of China (82203984), the Healthy Zhejiang Million Population Cohort Project (K-20230085), the Key Laboratory of Intelligent Preventive Medicine of Zhejiang Province (2020E10004), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2019R01007), the Key Research and Development Program of Zhejiang Province (2020C03002) and the basic science and technology project of Wenzhou Science and Technology Bureau (Project No. Y2020253). The funding source had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the paper for publication.
Institutional Review Board Statement
This study was approved by the Ethics Committee of Wenzhou Hospital of Integrative Medicine (2022-L079), following the principles of the Declaration of Helsinki. Due to the retrospective nature of this investigation, the Ethics Committee of Wenzhou Hospital of Integrative Medicine waived the requirement for informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to confidentiality requirements but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Pozuelo-Carrascosa, D.P.; Herráiz-Adillo, Á.; Alvarez-Bueno, C.; Añón, J.M.; Martínez-Vizcaíno, V.; Cavero-Redondo, I. Subglottic secretion drainage for preventing ventilator-associated pneumonia: An overview of systematic reviews and an updated meta-analysis. Eur. Respir. Rev. 2020, 29, 190107. [Google Scholar] [CrossRef] [PubMed]
- Chouhdari, A.; Shokouhi, S.; Bashar, F.R.; Azimi, A.V.; Shojaei, S.P.; Fathi, M.; Goharani, R.; Sahraei, Z.; Hajiesmaeili, M. Is a low incidence rate of ventilation associated pneumonia associated with lower mortality? A descriptive longitudinal study in Iran. Tanaffos 2018, 17, 110–116. [Google Scholar] [PubMed]
- Sadigov, A.; Mamedova, I.; Mammmadov, K. Ventilator-associated pneumonia and in-hospital mortality: Which risk factors may predict in-hospital mortality in such patients? J. Lung Health Dis. 2019, 3, 8–12. [Google Scholar] [CrossRef]
- Luckraz, H.; Manga, N.; Senanayake, E.L.; Abdelaziz, M.; Gopal, S.; Charman, S.C.; Giri, R.; Oppong, R.; Andronis, L. Cost of treating ventilator-associated pneumonia post cardiac surgery in the National Health Service: Results from a propensity-matched cohort study. J. Intensiv. Care Soc. 2018, 19, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Mathai, A.S.; Phillips, A.; Kaur, P.; Isaac, R. Incidence and attributable costs of ventilator-associated pneumonia (VAP) in a tertiary-level intensive care unit (ICU) in northern India. J. Infect. Public Health 2015, 8, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.d.A.d.; Vilela, C.A.P.; Cezário, R.C.; Almeida, A.B.; Filho, P.G. Ventilator-associated pneumonia in an adult clinical-surgical intensive care unit of a Brazilian university hospital: Incidence, risk factors, etiology, and antibiotic resistance. Braz. J. Infect. Dis. 2008, 12, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, C.; Zhang, S.; Zhong, Y. Risk factors of ventilator-associated pneumonia in critically III patients. Front. Pharmacol. 2019, 10, 482. [Google Scholar] [CrossRef]
- Cui, J.B.; Chen, Q.Q.; Liu, T.T.; Li, S.J. Risk factors for early-onset ventilator-associated pneumonia in aneurysmal subarachnoid hemorrhage patients. Braz. J. Med. Biol. Res. 2018, 51, e6830, Retraction in Braz. J. Med. Biol. Res. 2020, 53, e6830r.. [Google Scholar] [CrossRef]
- van der Kooi, T.I.I.; Boshuizen, H.; Wille, J.C.; de Greeff, S.C.; van Dissel, J.T.; Schoffelen, A.F.; van Gaalen, R.D. Using flexible methods to determine risk factors for ventilator-associated pneumonia in the Netherlands. PLoS ONE 2019, 14, e0218372. [Google Scholar] [CrossRef]
- Liu, Y.; Di, Y.; Fu, S. Risk factors for ventilator-associated pneumonia among patients undergoing major oncological surgery for head and neck cancer. Front. Med. 2017, 11, 239–246. [Google Scholar] [CrossRef]
- Ścisło, L.; Walewska, E.; Bodys-Cupak, I.; Gniadek, A.; Kózka, M. Nutritional status disorders and selected risk factors of ventilator-associated pneumonia (VAP) in patients treated in the intensive care ward—A retrospective study. Int. J. Environ. Res. Public Health 2022, 19, 602. [Google Scholar] [CrossRef] [PubMed]
- Zand, F.; Zahed, L.; Mansouri, P.; Dehghanrad, F.; Bahrani, M.; Ghorbani, M. The effects of oral rinse with 0.2% and 2% chlorhexidine on oropharyngeal colonization and ventilator associated pneumonia in adults’ intensive care units. J. Crit. Care 2017, 40, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, O.; Ersoy, Y.; Kuzucu, C.; Gedik, E.; Togal, T.; Yetkin, F. Assessment of the effectiveness of a ventilator associated pneumonia prevention bundle that contains endotracheal tube with subglottic drainage and cuff pressure monitorization. Braz. J. Infect. Dis. 2017, 21, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Guillamet, C.V.; Kollef, M.H. Is zero ventilatorassociated pneumonia achievable? practical approaches to ventilator-associated pneumonia prevention. Clin. Chest Med. 2018, 39, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. EPIC II group of investigators: International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Yang, R.; Huang, T.; Shen, L.; Feng, A.; Li, L.; Li, S.; Huang, L.; He, N.; Huang, W.; Liu, H.; et al. The use of antibiotics for ventilator-associated pneumonia in the MIMIC-IV database. Front. Pharmacol. 2022, 13, 869499. [Google Scholar] [CrossRef]
- Vincent, J.L.; Bihari, D.J.; Suter, P.M.; Bruining, H.A.; White, J.; Nicolas-Chanoin, M.H.; Wolff, M.; Spencer, R.C.; Hemmer, M. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. JAMA 1995, 274, 639–644. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Yamauchi, Y.; Yasunaga, H.; Takeshima, H.; Hasegawa, W.; Jo, T.; Sasabuchi, Y.; Matsui, H.; Fushimi, K.; Nagase, T. Development of a nomogram for predicting in-hospital mortality of patients with exacerbation of chronic obstructive pulmorary disease. Int. J. Chron. Obstruct Pulmon. Dis. 2017, 12, 1605–1611. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, C.; Zou, B.; Huang, D.; Yu, J.; Cheng, Y.; Meng, X. A nomogram to predict outcomes of lung cancer patients after pneumonectomy based on 47 indicators. Cancer Med. 2020, 9, 1430–1440. [Google Scholar] [CrossRef]
- Wang, S.; Yang, L.; Ci, B.; Maclean, M.; Gerber, D.E.; Xiao, G.; Xie, Y. Development and validation of a nomogram prognostic model for SCLC patients. J. Thorac. Oncol. 2018, 13, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhuo, H.; Yang, G.; Huang, H.; Li, C.; Wang, X.; Zhao, S.; Moliterno, J.; Zhang, Y. Postoperative pneumonia after craniotomy: Incidence, risk factors and prediction with a nomogram. J. Hosp. Infect. 2020, 105, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M. A novel nomogram to predict mortality in patients with stroke: A survival analysis based on the MIMIC-III clinical database. BMC Med. Inform. Decis. Mak. 2022, 22, 92. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Concato, J.; Peduzzi, P.; Holford, T.R.; Feinstein, A.R. Importance of events per independent variable in proportional hazards analysis I. Background, goals, and general strategy. J. Clin. Epidemiol. 1995, 48, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Huang, Y.; Zhang, T.-T.; Bin Cao, B.; Wang, H.; Zhuo, C.; Ye, F.; Su, X.; Fan, H.; Xu, J.-F.; et al. Chinese guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia in adults (2018 Edition). J. Thorac. Dis. 2019, 11, 2581–2616. [Google Scholar] [CrossRef]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Executive summary: Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin. Infect. Dis. 2016, 63, 575–582. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; Xu, F.; Han, D.; Zheng, S.; Zhang, F.; Li, L.; Wang, Z.; Lyu, J.; Yin, H. Risk factor analysis and nomogram for predicting in-hospital mortality in ICU patients with sepsis and lung infection. BMC Pulm. Med. 2022, 22, 17. [Google Scholar] [CrossRef]
- Vickers, A.J.; Van Calster, B.; Steyerberg, E.W. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ 2016, 352, i6. [Google Scholar] [CrossRef]
- Li, S.; Shang, L.; Yuan, L.; Li, W.; Kang, H.; Zhao, W.; Han, X.; Su, D. Construction and Validation of a Predictive Model for the Risk of Ventilator-Associated Pneumonia in Elderly ICU Patients. Can. Respir. J. 2023, 2023, 7665184. [Google Scholar] [CrossRef] [PubMed]
- Zahar, J.-R.; Nguile-Makao, M.; Français, A.; Schwebel, C.; Garrouste-Orgeas, M.; Goldgran-Toledano, D.; Azoulay, E.; Thuong, M.; Jamali, S.; Cohen, Y.; et al. Predicting the risk of documented ventilator-associated pneumonia for benchmarking: Construction and validation of a score. Crit. Care Med. 2009, 37, 2545–2551. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, Y.; Xu, J.; Xie, J.; Zhang, S.; Huang, L.; Huang, Y.; Yang, Y.; Qiu, H. A Ventilator-associated pneumonia prediction model in patients with acute respiratory distress syndrome. Clin. Infect. Dis. 2020, 71, S400–S408. [Google Scholar] [CrossRef] [PubMed]
- Rousson, V.; Zumbrunn, T. Decision curve analysis revisited: Overall net benefit, relationships to ROC curve analysis, and application to case-control studies. BMC Med. Inform. Decis. Mak. 2011, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, Y.; Yang, Z.; Wang, J.; Jin, A.; Wang, W.; Chen, R.; Zhan, S. Incidence, temporal trend and factors associated with ventilator-associated pneumonia in mainland China: A systematic review and meta-analysis. BMC Infect. Dis. 2017, 17, 468. [Google Scholar] [CrossRef]
- Sands, K.M.; Wilson, M.J.; Lewis, M.A.; Wise, M.P.; Palmer, N.; Hayes, A.J.; Barnes, R.A.; Williams, D.W. Respiratory pathogen colonization of dental plaque, the lower airways, and endotracheal tube biofilms during mechanical ventilation. J. Crit. Care 2017, 37, 30–37. [Google Scholar] [CrossRef]
- Machado, M.C.; Webster, T.J. Decreased pseudomonas aeruginosa biofilm formation on nanomodified endotracheal tubes: A dynamic lung model. Int. J. Nanomed. 2016, 11, 3825–3831. [Google Scholar] [CrossRef]
- Elliot, Z.J.; Elliot, S.C. An overview of mechanical ventilation in the intensive care unit. Nurs. Stand. 2018, 32, 41–49. [Google Scholar] [CrossRef]
- Charles, M.P.; Kali, A.; Easow, J.M.; Joseph, N.M.; Ravishankar, M.; Srinivasan, S.; Kumar, S.; Umadevi, S. Ventilator-associated pneumonia. Australas Med. J. 2014, 7, 334–344. [Google Scholar] [CrossRef]
- Othman, A.A.; Abdelazim, M.S. Ventilator-associated pneumonia in adult intensive care unit prevalence and complications. Egypt. J. Crit. Care Med. 2017, 5, 61–63. [Google Scholar] [CrossRef]
- Luyt, C.-E.; Bréchot, N.; Combes, A.; Trouillet, J.-L.; Chastre, J. Delivering antibiotics to the lungs of patients with ventilator-associated pneumonia: An update. Expert Rev. Anti-Infect. Ther. 2013, 11, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.S.; O’grady, N.P. Review: Short and long courses of antibiotics do not differ for mortality in ventilator-associated pneumonia. Ann. Intern. Med. 2014, 160, JC3. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Society of Critical Care Medicine American Society for Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically Ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar]
- Ren, M.; Liang, W.; Wu, Z.; Zhao, H.; Wang, J. Risk factors of surgical site infection in geriatric orthopedic surgery: A retrospective multicenter cohort study. Geriatr. Gerontol. Int. 2019, 19, 213–217. [Google Scholar] [CrossRef]
- Li, F.; Yuan, M.-Z.; Wang, L.; Wang, X.-F.; Liu, G.-W. Characteristics and prognosis of pulmonary infection in patients with neurologic disease and hypoproteinemia. Expert Rev. Anti-Infect. Ther. 2015, 13, 521–526. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Altman, D.G.; Vergouwe, Y.; Royston, P.; Moons, K.G.M. Prognosis and prognostic research: Validating a prognostic model. BMJ 2009, 338, b605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).