Impact of a Food Rebalancing Program Associated with Plant-Derived Food Supplements on the Biometric, Behavioral, and Biological Parameters of Obese Subjects
Abstract
:1. Introduction
2. Materials and Methods
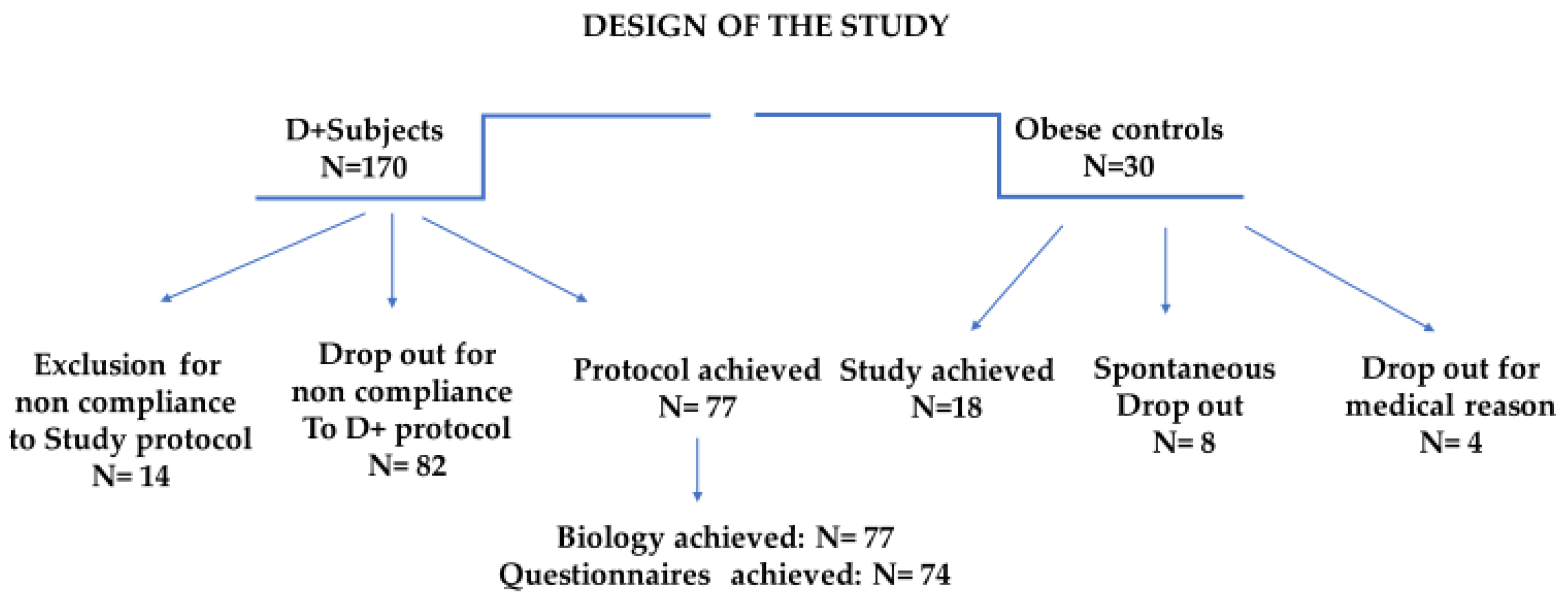
2.1. Study Population, Inclusion Criteria and Enrollment
2.2. The Dietplus® Program
2.3. Exclusion Criteria, Dropout, and Protocol Discontinuation
2.4. Medical and Anthropometric Evaluation
2.5. Biological Evaluation
2.6. Behavioral Evaluation and Monitoring
2.7. Statistical Analysis
3. Results
3.1. Dropout: Analysis and Interpretation
3.2. Comparison of the Two Cohorts
3.3. Evolution of the Control Group
3.4. Effect of The Dietplus® Cure
3.4.1. Anthropometric and Hemodynamic Effects
3.4.2. Behavioral Parameters
- ▪
- Quality of Life
- ▪
- Nutriscore
- ▪
- Physical Activity
- ▪
- Prochaska and Di Clemente scale
3.4.3. Biological and Metabolic Effects
- ▪
- Hematology and protein metabolism
- ▪
- Hydromineral metabolism
- ▪
- Liver enzymes
- ▪
- Renal function
- ▪
- Glucose metabolism
- ▪
- Lipid metabolism
- ▪
- Inflammatory syndrome
- ▪
- Vitamin D
3.4.4. Late Morphometric Measurements (24 Weeks)
4. Discussion
4.1. Common Clinical Problems Observed in Obese Subjects
4.2. Morphometric Parameters
4.3. Behavioral Parameters
4.4. Metabolic Parameters
4.4.1. Glucose Metabolism
4.4.2. Lipid Metabolism
4.4.3. Liver Function
4.4.4. Inflammatory Syndrome
4.4.5. 25-OH-Vitamin D
4.4.6. The Role of the Different Factors of the Dietplus® Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peeters, A.; Barendregt, J.J.; Willekens, F.; Mackenbach, J.P.; Al Mamun, A.; Bonneux, L. NEDCOM, the Netherlands Epidemiology and Demography Compression of Morbidity Research Group. Obesity in adulthood and its consequences for life expectancy: A life-table analysis. Ann. Intern. Med. 2003, 138, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, K.R.; Redden, D.T.; Wang, C.; Westfall, A.O.; Allison, D.B. Years of life lost due to obesity. JAMA 2003, 289, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Drieskens, S. Etat Nutritionnel. Enquête de Santé 2018. Sciensano. Available online: https://www.sciensano.be/en/health-topics/obesity/numbers#overweight-and-obesity-in-belgium (accessed on 15 May 2023).
- Aceves-Martins, M.; López-Cruz, L.; García-Botello, M.; Gutierrez-Gómez, Y.Y.; Moreno-García, C.F. Interventions to Prevent Obesity in Mexican Children and Adolescents: Systematic Review. Prev Sci. 2022, 23, 563–586. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Wu, H.X.; Hu, N.; Zhou, Y.H.; Li, L.; Xiao, F.; Wang, T.; Jiang, H.L.; Xu, S.N.; Huang, B.L.; et al. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus—A systematic review and meta-analysis of randomized control trials. Obes. Rev. 2022, 23, e13435. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Closset, J.; Mehdi, A.; Barea, M.; Buedts, K.; Gelin, M.; Houben, J.J. Results of silastic ring vertical gastroplasty more than 6 years after surgery: Analysis of a cohort of 214 patients. Obes. Surg. 2004, 14, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Shikora, S.A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R.V.; De Luca, M.; Faria, S.L.; Goodpaster, K.P.S.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surg. Obes. Relat. Dis. 2022, 18, 1345–1356. [Google Scholar] [CrossRef]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef]
- Cobiac, L.; Vos, T.; Veerman, L. Cost-effectiveness of Weight Watchers and the Lighten Up to a Healthy Lifestyle program. Aust. N. Z. J. Public 2010, 10, 16–23. [Google Scholar] [CrossRef]
- Thorning, T.K.; Fabre, O.; Legrand, R.; Astrup, A.; Hjorth, M.F. Weight loss and weight loss maintenance efficacy of a novel weight loss program: The retrospective RNPC® cohort. Obes. Med. 2018, 10, 16–23. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA). Safety of pasteurised Akkermansia muciniphila as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 6780. [Google Scholar] [CrossRef]
- Directive 2002/46/CE du Parlement Européen et du Conseil du 10 Juin 2002 Relative au Rapprochement des Législations des États Membres Concernant les Compléments Alimentaires (Version Consolidée du 20 Mars 2021). Available online: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/directive_2002_46_fr_consol_3_2021_0.pdf (accessed on 15 May 2023).
- Saper, R.B.; Eisenberg, D.M.; Phillips, R.S. Common dietary supplements for weight loss. Am. Fam. Phys. 2004, 70, 1731–1738. [Google Scholar]
- Ríos-Hoyo, A.; Gutiérrez-Salmeán, G. New Dietary Supplements for Obesity: What We Currently Know. Curr. Obes. Rep. 2016, 5, 262–270. [Google Scholar] [CrossRef]
- Pourhabibi-Zarandi, F.; Rafraf, M.; Zayeni, H.; Asghari-Jafarabadi, M.; Ebrahimi, A.A. Effects of curcumin supplementation on metabolic parameters, inflammatory factors and obesity values in women with rheumatoid arthritis: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2022, 36, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Messina, V.; Rowland, I.; Frankowska, A.; Bradbury, J.; Smetana, S.; Medici, E. Plant-Based Dairy Alternatives Contribute to a Healthy and Sustainable Diet. Nutrients 2023, 15, 3393. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, P.A.; Granner, M.L.; Conway, J.M.; Ainsworth, B.E.; Dobre, M. Availability of weight-loss supplements: Results of an audit of retail outlets in a southeastern city. J. Am. Diet. Assoc. 2006, 106, 2045–2051. [Google Scholar] [CrossRef]
- Ngondi, J.L.; Etoundi, B.C.; Nyangono, C.B.; Mbofung, C.M.; Oben, J.E. IGOB131, a novel seed extract of the West African plant Irvingia gabonensis, significantly reduces body weight and improves metabolic parameters in overweight humans in a randomized double-blind placebo-controlled investigation. Lipids Health Dis. 2009, 8, 7. [Google Scholar] [CrossRef]
- Onakpoya, I.; Davies, L.; Posadzki, P.; Ernst, E. The efficacy of Irvingia gabonensis supplementation in the management of overweight and obesity: A systematic review of randomized controlled trials. J. Diet Suppl. 2013, 10, 29–38. [Google Scholar] [CrossRef]
- Haaz, S.; Fontaine, K.R.; Cutter, G.; Limdi, N.; Perumean-Chaney, S.; Allison, D.B. Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: An update. Obes Rev. 2006, 7, 79–88. [Google Scholar] [CrossRef]
- Manore, M.M. Dietary supplements for improving body composition and reducing body weight: Where is the evidence? Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 139–154. [Google Scholar] [CrossRef]
- Icken, D.; Feller, S.; Engeli, S.; Mayr, A.; Müller, A.; Hilbert, A.; de Zwaan, M. Caffeine intake is related to successful weight loss maintenance. Eur. J. Clin. Nutr. 2016, 70, 532–534. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Randell, R. Fat burners: Nutrition supplements that increase fat metabolism. Obes. Rev. 2011, 12, 841–851. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R. Impact of the COVID-19 pandemic on clinical research. Nat. Rev. Nephrol. 2020, 16, 562–564. [Google Scholar] [CrossRef]
- AFSCA Allégations Nutritionnelles et de Santé. Bulletin 2014, 58, 1–12. Available online: https://www.favv-afsca.be/viepratique/allegationsnutritionnelles/dossier/ (accessed on 15 May 2023).
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Podstawski, R.; Omelan, A.; Borysławski, K.; Wąsik, J. Relationships between anthropometric and body composition characteristics and age in Polish women over 60 as affected by their socioeconomic and health status and physical activity levels. Front. Physiol. 2023, 14, 1198485. [Google Scholar] [CrossRef]
- Alwash, S.M.; McIntyre, H.D.; Mamun, A. The association of general obesity, central obesity and visceral body fat with the risk of gestational diabetes mellitus: Evidence from a systematic review and meta-analysis. Obes. Res. Clin. Pract. 2021, 15, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Ingenbleek, Y.; Carpentier, Y.A. A prognostic inflammatory and nutritional index scoring critically ill patients. Int. J. Vitam. Nutr. Res. 1985, 55, 91–101. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Chen, H.; Sullivan, G.; Yue, L.Q.; Katz, A.; Quon, M.J. QUICKI is a useful index of insulin sensitivity in subjects with hypertension. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E804–E812. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; DiClemente, C.C.; Norcross, J.C. In search of how people change. Applications to addictive behaviors. Am. Psychol. 1992, 47, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; DiClemente, C.C. Stages of change in the modification of problem behaviors. Prog. Behav. Modif. 1992, 28, 183–218. [Google Scholar] [PubMed]
- Cohen, S.R.; Russell, L.B.; Leis, A.; Shahidi, J.; Porterfield, P.; Kuhl, D.R.; Gadermann, A.M.; Sawatzky, R. More comprehensively measuring quality of life in life-threatening illness: The McGill Quality of Life Questionnaire—Expanded. BMC Palliat. Care 2019, 18, 92. [Google Scholar] [CrossRef]
- Dalle Grave, R.; Soave, F.; Ruocco, A.; Dametti, L.; Calugi, S. Quality of Life and Physical Performance in Patients with Obesity: A Network Analysis. Nutrients 2020, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Cowan, R.; Britton, P.J.; Logue, E.; Smucker, W.; Milo, L. The relationship among the transtheoretical model of behavioral change, psychological distress, and diet attitudes in obesity: Implications for primary care intervention. J. Clin. Psychol. Med. Settings 1995, 2, 249–267. [Google Scholar] [CrossRef]
- Pietrabissa, G.; Sorgente, A.; Rossi, A.; Simpson, S.; Riva, G.; Manzoni, G.M.; Prochaska, J.O.; Prochaska, J.M.; Cattivelli, R.; Castelnuovo, G. Stages of change in obesity and weight management: Factorial structure of the Italian version of the University of Rhode Island Change Assessment Scale. Eat Weight Disord. 2017, 22, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Carrard, I.; Kruseman, M.; Chappuis, M.; Schmutz, N.; Volery, M. Un outil pour évaluer les comportements alimentaires: ESSCA [A tool for assessing eating behaviors: ESSCA]. Rev. Med. Suisse 2016, 12, 591–596. (In French) [Google Scholar]
- Balani, R.; Herrington, H.; Bryant, E.; Lucas, C.; Kim, S.C. Nutrition knowledge, attitudes, and self-regulation as predictors of overweight and obesity. J. Am. Assoc. Nurse Pract. 2019, 31, 502–510. [Google Scholar] [CrossRef]
- De Cosmi, V.; Scaglioni, S.; Agostoni, C. Early Taste Experiences and Later Food Choices. Nutrients 2017, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Zheng, H. Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol. Behav. 2012, 107, 527–532. [Google Scholar] [CrossRef]
- Hercberg, S.; Touvier, M.; Salas-Salvado, J. Group of European scientists supporting the implementation of Nutri-Score in Europe. The Nutri-Score nutrition label. Int. J. Vitam. Nutr. Res. 2022, 92, 147–157. [Google Scholar] [CrossRef]
- Burgess, E.; Hassmén, P.; Pumpa, K.L. Determinants of adherence to lifestyle intervention in adults with obesity: A systematic review. Clin. Obes. 2017, 7, 123–135. [Google Scholar] [CrossRef]
- Monnier, L.; Schlienger, J.L.; Colette, C.; Bonnet, F. The obesity treatment dilemma: Why dieting is both the answer and the problem? A mechanistic overview. Diabetes Metab. 2021, 47, 101192. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.C.; Kenny, P.J. Food addiction: A valid concept? Neuropsychopharmacology 2018, 43, 2506–2513, Erratum in Neuropsychopharmacology 2019, 44, 834. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.; Dalle Grave, R.; Mannucci, E.; Molinari, E.; Compare, A.; Cuzzolaro, M.; Marchesini, G. Complexity of attrition in the treatment of obesity: Clues from a structured telephone interview. Int. J. Obes. Lond. 2006, 30, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Shabana, H.S. Obesity, More than a ‘Cosmetic’ Problem. Current Knowledge and Future Prospects of Human Obesity Genetics. Biochem. Genet. 2016, 54, 1–28. [Google Scholar] [CrossRef]
- Gudzune, K.A.; Beach, M.C.; Roter, D.L.; Cooper, L.A. Physicians build less rapport with obese patients. Obes. Silver Spring 2013, 21, 2146–2152. [Google Scholar] [CrossRef]
- Fox, C.S.; Massaro, J.M.; Hoffmann, U.; Pou, K.M.; Maurovich-Horvat, P.; Liu, C.Y.; Vasan, R.S.; Murabito, J.M.; Meigs, J.B.; Cupples, L.A.; et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 2007, 116, 39–48. [Google Scholar] [CrossRef]
- Zhang, C.; Rexrode, K.M.; van Dam, R.M.; Li, T.Y.; Hu, F.B. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: Sixteen years of follow-up in US women. Circulation 2008, 117, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; Di Clemente, C.C. Toward a Comprehensive Model of Change. In Treating Addictive Behaviors: Processes of Change; Miller, W.R., Heather, N., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 3–27. [Google Scholar]
- King, R.I.; Florkowski, C.M.; Yeo, J.; Walmsley, T.A.; Shand, B.I.; Scott, R.S.; George, P.M. What is the best predictor of the atherogenic LDL subclass phenotype ‘pattern B’ in patients with type 2 diabetes mellitus? Ann. Clin. Biochem. 2011, 48 Pt 2, 166–169. [Google Scholar] [CrossRef]
- Martínez-Fernández, L.; Laiglesia, L.M.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015, 121 Pt A, 24–41. [Google Scholar] [CrossRef]
- Carpentier, Y.A.; Portois, L.; Malaisse, W.J. n-3 fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83 (Suppl. S6), 1499S–1504S. [Google Scholar] [CrossRef] [PubMed]
- Milić, S.; Lulić, D.; Štimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Kastrati, A. Gamma-glutamyl transferase and cardiovascular disease. Ann. Transl. Med. 2016, 4, 481. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, S.; Bouzas, C.; Mascaró, C.M.; Casares, M.; Llompart, I.; Abete, I.; Angullo-Martinez, E.; Zulet, M.Á.; Martínez, J.A.; Tur, J.A. Effect of Dietary and Lifestyle Interventions on the Amelioration of NAFLD in Patients with Metabolic Syndrome: The FLIPAN Study. Nutrients 2022, 14, 2223. [Google Scholar] [CrossRef]
- Di Napoli, M.; Papa, F. Villa Pini Stroke Data Bank Investigators. Inflammation, hemostatic markers, and antithrombotic agents in relation to long-term risk of new cardiovascular events in first-ever ischemic stroke patients. Stroke 2002, 33, 1763–1771. [Google Scholar] [CrossRef]
- Neves, C.V.B.; Mambrini, J.V.M.; Torres, K.C.L.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Lima-Costa, M.F.; Peixoto, S.V. Association of metabolic syndrome with inflammatory markers in a sample of community-dwelling older adults. Cad. Saude Publ. 2019, 35, e00129918. [Google Scholar] [CrossRef]
- Vranić, L.; Mikolašević, I.; Milić, S. Vitamin D Deficiency: Consequence or Cause of Obesity? Med. Kaunas 2019, 55, 541. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Slusher, A.L.; McAllister, M.J.; Huang, C.J. A therapeutic role for vitamin D on obesity-associated inflammation and weight-loss intervention. Inflamm. Res. 2015, 64, 565–575. [Google Scholar] [CrossRef]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of Association between Vitamin D Deficiency and Insulin Resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef]
- EFSA. Vitamine D: L’EFSA Définit des Valeurs Nutritionnelles de Référence pour la Vitamine D. Available online: https://www.efsa.europa.eu.oct.2016 (accessed on 15 May 2023).
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Arihiro, S.; Nakashima, A.; Matsuoka, M.; Suto, S.; Uchiyama, K.; Kato, T.; Mitobe, J.; Komoike, N.; Itagaki, M.; Miyakawa, Y.; et al. Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza and Upper Respiratory Infection in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, Z.S.; Kafeshani, M.; Tavasoli, P.; Zadeh, A.H.; Entezari, M.H. Effect of Vitamin D Supplementation on Weight Loss, Glycemic Indices, and Lipid Profile in Obese and Overweight Women: A Clinical Trial Study. Int. J. Prev. Med. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Bosomworth, N.J. Atténuer la carence épidémique en vitamine D: La tourmente des données scientifiques. Can. Fam. Phys. 2011, 57, e1–e6. (In French) [Google Scholar]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.S.; Magkos, F.; Dalamaga, M. Vitamin D and Obesity: Current Evidence and Controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]
- Contento, I.R. Nutrition education: Linking research, theory, and practice. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 176–179. [Google Scholar]
- Van Rinsum, C.; Gerards, S.; Rutten, G.; Johannesma, M.; van de Goor, I.; Kremers, S. The implementation of the coaching on lifestyle (CooL) intervention: Lessons learnt. BMC Health Serv. Res. 2019, 19, 667. [Google Scholar] [CrossRef]
- Rice, K.G.; Jumamil, R.B.; Jabour, S.M.; Cheng, J.K. Role of Health Coaches in Pediatric Weight Management. Clin. Pediatr. Phila. 2017, 56, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Nicklas, B.J. Effects of lifestyle interventions on inflammatory markers in the metabolic syndrome. Front. Biosci. Schol. Ed. 2011, 3, 168–177. [Google Scholar] [CrossRef]
- John, N.A.; John, J.; Tarnikanti, M.; Kalpana, M.; Kamble, P.; Singhal, A.; Ganji, V.; Gaur, A.; Umesh, M.; Katta, R.; et al. Implications of lifestyle medicine in medical practice. J. Fam. Med. Prim. Care. 2023, 12, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Andreyeva, T.; Long, M.W.; Henderson, K.E.; Grode, G.M. Trying to lose weight: Diet strategies among Americans with overweight or obesity in 1996 and 2003. J. Am. Diet. Assoc. 2010, 10, 535–542. [Google Scholar] [CrossRef]
- Astell, K.J.; Mathai, M.L.; Su, X.Q. Plant extracts with appetite suppressing properties for body weight control: A systematic review of double blind randomized controlled clinical trials. Complement. Ther. Med. 2013, 21, 407–416. [Google Scholar] [CrossRef]
- Allison, D.B.; Fontaine, K.R.; Heshka, S.; Mentore, J.L.; Heymsfield, S.B. Alternative treatments for weight loss: A critical review. Crit. Rev. Food Sci. Nutr. 2001, 41, 1–28; discussion 39–40. [Google Scholar] [CrossRef]
- Rodondi, P.Y.; Degoumois, F.; Marques-Vidal, P.; Rodondi, N. Peut-on abaisser son taux de cholestérol avec des compléments alimentaires? [Is it possible to decrease cholesterol levels with dietary supplements?]. Rev. Med. Suisse 2016, 12, 451–453. (In French) [Google Scholar] [PubMed]
- Egras, A.M.; Hamilton, W.R.; Lenz, T.L.; Monaghan, M.S. An evidence-based review of fat modifying supplemental weight loss products. J. Obes. 2011, 2011, 297315. [Google Scholar] [CrossRef]
- Shaik Mohamed Sayed, U.F.; Moshawih, S.; Goh, H.P.; Kifli, N.; Gupta, G.; Singh, S.K.; Chellappan, D.K.; Dua, K.; Hermansyah, A.; Ser, H.L.; et al. Natural products as novel anti-obesity agents: Insights into mechanisms of action and potential for therapeutic management. Front. Pharmacol. 2023, 14, 1182937. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.; Welch, S. Comparing effectiveness of fat burners and thermogenic supplements to diet and exercise for weight loss and cardiometabolic health: Systematic review and meta-analysis. Nutr. Health 2021, 27, 445–459. [Google Scholar] [CrossRef]
- Grohmann, T.; Litts, C.; Horgan, G.; Zhang, X.; Hoggard, N.; Russell, W.; de Roos, B. Efficacy of Bilberry and Grape Seed Extract Supplement Interventions to Improve Glucose and Cholesterol Metabolism and Blood Pressure in Different Populations—A Systematic Review of the Literature. Nutrients 2021, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Polansky, M.M.; Sato, Y.; Adeli, K.; Anderson, R.A. Cinnamon extract inhibits the postprandial overproduction of apolipoprotein B48-containing lipoproteins in fructose-fed animals. J. Nutr. Biochem. 2009, 20, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.C.; Thi Phuong, T.N.; Minh, L.B.; Minh Thuc, V.T.; Bac, N.D.; Van Tien, N.; Pham, V.H.; Show, P.L.; Tao, Y.; Nhu Ngoc, V.T.; et al. The effects of green tea on lipid metabolism and its potential applications for obesity and related metabolic disorders—An existing update. Diabetes Metab. Syndr. 2019, 13, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Wider, B.; Pittler, M.H.; Thompson-Coon, J.; Ernst, E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst. Rev. 2013, 28, CD003335. [Google Scholar] [CrossRef]
- Mhurchu, C.N.; Poppitt, S.D.; McGill, A.T.; Leahy, F.E.; Bennett, D.A.; Lin, R.B.; Ormrod, D.; Ward, L.; Strik, C.; Rodgers, A. The effect of the dietary supplement, Chitosan, on body weight: A randomised controlled trial in 250 overweight and obese adults. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1149–1156. [Google Scholar] [CrossRef]
- Corona-Cervantes, K.; Parra-Carriedo, A.; Hernández-Quiroz, F.; Martínez-Castro, N.; Vélez-Ixta, J.M.; Guajardo-López, D.; García-Mena, J.; Hernández-Guerrero, C. Physical and Dietary Intervention with Opuntia ficus-indica (Nopal) in Women with Obesity Improves Health Condition through Gut Microbiota Adjustment. Nutrients 2022, 14, 1008. [Google Scholar] [CrossRef]
- Emamat, H.; Zahedmehr, A.; Asadian, S.; Nasrollahzadeh, J. The effect of barberry (Berberis integerrima) on lipid profile and systemic inflammation in subjects with cardiovascular risk factors: A randomized controlled trial. BMC Complement. Med. Ther. 2022, 22, 59. [Google Scholar] [CrossRef]
- Greenway, F.; de Jonge-Levitan, L.; Martin, C.; Roberts, A.; Grundy, I.; Parker, C. Dietary herbal supplements with phenylephrine for weight loss. J. Med. Food 2006, 9, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Stuby, J.; Gravestock, I.; Wolfram, E.; Pichierri, G.; Steurer, J.; Burgstaller, J.M. Appetite-Suppressing and Satiety-Increasing Bioactive Phytochemicals: A Systematic Review. Nutrients 2019, 11, 2238. [Google Scholar] [CrossRef]
- Chin, Y.H.; Ng, C.H.; Chew, N.W.; Kong, G.; Lim, W.H.; Tan, D.J.H.; Chan, K.E.; Tang, A.; Huang, D.Q.; Chan, M.Y.; et al. The placebo response rate and nocebo events in obesity pharmacological trials. A systematic review and meta-analysis. E Clin. Med. 2022, 54, 101685. [Google Scholar] [CrossRef]
- Stephens, S.K.; Cobiac, L.J.; Veerman, J.L. Improving diet and physical activity to reduce population prevalence of overweight and obesity: An overview of current evidence. Prev. Med. 2014, 62, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, E.A.; Verghese, N.R. Incremental cost-effectiveness of evidence-based non-surgical weight loss strategies. Clin. Obes. 2019, 9, e12294. [Google Scholar] [CrossRef] [PubMed]
| Cause of Abandonment | Dietplus® Group (n = 80/170) | Control Group (n = 12/30) |
|---|---|---|
| Lack of compliance (cure) | 21 (26.2%) | 5 (41.7%) |
| Medical exclusion criteria | 12 (15.0%) | 4 (33.4%) |
| Family constraints | 12 (15.0%) | 1 (8.3%) |
| Financial constraints | 11 (13.7%) | 1 (8.3%) |
| Logistic (Prof) incompatibility | 10 (12.5%) | 0 (0%) |
| Clinical study | 6 (7.5%) | 1 (8.3%) |
| Vegetarian | 3 (3.8%) | 0 (0%) |
| Psychological disorder | 3 (3.8%) | 0 (0%) |
| Family practice doctor refusal | 2 (2.5%) | 0 (0%) |
| Parameter | Dietplus® Group (n = 170) | Control Group (n = 30) | p-Value |
|---|---|---|---|
| Age (y) | 45 ± 12 | 45 ± 12 | n.s.* |
| Sex ratio (f/m%) | 89/11 | 89/11 | n.s. |
| Weight (kg) | 94.1 ± 14.2 | 100.0 ± 15.3 | n.s. |
| Excess weight (%) | 39.6 ± 4.0 | 43.5 ± 4.2 | p < 0.05 |
| BMI (kg/m2) | 34.9 ± 3.7 | 36 ± 4 | n.s. |
| Abd. Circumference (cm) | 110 ± 132 | 109 ± 12.5 | n.s. |
| Comorbidity % | 46 | 62 | p < 0.001 |
| Dropout % | 59 | 40 | p < 0.05 |
| Parameter | Dietplus® Group (n = 77/170) | Control Group (n = 18/30) | p-Value |
|---|---|---|---|
| Quality of Life (score/60) | 34.4 ± 6.0 | 29 ± 5.4 | n.s. |
| Nutriscore (score/100) | 43.5 ± 4.2 | 59 ± 5.7 | p < 0.05 |
| Physical Activity (score/100) | 40.9 ± 9.9 | 40 ± 9.3 | n.s. |
| Prochaska Di Clemente Scale | 7.8 ± 4.1 | 4.0 ± 1.3 | p < 0.05 |
| Parameter | At Enrollment (n = 18) | 12-Week Work-up (n = 18) | p-Value |
|---|---|---|---|
| Quality of Life (score/60) | 29.0 ± 5.4 | 29.9 ± 5.3 | n.s. |
| Nutriscore (score/100) | 58.9 ± 5.7 | 61.5 ± 7.4 | n.s. |
| Physical Activity (score/100) | 39.5 ± 9.6 | 40.8 ±9.3 | n.s. |
| Prochaska Di Clemente Scale | 4.0 ± 1.3 | 7.0 ± 4.5 | p < 0.05 |
| Parameter | At Enrollment (n = 77) | 12-Week Work-Up (n = 77) | p-Value |
|---|---|---|---|
| FAT % | 42.45 ± 10.11 | 35.06 ±9.40 | p < 0.001 |
| WATER % | 38.79 ± 7.44 | 37.65 ±7.13 | n.s. |
| MUSCLE % | 29.43 ± 6.10 | 28.52 ± 5.83 | n.s. |
| Precontemplation | Contemplation | Determination | Action | |
|---|---|---|---|---|
| Dietplus® Subjects’ Score at enrollment (n = 74) | 38 | 32 | 4 | 0 |
| Cure and study completed. (n = 74) | 12 | 43 | 18 | 1 |
| Parameter | At Enrollment | 12-Week Work-Up | p-Value |
|---|---|---|---|
| High Glucose concentration | 7/77 (10.0%) | 5/77 (6.5%) | n.s. |
| Insulin increased | 11/49 (22.4%) | 3/49 (6.1%) | p < 0.05. |
| HOMA > 2.26 | 26/49 (53.1%) | 11/49 (22%) | p < 0.01 |
| QUICKI | 28/49 (57.1%) | 15/49 (30.6%) | p < 0.01 |
| Parameter | At Enrollment (n = 77) | 12-Week Work-Up (n = 77) | p-Value |
|---|---|---|---|
| Total cholesterol (mg/dL) | 202.8 ± 47.1 | 190.0 ± 43.7 | p < 0.001 |
| Triglycerides (mg/dL) | 135.5 ± 68.3 | 118.1 ± 52.7 | p < 0.005 |
| HDL (mg/dL) | 54.0 ± 15.4 | 52.9 ± 12.6 | n.s. |
| LDL (mg/dL) | 121.6 ± 40.4 | 113.5 ± 38.7 | p < 0.001 |
| VLDL (mg/dL) | 27.2 ± 13.7 | 23.6 ± 10.5 | p < 0.005 |
| computed non-HDL (mg/dL) | 149.8 ± 45.8 | 137.1 ± 42.8 | p < 0.001 |
| Phenotype A Ln (Tg/HDL) | 45/77 (58%) | 28/77 (36%) | p < 0.01 |
| Parameter | At Enrollment (n = 77) | 12-Week Work-Up (n = 77) | p-Value |
|---|---|---|---|
| Fibrinogen (mg/dL) | 355.57 ± 75.99 | 357.92 ± 66.19 | n.s. |
| CRP (mg/dL) | 6.42 ± 6.67 | 5.70 ± 5.89 | p < 0.001 |
| usCRP (mg/dL) | 2.55 ± 1.98 | 2.30 ± 1.84 | p < 0.001 |
| Orosomucoid (g/L) | 0.89 ± 0.19 | 0.84 ± 0.20 | p < 0.001 |
| PINI 1 index | 0.56 ± 0.65 | 0.46 ± 0.53 | p < 0.001 |
| PINI 2 index | 0.35 ± 0.34 | 0.30 ± 0.33 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houben, J.-J.; Carpentier, Y.; Paulissen, G.; Snick, G.V.; Soetewey, A. Impact of a Food Rebalancing Program Associated with Plant-Derived Food Supplements on the Biometric, Behavioral, and Biological Parameters of Obese Subjects. Nutrients 2023, 15, 4780. https://doi.org/10.3390/nu15224780
Houben J-J, Carpentier Y, Paulissen G, Snick GV, Soetewey A. Impact of a Food Rebalancing Program Associated with Plant-Derived Food Supplements on the Biometric, Behavioral, and Biological Parameters of Obese Subjects. Nutrients. 2023; 15(22):4780. https://doi.org/10.3390/nu15224780
Chicago/Turabian StyleHouben, Jean-Jacques, Yvon Carpentier, Genevieve Paulissen, Georges Van Snick, and Antoine Soetewey. 2023. "Impact of a Food Rebalancing Program Associated with Plant-Derived Food Supplements on the Biometric, Behavioral, and Biological Parameters of Obese Subjects" Nutrients 15, no. 22: 4780. https://doi.org/10.3390/nu15224780
APA StyleHouben, J.-J., Carpentier, Y., Paulissen, G., Snick, G. V., & Soetewey, A. (2023). Impact of a Food Rebalancing Program Associated with Plant-Derived Food Supplements on the Biometric, Behavioral, and Biological Parameters of Obese Subjects. Nutrients, 15(22), 4780. https://doi.org/10.3390/nu15224780








