Durable Self-Cleaning Coatings for Architectural Surfaces by Incorporation of TiO2 Nano-Particles into Hydroxyapatite Films
Abstract
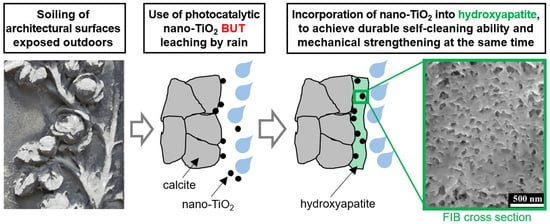
:1. Introduction
2. Results and Discussion
2.1. Coating Morphology and Microstructure
2.2. Coating Composition
2.3. Consolidating Ability
2.4. Compatibility
2.5. Self-Cleaning Ability
2.6. Resistance to Simulated Rain
3. Materials and Methods
3.1. Marble
3.2. Treatments
- “H”, consisting in the HAP treatment alone. A 3M aqueous solution of DAP (Sigma-Aldrich, Milan, Italy, assay ≥ 98%, reagent grade) was applied by brushing until apparent refusal (8 brush strokes). Then, samples were wrapped in a plastic film to avoid evaporation and left to react for 48 h. After removal of the plastic film and rinsing with deionized water, samples were left to dry at room temperature for 4 days. Afterwards, a poultice of so-called limewater (i.e., a saturated solution of calcium hydroxide) was prepared using cellulose pulp (MH300 Phase, Italy) and Ca(OH)2 (Sigma-Aldrich, reagent grade), with a limewater:cellulose pulp weight ratio of 6:1. The limewater poultice was applied with the twofold aim of (i) supplying additional calcium ions for reaction with unreacted DAP and (ii) removing unreacted residues at the end of the treatment, by drying in contact with the samples [64]. A sheet of Japanese paper was inserted between the samples and the poultice to avoid sticking. After covering with the poultice, samples were wrapped in a plastic film for 24 h, then the film was removed and the poultice was left to dry in contact with the samples until constant weight. After removal of the dried poultice, samples were rinsed with deionized water and finally left to dry at room temperature.
- “T”, consisting in the TiO2 treatment alone. A 2 wt. % suspension of TiO2 particles (98.1% anatase, 1.9% brookite, crystallite average size between 10 and 20 nm) in 80-20 wt. % water-isopropanol medium was applied by a single brush stroke. A maximum concentration of 2 wt. % was recommended in the literature to avoid particle agglomeration [24].
- “H+T”, consisting in the application of treatments “H” and “T” in sequence, applied exactly as described above for the single treatments.
- “HT”, consisting in the application of a single treatment combining HAP and nano-TiO2. The combined treatment was obtained by mixing the 2 wt. % TiO2 suspension and the 3M DAP solution in the weight proportion 1.5:98.5, respectively. This proportion was selected based on the number of brush strokes adopted for treatment application: 8 strokes in the case of “HT” (like the “H” samples), instead of a single stroke in the “T” samples. Because repeated application leads to particle accumulation over the surface, a lower particle concentration than in the “T” treatment was selected. After treatment application by brushing and after reaction for 48 h wrapped in a plastic film, samples were dried at room temperature and then subjected to application of the limewater poultice, as described for the “H” samples. After drying of the poultice, samples were finally rinsed with deionized water and dried at room temperature.
3.3. Characterization
3.3.1. Coating Morphology and Microstructure
3.3.2. Coating Composition
3.3.3. Consolidating Ability
3.3.4. Aesthetic Compatibility
3.3.5. Self-Cleaning Ability
3.3.6. Resistance to Simulated Rain
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grossi, C.M.; Esbert, R.M.; Dìaz-Pache, F.; Alonso, F.J. Soiling of building stones in urban environments. Build. Environ. 2003, 38, 147–159. [Google Scholar] [CrossRef]
- Moropoulou, A.; Bisbikou, K.; Torfs, K.; Van Grieken, R.; Zezza, F.; Macri, F. Origin and growth of weathering crusts on ancient marbles in industrial atmosphere. Atmos. Environ. 1998, 32, 967–982. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P.; Biscontin, G. Origin, characteristics and morphology of weathering crusts on Istria stone in Venice. Atmos. Environ. 1999, 33, 1699–1709. [Google Scholar] [CrossRef]
- Amoroso, G.G.; Fassina, V. Stone Decay and Conservation; Elsevier: New York, NY, USA, 1983; ISBN 0-444-42146-7. [Google Scholar]
- Camuffo, D.; Del Monte, M.; Sabbioni, C. Origin and growth mechanisms of the sulphated crusts on urban limestone. Water Air Soil Pollut. 1983, 19, 351–359. [Google Scholar] [CrossRef]
- Lazzarini, L.; Laurenzi Tabasso, M. Il Restauro Della Pietra; CEDAM: Padua, Italy, 1986; ISBN 8813159587. [Google Scholar]
- Kucera, V. EU 5FP Project MULTI-ASSESS “Model for Multi-Pollutant Impact and Assessment of Threshold Levels for Cultural Heritage”; Publishable Final Report; European Commission: Brussels, Belgium, 2005. [Google Scholar]
- Kucera, V. EU 6FP Project CULT-STRAT “Assessment of Air Pollution Effects on Cultural Heritage—Management Strategies”; Publishable Final Report; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Grossi, M.C.; Brimblecombe, P. Effect of long-term changes in air pollution and climate on the decay and blackening of European stone buildings. In Building Stone Decay: From Diagnosis to Conservation; Prikryl, R., Smith, B.J., Eds.; Geological Society Special Publications: London, UK, 2007; Volume 271, pp. 117–130. [Google Scholar]
- Bernardi, A.; Becherini, F.; Bonazza, A.; Kupriska, B.; Pockelè, L.; Van Grieken, R.; de Grandi, S.; Ozga, I.; Veiga Rico, A.J.; Garcia Mercero, O.; et al. A methodology to monitor pollution impact on historic buildings surfaces: The TeACH project. In EuroMed 2012: Progress in Cultural Heritage Preservation; Ioannides, M., Fritsch, D., Leissner, J., Davies, R., Remondino, F., Caffo, R., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7616, pp. 765–775. [Google Scholar]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. AAPPS Bull. 2007, 17, 12–28. [Google Scholar] [CrossRef]
- Maggos, T.; Bartzis, J.G.; Liakou, M.; Gobin, C. Photocatalytic degradation of NOx gases using TiO2-containing paint: A real scale study. J. Hazard. Mater. 2007, 146, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.M.; Demeestere, K.; de Belie, N.; Mäntylä, T.; Levänen, E. Titanium dioxide coated cementitious materials for air purifying purposes: Preparation, characterization and toluene removal potential. Build. Environ. 2010, 45, 832–838. [Google Scholar] [CrossRef]
- Babaizadech, H.; Hassan, M. Life cycle assessment of nano-sized titanium dioxide coating on residential windows. Constr. Build. Mater. 2013, 40, 314–321. [Google Scholar] [CrossRef]
- Licciulli, A.; Calia, A.; Lettieri, M.; Diso, D.; Masieri, M.; Franza, S.; Amadelli, R.; Casarano, G. Photocatalytic TiO2 coatings on limestone. J. Sol-Gel Sci. Technol. 2011, 60, 437–444. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Cordoni, C.; Munafò, P. Self-cleaning and de-polluting stone surfaces: TiO2 nanoparticles for limestone. Constr. Build. Mater. 2012, 37, 51–57. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Smart surfaces for architectural heritage: Preliminary results about the application of TiO2-based coatings on travertine. J. Cult. Heritage 2012, 13, 204–209. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Self-cleaning materials on Architectural Heritage: Compatibility of photo-induced hydrophilicity of TiO2 coatings on stone surfaces. J. Cult. Heritage 2013, 14, 1–7. [Google Scholar] [CrossRef]
- Franzoni, E.; Fregni, A.; Gabrielli, R.; Graziani, G.; Sassoni, E. Compatibility of photocatalytic TiO2-based finishing for renders in architectural restoration: A preliminary study. Build. Environ. 2014, 80, 125–135. [Google Scholar] [CrossRef]
- Munafò, P.; Quagliarini, E.; Goffredo, G.B.; Bondioli, F.; Licciulli, A. Durability of nano-engineered TiO2 self-cleaning treatments on limestone. Constr. Build. Mater. 2014, 65, 218–231. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Bondioli, F.; D’Orazio, M. Durability of self-cleaning TiO2 coatings on fired clay brick façades: Effects of UV exposure and wet & dry cycles. Build. Environ. 2014, 71, 193–203. [Google Scholar] [CrossRef]
- Luvidi, L.; Mecchi, A.M.; Ferretti, M.; Sidoti, G. Treatments with self-cleaning products for the maintenance and conservation of stone surfaces. Int. J. Conserv. Sci. 2016, 7, 311–322. [Google Scholar]
- Bergamonti, L.; Bondioli, F.; Alfieri, I.; Lorenzi, A.; Mattarozzi, M.; Predieri, G.; Lottici, P.P. Photocatalytic self-cleaning TiO2 coatings on carbonatic stones. Appl. Phys. A 2016, 122. [Google Scholar] [CrossRef]
- Aldoasri, M.A.; Darwish, S.S.; Adam, M.A.; Emalrzugi, N.A.; Ahmed, S.M. Protecting of marble stone facades of historic buildings using multifunctional TiO2 nanocoatings. Sustainability 2017, 9, 2002. [Google Scholar] [CrossRef]
- Quagliarini, E.; Graziani, L.; Diso, D.; Licciulli, A.; D’Orazio, M. Is nano-TiO2 alone an effective strategy for the maintenance of stones in Cultural Heritage? J. Cult. Heritage 2017. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Franzoni, E.; Gabrielli, R.; Sassoni, E.; Fregni, A.; Graziani, G.; Roveri, N.; D’Amen, E. Performance and permanence of TiO2-based surface treatments for architectural heritage: Some experimental findings from on-site and laboratory testing. In Science and Art: A Future for Stone: Proceedings of the 13th International Congress on the Deterioration and Conservation of Stone, Glasgow, UK, 6–10 September 2016; Hughes, J., Howind, T., Eds.; University of the West of Scotland: Paisley, UK, 2016; Volume 2, pp. 761–768. ISBN 978-1-903978-58-0. [Google Scholar]
- Blocken, B.; Derome, D.; Carmeliet, J. Rainwater runoff from building facades: A review. Build. Environ. 2013, 60, 339–361. [Google Scholar] [CrossRef]
- Pinho, L.; Mosquera, M.J. Titania-silica nanocomposite photocatalysts with application in stone self-cleaning. J. Phys. Chem. C 2011, 115, 22851–22862. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Rovella, N.; Belfiore, C.M.; Palermo, A.M.; Guzzi, M.T.; Crisci, G.M. Multifunctional TiO2 coatings for Cultural Heritage. Prog. Org. Coat. 2012, 74, 186–191. [Google Scholar] [CrossRef]
- La Russa, M.F.; Rovella, N.; Alvarez de Buergo, M.; Belfiore, C.M.; Pezzino, A.; Crisci, G.M.; Ruffolo, S.A. Nano-TiO2 coatings for cultural heritage protection: The role of the binder on hydrophobic and self-cleaning efficacy. Prog. Org. Coat. 2016, 91, 1–8. [Google Scholar] [CrossRef]
- Colangiuli, D.; Calia, A.; Bianco, N. Novel multifunctional coatings with photocatalytic and hydrophobic properties for the preservation of the stone building heritage. Constr. Build. Mater. 2015, 93, 189–196. [Google Scholar] [CrossRef]
- Gherardi, F.; Roveri, M.; Goidanich, S.; Toniolo, L. Photocatalytic Nanocomposites for the Protection of European Architectural Heritage. Materials 2018, 11, 65. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2-SiO2-PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Naidu, S.; Scherer, G.W. Nucleation, growth and evolution of calcium phosphate films on calcite. J. Colloid Interface Sci. 2014, 435, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.; Blair, J.; Scherer, G.W. Acid-resistant coatings on marble. J. Am. Ceram. Soc. 2016, 99, 3421–3428. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Y. Artificial hydroxyapatite film for the conservation of outdoor marble artworks. Mater. Lett. 2014, 124, 201–203. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Franzoni, E.; Scherer, G.W. Hydroxyapatite coatings for marble protection: Optimization of calcite covering and acid resistance. Appl. Surf. Sci. 2016, 368, 241–257. [Google Scholar] [CrossRef]
- Graziani, G.; Sassoni, E.; Franzoni, E.; Scherer, G.W. Resistance to simulated rain of hydroxyapatite- and calcium oxalate-based coatings for protection of marble against corrosion. Corros. Sci. 2017, 127, 168–174. [Google Scholar] [CrossRef]
- Sassoni, E.; Franzoni, E. Sugaring marble in the Monumental Cemetery in Bologna (Italy): Characterization of naturally and artificially weathered samples and first results of consolidation by hydroxyapatite. Appl. Phys. A Mater. 2014, 117, 1893–1906. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E. Repair of sugaring marble by ammonium phosphate: Comparison with ethyl silicate and ammonium oxalate and pilot application to historic artifact. Mater. Des. 2015, 88, 1145–1157. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, B. Synthesis and characterization of a novel biomaterial for the conservation of historic stone building and sculptures. Mater. Sci. Forum 2011, 675–677, 317–320. [Google Scholar] [CrossRef]
- Sassoni, E.; Naidu, S.; Scherer, G.W. The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. J. Cult. Heritage 2011, 12, 346–355. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E. An innovative phosphate-based consolidant for limestone. Part 1: Effectiveness and compatibility in comparison with ethyl silicate. Constr. Build. Mater. 2016, 102, 918–930. [Google Scholar] [CrossRef]
- Sassoni, E.; Graziani, G.; Franzoni, E. An innovative phosphate-based consolidant for limestone. Part 2: Durability in comparison with ethyl silicate. Constr. Build. Mater. 2016, 102, 931–942. [Google Scholar] [CrossRef]
- Matteini, M.; Rescic, S.; Fratini, F.; Botticelli, G. Ammonium phosphates as consolidating agents for carbonatic stone materials used in architecture and cultural heritage: Preliminary research. Int. J. Archit. Heritage 2011, 5, 717–736. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, B.; Liu, Y.; Guofeng, W.; Zhang, H.; Cheng, W.; Xu, Z. Biomimic conservation of weathered calcareous stones by apatite. New J. Chem. 2011, 35, 887–892. [Google Scholar] [CrossRef]
- Ma, X.; Balonis, M.; Pasco, H.; Toumazou, M.; Counts, D.; Kakoulli, I. Evaluation of hydroxyapatite effects for the consolidation of a Hellenistic-Roman rock-cut chamber tomb at Athienou-Malloura in Cyprus. Constr. Build. Mater. 2017, 150, 333–344. [Google Scholar] [CrossRef]
- Molina, E.; Rueda-Quero, L.; Benavente, D.; Burgos-Cara, A.; Ruiz-Agudo, E.; Cultrone, G. Gypsum crust as a source of calcium for the consolidation of carbonate stones using a calcium phosphate-based consolidant. Constr. Build. Mater. 2017, 143, 298–311. [Google Scholar] [CrossRef]
- Xu, F.; Li, D. The use of CTAB as an addition of DAP for improvement resisting acid rain on limestone. Appl. Surf. Sci. 2017, 422, 1059–1066. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophophates. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef] [PubMed]
- Possenti, E.; Colombo, C.; Bersani, D.; Bertasa, M.; Botteon, A.; Conti, C.; Lottici, P.P.; Realini, M. New insight on the interaction of diammonium hydrogenphosphate conservation treatment with carbonatic substrates: A multi-analytical approach. Microchem. J. 2016, 127, 79–86. [Google Scholar] [CrossRef]
- Sassoni, E. Phosphate-based treatments for conservation of stone. RILEM Tech. Lett. 2017, 2, 14–19. [Google Scholar] [CrossRef]
- Barriuso, B.C.; Botticelli, G.; Cuzman, O.A.; Osticioli, I.; Tiano, P.; Matteini, M. Conservation of calcareous stone monuments: Screening different diammonium phosphate-based formulations for countering phototrophic colonization. J. Cult. Heritage 2017, 27, 97–106. [Google Scholar] [CrossRef]
- Joseph Nathanael, A.; Mangalaraj, D.; Chi Chen, P.; Ponpandian, N. Mechancial and photocatalytic properties of hydroxyapatite/titania nanocomposites prepared by combined high gravity and hydrothermal process. Compos. Sci. Technol. 2010, 70, 419–426. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Todorova, N.; Romanos, G.; Vaimakis, T.; Dillert, R.; Bahnemann, D.; Trapalis, C. Composite hydroxyapatite/TiO2 materials for photocatalytic oxidation of NOx. Mater. Sci. Eng. B 2012, 177, 1046–1052. [Google Scholar] [CrossRef]
- Anmin, H.; Tong, L.; Ming, L.; Chengkang, C.; Huiqin, L.; Dali, M. Preparation of nanocrystals hydroxyapatite/TiO2 compound by hydrothermal treatment. App. Catal. A 2006, 63, 41–44. [Google Scholar] [CrossRef]
- Moreno, E.C.; Zahradnik, R.T.; Glazman, A.; Hwu, R. Precipitation of hydroxyapatite from dilute solutions upon seeding. Calcif. Tissue Res. 1977, 24, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Miliani, C.; Velo-Simpson, M.L.; Scherer, G.W. Particle-modified consolidants: A study on the effect of particles on sol–gel properties and consolidation effectiveness. J. Cult. Heritage 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Franzoni, E.; Sassoni, E.; Scherer, G.W.; Naidu, S. Artificial weathering of stone by heating. J. Cult. Heritage 2013, 14S, e85–e93. [Google Scholar] [CrossRef]
- Sassoni, E.; Franzoni, E. Influence of porosity on artificial deterioration of marble and limestone by heating. Appl. Phys. A Mater. 2014, 115, 809–816. [Google Scholar] [CrossRef]
- Evans, A.G.; Drory, M.D.; Hu, M.S. The cracking and decohesion of thin films. J. Mater. Res. 1988, 3, 1043–1049. [Google Scholar] [CrossRef]
- Franzoni, E.; Sassoni, E.; Graziani, G. Brushing, poultice or immersion? Role of the application technique on the performance of a novel hydroxyapatite-based consolidating treatment for limestone. J. Cult. Heritage 2015, 16, 173–184. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 15, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, U.; Eror, N.G. Raman spectra of titanium dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Maeda, H.; Tamura, T.; Kasuga, T. Experimental and theoretical investigation of the structural role of titanium oxide in CaO-P2O5−TiO2 invert glass. J. Phys. Chem. B 2017, 121, 5433–5438. [Google Scholar] [CrossRef] [PubMed]
- Ruedrich, J.; Knell, C.; Enseleit, J.; Rieffel, Y.; Siegesmund, S. Stability assessment of marble statuaries of the Schlossbrücke (Berlin, Germany) based on rock strength measurement and ultrasonic wave velocities. Environ. Earth Sci. 2013, 69, 1451–1469. [Google Scholar] [CrossRef]
- Sharma, G. Color fundamentals for digital imaging. In Digital Color Imagining Handbook; Sharma, G., Ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Weiss, T.; Rasolofosaon, P.N.J.; Siegesmund, S. Ultrasonic wave velocities as a diagnostic tool for the quality assessment of marble. In Natural Stone, Weathering Phenomena, Conservation Strategies and Case Studies; Siegesmund, S., Weiss, T., Vollbrecht, A., Eds.; Geological Society Special Publications: London, UK, 2002; Volume 205, pp. 149–164. [Google Scholar] [CrossRef]
- Sjöberg, E.L.; Rickard, D.T. The effect of added calcium on calcite dissolution kinetics in aqueous solutions at 25 °C. Chem. Geol. 1985, 49, 405–413. [Google Scholar] [CrossRef]
- Bonazza, A.; Messina, P.; Sabbioni, C.; Grossi, C.M.; Brimblecombe, P. Mapping the impact of climate change on surface recession of carbonate buildings in Europe. Sci. Total Environ. 2009, 407, 2039–2050. [Google Scholar] [CrossRef] [PubMed]







| Sample | Ti wt. % (EDS) | Ti ppm (ICP) | |
|---|---|---|---|
| Before Rain | After Rain | Runoff Solution | |
| T | 31.9 | 1.1 | 0.256 |
| H+T | 32.4 | 33.1 | 0.085 |
| HT | 0.1 | 0.1 | 0.025 |
| Sample | UPV (km/s) | % of Initial UPV * |
|---|---|---|
| UT | 0.7 (±0.1) | 23 |
| H | 3.1 (±0.2) | 106 |
| T | 0.7 (±0.1) | 24 |
| H+T | 3.0 (±0.5) | 103 |
| HT | 3.1 (±0.2) | 106 |
| Sample | ΔL* | Δa* | Δb* | ΔE* |
|---|---|---|---|---|
| H | 1.83 | −0.35 | −2.25 | 2.18 |
| T | −0.97 | −0.24 | 0.60 | 1.94 |
| H+T | 1.21 | −0.23 | −1.50 | 1.25 |
| HT | −0.19 | −0.35 | −1.72 | 0.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassoni, E.; D’Amen, E.; Roveri, N.; Scherer, G.W.; Franzoni, E. Durable Self-Cleaning Coatings for Architectural Surfaces by Incorporation of TiO2 Nano-Particles into Hydroxyapatite Films. Materials 2018, 11, 177. https://doi.org/10.3390/ma11020177
Sassoni E, D’Amen E, Roveri N, Scherer GW, Franzoni E. Durable Self-Cleaning Coatings for Architectural Surfaces by Incorporation of TiO2 Nano-Particles into Hydroxyapatite Films. Materials. 2018; 11(2):177. https://doi.org/10.3390/ma11020177
Chicago/Turabian StyleSassoni, Enrico, Eros D’Amen, Norberto Roveri, George W. Scherer, and Elisa Franzoni. 2018. "Durable Self-Cleaning Coatings for Architectural Surfaces by Incorporation of TiO2 Nano-Particles into Hydroxyapatite Films" Materials 11, no. 2: 177. https://doi.org/10.3390/ma11020177
APA StyleSassoni, E., D’Amen, E., Roveri, N., Scherer, G. W., & Franzoni, E. (2018). Durable Self-Cleaning Coatings for Architectural Surfaces by Incorporation of TiO2 Nano-Particles into Hydroxyapatite Films. Materials, 11(2), 177. https://doi.org/10.3390/ma11020177