Improvement in Slag Resistance of No-Cement Refractory Castables by Matrix Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composition Design
2.2. Sample Preparation and Characterization
3. Results and Discussion
3.1. Wet-Out Time, Flow, and Mechanical Properties
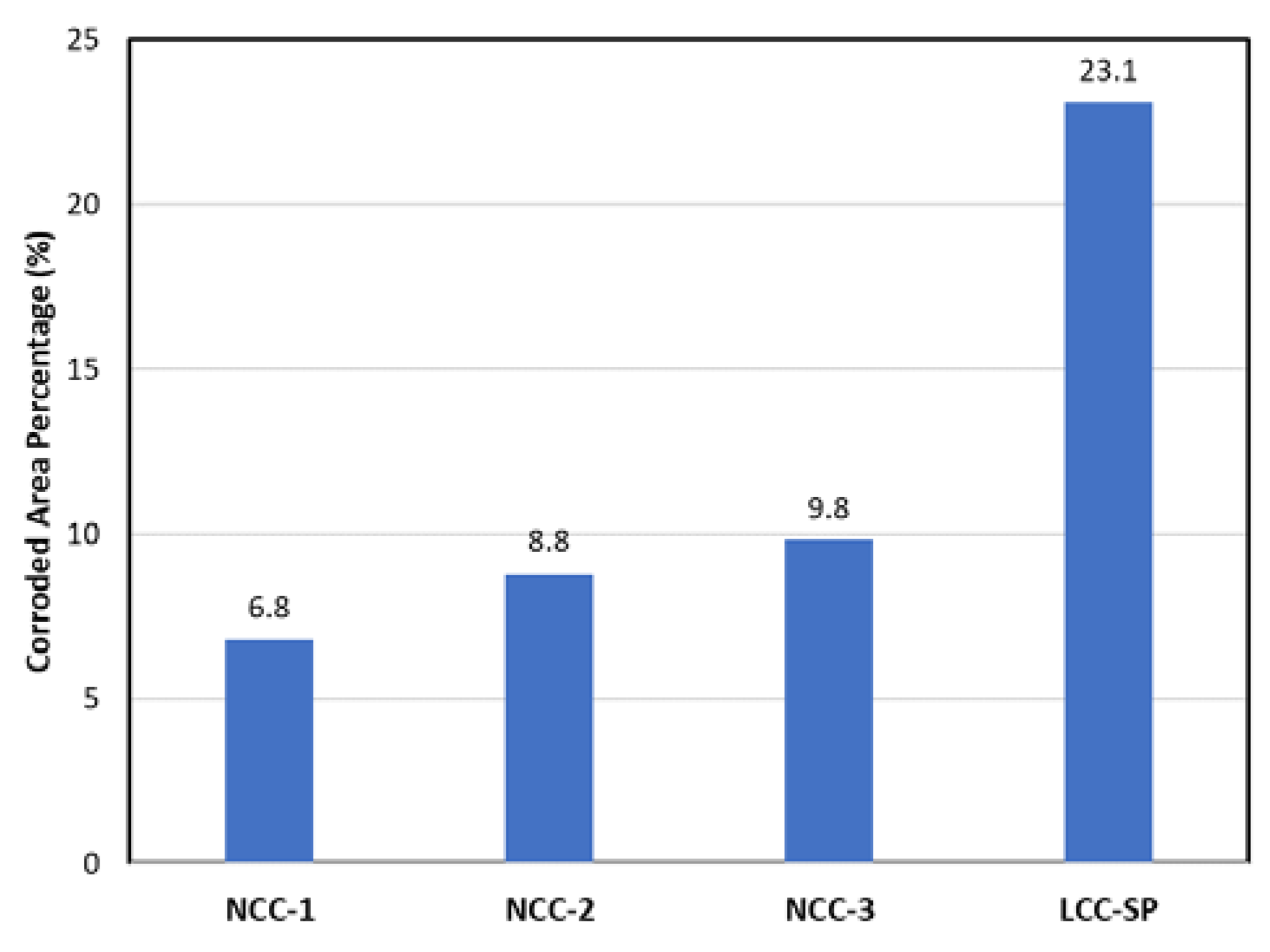
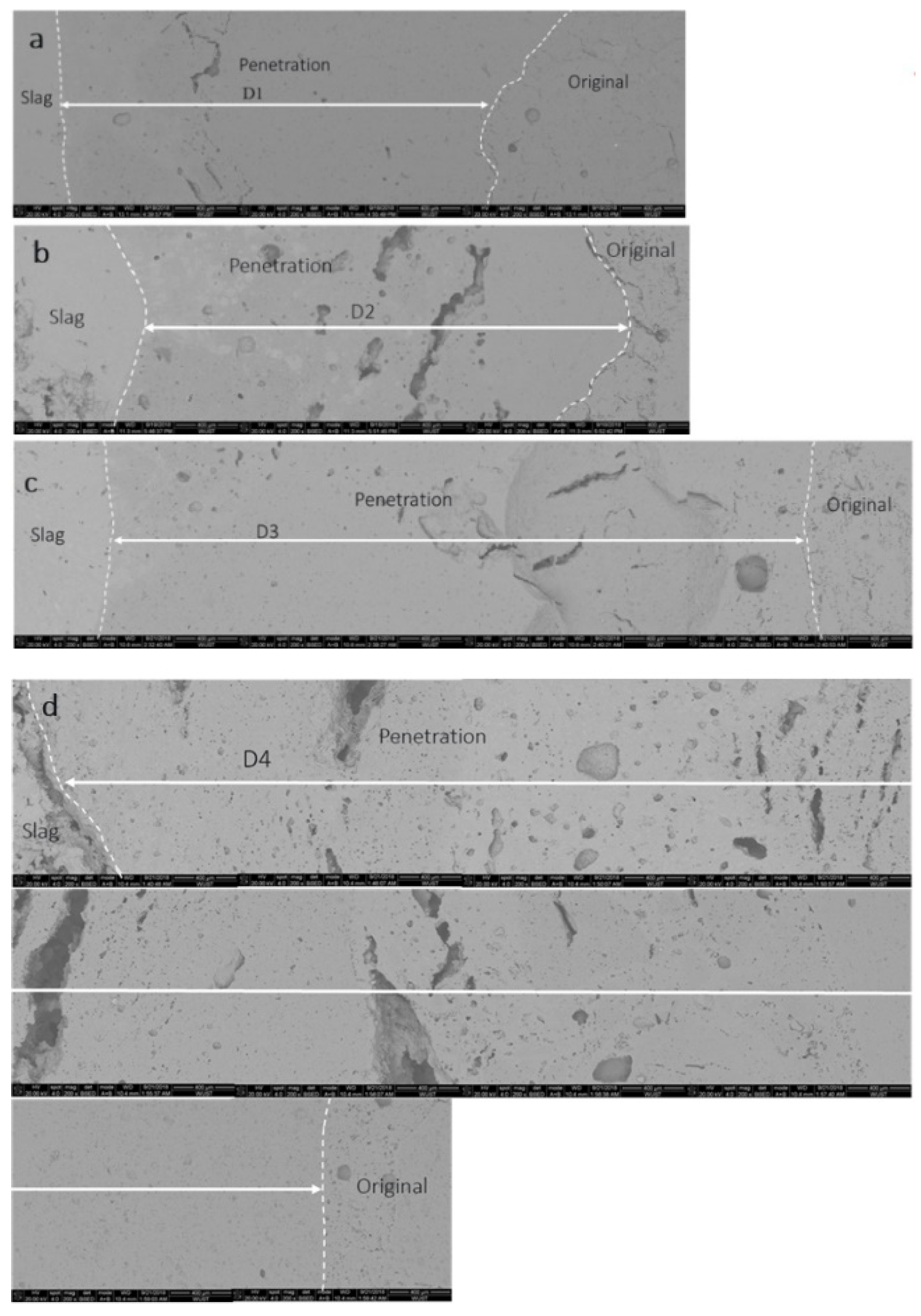
3.2. Corrosion Cup Test
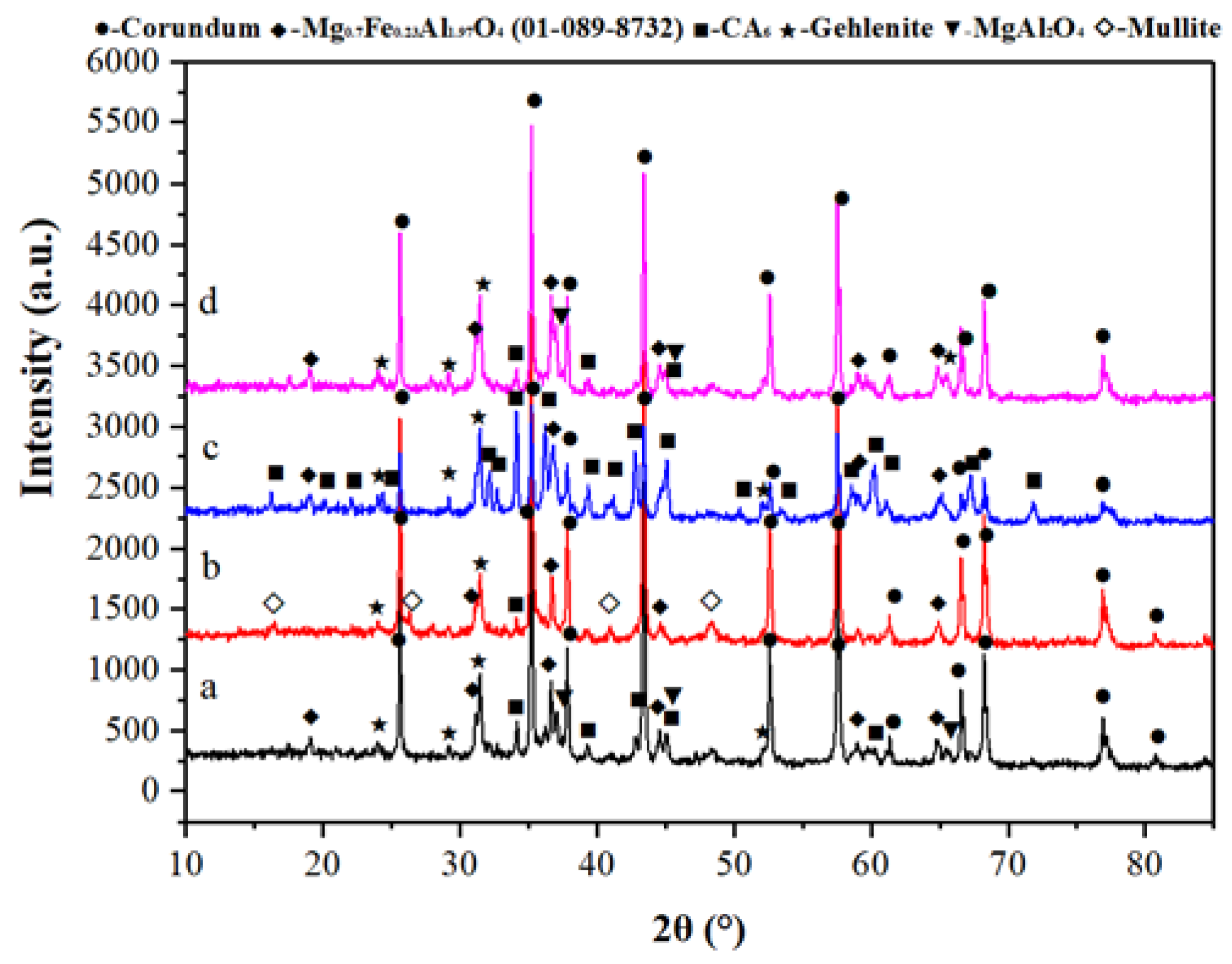
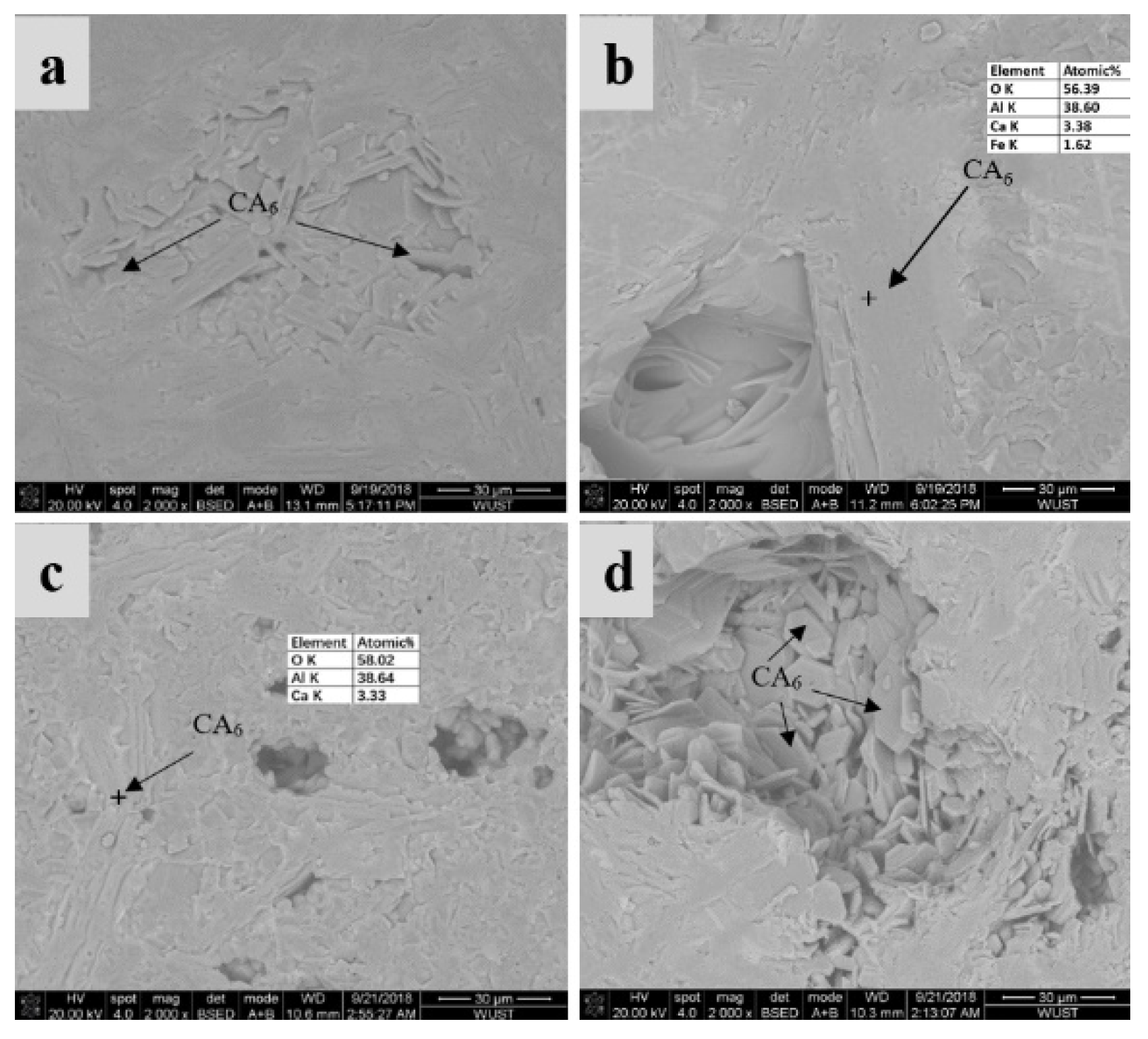
3.3. SEM and XRD Characterization
3.4. Thermodynamic Simulation
4. Conclusions
- Selection of the optimal type of binder was a key factor in refractory design. All three types of tested NCCs outperformed the LCC with pre-formed spinel in terms of corrosion resistance against the tested ladle slag.
- CAC binder would not only produce a large amount of liquid phase, but also lead to a lowering of the viscosity. Consequently, the resistance to slag penetration in the LCC-SP was inferior to the NCCs.
- The no-cement binders composed of Al2O3 + MgO (alumina bond with in situ spinel formation) and Al2O3 + SiO2 (microsilica-gel bond with mullite formation) showed better corrosion resistance than the magnesium silicate hydrate (M-S-H) bonded NCC.
- The viscosity of the liquid formed during the interaction between the slag and the refractory had a strong impact on the corrosion resistance. The viscosity increased with the increase of microsilica addition, contributing to better penetration resistance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sako, E.; Braulio, M.; Pandolfelli, V. The corrosion and microstructure relationship for cement-bonded spinel refractory castables. Ceram. Int. 2012, 38, 2177–2185. [Google Scholar] [CrossRef]
- Nakagawa, Z.; Enomoto, N.; Yi, I.S.; Asano, K. Effect of corundum periclase sizes on expansion behaviour during synthesis of spinel. In Proceedings of the UNITECR’95, Kyoto, Japan, 19–22 November 1995; pp. 1312–1319. [Google Scholar]
- Rigaud, M.; Palco, S.; Wang, N. Spinel formation in the MgO-Al2O3 system relevant to basic oxides. In Proceedings of the UNITECR’95, Kyoto, Japan, 19–22 November 1995; pp. 387–394. [Google Scholar]
- Carter, R.E. Mechanism of solid-state reaction between magnesium oxide and aluminum oxide and between magnesium oxide and ferric oxide. J. Am. Ceram. Soc. 1997, 44, 116–120. [Google Scholar] [CrossRef]
- Korgul, P.; Wilson, D.; Lee, W. Microstructural analysis of corroded alumina-spinel castable refractories. J. Eur. Ceram. Soc. 1997, 17, 77–84. [Google Scholar] [CrossRef]
- Braulio, M.; Rigaud, M.; Buhr, A.; Parr, C.; Pandolfelli, V. Spinel-containing alumina-based refractory castables. Ceram. Int. 2011, 37, 1705–1724. [Google Scholar] [CrossRef]
- Luz, A.P.; Braulio, M.A.L.; Martinez, A.G.T.; Pandolfelli, V. Slag attack evaluation of in situ spinel-containing refractory castables via experimental tests and thermodynamic simulations. Ceram. Int. 2012, 38, 1497–1505. [Google Scholar] [CrossRef]
- Martinez, A.T.; Luz, A.; Braulio, M.; Pandolfelli, V. Al2O3-based binders for corrosion resistance optimization of Al2O3-MgAl2O4 and Al2O3-MgO refractory castables. Ceram. Int. 2015, 41, 9947–9956. [Google Scholar] [CrossRef]
- Schnabel, M.; Buhr, A.; Exenberger, R.; Rampitsch, C. Spinel: In situ versus preformed—clearing the myth. Refract. Worldforum 2010, 2, 87–93. [Google Scholar]
- Myhre, B.; Peng, H. The hydration behaviour of MgO-SiO2-H2O gel bonded MgO castables. Refract. Worldforum 2015, 7, 95–100. [Google Scholar]




| Wt% | NCC-1 | NCC-2 | NCC-3 | LCC-SP |
|---|---|---|---|---|
| Tabular 0–5 mm | 79.1 | 82.5 | 79.7 | 52.2 |
| Spinel AR 78 0–1 mm | 24.9 | |||
| Calcined alumina fines | 4.9 | 9.5 | 3.4 | 15.4 |
| Calcium aluminate cement (70% Al2O3) | 0.5 | 7.0 | ||
| Spherical alumina fines | 12.6 | 8.7 | 0.5 | |
| MgO (325 mesh) | 2.9 | 3.9 | ||
| Elkem MS971U | 5.0 | 2.4 | ||
| SioxX-Zero | 3.0 | |||
| SioxX-Mag | 2.0 | |||
| Water % | 4.1 | 4.4 | 4.3 | 5.0 |
| (wt%) | CaO/SiO2 mass | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | MnO |
|---|---|---|---|---|---|---|---|---|---|---|
| Slag | 1.47 | 16.4 | 32.62 | 15.4 | 24.1 | 6.1 | 0.07 | 0.4 | 0.35 | 3.15 |
| wt% | Al2O3 | MgO | SiO2 | CaO |
|---|---|---|---|---|
| NCC-1 | 92.1 | 7.5 | 0.5 | |
| NCC-2 | 82.5 | 0.1 | 17.5 | |
| NCC-3 | 82.5 | 10 | 7.5 | |
| LCC-SP | 80 | 13.8 | 6.2 |
| Sample | NCC-1 | NCC-2 | NCC-3 | LCC-SP |
|---|---|---|---|---|
| Self-flow (%) | 54 | 80 | 60 | 152 |
| Vibration-flow (%) | 124 | 124 | 120 | 172 |
| 110 °C/24 h | ||||
| CMOR | 3.68 | 6.26 | 14.8 | 17.3 |
| CCS | 16.1 | 38.7 | 110.1 | 151.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Liu, J.; Wang, Q.; Li, Y. Improvement in Slag Resistance of No-Cement Refractory Castables by Matrix Design. Ceramics 2020, 3, 31-39. https://doi.org/10.3390/ceramics3010004
Peng H, Liu J, Wang Q, Li Y. Improvement in Slag Resistance of No-Cement Refractory Castables by Matrix Design. Ceramics. 2020; 3(1):31-39. https://doi.org/10.3390/ceramics3010004
Chicago/Turabian StylePeng, Hong, Jun Liu, Qinghu Wang, and Yawei Li. 2020. "Improvement in Slag Resistance of No-Cement Refractory Castables by Matrix Design" Ceramics 3, no. 1: 31-39. https://doi.org/10.3390/ceramics3010004




