Phase Formation in Heterovalent Equimolar Quinary Oxide Systems of ZrO2-HfO2-CeO2-Nb2O5-RE2O3 Type (RE = Y, Yb, Nd, Gd)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Processing
2.2. Materials Characterization
3. Results and Discussion
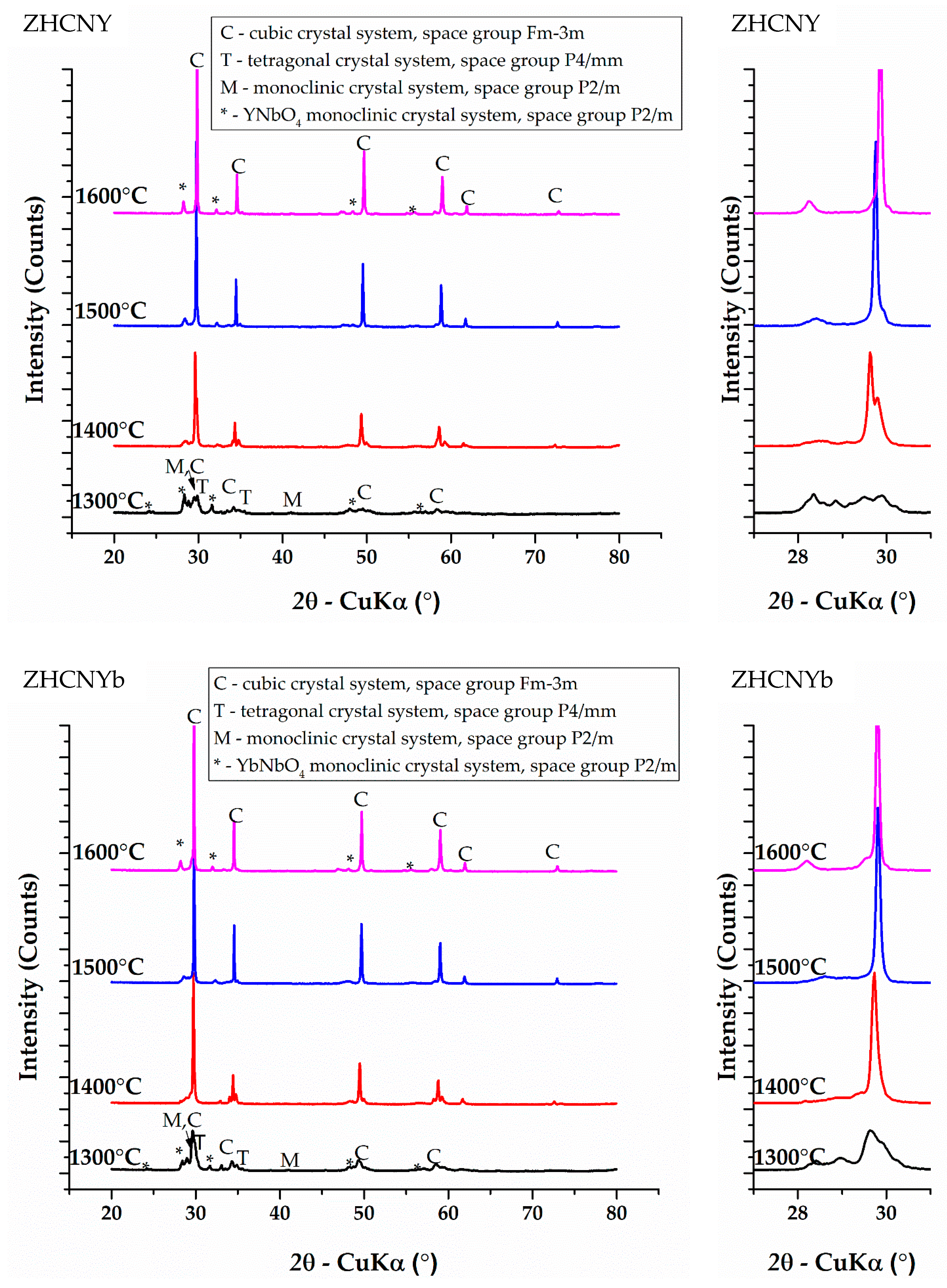
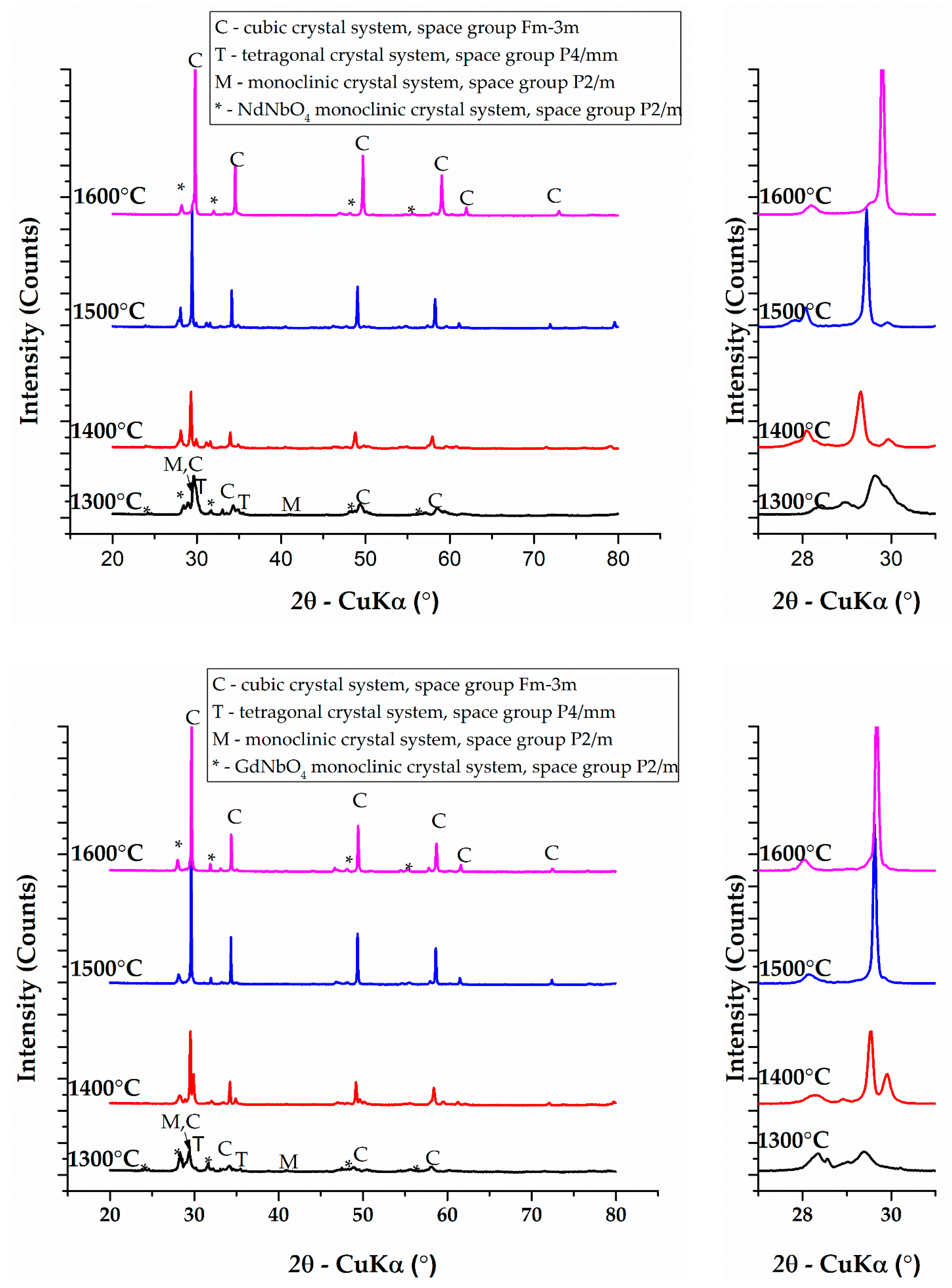
3.1. Phase Composition
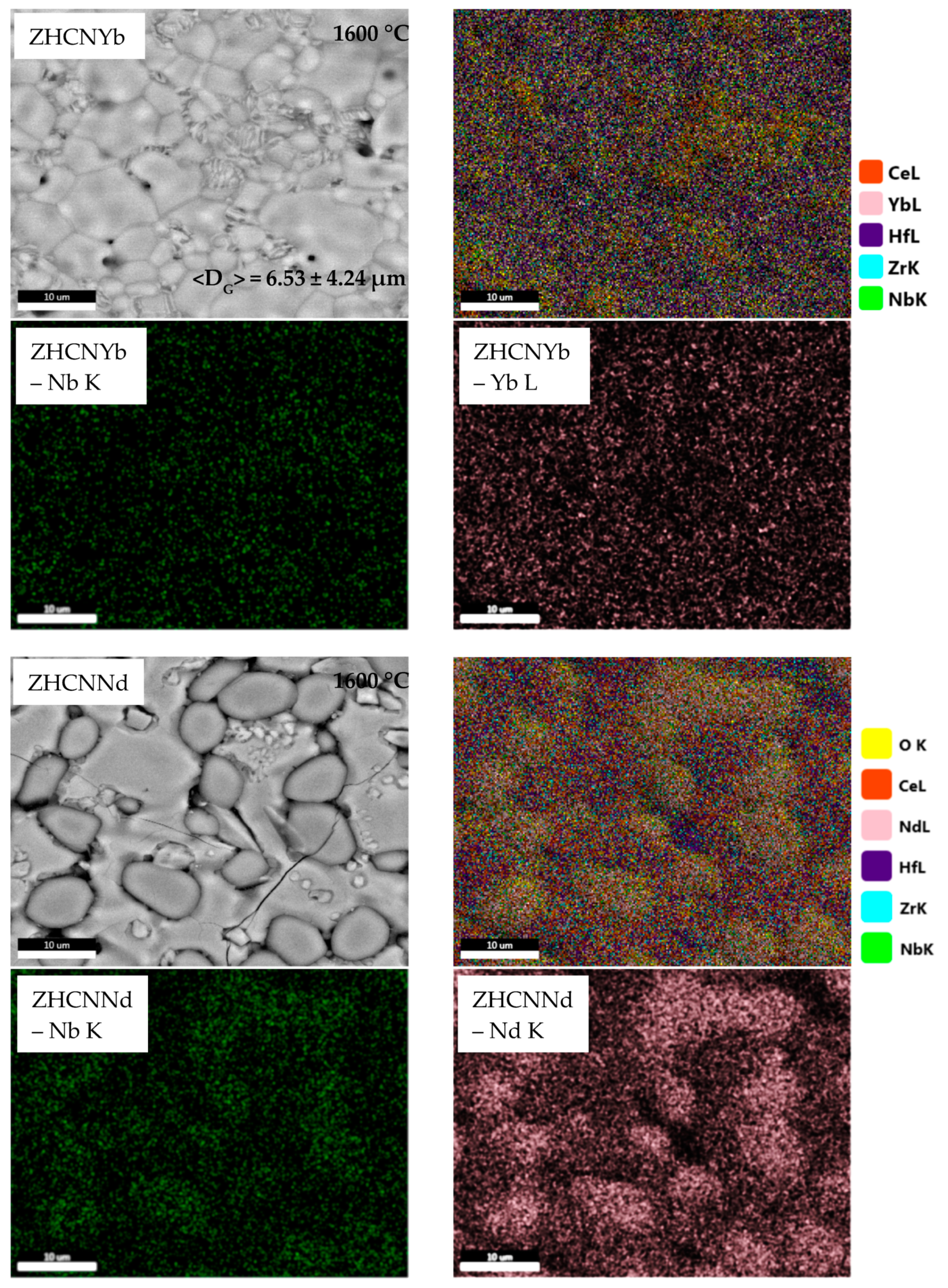
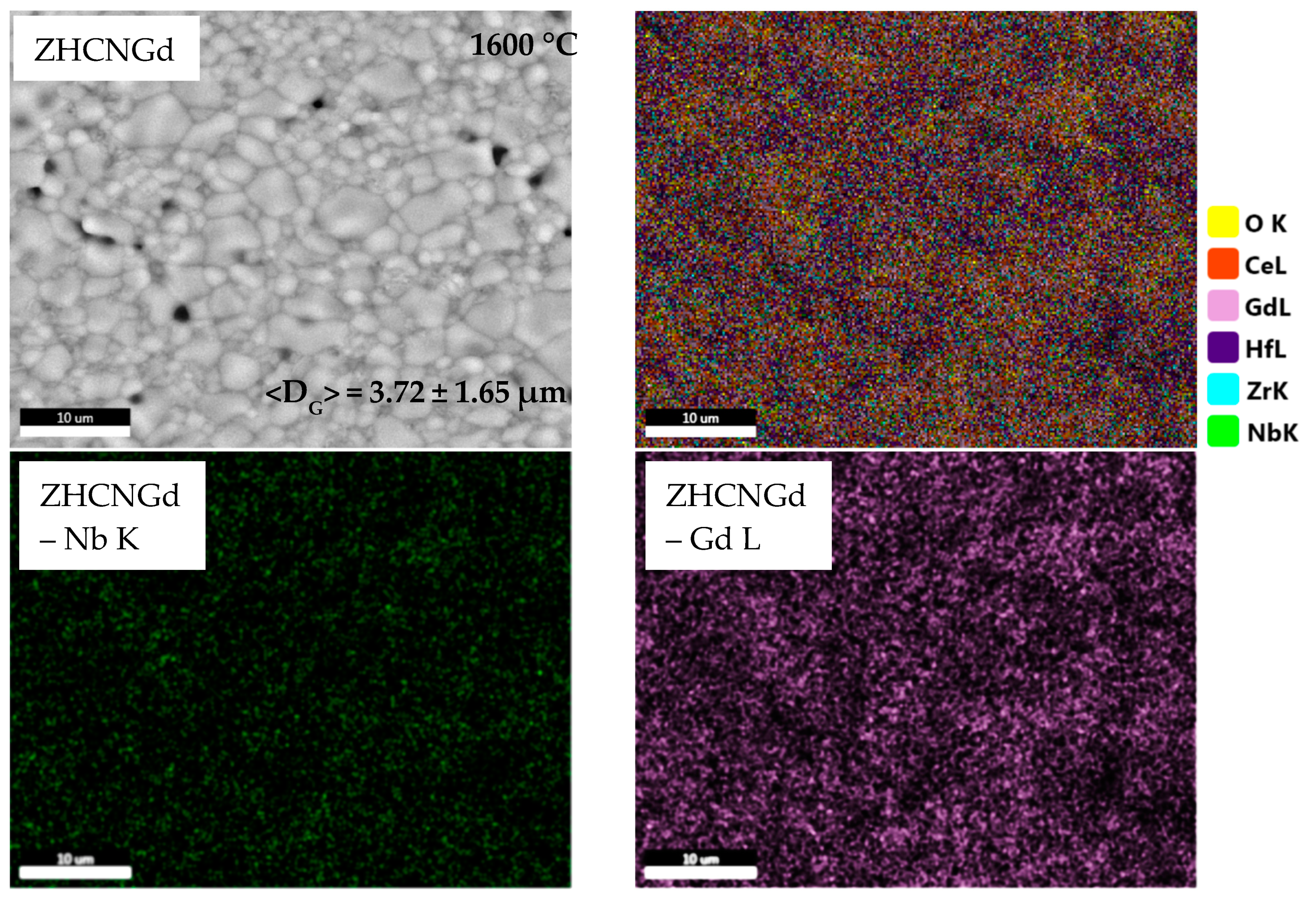
3.2. Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parras, J.P.; De Souza, R.A. Grain-boundary diffusion of cations in fluorite-type oxides is faster but not always easier. Acta Mater. 2020, 195, 383–391. [Google Scholar] [CrossRef]
- Solomon, J.M.; Shamblin, J.; Lang, M.; Navrotsky, A.; Asta, M. Chemical ordering in substituted fluorite oxides: A computational investigation of Ho2Zr2O7 and RE2Th2O7 (RE = Ho, Y, Gd, Nd, La). Sci. Rep. 2016, 6, 38772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Liu, Z.; Pang, L.; Han, Y.; Yao, S.; Wang, X. Preparation of Y2O3–ZrO2–CeO2 solid solution by co-precipitation and its electrical property. Phys. B Condens. Matter 2021, 612, 412972. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, L.; Liu, T.; Sun, Q.; Jin, E.; Tian, C.; Yu, J. Enhanced ionic conductivity and thermal shock resistance of MgO stabilized ZrO2 doped with Y2O3. Ceram. Int. 2020, 46, 19835–19842. [Google Scholar] [CrossRef]
- Guo, L.; Li, G.; Zhilin, G. Effects of surface roughness on CMAS corrosion behavior for thermal barrier coating applications. J. Adv. Ceram. 2021, 10, 472–481. [Google Scholar] [CrossRef]
- Fan, W.; Bai, Y.; Wang, Y.; He, T.; Gao, Y.; Zhang, Y.; Zhong, X.; Li, B.; Chang, Z.; Ma, Y. Microstructural design and thermal cycling performance of a novel layer-gradient nanostructured Sc2O3–Y2O3 co-stabilized ZrO2 thermal barrier coating. J. Alloys Compd. 2020, 829, 154525. [Google Scholar] [CrossRef]
- Fang, H.; Wang, W.; Huang, J.; Li, Y.; Ye, D. Corrosion behavior and thermos-physical properties of a promising Yb2O3 and Y2O3 co-stabilized ZrO2 ceramic for thermal barrier coatings subject to calcium-magnesium-aluminum-silicate (CMAS) deposition: Experiments and first-principles calculation. Corros. Sci. 2021, 182, 109230. [Google Scholar] [CrossRef]
- Park, M.H.; Hwang, C.S. Fluorite-structure antiferroelectrics. Rep. Prog. Phys. 2019, 82, 124502. [Google Scholar] [CrossRef]
- Ali, F.; Zhou, D.; Sun, N.; Ali, H.W.; Abbas, A.; Iqbal, F.; Dong, F.; Kim, K.-H. Fluorite-structured ferroelectric-/antiferroelectric-based electrostatic nanocapacitors for energy storage applications. ACS Appl. Energy Mater. 2020, 3, 6036–6055. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Qin, C.; Han, C.; Sun, L.; Wang, Y. Band-gap-tunable CeO2 nanoparticles for room-temperature NH3 gas sensors. Ceram. Int. 2020, 46, 19232–19240. [Google Scholar] [CrossRef]
- Wright, A.J.; Wang, Q.; Huang, C.; Nieto, A.; Chen, R.; Luo, J. From high-entropy ceramics to compositionally-complex ceramics: A case study of fluorite oxides. J. Eur. Ceram. Soc. 2020, 40, 2120–2129. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowa, J.; Szymczak, M.; Zajusz, M.; Mikuła, A.; Moździerz, M.; Berent, K.; Wytrwal-Sarna, M.; Bernasik, A.; Stygar, M.; Świerczek, K. Stabilizing fluorite structure in ceria-based high-entropy oxides: Influence of Mo addition on crystal structure and transport properties. J. Eur. Ceram. Soc. 2020, 40, 5870–5881. [Google Scholar] [CrossRef]
- Gild, J.; Samiee, M.; Braun, J.L.; Harrington, T.; Vega, H.; Hopkins, P.E.; Vecchio, K.; Luo, J. High-entropy fluorite oxides. J. Eur. Ceram. Soc. 2018, 38, 3578–3584. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.; Liu, J.; Do-Thanh, C.-L.; Chen, H.; Xu, S.; Lin, Q.; Jiao, Y.; Wang, J.; Wang, Y. Entropy-stabilized single-atom Pd catalysts via high-entropy fluorite oxide supports. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Spiridigliozzi, L.; Ferone, C.; Cioffi, R.; Dell’Agli, G. A simple and effective predictor to design novel fluorite-structured High Entropy Oxides (HEOs). Acta Mater. 2021, 202, 181–189. [Google Scholar] [CrossRef]
- Ruh, R.; Garrett, H.J.; Domagala, R.F.; Tallan, N.M. The System Zirconia-Hafnia. J. Am. Ceram. Soc. 1968, 51, 23–27. [Google Scholar] [CrossRef]
- Andrievskaya, E.R.; Gerasimyuk, G.I.; Kornienko, O.A.; Samelyuk, A.V.; Lopato, L.M. Phase equilibria in the HfO2-ZrO2-CeO2 system at 1250 °C. Inorg. Mater. 2006, 42, 1352–1359. [Google Scholar] [CrossRef]
- Andrievskaya, E.R.; Samelyuk, A.V.; Lopato, L.M. Reactions of Ceria with Zirconia and Yttria at 1250 °C. Poroshk. Met. 2002, 41, 1–2. [Google Scholar] [CrossRef]
- Andrievskaya, E.R.; Gerasimyuk, G.I.; Kornienko, O.A.; Samelyuk, A.V.; Lopato, L.M.; Red’ko, V.P. Phase equilibria in the system HfO2-ZrO2-CeO2 at 1500 °C. Powder Metall. Met. Ceram. 2006, 45, 448–456. [Google Scholar] [CrossRef]
- Wang, Z.; Saxena, S.K.; Pischedda, V.; Liermann, H.P.; Zha, C.S. In situ X-ray diffraction study of the pressure-induced phase transformation in nanocrystalline CeO2. Phys. Rev. B 2001, 64, 12102. [Google Scholar] [CrossRef]
- Xia, X.; Oldman, R.; Catlow, R. Computational modeling study of bulk and surface of yttria-stabilized cubic zirconia. Chem. Mater. 2009, 21, 3576–3585. [Google Scholar] [CrossRef]
- Adams, D.M.; Leonard, S.; Russell, D.R.; Cernik, R.J. X-ray diffraction study of hafnia under high pressure using synchrotron radiation. J. Phys. Chem. Solids 1991, 52, 1181–1186. [Google Scholar] [CrossRef]
- Emeka, N.C.; Imoisili, P.E.; Jen, T.-C. Preparation and Characterization of NbxOy Thin Films: A Review. Coatings 2020, 10, 1246. [Google Scholar] [CrossRef]
- Henriques, M.S.; Ferreira, A.C.; Cruz, A.; Ferreira, L.M.; Branco, J.B.; Brazda, P.; Jurek, K.; Stora, T.; Goncalves, A.P. Preparation of Yb2O3 submicron-and nano-materials via electrospinning. Ceram. Int. 2015, 41, 10795–10802. [Google Scholar] [CrossRef]
- Delice, S.; Isik, M.; Gasanly, N.M. Effect of heating rate on thermoluminescence characteristics of Y2O3 nanoparticles. J. Lumin. 2019, 212, 233–237. [Google Scholar] [CrossRef]

| Composition | 0.2ZrO2-0.2HfO2-0.2CeO2-0.2Nb2O3-0.2Y2O3 (ZHCNY) | 0.2ZrO2-0.2HfO2-0.2CeO2-0.2Nb2O3-0.2Yb2O3 (ZHCNYb) | 0.2ZrO2-0.2HfO2-0.2CeO2-0.2Nb2O3-0.2Nd2O3 (ZHCNNd) | 0.2ZrO2-0.2HfO2-0.2CeO2-0.2Nb2O3-0.2Gd2O3 (ZHCNGd) | |
|---|---|---|---|---|---|
| Precursor | |||||
| ZrO2 | 3.2787 g | 2.9486 g | 3.0539 g | 3.0055 g | |
| HfO2 | 5.6009 g | 5.0370 g | 5.2168 g | 5.1341 g | |
| CeO2 | 4.5796 g | 4.1185 g | 4.2656 g | 4.1979 g | |
| Nb2O5 | 3.5364 g | 3.1804 g | 3.2939 g | 3.2417 g | |
| Y2O3 | 3.0043 g | - | - | - | |
| Yb2O3 | - | 4.7155 g | - | - | |
| Nd2O3 | - | - | 4.1697 g | - | |
| Gd2O3 | - | - | - | 4.4209 g | |
| Sample | Thermal Treatment Temperature | Monoclinic—P2/m | Tetragonal—P4/mmm | Cubic—Fm-3m | RENbO4—Monoclinic—P2/m |
|---|---|---|---|---|---|
| ZHCNY | 1300 °C | 17.1% ± 0.5% | 22.3% ± 0.5% | 5.9% ± 0.5% | 54.6% ± 0.5% |
| 1400 °C | 0.8% ± 0.5% | 64.9% ± 0.5% | 3.2% ± 0.5% | 31.0% ± 0.5% | |
| 1500 °C | 0.0% | 68.0% ± 0.50% | 0.0% | 32.0% ± 0.5% | |
| 1600 °C | 0.0% | 0.0% | 76.2% ± 0.5% | 23.8% ± 0.5% | |
| ZHCNYb | 1300 °C | 7.0% ± 0.5% | 29.6% ± 0.5% | 16.0% ± 0.5% | 47.4% ± 0.5% |
| 1400 °C | 0.0% | 22.3% ± 0.5% | 45.3% ± 0.5% | 32.4% ± 0.5% | |
| 1500 °C | 0.0% | 0.0% | 68.2% ± 0.5% | 31.8% ± 0.5% | |
| 1600 °C | 0.0% | 0.0% | 70.9% ± 0.5% | 29.1% ± 0.5% | |
| ZHCNNd | 1300 °C | 9.5% ± 0.5% | 56.1% ± 0.5% | 34.4% ± 0.5% | 0.0% |
| 1400 °C | 33.7% ± 0.5% | 6.1% ± 0.5% | 41.3% ± 0.5% | 18.9% ± 0.5% | |
| 1500 °C | 25.5% ± 0.5% | 2.4% ± 0.5% | 49.6% ± 0.5% | 22.5% ± 0.5% | |
| 1600 °C | 0.0% | 0.0% | 70.0% ± 0.5% | 30.0% ± 0.5% | |
| ZHCNGd | 1300 °C | 18.4% ± 0.5% | 30.8% ± 0.5% | 2.0% ± 0.5% | 48.8% ± 0.5% |
| 1400 °C | 0.0% | 28.5% ± 0.5% | 28.6% ± 0.5% | 42.9% ± 0.5% | |
| 1500 °C | 0.0% | 0.0% | 59.5% ± 0.5% | 40.5% ± 0.5% | |
| 1600 °C | 0.0% | 0.0% | 63.4% ± 0.5% | 36.6% ± 0.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surdu, V.-A.; Andronescu, E. Phase Formation in Heterovalent Equimolar Quinary Oxide Systems of ZrO2-HfO2-CeO2-Nb2O5-RE2O3 Type (RE = Y, Yb, Nd, Gd). Ceramics 2021, 4, 476-485. https://doi.org/10.3390/ceramics4030035
Surdu V-A, Andronescu E. Phase Formation in Heterovalent Equimolar Quinary Oxide Systems of ZrO2-HfO2-CeO2-Nb2O5-RE2O3 Type (RE = Y, Yb, Nd, Gd). Ceramics. 2021; 4(3):476-485. https://doi.org/10.3390/ceramics4030035
Chicago/Turabian StyleSurdu, Vasile-Adrian, and Ecaterina Andronescu. 2021. "Phase Formation in Heterovalent Equimolar Quinary Oxide Systems of ZrO2-HfO2-CeO2-Nb2O5-RE2O3 Type (RE = Y, Yb, Nd, Gd)" Ceramics 4, no. 3: 476-485. https://doi.org/10.3390/ceramics4030035
APA StyleSurdu, V.-A., & Andronescu, E. (2021). Phase Formation in Heterovalent Equimolar Quinary Oxide Systems of ZrO2-HfO2-CeO2-Nb2O5-RE2O3 Type (RE = Y, Yb, Nd, Gd). Ceramics, 4(3), 476-485. https://doi.org/10.3390/ceramics4030035







