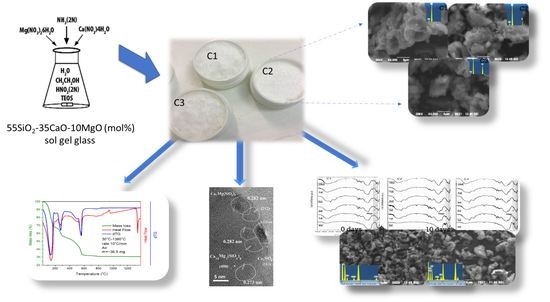
Synthesis and Characterization of Novel Calcium-Silicate Nanobioceramics with Magnesium: Effect of Heat Treatment on Biological, Physical and Chemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glass-Ceramic Synthesis of Bioceramic Nanopowder
2.2. Morphological and Structural Characterization of Synthesized Bioceramic Nanopowder
2.2.1. Differential Thermal and Thermogravimetric Analysis (TG-DTA)
2.2.2. Structural Characterization
2.3. In Vitro Apatite-Forming Ability in Simulated Body Fluid
2.4. Preparation of Cell Lines of Human Fibroblasts (Human Gingival Fibrοblasts, HGFs)
Mitochondrial Activity—MTT Assay
2.5. Antibacterial Assay
2.5.1. Growth Experiments
2.5.2. Statistical Data Analysis of the Bacterial Cultures
2.6. Fluorescence Analysis for the Detection of ROS Levels
3. Results
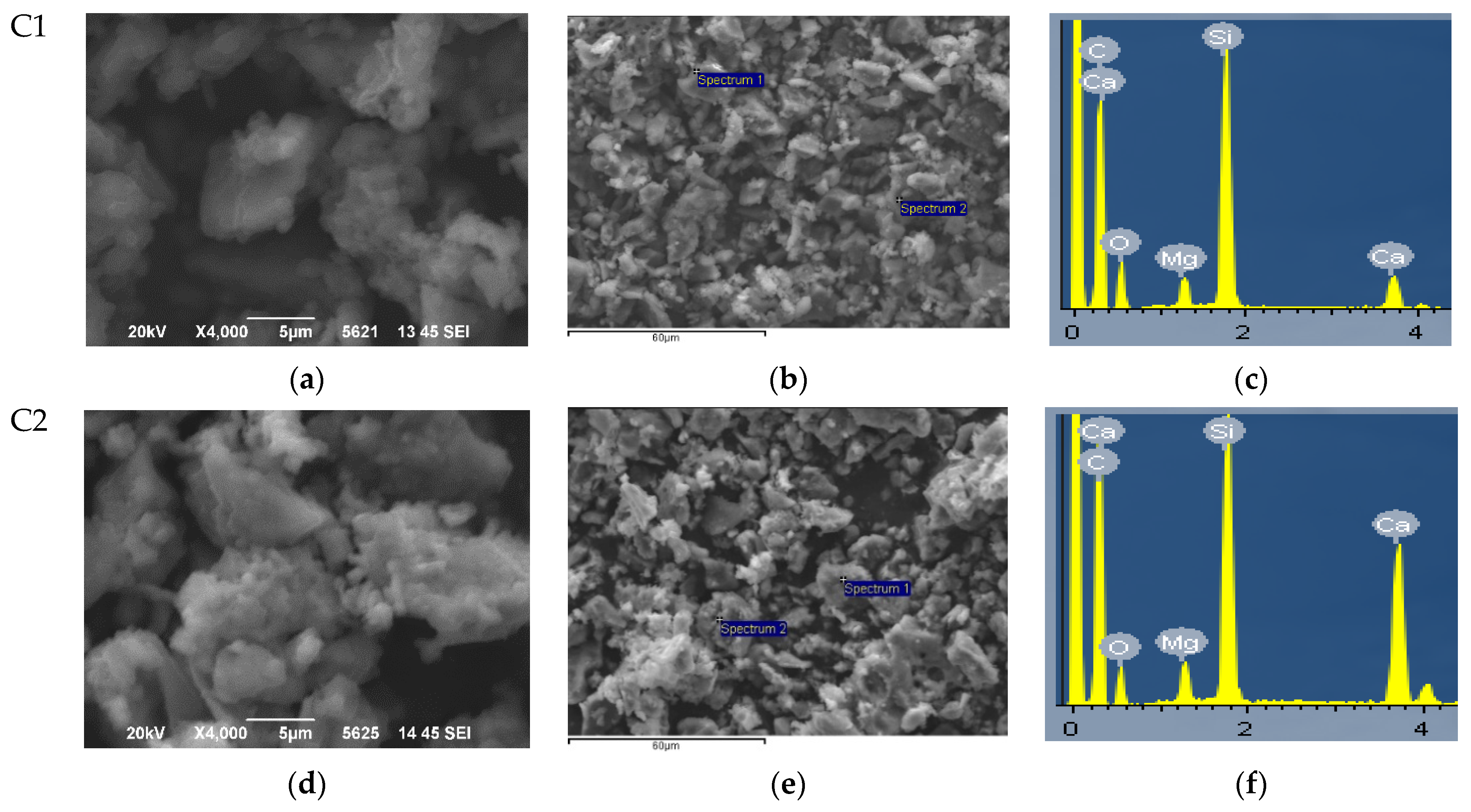
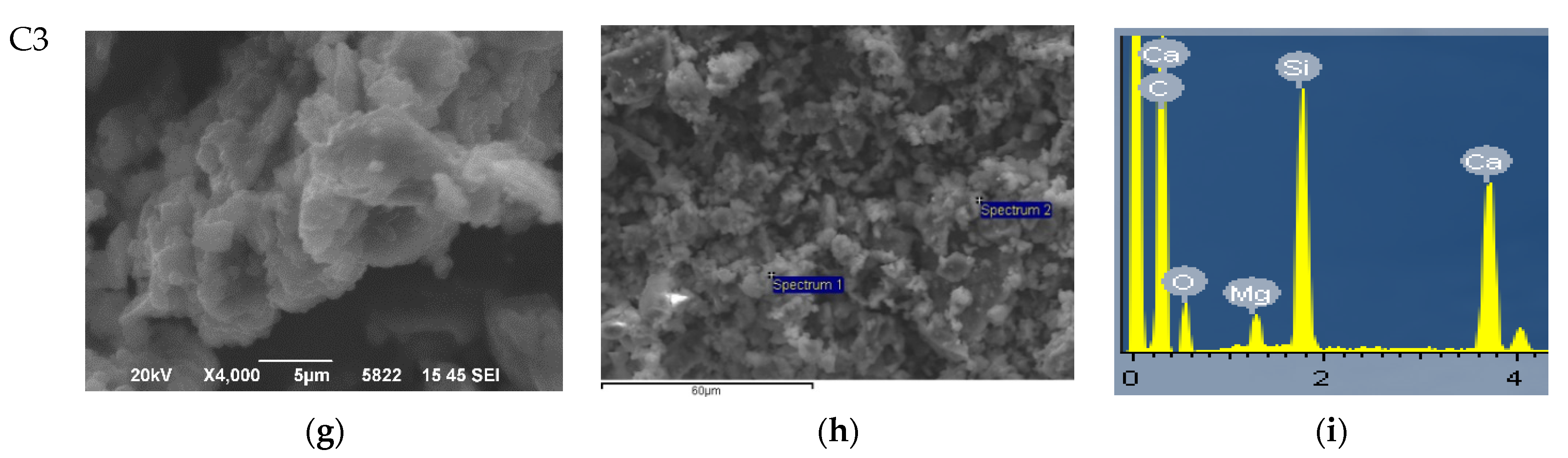
3.1. Morphological and Structural Characterization of Synthesized Bioceramic Nanopowders
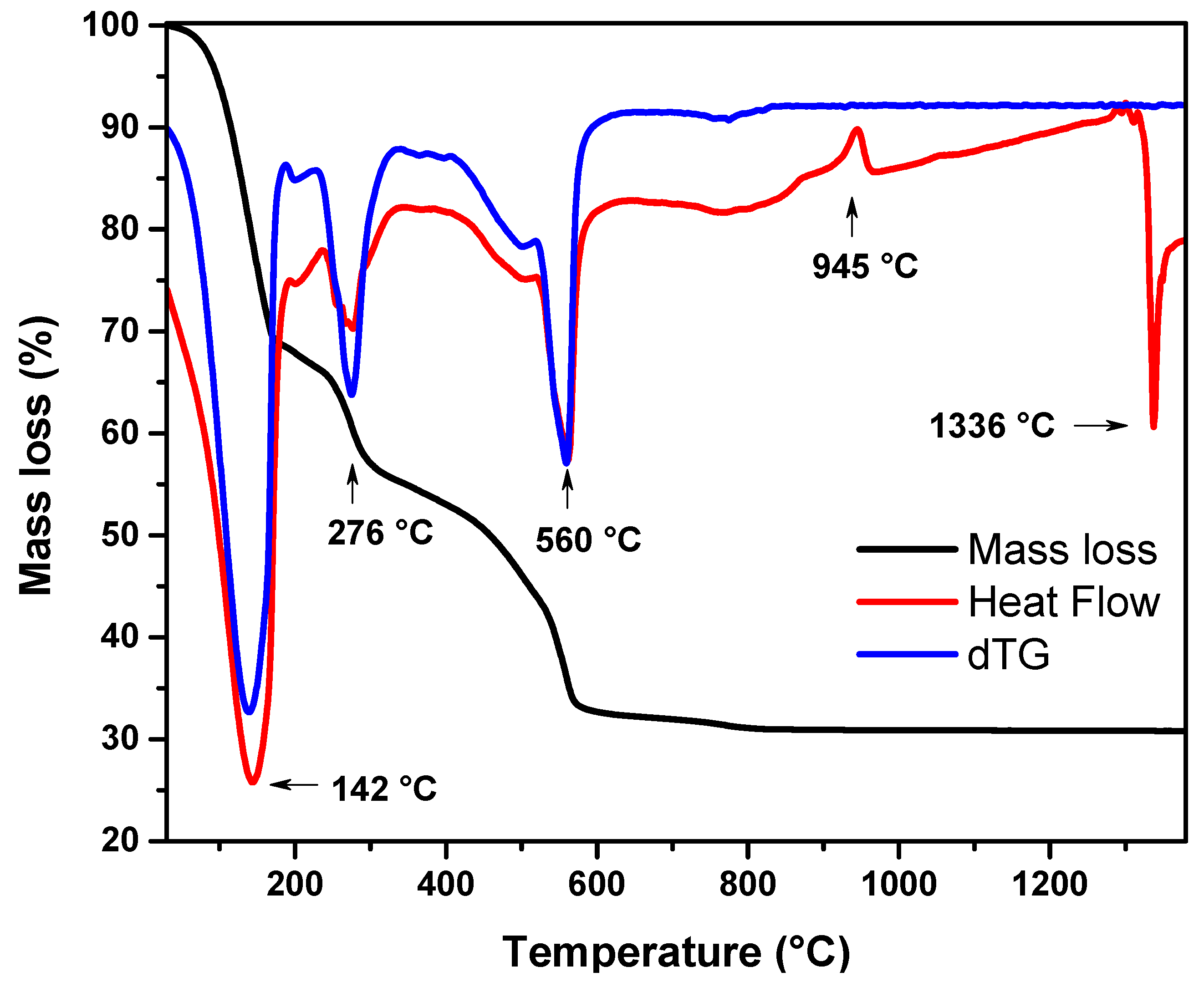
3.1.1. Thermogravimetric Results
- (a)
- 25–190 °C: Mass loss of about 31.6%, which was associated with an endothermic peak at 142 °C. It was attributed to the loss of adsorbed water and the degradation of HNO3. As reported in the literature, there are two forms of water “trapped” in the crystalline cell structure of the sol–gel-based nanopowder when it is in the gel state: the unbound water inside the gel structure and water [81,82,83].
- (b)
- 190–333 °C: Mass loss of about 13%, which was associated with an endothermic peak at 276 °C. It was attributed to incineration of organic residues of ethanol and acetone [84]. In addition, in this temperature range, the decomposition of the chemically adsorbed water was attributed to the decomposition of the polycondensation reaction.
- (c)
- 333–640 °C: Mass loss of about 23.1%, associated with an endothermic peak at 560 °C. According to the literature, it is attributed to the decomposition of calcium nitrate tetrahydrate, which is first converted to anhydrous calcium nitrate Ca(NO3)2 (eliminating H2O at about 350 °C) and then it decomposes into CaO and NO2 [85,86].
- (d)
- 640–835 °C/1000 °C: Mass loss of 1.4% due to dehydroxylation [81,83], achieved above 850 °C. When the viscous flow began and the hydroxyl groups reacted, the particles came closer together on the surfaces of the gels, thereby eliminating the inner pores and gaps. This was consistent with the Heat Flow curve, since there was no indication of a decomposition process, while at the same time there was no significant loss of mass at that particular temperature range and above.
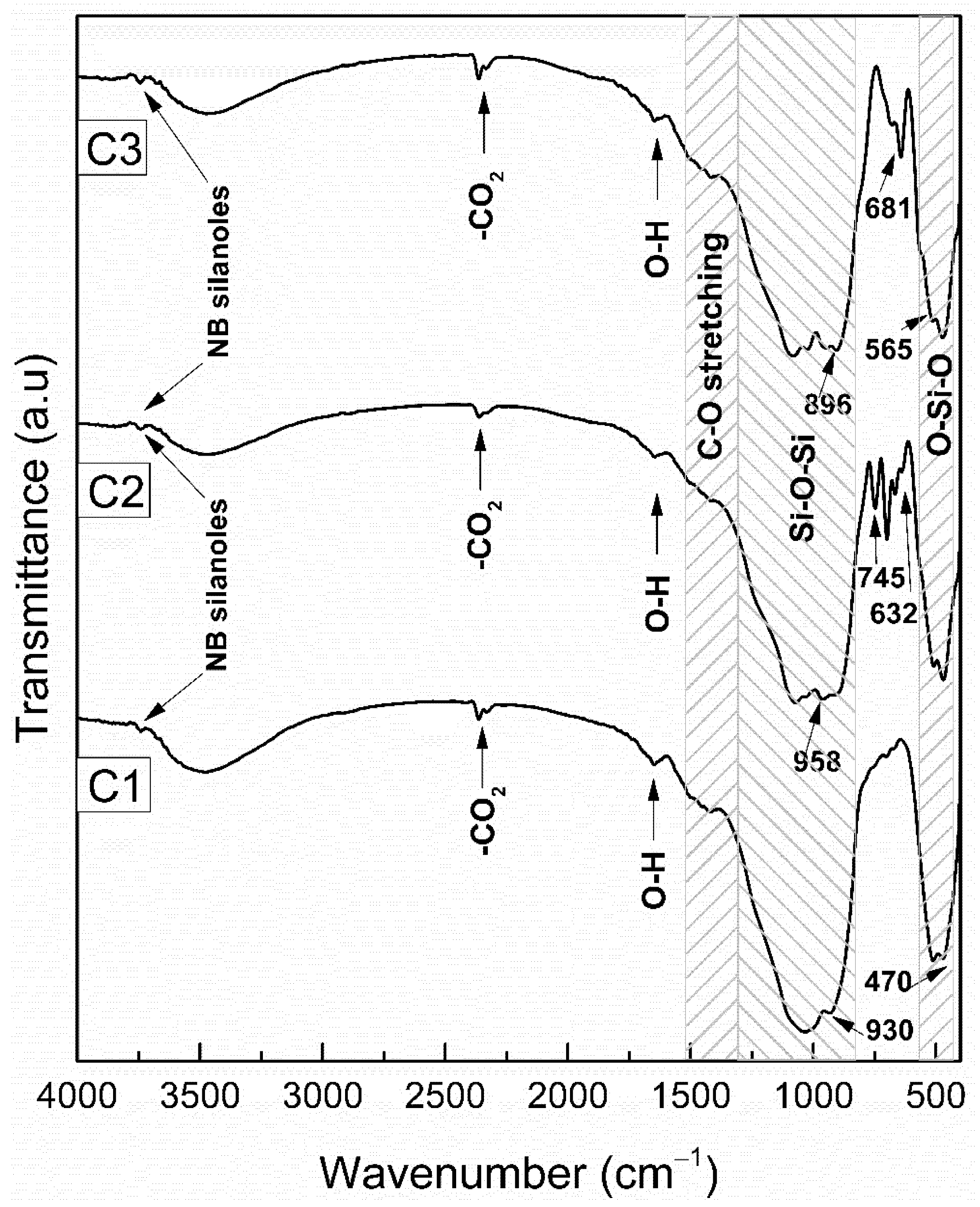
3.1.2. FTIR Analysis of Heat-Treated Bioceramic Materials
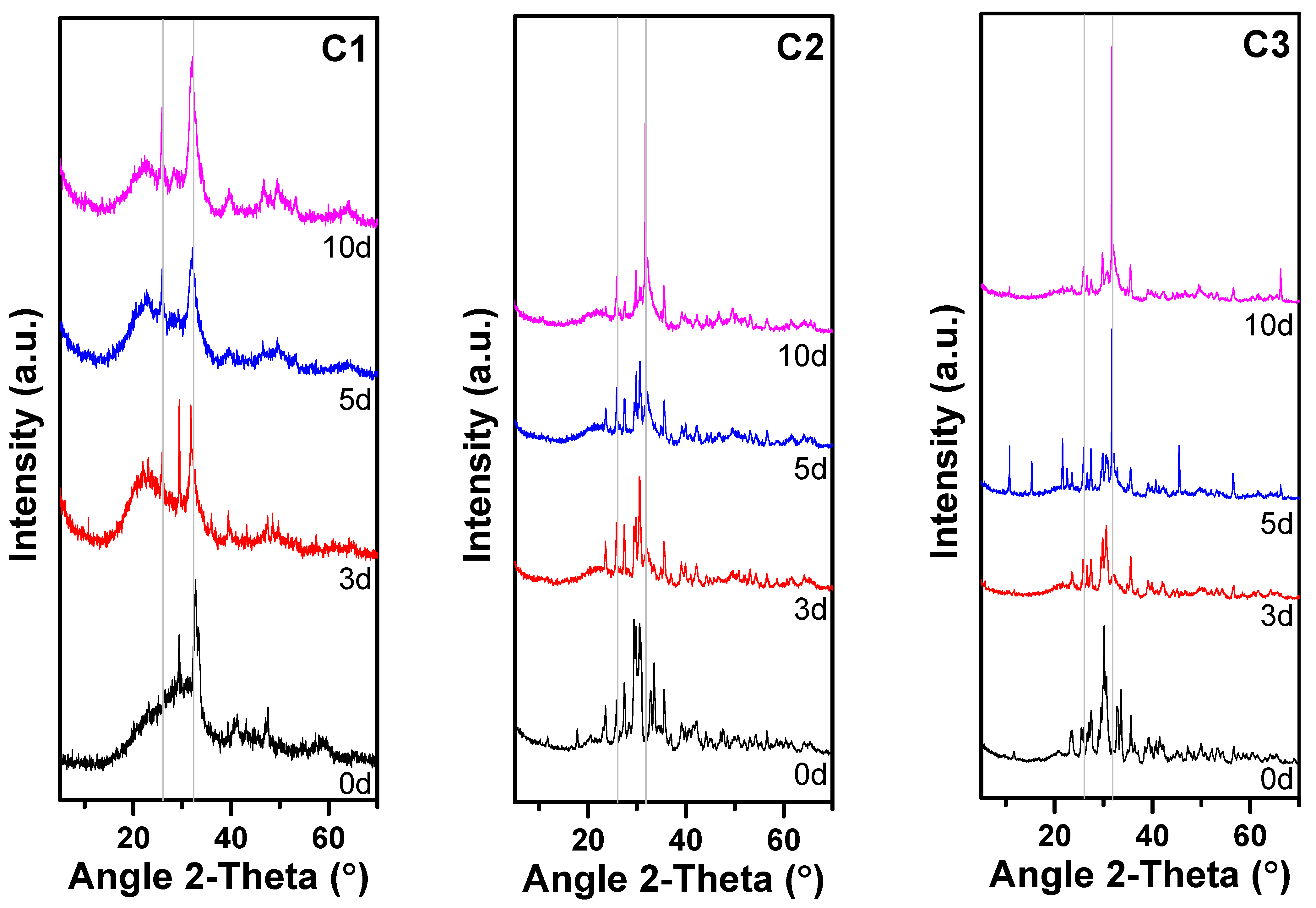
3.1.3. XRD Results
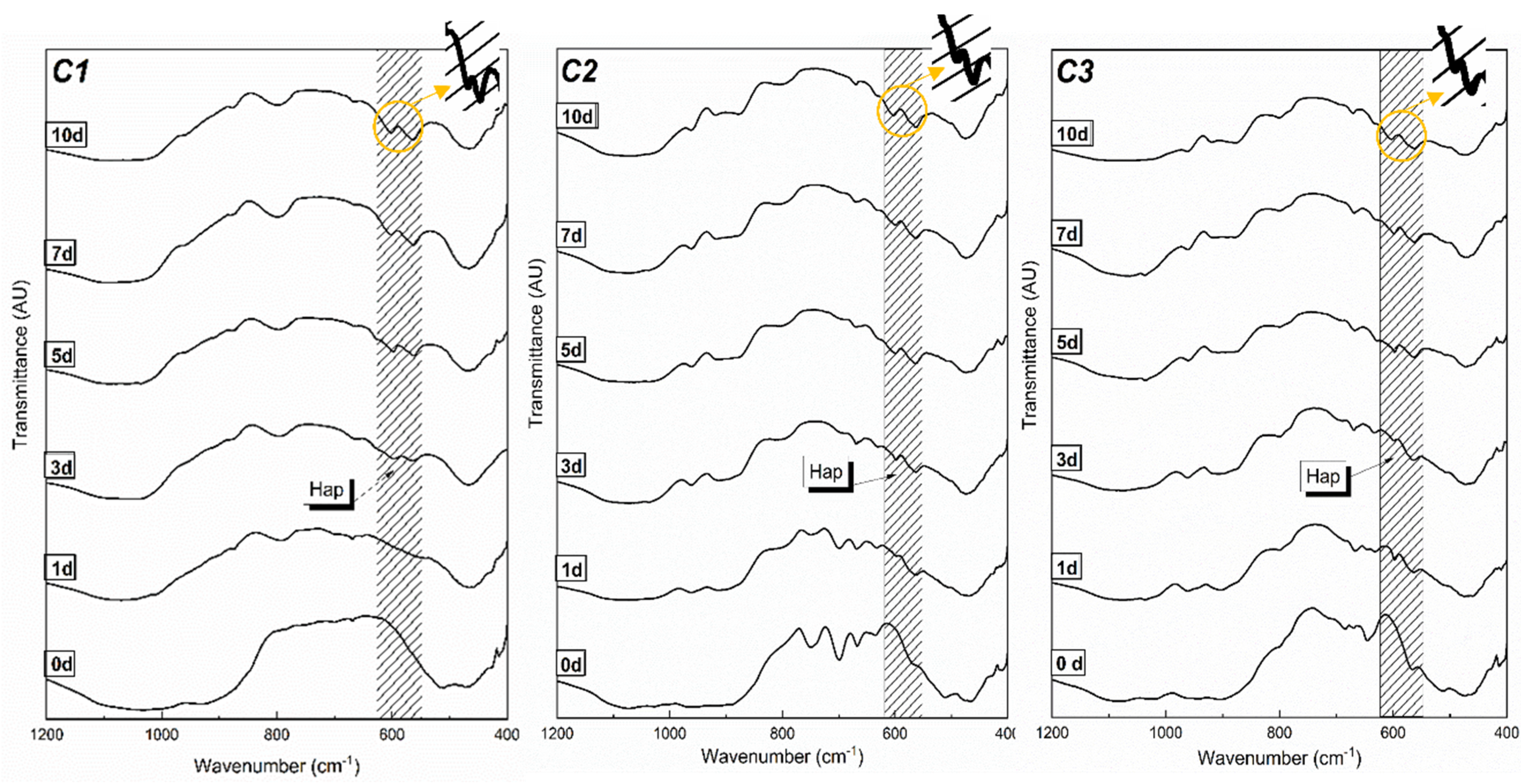
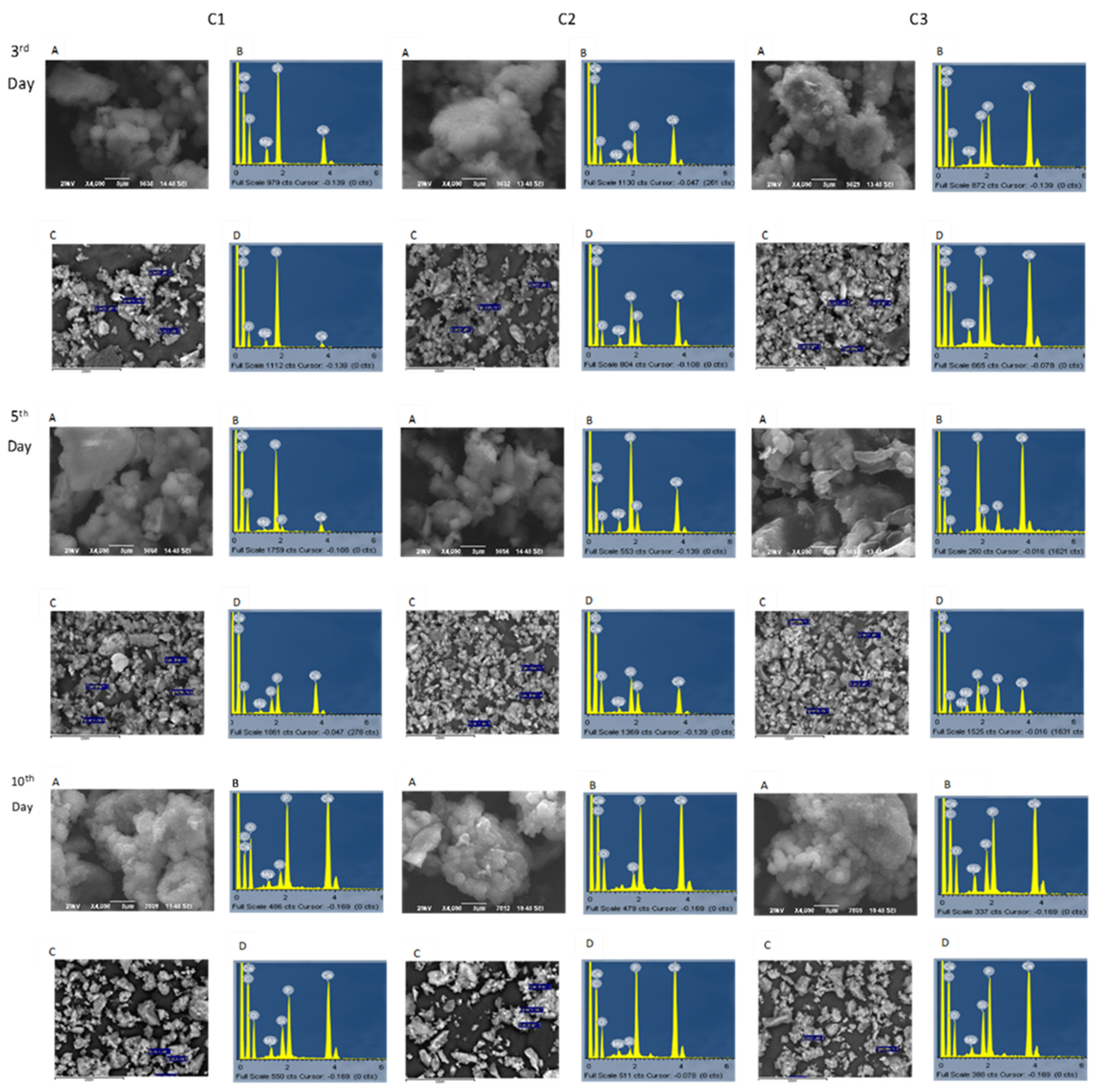
3.2. In Vitro Apatite-Forming Ability in Simulated Body Fluid (SBF)
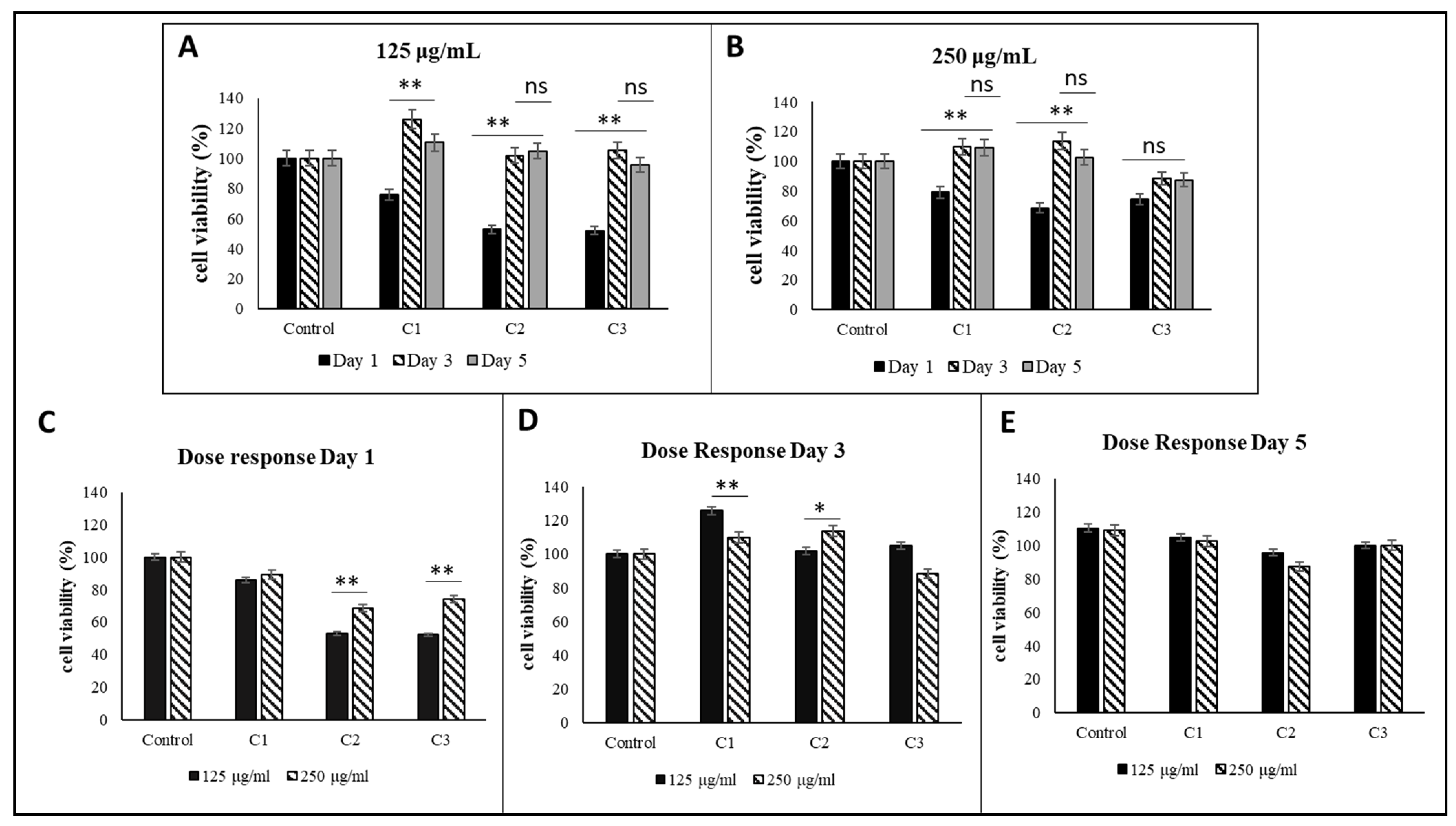
3.3. Mitochondrial Activity-MTT Assay
3.4. Antibacterial Assay
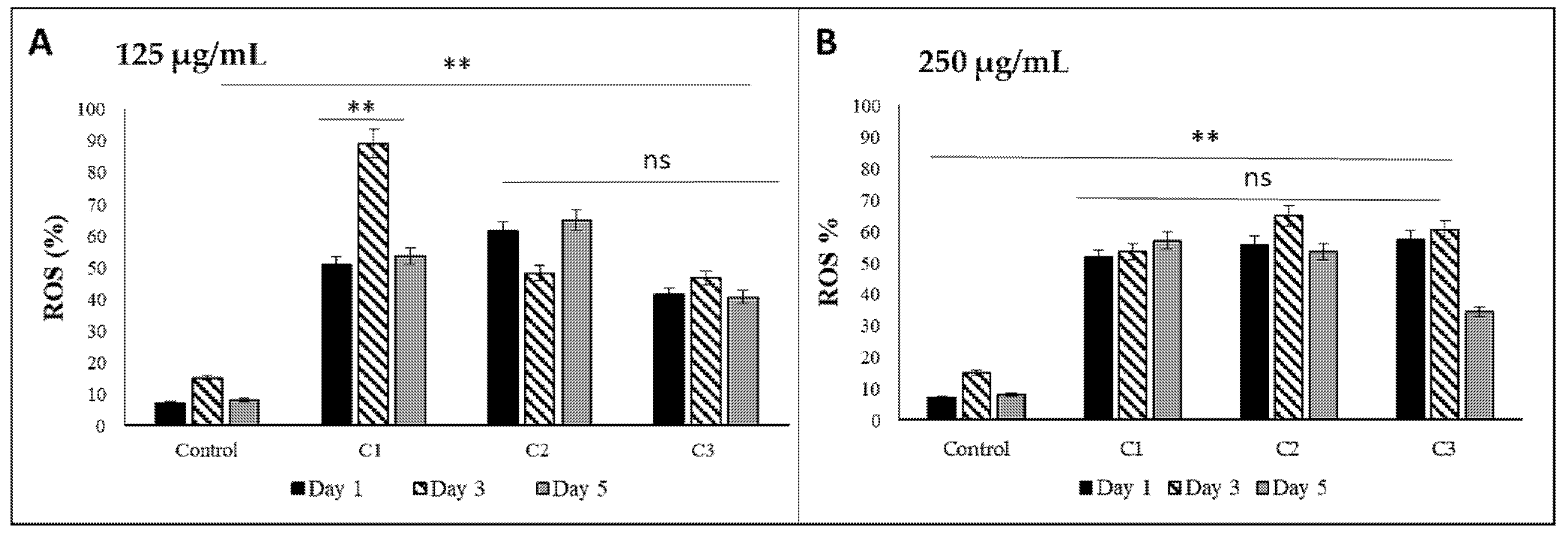
3.5. Oxidative Activity of Bioactive Glass Nanoceramics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Papadopoulou, L.; Kontonasaki, E.; Zorba, T.; Chatzistavrou, X.; Pavlidou, E.; Paraskevopoulos, K.; Sklavounos, S.; Koidis, P. Dental ceramics coated with bioactive glass: Surface changes after exposure in a simulated body fluid under static and dynamic conditions. Phys. Status Solidi 2003, 198, 65–75. [Google Scholar] [CrossRef]
- Goudouri, O.M. Solid State Section Novel Bioactive Ceramics for Dental Ourania Menti Goudouri. 2012. Available online: http://ikee.lib.auth.gr/record/129556/files/GRI-2012-8967.pdf (accessed on 4 June 2019).
- Park, J.B.; Lakes, R.S. Soft Tissue Replacement—I: Sutures, Skin, and Maxillofacial Implants. In Biomaterials; Springer: New York, NY, USA, 2007; pp. 291–329. [Google Scholar]
- Gabriel, S.E. Soft Tissue Response to Silicones. In Handbook of Biomaterial Properties; Springer: Boston, MA, USA, 1998; pp. 556–571. [Google Scholar]
- Papynov, E.; Shichalin, O.; Apanasevich, V.; Portnyagin, A.; Yu, M.V.; Yu, B.I.; Merkulov, E.; Kaidalova, T.; Modin, E.; Afonin, I.; et al. Sol-gel (template) synthesis of osteoplastic CaSiO3/HAp powder biocomposite: “In vitro” and “in vivo” biocompatibility assessment. Powder Technol. 2020, 367, 762–773. [Google Scholar] [CrossRef]
- Apanasevich, V.; Papynov, E.; Plekhova, N.; Zinoviev, S.; Kotciurbii, E.; Stepanyugina, A.; Korshunova, O.; Afonin, I.; Evdokimov, I.; Shichalin, O.; et al. Morphological Characteristics of the Osteoplastic Potential of Synthetic CaSiO3/HAp Powder Biocomposite. J. Funct. Biomater. 2020, 11, 68. [Google Scholar] [CrossRef]
- Mucalo, M. Hydroxyapatite (HAp) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9781782420415. [Google Scholar]
- Ni, S.; Chang, J. In vitro Degradation, Bioactivity, and Cytocompatibility of Calcium Silicate, Dimagnesium Silicate, and Tricalcium Phosphate Bioceramics. J. Biomater. Appl. 2009, 24, 139–158. [Google Scholar] [CrossRef]
- Park, J.B.; Lakes, R.S. Hard Tissue Replacement—I: Long Bone Repair. In Biomaterials; Springer: New York, NY, USA, 2007; pp. 369–394. [Google Scholar]
- Beck, G.R.; Ha, S.-W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.-K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 793–803. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Apanasevich, V.I.; Afonin, I.S.; Evdokimov, I.O.; Mayorov, V.; Portnyagin, A.S.; Agafonova, I.G.; Skurikhina, Y.; Medkov, M.A. Synthetic CaSiO3 sol-gel powder and SPS ceramic derivatives: “In vivo” toxicity assessment. Prog. Nat. Sci. 2019, 29, 569–575. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Belov, A.A.; Portnyagin, A.S.; Mayorov, V.Y.; Merkulov, E.B.; Kaydalova, T.I.; Skurikhina, Y.E.; Turkutyukov, V.B.; et al. CaSiO3-HAp Structural Bioceramic by Sol-Gel and SPS-RS Techniques: Bacteria Test Assessment. J. Funct. Bioceram. 2020, 11, 41. [Google Scholar] [CrossRef]
- Black, J.; Hastings, G.W. Handbook of Biomaterial Properties; Chapman & Hall: London, UK, 1998; ISBN 0412603306. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Cambridge, MA, USA, 2013; ISBN 9780123746269. [Google Scholar]
- Doostmohammadi, A.; Monshi, A.; Fathi, M.; Braissant, O. A comparative physico-chemical study of bioactive glass and bone-derived hydroxyapatite. Ceram. Int. 2011, 37, 1601–1607. [Google Scholar] [CrossRef]
- Gupta, R.; Kumar, A. Bioactive materials for biomedical applications using sol–gel technology. Biomed. Mater. 2008, 3, 034005. [Google Scholar] [CrossRef]
- Paulo, C.; Marques, J.; Sequeira, D.; Diogo, P.; Paiva, R.; Palma, P.; Santos, J. Influence of Blood Contamination on Push-Out Bond Strength of Three Calcium Silicate-Based Materials to Root Dentin. Appl. Sci. 2021, 11, 6849. [Google Scholar] [CrossRef]
- Lim, M.; Jung, C.; Shin, D.-H.; Cho, Y.-B.; Song, M. Calcium silicate-based root canal sealers: A literature review. Restor. Dent. Endod. 2020, 45, e35. [Google Scholar] [CrossRef]
- El-Kady, A.; Farag, M. Bioactive Glass Nanoparticles as a New Delivery System for Sustained 5-Fluorouracil Release: Characterization and Evaluation of Drug Release Mechanism. J. Nanomater. 2015, 2015, 839207. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, V.; Nahrkhalaji, M.M.; Fathi, M.H.; Mousavi, S.B.; Esfahani, B.N. Antibacterial effects of sol-gel-derived bioactive glass nanoparticle on aerobic bacteria. J. Biomed. Mater. Res. Part A 2010, 94, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Jittiarporn, P.; Badilescu, S.; Al Sawafta, M.N.; Sikong, L.; Truong, V.-V. Electrochromic properties of sol–gel prepared hybrid transition metal oxides—A short review. J. Sci. Adv. Mater. Devices 2017, 2, 286–300. [Google Scholar] [CrossRef]
- Ullah, M.; Ali, M.E.; Hamid, S.B.A. Surfactant-Assisted Ball Milling: A Novel Route to Novel Materials with Controlled Nanostructure—A Review. Rev. Adv. Mater. Sci. 2014, 37, 1–14. [Google Scholar]
- Ramesh, K.T. Nanomaterials. In Nanomaterials; Springer: Boston, MA, USA, 2009; pp. 1–20. [Google Scholar]
- Chapelle, A.; Hassani, F.O.; Presmanes, L.; Barnabe, A.; Tailhades, P. CO2 sensing properties of semiconducting copper oxide and spinel ferrite nanocomposite thin film. Appl. Surf. Sci. 2010, 256, 4715–4719. [Google Scholar] [CrossRef] [Green Version]
- Ennas, G.; Marongiu, G.; Marras, S.; Piccaluga, G. Mechanochemical Route for the Synthesis of Cobalt Ferrite–Silica and Iron–Cobalt Alloy–Silica Nanocomposites. J. Nanopart. Res. 2004, 6, 99–105. [Google Scholar] [CrossRef]
- Clark, A.E.; Hench, L.L.; Paschall, H.A. The influence of surface chemistry on implant interface histology: A theoretical basis for implant materials selection. J. Biomed. Mater. Res. 1976, 10, 161–174. [Google Scholar] [CrossRef]
- Rahman, A.; Seth, D.; Mukhopadhyaya, S.K.; Brahmachary, R.L.; Ulrichs, C.; Goswami, A. Surface functionalized amorphous nanosilica and microsilica with nanopores as promising tools in biomedicine. Naturwissenschaften 2009, 96, 31–38. [Google Scholar] [CrossRef]
- Decan, N.; Wu, D.; Williams, A.; Bernatchez, S.; Johnston, M.; Hill, M.; Halappanavar, S. Characterization of in vitro genotoxic, cytotoxic and transcriptomic responses following exposures to amorphous silica of different sizes. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 796, 8–22. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Barrioni, B.R.; Aline, A.; De Oliveira, R.; Materials, A.S. Biocompatible Glasses; Marchi, J., Ed.; Centro de Ciências Naturais e Humanas Universidade Federal do ABC: Santo André, Brazil, 2016; Volume 53, ISBN 978-3-319-44247-1. [Google Scholar]
- Jaganathan, H.; Godin, B. Biocompatibility assessment of Si-based nano- and micro-particles. Adv. Drug Deliv. Rev. 2012, 64, 1800–1819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiocco, L.; Li, S.; Stevens, M.; Bernardo, E.; Jones, J. Biocompatibility and bioactivity of porous polymer-derived Ca-Mg silicate ceramics. Acta Biomater. 2017, 50, 56–67. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of Mesoporous Silica Nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef]
- Eom, H.-J.; Choi, J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol. Vitr. 2009, 23, 1326–1332. [Google Scholar] [CrossRef]
- ECETOC Synthetic Amorphous Silica-ECETOC JACC REPORT No. 51. 2006. Available online: http://www.ecetoc.org/publication/jacc-report-51-synthetic-amorphous-silica/ (accessed on 15 August 2019).
- Lin, Y.-S.; Haynes, C. Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity. J. Am. Chem. Soc. 2010, 132, 4834–4842. [Google Scholar] [CrossRef]
- Razzaboni, B.L.; Bolsaitis, P. Evidence of an oxidative mechanism for the hemolytic activity of silica particles. Environ. Health Perspect. 1990, 87, 337–341. [Google Scholar] [CrossRef]
- Bellantone, M.; Coleman, N.J.; Hench, L.L. Bacteriostatic Action of a Novel Four-Componenr Bioactive Glass. J. Biomed. Mater. Res. 2000, 51, 484–490. [Google Scholar] [CrossRef]
- Stoor, P.; Söderling, E.; Salonen, J.I. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol. Scand. 1998, 56, 161–165. [Google Scholar] [CrossRef]
- Rivadeneira, J.; Luz, G.M.; Audisio, M.C.; Mano, J.F.; Gorustovich, A.A. Novel antibacterial bioactive glass nanocomposite functionalized with tetracycline hydrochloride. Biomed. Glas. 2015, 1, 128–135. [Google Scholar] [CrossRef]
- Catauro, M.; Raucci, M.G.; De Gaetano, F.; Marotta, A. Antibacterial and bioactive silver-containing Na2O·CaO·2SiO2 glass prepared by sol–gel method. J. Mater. Sci. Mater. Med. 2004, 15, 831–837. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Madras, G.; Basu, B. In vitro/In vivo assessment and mechanisms of toxicity of bioceramic materials and its wear particulates. RSC Adv. 2014, 4, 12763–12781. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.; Reis, R.L.; Hunt, J. The biocompatibility of novel starch-based polymers and composites: In vitro studies. Biomaterials 2002, 23, 1471–1478. [Google Scholar] [CrossRef]
- Doostmohammadi, A.; Monshi, A.; Salehi, R.; Fathi, M.H.; Golniya, Z.; Daniels, A.U. Bioactive glass nanoparticles with negative zeta potential. Ceram. Int. 2011, 37, 2311–2316. [Google Scholar] [CrossRef]
- Serrano, E.; Linares, N.; Garcia-Martinez, J.; Berenguer, J.R. Sol-Gel Coordination Chemistry: Building Catalysts from the Bottom-Up. ChemCatChem 2013, 5, 844–860. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 1990; ISBN 0121349705. [Google Scholar]
- Xu, S.; Lin, K.; Wang, Z.; Chang, J.; Wang, L.; Lu, J.; Ning, C. Reconstruction of calvarial defect of rabbits using porous calcium silicate bioactive ceramics. Biomaterials 2008, 29, 2588–2596. [Google Scholar] [CrossRef]
- Iimori, Y.; Kameshima, Y.; Yasumori, A.; Okada, K. Effect of solid/solution ratio on apatite formation from CaSiO3 ceramics in simulated body fluid. J. Mater. Sci. Mater. Med. 2004, 15, 1247–1253. [Google Scholar] [CrossRef]
- Ni, S.; Chang, J.; Chou, L.; Zhai, W. Comparison of osteoblast-like cell responses to calcium silicate and tricalcium phosphate ceramicsin vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80, 174–183. [Google Scholar] [CrossRef]
- Bouler, J.-M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Iimori, Y.; Kameshima, Y.; Okada, K.; Hayashi, S. Comparative study of apatite formation on CaSiO3 ceramics in simulated body fluids with different carbonate concentrations. J. Mater. Sci. Mater. Med. 2005, 16, 73–79. [Google Scholar] [CrossRef]
- Srinath, P.; Azeem, P.A.; Reddy, K.V. Review on calcium silicate-based bioceramics in bone tissue engineering. Int. J. Appl. Ceram. Technol. 2020, 17, 2450–2464. [Google Scholar] [CrossRef]
- Ni, S.; Chang, J.; Chou, L. In vitro studies of novel CaO-SiO2-MgO system composite bioceramics. J. Mater. Sci. Mater. Med. 2008, 19, 359–367. [Google Scholar] [CrossRef]
- Liu, C.C. Magnesium Directly Stimulates Osteoblast Proliferation. J. Bone Miner. Res. 1988, 3, S104. [Google Scholar]
- Vallet-Regí, M.; Salinas, A.J.; Román, J.; Gil, M. Effect of magnesium content on the in vitro bioactivity of CaO-MgO-SiO2-P2O5 sol-gel glasses. J. Mater. Chem. 1999, 9, 515–518. [Google Scholar] [CrossRef]
- Chen, X.; Ou, J.; Kang, Y.; Huang, Z.; Zhu, H.; Yin, G.; Wen, H. Synthesis and characteristics of monticellite bioactive ceramic. J. Mater. Sci. Mater. Electron. 2008, 19, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Goudouri, O.-M.; Vogel, C.; Grünewald, A.; Detsch, R.; Kontonasaki, E.; Boccaccini, A.R. Sol–gel processing of novel bioactive Mg-containing silicate scaffolds for alveolar bone regeneration. J. Biomater. Appl. 2016, 30, 740–749. [Google Scholar] [CrossRef] [Green Version]
- Bakopoulou, A.; Papachristou, E.; Bousnaki, M.; Hadjichristou, C.; Kontonasaki, E.; Theocharidou, A.; Papadopoulou, L.; Kantiranis, N.; Zachariadis, G.; Leyhausen, G.; et al. Human treated dentin matrices combined with Zn-doped, Mg-based bioceramic scaffolds and human dental pulp stem cells towards targeted dentin regeneration. Dent. Mater. 2016, 32, e159–e175. [Google Scholar] [CrossRef]
- Chen, I.; Salhab, I.; Setzer, F.; Kim, S.; Nah, H.-D. A New Calcium Silicate–based Bioceramic Material Promotes Human Osteo- and Odontogenic Stem Cell Proliferation and Survival via the Extracellular Signal-regulated Kinase Signaling Pathway. J. Endod. 2016, 42, 480–486. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, Z.; Chen, L.; Zhang, W.; Zeng, D.; Zhang, X.; Chang, J.; Jiang, X. Proliferation and osteogenic differentiation of human periodontal ligament cells on akermanite and β-TCP bioceramics. Eur. Cells Mater. 2011, 22, 68–83. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wu, C.; Xiao, Y. The stimulation of proliferation and differentiation of periodontal ligament cells by the ionic products from Ca7Si2P2O16 bioceramics. Acta Biomater. 2012, 8, 2307–2316. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Zhang, X.; Han, P.; Xiao, Y. The ionic products from bredigite bioceramics induced cementogenic differentiation of periodontal ligament cells via activation of the Wnt/β-catenin signalling pathway. J. Mater. Chem. B 2013, 1, 3380–3389. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, H.; Romero, A.R.; Ferroni, L.; Gardin, C.; Zavan, B.; Bernardo, E. Bioactive Glass-Ceramic Scaffolds from Novel ‘Inorganic Gel Casting’ and Sinter-Crystallization. Materials 2017, 10, 171. [Google Scholar] [CrossRef]
- Srinath, P.; Azeem, P.A.; Reddy, K.V.; Chiranjeevi, P.; Bramanandam, M.; Rao, R.P. A novel cost-effective approach to fabricate diopside bioceramics: A promising ceramics for orthopedic applications. Adv. Powder Technol. 2021, 32, 875–884. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Kazeli, K.; Lymperaki, E.; Pouroutzidou, G.K.; Oikonomou, I.M.; Komninou, P.; Zachariadis, G.; Reybier, K.; Pantaleo, A.; Kontonasaki, E. Effect of Sintering Temperature of Bioactive Glass Nanoceramics on the Hemolytic Activity and Oxidative Stress Biomarkers in Erythrocytes. Cell. Mol. Bioeng. 2020, 13, 201–218. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Greasley, S.L.; Page, S.J.; Sirovica, S.; Chen, S.; Martin, R.; Riveiro, A.; Hanna, J.V.; Porter, A.E.; Jones, J.R. Controlling particle size in the Stöber process and incorporation of calcium. J. Colloid Interface Sci. 2016, 469, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Kokubo, T.; Ito, S.; Huang, Z.T.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Pouroutzidou, G.K.; Lymperaki, E.; Kazeli, K.; Lioutas, C.B.; Christodoulou, E.; Kontonasaki, E. Effect of ion doping in silica-based nanoparticles on the hemolytic and oxidative activity in contact with human erythrocytes. Chem.-Biol. Interact. 2020, 318, 108974. [Google Scholar] [CrossRef]
- Saravanan, P.; Raja, M.M.; Gopalan, R.; Rao, N.R.; Suresh, K.; Rao, D.S.; Chandrasekaran, V. Structural and Mössbauer studies on mechanical milled SmCo5/α-Fe nanocomposite magnetic powders. Intermetallics 2008, 16, 636–641. [Google Scholar] [CrossRef]
- Lu, K.; Zhang, H.Y.; Zhong, Y.; Fecht, H.J. Grain Size Dependence of Mechanical Properties in Nanocrystalline Selenium. J. Mater. Res. 1997, 12, 923–930. [Google Scholar] [CrossRef]
- Hench, L.L.; Ulrich, D.R.; University of Florida, Department of Materials Science and Engineering; University of Florida, College of Engineering. Ultrastructure Processing of Ceramics, Glasses, and Composites; Wiley: Hoboken, NJ, USA, 1984; ISBN 0471896691. [Google Scholar]
- Zhou, L.; Zhang, H.; Zhang, Z. Homogeneous nanoparticle dispersion prepared with impurity-free dispersant by the ball mill technique. Particuology 2013, 11, 441–447. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Javidi, J.; Dadkhah, M. Ball Milling Synthesis of Silica Nanoparticle from Rice Husk Ash for Drug Delivery Application. Salavati-Niasari M, Javidi J, Dadkhah M. Ball milling synthesis of silica nanoparticle from rice husk ash for drug delivery application. Comb. Chem. High Throughput Screen. 2013, 16, 458–462. [Google Scholar] [CrossRef]
- Mattos, B.; Rojas, O.; Magalhaes, W. Biogenic SiO2 in colloidal dispersions via ball milling and ultrasonication. Powder Technol. 2016, 301, 58–64. [Google Scholar] [CrossRef]
- Díaz-Flores, L.L.; Pérez-Bueno, J.J.; Espinoza-Beltrán, F.J.; Pérez-Robles, J.F.; Ramírez-Bon, R.; Vorobiev, Y.V.; González-Hernández, J. Effect of gelation and ball milling on the optical absorption and emission of macrilon yellow dye molecules trapped in silica prepared by the sol–gel method. Microelectron. Eng. 2000, 51, 659–666. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Kazeli, K.; Pouroutzidou, G.; Reybier, K.; Pantaleo, A.; Lymperaki, E.; Kontonasaki, E. Evaluation of Hemolytic Activity and Oxidative Stress Biomarkers in Erythrocytes after Exposure to Bioactive Glass Nanoceramics. Beilstein Arch. 2019, 1, 201985. [Google Scholar] [CrossRef] [Green Version]
- PC Powder Diffraction Files. 2003. Joint Committee on Powder Diffraction Standards. International Centre for Diffraction Data (PC PDF, JCPDS-ICDD). Available online: https://www.icdd.com/ (accessed on 5 April 2019).
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Hench, L.L.; Wang, S.H. The sol-gel glass transformation of silica. Phase Transit. 1990, 24–26, 785–834. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Goudouri, O.M.; Kontonasaki, E.; Theocharidou, A.; Papadopoulou, L.; Kantiranis, N.; Chatzistavrou, X.; Koidis, P.; Paraskevopoulos, K.M. Investigation of the Bioactivity of Dental Ceramic/Bioactive Glass Composites Prepared by the Sol Gel Route. Mater. Chem. Phys. 2011, 125, 309–313. [Google Scholar] [CrossRef]
- Hollinger, J.O. Bone Tissue Engineering; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780849316210. [Google Scholar]
- L’vov, B.V. Decomposition Mechanism; Springer: Berlin/Heidelberg, Germany, 2007; pp. 11–32. [Google Scholar]
- Ettarh, C.; Galwey, A.K. A kinetic and mechanistic study of the thermal decomposition of calcium nitrate. Thermochim. Acta 1996, 288, 203–219. [Google Scholar] [CrossRef]
- Viczián, I. Földvári, Mária: Handbook of the thermogravimetric system of minerals and its use in geological practice. Central Eur. Geol. 2013, 56, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Mirhadi, S.; Tavangarian, F.; Emadi, R. Synthesis, characterization and formation mechanism of single-phase nanostructure bredigite powder. Mater. Sci. Eng. C 2012, 32, 133–139. [Google Scholar] [CrossRef]
- Goudouri, O.M.; Kontonasaki, E.; Chatzistavrou, X.; Papadopoulou, L.; Koidis, P.; Paraskevopoulos, K.M. Investigation of the Bioactivity of Dental Ceramic/Bioactive Glass Composites Prepared by the Sol Gel Route. Key Eng. Mater. 2009, 396–398, 119–122. [Google Scholar] [CrossRef]
- Zhong, J.; Greenspan, D.C. Processing and properties of sol-gel bioactive glasses. J. Biomed. Mater. Res. 2000, 53, 694–701. [Google Scholar] [CrossRef]
- Tomozawa, M.; Hong, J.-W.; Ryu, S.-R. Infrared (IR) investigation of the structural changes of silica glasses with fictive temperature. J. Non-Cryst. Solids 2005, 351, 1054–1060. [Google Scholar] [CrossRef]
- Farmer, V.C. The Anhydrous Oxide Minerals. In Infrared Spectra of Minerals in Mineralogical, Society Monograph 4; Mineralogical Society: London, UK, 1974; pp. 183–204. [Google Scholar]
- Gervais, F.; Blin, A.; Massiot, D.; Coutures, J.; Chopinet, M.; Naudin, F. Infrared reflectivity spectroscopy of silicate glasses. J. Non-Crystalline Solids 1987, 89, 384–401. [Google Scholar] [CrossRef]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Lenza, R.; Vasconcelos, W. Preparation of silica by sol-gel method using formamide. Mater. Res. 2001, 4, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Simon, I.; McMahon, H.O. Study of Some Binary Silicate Glasses by Means of Reflection in Infrared. J. Am. Ceram. Soc. 1953, 36, 160–164. [Google Scholar] [CrossRef]
- Gutzow, I.; Schmelzer, J. The Vitreous State: Thermodynamics, Structure, Rheology, and Crystallization; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9783642346330. [Google Scholar]
- Bonfield, W.; Hastings, G.W.; Tanner, K.E. International Symposium on Ceramics in Medicine (4th: 1991: London, G.B. Bioceramics. Volume 4. In Proceedings of the 4th International Symposium on Ceramics in Medicine, London, UK, 11–13 September 1991; Butterworth Heinemann: Oxford, UK, 1991; Volume 4, ISBN 9781483193649. [Google Scholar]
- Goudouri, O.M.; Kontonasaki, E.; Theocharidou, A.; Papadopoulou, L.; Chatzistavrou, X.; Koidis, P.; Paraskevopoulos, K.M. In Vitro Bioactivity Studies of Sol-Gel Derived Dental Ceramics/Bioactive Glass Composites in Periodically Renewed Biomimetic Solution. Bioceram. Dev. Appl. 2011, 1, 1–4. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regí, M. Bioactive ceramics: From bone grafts to tissue engineering. RSC Adv. 2013, 3, 11116–11131. [Google Scholar] [CrossRef]
- Hafezi-Ardakani, M.; Moztarzadeh, F.; Rabiee, M.; Talebi, A.R. Synthesis and characterization of nanocrystalline merwinite (Ca3Mg(SiO4)2) via sol–gel method. Ceram. Int. 2011, 37, 175–180. [Google Scholar] [CrossRef]
- Rahmati, M.; Fathi, M.; Ahmadian, M. Preparation and structural characterization of bioactive bredigite (Ca7MgSi4O16) nanopowder. J. Alloys Compd. 2018, 732, 9–15. [Google Scholar] [CrossRef]
- Hou, X.; Yin, G.; Chen, X.; Liao, X.; Yao, Y.; Huang, Z. Effect of akermanite morphology on precipitation of bone-like apatite. Appl. Surf. Sci. 2011, 257, 3417–3422. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J.; Zhai, W.; Ni, S. A novel bioactive porous bredigite (Ca7MgSi4O16) scaffold with biomimetic apatite layer for bone tissue engineering. J. Mater. Sci. Mater. Med. 2007, 18, 857–864. [Google Scholar] [CrossRef]
- Prabhu, M.; Kavitha, K.; Manivasakan, P.; Rajendran, V.; Kulandaivelu, P. Synthesis, characterization and biological response of magnesium-substituted nanobioactive glass particles for biomedical applications. Ceram. Int. 2013, 39, 1683–1694. [Google Scholar] [CrossRef]
- Ferguson, J.B.; Merwin, H.E. The ternary system CaO-MgO-SiO2. Proc. Natl. Acad. Sci. USA 1904, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chang, J.; Wang, J.; Ni, S.; Zhai, W. Preparation and characteristics of a calcium magnesium silicate (bredigite) bioactive ceramic. Biomaterials 2005, 26, 2925–2931. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Theodorou, G.S.; Kontonasaki, E.; Papadopoulou, L.; Kantiranis, N.; Patsiaoura, D.; Chrissafis, K.; Lioutas, C.B.; Paraskevopoulos, K.M. Synthesis of a bioactive nanomaterial in the ternary system SiO2-CaO-MgO doped with CuO: The effect of Ball milling on the particle size, morphology and bioactive behavior. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2019; Volume 2075, p. 200005. [Google Scholar]
- Goudouri, O.M.; Chatzistavrou, X.; Kontonasaki, E.; Kantiranis, N.; Papadopoulou, L.; Chrissafis, K.; Paraskevopoulos, K.M. Study of the Bioactive Behavior of Thermally Treated Modified 58S Bioactive Glass. Key Eng. Mater. 2009, 396–398, 131–134. [Google Scholar] [CrossRef]
- Yamagata, C.; Paiva, M.R.S.; Higa, O.Z.; Rodas, A.D.; Silveira, A.C.F.; Reis, S.T.; Paulo, S. Sol Gel Modified Derived CaO-MgO-SiO2 Ceramic Glass System: Preparation and In Vitro. 1969. Available online: https://www.ipen.br/biblioteca/2013/eventos/19086.pdf (accessed on 23 October 2019).
- Goudouri, O.-M.; Kontonasaki, E.; Papadopoulou, L.; Kantiranis, N.; Lazaridis, N.; Chrissafis, K.; Chatzistavrou, X.; Koidis, P.; Paraskevopoulos, K. Towards the synthesis of an experimental bioactive dental ceramic. Part I: Crystallinity characterization and bioactive behavior evaluation. Mater. Chem. Phys. 2014, 145, 125–134. [Google Scholar] [CrossRef]
- Cacciotti, I.; Lombardi, M.; Bianco, A.; Ravaglioli, A.; Montanaro, L. Sol–gel derived 45S5 bioglass: Synthesis, microstructural evolution and thermal behaviour. J. Mater. Sci. Mater. Med. 2012, 23, 1849–1866. [Google Scholar] [CrossRef]
- Groh, D.; Döhler, F.; Brauer, D.S. Bioactive glasses with improved processing. Part 1. Thermal properties, ion release and apatite formation. Acta Biomater. 2014, 10, 4465–4473. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and Anionic Substitutions in Hydroxyapatite; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319124605. [Google Scholar]
- Mohammadi, H.; Hafezi, M.; Nezafati, N.; Heasarki, S.; Nadernezhad, A.; Ghazanfari, S.M.H.H.; Sepantafar, M. Bioinorganics in Bioactive Calcium Silicate Ceramics for Bone Tissue Repair: Bioactivity and Biological Properties. J. Ceram. Sci. Technol. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Rismanchian, M.; Khodaeian, N.; Bahramian, L.; Fathi, M.H.; Sadeghi-Aliabadi, H. In-vitro Comparison of Cytotoxicity of Two Bioactive Glasses in Micropowder and Nanopowder forms. Iran. J. Pharm. Res. 2013, 12, 437–443. [Google Scholar]
- Abiraman, S.; Varma, H.K.; Kumari, T.V.; Umashankar, P.R.; John, A. Preliminaryin vitro andin vivo characterizations of a sol-gel derived bioactive glass-ceramic system. Bull. Mater. Sci. 2002, 25, 419–429. [Google Scholar] [CrossRef]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cazzola, M.; Cristallini, C.; Miola, M.; Vernè, E.; Spriano, S. Bioactive materials: In vitro investigation of different mechanisms of hydroxyapatite precipitation. Acta Biomater. 2020, 102, 468–480. [Google Scholar] [CrossRef]
- Brito, A.F.; Antunes, B.; Dos Santos, F.; Fernandes, H.R.; Ferreira, J.M.F. Osteogenic capacity of alkali-free bioactive glasses. In vitro studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 2360–2365. [Google Scholar] [CrossRef]
- Hench, L.L.; Jones, J.R.; Sepulveda, P. Bioactive Materials for Tissue Engineering Scaffolds. In Future Strategies for Tissue and Organ Replacement; Imperial College Press: London, UK, 2002; pp. 3–24. [Google Scholar]
- Karadjian, M.; Essers, C.; Tsitlakidis, S.; Reible, B.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. Biological Properties of Calcium Phosphate Bioactive Glass Composite Bone Substitutes: Current Experimental Evidence. Int. J. Mol. Sci. 2019, 20, 305. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, S.; Widholz, B.; Essers, C.; Becker, M.; Tulyaganov, D.; Moghaddam, A.; de Juan, I.G.; Westhauser, F. Superior biocompatibility and comparable osteoinductive properties: Sodium-reduced fluoride-containing bioactive glass belonging to the CaO–MgO–SiO2 system as a promising alternative to 45S5 bioactive glass. Bioact. Mater. 2020, 5, 55–65. [Google Scholar] [CrossRef]
- Detsch, R.; Guillon, O.; Wondraczek, L.; Boccaccini, A.R. Initial Attatchment of rMSC and MG-63 Cells on Patterned Bioglass® Substrates. Adv. Eng. Mater. 2012, 14, B38–B44. [Google Scholar] [CrossRef]
- Aljarah, A.K.; Al-Saadi, A.H.; Auda, N.A. ELK1 Gene Transfection Effect in Prostate Cancer Cell Line Proliferation Activity. Adv. Life Sci. Technol. 2014, 6597, 1–6. [Google Scholar]
- Chang, J.-S.; Chang, K.L.B.; Hwang, D.-F.; Kong, Z.-L. In Vitro Cytotoxicitiy of Silica Nanoparticles at High Concentrations Strongly Depends on the Metabolic Activity Type of the Cell Line. Environ. Sci. Technol. 2007, 41, 2064–2068. [Google Scholar] [CrossRef]
- Tsigkou, O.; Jones, J.; Polak, J.M.; Stevens, M.M. Differentiation of fetal osteoblasts and formation of mineralized bone nodules by 45S5 Bioglass® conditioned medium in the absence of osteogenic supplements. Biomaterials 2009, 30, 3542–3550. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.E.; Morris, A.S.; Mueller, P.S.; Salem, A.; Grassian, V.H.; Larsen, S.C. Silica nanoparticle-generated ROS as a predictor of cellular toxicity: Mechanistic insights and safety by design. Environ. Sci. Nano 2016, 3, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.-W.; Sikorski, J.A.; Weitzmann, M.N.; Beck, G.R. Bio-active engineered 50nm silica nanoparticles with bone anabolic activity: Therapeutic index, effective concentration, and cytotoxicity profile in vitro. Toxicol. Vitr. 2014, 28, 354–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcaide, M.; Portolés, P.; López-Noriega, A.; Arcos, D.; Vallet-Regí, M.; Portolés, M. Interaction of an ordered mesoporous bioactive glass with osteoblasts, fibroblasts and lymphocytes, demonstrating its biocompatibility as a potential bone graft material. Acta Biomater. 2010, 6, 892–899. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Xie, W.; Chen, X.; Miao, G.; Tang, J.; Fu, X. Regulation of cellular behaviors of fibroblasts related to wound healing by sol-gel derived bioactive glass particles. J. Biomed. Mater. Res. Part A 2016, 104, 2420–2429. [Google Scholar] [CrossRef] [PubMed]
- Ciraldo, F.E.; Boccardi, E.; Melli, V.; Westhauser, F.; Boccaccini, A.R. Tackling Bioactive Glass Excessive in Vitro Bioreactivity: Preconditioning Approaches for Cell Culture Tests. Acta Biomater. 2018, 75, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hohenbild, F.; Arango-Ospina, M.; Moghaddam, A.; Boccaccini, A.R.; Westhauser, F. Preconditioning of Bioactive Glasses before Introduction to Static Cell Culture: What Is Really Necessary? Methods Protoc. 2020, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Torres-lagares, D. Biocompatibility of Polymer and Ceramic CAD/CAM. Polymers 2019, 11, 446. [Google Scholar]
- Laranjeira, M.S.; Carvalho, Â.; Pelaez-Vargas, A.; Hansford, D.; Ferraz, M.P.; Coimbra, S.; Monteiro, F.J. Modulation of human dermal microvascular endothelial cell and human gingival fibroblast behavior by micropatterned silica coating surfaces for zirconia dental implant applications. Sci. Technol. Adv. Mater. 2014, 15, 25001. [Google Scholar] [CrossRef] [Green Version]
- Wananuruksawong, R.; Wasanapiarnpong, T.; Dhanesuan, N.; Didron, P.P. Microhardness and Biocompatibility of Silicon Nitride Ceramic Developed for Dental Applications. Mater. Sci. Appl. 2014, 5, 1034–1039. [Google Scholar] [CrossRef] [Green Version]
- Atay, A.; Gürdal, I.; Cetintas, V.B.; Üşümez, A.; Cal, E. Effects of New Generation All-Ceramic and Provisional Materials on Fibroblast Cells. J. Prosthodont. 2018, 28, e383–e394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Peng, J.; Xu, Y.; Chang, J.; Li, H. Bioglass Activated Skin Tissue Engineering Constructs for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-H.; Wu, S.-H.; Yao, M.; Lu, C.-W.; Lin, Y.-S.; Hung, Y.; Mou, C.-Y.; Chen, Y.-C.; Huang, D.-M. The effect of surface charge on the uptake and biological function of mesoporous silica nanoparticles in 3T3-L1 cells and human mesenchymal stem cells. Biomaterials 2007, 28, 2959–2966. [Google Scholar] [CrossRef]
- Tavakoli, M.; Bateni, E.; Rismanchian, M.; Fathi, M.; Doostmohammadi, A.; Rabiei, A.; Sadeghi, H.; Etebari, M.; Mirian, M. Genotoxicity Effects of Nano Bioactive Glass and Novabone Bioglass on Gingival Fibroblasts Using Single Cell Gel Electrophoresis (Comet Assay): An in Vitro Study. Dent. Res. J. 2012, 9, 314–320. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Greenspan, D.; Hench, L.L.; Boccaccini, A.R. Bioactive Glasses in Soft Tissue Repair. Biomaterials 2015, 94, 27–31. [Google Scholar]
- Kargozar, S.; Hamzehlou, S.; Baino, F. Potential of bioactive glasses for cardiac and pulmonary tissue engineering. Materials 2017, 10, 1429. [Google Scholar] [CrossRef] [Green Version]
- Hensley, K.; A Robinson, K.; Gabbita, S.; Salsman, S.; A Floyd, R. Reactive oxygen species, cell signaling, and cell injury. Free. Radic. Biol. Med. 2000, 28, 1456–1462. [Google Scholar] [CrossRef]
- Zhang, L.; Laug, L.; Münchgesang, W.; Pippel, E.; Gösele, U.; Brandsch, M.; Knez, M. Reducing Stress on Cells with Apoferritin-Encapsulated Platinum Nanoparticles. Nano Lett. 2010, 10, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Gogvadze, V.; Laffranchi, R.; Schlapbach, R.; Schweizer, M.; Suter, M.; Walter, P.; Yaffee, M. Oxidants in mitochondria: From physiology to diseases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 1995, 1271, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Lamkhao, S.; Phaya, M.; Jansakun, C.; Chandet, N.; Thongkorn, K.; Rujijanagul, G.; Bangrak, P.; Randorn, C. Synthesis of Hydroxyapatite with Antibacterial Properties Using a Microwave-Assisted Combustion Method. Sci. Rep. 2019, 9, 4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Chang, J.; Liu, M.; Ning, C. Study on antibacterial effect of 45S5 Bioglass®. J. Mater. Sci. Mater. Med. 2009, 20, 281–286. [Google Scholar] [CrossRef]
- Ma, N.; Fan, X.; Quan, X.; Zhang, Y. Ag–TiO2/HAP/Al2O3 bioceramic composite membrane: Fabrication, characterization and bactericidal activity. J. Membr. Sci. 2009, 336, 109–117. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-ró, D. Buttonwood: All It Needs Is Love. Economist 2014, 3–16. Available online: www.theguardian.com/environment/2014/oct/02/uk-renewable-energy-subsidy-changes-anger-solar-industry (accessed on 6 March 2015).
- Hancock, J.T.; Desikan, R.; Neill, S. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001, 29, 345–349. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef]
- Wellen, K.E.; Thompson, C.B. Cellular Metabolic Stress: Considering How Cells Respond to Nutrient Excess. Mol. Cell 2010, 40, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zhuang, J.; Teng, X.; Li, L.; Chen, D.; Yan, X.; Tang, F. The promotion of human malignant melanoma growth by mesoporous silica nanoparticles through decreased reactive oxygen species. Biomaterials 2010, 31, 6142–6153. [Google Scholar] [CrossRef]
- He, Q.; Shi, J. Mesoporous silica nanoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- He, Q.; Zhang, J.; Chen, F.; Guo, L.; Zhu, Z.; Shi, J. An Anti-ROS/Hepatic Fibrosis Drug Delivery System Based on Salvianolic Acid B Loaded Mesoporous Silica Nanoparticles. Biomaterials 2010, 31, 7785–7796. [Google Scholar] [CrossRef]
- Asharani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Willson, R.L. Hydroxyl Radicals and Biological Damage in Vitro: What Relevance in Vivo? Ciba Foundation symposium. In Oxygen Free Radicals and Tissue Damage; Excerpta Medica Amsterdam: Amsterdam, The Netherlands, 1979; pp. 19–42. [Google Scholar]
- Jiang, L.; Yu, Y.; Li, Y.; Yu, Y.; Duan, J.; Zou, Y.; Li, Q.; Sun, Z. Oxidative Damage and Energy Metabolism Disorder Contribute to the Hemolytic Effect of Amorphous Silica Nanoparticles. Nanoscale Res. Lett. 2016, 11, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napierska, D.; Rabolli, V.; Thomassen, L.C.J.; Dinsdale, D.; Princen, C.; Gonzalez, L.; Poels, K.L.C.; Kirsch-Volders, M.; Lison, D.; Martens, J.; et al. Oxidative Stress Induced by Pure and Iron-Doped Amorphous Silica Nanoparticles in Subtoxic Conditions. Chem. Res. Toxicol. 2012, 25, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Mihailova, I.K.; Radev, L.; Aleksandrova, V.A.; Colova, I.V. Novel Merwinite/Akermanite Ceramics: In Vitro Bioactivity. Bulg. Chem. Commun. 2015, 47, 253–260. [Google Scholar]

| Compound C1 | Compound C2 | Compound C3 | ||||
|---|---|---|---|---|---|---|
| Bacterium | Ratio Test: Control | t Test 1 | Ratio Test: Control | t Test 1 | Ratio Test: Control | t Test 1 |
| Bacillus cereus | 0.86 | 0.001 | 0.87 | 0.001 | 0.87 | 0.001 |
| Staphylococcus aureus | 0.83 | 0.049 | 0.88 | 0.096 | 0.86 | 0.071 |
| Listeria monocytogenes | 1.01 | 0.932 | 1.00 | 0.987 | 0.90 | 0.165 |
| Escherichia coli | 0.97 | 0.238 | 1.03 | 0.226 | 0.94 | 0.071 |
| Salmonella enterica ser. Typhimurium | 0.79 | 0.095 | 0.91 | 0.011 | 0.97 | 0.235 |
| Salmonella enterica ser. Enteritidis | 0.90 | 0.013 | 0.81 | 0.000 | 0.87 | 0.000 |
| Pseudomonas aeruginosa | 0.93 | 0.000 | 0.89 | 0.002 | 0.97 | 0.234 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazeli, K.; Tsamesidis, I.; Theocharidou, A.; Malletzidou, L.; Rhoades, J.; Pouroutzidou, G.K.; Likotrafiti, E.; Chrissafis, K.; Lialiaris, T.; Papadopoulou, L.; et al. Synthesis and Characterization of Novel Calcium-Silicate Nanobioceramics with Magnesium: Effect of Heat Treatment on Biological, Physical and Chemical Properties. Ceramics 2021, 4, 628-651. https://doi.org/10.3390/ceramics4040045
Kazeli K, Tsamesidis I, Theocharidou A, Malletzidou L, Rhoades J, Pouroutzidou GK, Likotrafiti E, Chrissafis K, Lialiaris T, Papadopoulou L, et al. Synthesis and Characterization of Novel Calcium-Silicate Nanobioceramics with Magnesium: Effect of Heat Treatment on Biological, Physical and Chemical Properties. Ceramics. 2021; 4(4):628-651. https://doi.org/10.3390/ceramics4040045
Chicago/Turabian StyleKazeli, Konstantina, Ioannis Tsamesidis, Anna Theocharidou, Lamprini Malletzidou, Jonathan Rhoades, Georgia K. Pouroutzidou, Eleni Likotrafiti, Konstantinos Chrissafis, Theodoros Lialiaris, Lambrini Papadopoulou, and et al. 2021. "Synthesis and Characterization of Novel Calcium-Silicate Nanobioceramics with Magnesium: Effect of Heat Treatment on Biological, Physical and Chemical Properties" Ceramics 4, no. 4: 628-651. https://doi.org/10.3390/ceramics4040045
APA StyleKazeli, K., Tsamesidis, I., Theocharidou, A., Malletzidou, L., Rhoades, J., Pouroutzidou, G. K., Likotrafiti, E., Chrissafis, K., Lialiaris, T., Papadopoulou, L., Kontonasaki, E., & Lymperaki, E. (2021). Synthesis and Characterization of Novel Calcium-Silicate Nanobioceramics with Magnesium: Effect of Heat Treatment on Biological, Physical and Chemical Properties. Ceramics, 4(4), 628-651. https://doi.org/10.3390/ceramics4040045