1. Introduction
Fish scales, as wastes of the fishing and food industries, are a serious environmental and economic problem [
1,
2]. Some quantity of produced fish scales is used for the production of collagen [
3], hydroxyapatite [
4,
5], guanine [
6], animal feed [
2,
7], fertilizers [
8], food [
9], cosmetics [
10], adsorbents [
11], biomaterials [
12], etc.
The main constituents of fish scales are proteins (mainly collagen) and minerals [
13,
14]. The main component of the inorganic mineral constituents of fish scales is hydroxyapatite [
15]. Most of the known methods of separating constituents of organic and inorganic nature from each other consist of transforming fish scale components into a soluble state; using heating; and treating them with enzymes, acids, alkalis, or organic solvents [
16,
17,
18,
19]. All of these methods are rather long processes (up to two weeks) accompanied by a significant consumption of washing liquids and high energy.
Different methods have been used for the extraction of hydroxyapatite from fish scales. Hydroxyapatite can be prepared via deproteinization, microwave irradiation, alkaline heat treatment, and thermal decomposition methods [
20,
21,
22]. Hydroxyapatite was prepared via calcination at 1000 °C from fish scales after cleaning, deproteinization through external washing with 1 N HCl, thoroughly washing several times with distilled water, treatment with 1 N NaOH solution, filtration, washing with distilled water, and drying at 60 °C in a hot air oven for several hours [
22].
Preparation of inorganic powders containing chlorapatite and some quantity of sodium rhenanite from fish scales included washing, drying, deproteinization using a water solution of HCl, neutralization using a water solution of NaOH, separation of the solution, drying, and heat treatment at 600, 800, and 1000 °C [
23]. Treating fish scales with the mentioned reagents led to the formation of sodium chloride (NaCl). High-temperature treatment of fish scales in the presence of sodium chloride (NaCl) led to the formation of chlorapatite/rhenanite powder consisting of quite large particles with dimensions of 1–5 μm after heat treatment at 1000 °C. According to the thermal analysis data, the total mass loss of this powder was greater than 60%. The composition of fish scale powder, as a reason for the quite remarkable mass loss and particle growth, can be treated as a negative factor in the aspect of its possible use for ceramics production. At the same time, the possibility of changing the phase composition of inorganic powder based on fish scales via pretreatment with different solutions of inorganic salts was shown.
Powders of hydroxyapatite were prepared from fish scales using heat treatment in air at 200, 400, 800, 1000, and 1200 °C after washing, drying, and crushing them into small pieces using a mortar grinder [
24]. Based on the thermal analysis data, the mass loss of crushed fish scales was estimated at 35 wt.% when heated up to 1000 °C. The phase composition of powders after heat treatment at temperatures 1000 and 1200 °C included hydroxyapatite and 6–16 wt.% β-tricalcium phosphate. Based on data of the relative density (RD) of ceramics based on fish scale powder (2.89 ± 0.04 g/cm
3, RD 91.6%) and ceramics based on synthetic powder of hydroxyapatite (2.24 ± 0.05 g/cm
3, RD ~71%), a conclusion about the positive influence of trace elements such as Mg, Na, Sr, and K on the sinterability of fish scale hydroxyapatite was drawn.
Inorganic powders were prepared via heat treatment at 600, 800, 1000, and 1200 °C from boiled (in water), dried, and milled fish scales [
25]. All calcined samples consisted of a mixture of hydroxyapatite and β-tricalcium phosphate. It was found that the phase composition of heat-treated fish scale powders changed with the growth of the calcination temperature. The higher the temperature, the lower the content of hydroxyapatite and the higher the content of β-tricalcium phosphate.
All of the above-mentioned examples of the preparation of inorganic powders from fish scales are multistage. Washing, drying, and thermal treatment are the mandatory stages. The properties of inorganic powders prepared from fish scales depend on a range of factors. Among them are the fishing region, the sort of fish, the method of extraction, solutions used, and the temperature of heat treatment.
An economically efficient method was developed for obtaining powder enriched with inorganic components and fluff enriched with collagen from fish scales [
26,
27,
28]. The main feature of the preparation of this fish scale powder enriched with inorganic components consisted of using high-speed mechanical grinding of a clean dry mixture of fish scales and the separation of ground cotton-like product via vibration sieving. This separation was possible due to the difference between the collagen (1.33 g/cm
3) and hydroxyapatite (3.16 g/cm
3) densities. In our investigation, we started with a fish scale powder enriched with inorganic components prepared from fish scale wastes of the fishing and food industries available in the Baltic Region of the Russian Federation via a method suggested earlier [
26,
27,
28].
The aim of this work consisted of the investigation of the properties of inorganic powders prepared using heat treatment at temperatures of 400–1000 °C from fish scale powder enriched with inorganic components.
2. Materials and Methods
2.1. Preparation of Fish Scale Powder Enriched with Inorganic Components
Preparation of fish scale powder enriched with inorganic components was performed according to the method of complex processing of fish scales described previously [
26,
27,
28]. Fish scales of
abramis brama (freshwater bream),
carassius carassius (crucian carp), and
sander lucioperca (pike perch) (
Figure 1) were obtained using an electric fish cleaning machine (RF-0.1, Uralspetsmash LLC, Miass, Russia).
A mixture containing 20 wt.% abramis brama fish scales, 20 wt.% carassius carassius fish scales, and 60 wt.% sander lucioperca fish scales was placed in a mesh bag with the mesh size 3 × 3 mm and loaded into a perforated centrifuge of an automatic washing machine (LG model F2WN2S6S3E; LG, Wroclaw, Poland) to remove organic impurities by stirring in water at a temperature of 20 °C for 20 min. Then, the liquid was drained, and the fish scales were washed with water and wrung out at 800 rpm.
A total of 400 g of the fish scales after treatment in a washing centrifuge, 20 g of sodium chloride (NaCl), 4 g of sodium hydro carbonate (NaHCO3), and 80 g of crushed ice were loaded into a Moulinex Delico FP203 two-speed mixer (500 W, SEB Group, London, UK) and processed for 10 min at 1000 rpm. Then, the fish scales were washed with water to remove salts and organic impurities.
The washed fish scales and water at a weight ratio of 1:4 were placed into a container and kept there at room temperature for 30 min with the addition of 5% sodium chloride (NaCl) and 1% sodium hydro carbonate (NaHCO3) to the mass of fish scales. After the separation of sodium salts in a water solution containing impurities of organic nature using a sieve (mesh dimension 2 × 2 mm), the fish scales were washed with water until the smell disappeared.
The fish scales cleaned as described above were dried with a hot air fan at a temperature ≤ 50 °C, up to a residual humidity of less than 10%, using a Spectr-Pribor ESOF-2-0,6/220 Veterok-2 electric dryer (6 pallets) (seller of household electrical products, Spectr-Pribor LLC, Kursk, Russia).
Dried fish scales in portions of 50 g were placed in a container with a capacity of 0.8 L of a high-speed multifunctional grinder (Zhejiang Winki Plastic Co., Ltd., Wuyi, China) and crushed at 36,000 for 5 min.
The ground fish scale cotton-like product was separated into several fractions using an electric vibrating flour sifter (model PS-300B, Yonkang WD Industry and Trade Ltd., Yongkang, China). The fraction of ground fish scale powder enriched with inorganic components was separated using a 170-mesh sieve by passing it through grid cells with a size of 0.088 mm.
The scheme of the preparation of fish scale powder enriched with inorganic components is shown in
Figure 2.
2.2. Thermal Evolution of Fish Scale Powder Enriched with Inorganic Components
Heat treatment of the powder enriched with inorganic components prepared from the mixture of fish scales using high-speed grinding and vibration sieving was conducted at 400 °C, 700 °C, 800 °C, 900 °C, and 1000 °C for 2 h at the specified temperatures to investigate the thermal evolution of its microstructure and phase composition. The heating rate was 5 °C/min.
2.3. Characterization Methods
The phase composition of the fish scale powder enriched with inorganic components and powders after heat treatment at 400 °C, 700 °C, 800 °C, 900 °C, and 1000 °C was determined by X-ray powder diffraction (XRD) analysis using a Rigaku Miniflex 600 diffractometer (CuKα radiation, Kβ filter, and D/teX Ultra detector) in Bragg–Brentano geometry (Rigaku Corporation, Tokyo, Japan) with an angle interval 2Ѳ from 3° to 70° (step 2Ѳ−0.02°). Phase analysis was performed using the ICDD PDF2 database [
29] and Match! software (
https://www.crystalimpact.com/, accessed on 25 June 2022).
Determination of the total contents of potassium, sodium, calcium, magnesium, and phosphorus was carried out for (sample 1) fish scale powder after heat treatment at 1000 °C and for (sample 2) fish scale powder enriched with inorganic components. To determine the total contents of potassium, sodium, calcium, magnesium, and phosphorus in the samples, 0.6–2.5 g of materials was weighed with a deviation ≤0.001 g and placed into a conical flask with a capacity of 250 mL. Then, 10 mL of distilled water and 50 mL of nitric acid (GOST 4461-77) were added to each sample. The flasks containing powders and a solution of nitric acid were boiled for ~10 min until the material was completely dissolved. Then, the solutions were quantitatively transferred into measuring flasks with a capacity of 250 mL, and distilled water was added to reach the specified volume. The solutions prepared from reference sample 1 (fish scale powder after heat treatment at 1000 °C) turned out to be transparent and colorless, and sample 2 (fish scale powder enriched with inorganic components) was transparent but light yellow. The prepared solutions were used to determine the named mineral components of the powders under investigation.
The contents of sodium and potassium in the samples were determined by the flame photometric method described earlier [
30]. The concentrations of K
+ and Na
+ ions in solutions were determined by emission flame photometry on a FLAPHO 4 device (VEB Carl Zeiss Jena, Dresden, DDR). The measurements were carried out at a fixed wavelength of 589 nm. Calibration solutions of 0.1, 0.25, 0.50, and 0.75 mmol/l were used for determination. The error in determining concentrations was 7%.
The content of phosphorus was determined by a differential photometric method according to GOST 20851.2-75, consisting of photometry of a colored solution of a phosphor vanadium–molybdenum complex via comparison with solutions containing a known amount of phosphorus. Measurements were carried out at a wavelength of 440 nm in cuvettes with a light-absorbing layer thickness of 10 mm on a KFK-2 photovoltaic concentration colorimeter (JSC “Zagorsk Optical and Mechanical Plant” (JSC “ZOMZ”), Sergiev Posad, Russia). The error in determining the content calculated for P2O5 was 0.5%.
The contents of calcium and magnesium ions were determined by complexometric titration according to [
31]. The essence of the method consists of the formation of a poorly dissociated complex compound of both calcium and magnesium with the disodium salt of ethylenediamine-
N′,
N′,
N′,
N′-tetraacetic acid (trilon B) in a highly alkaline medium and the determination of the equivalence point during titration using a metal indicator. The stability constants of calcium and magnesium ethylenediamine tetraacetates are close in value, so the separate determination of calcium and magnesium when they are both present in the solution is based on the preliminary determination of their total content by titration of one aliquot of the analyzed solution in an ammonium buffer medium with a pH of 10.5 and with the indicator acid chromium dark blue. In another aliquot, pH > 12 was created by introducing 4h KOH; while magnesium was precipitated as hydroxide, it was not filtered out, and calcium was determined complexometrically in the solution. In our case, the indicator was fluorexone. Titration was conducted on a dark background. The error in determining the content calculated for Ca was 2%.
Thermal analysis (TA) was performed to determine the total mass loss of fish scale powder enriched with inorganic components by heating it up to 1000 °C in the air using a NETZSCH STA 449 F3 Jupiter thermal analyzer (NETZSCH, Selb, Germany). The gas-phase composition was monitored by a QMS 403 Quadro quadrupole mass spectrometer (NETZSCH, Selb, Germany) combined with a NETZSCH STA 449 F3 Jupiter thermal analyzer. The mass spectra (MS) were registered for the following m/Z values: 15 (NH); 16 (CH4); 17 (NH3, HO); 18 (H2O); 44 (CO2); and 30 (NO). The heating rate was 10 °C/min.
Fish scale powders before and after heat treatment at 400 °C were examined by scanning electron microscopy (SEM) using a Helios Nanolab 600i electron microscope (FEI, Hillsboro, OR, USA) at an accelerating voltage of 2 kV. Fish scale powders before and after heat treatment at 700 °C, 800 °C, 900 °C, and 1000 °C were also examined by SEM on a LEO SUPRA 50VP electron microscope (Carl Zeiss, Jena, Germany; auto-emission source). This investigation was carried out at an accelerating voltage of 3–20 kV using SE2 detectors. The surface of the powders was coated with a layer of chromium (up to 10 nm).
3. Results and Discussion
A micrograph of fish scale powder enriched with inorganic components prepared from the mixture of fish scales using high-speed grinding and vibration sieving is presented in
Figure 3. According to this micrograph, the powder consists of quite large particles of irregular forms and includes elongated fibrous fragments inheriting the collagen fiber structure. The reason for the enrichment of ground fish scale powder with inorganic components during vibration sieving is the remarkable difference between the densities of organic (collagen, density about 1.33 g/cm
3) and inorganic (calcium phosphates, density about 3.16 g/cm
3) constituents of fish scales.
Data from the TA of fish scale powder enriched with inorganic components prepared from the mixture of fish scales using high-speed grinding and vibration sieving is presented in
Figure 4.
The total mass loss of the fish scale powder enriched with inorganic components prepared from the mixture of fish scales using high-speed grinding and vibration sieving when heated up to 1000 °C was 36.5%. There are three noticeable steps in the curve of mass loss (
Table 1).
The mass loss in the first step (40–200 °C, with the maximum at 105 °C) is estimated at 7.5% and, according to MS data, can be attributed to both adsorbed H
2O (
m/z = 18) and NH
3 (m/Z=15) evolving. The mass loss in the second step (200–570 °C, with the maximum at 345 °C) is estimated at 26.0%, and in the third step (570–850 °C, with the maximum at 630 °C), it is estimated at 3.0%. The mass loss in the second and third steps can be explained by the decomposition of organic components (especially collagen) present in fish scale powder. According to MS data, the maximum values of ion current were 1.1 × 10
−8 A for CH
4 (
m/z = 16), 4.7 × 10
−10 A for H
2O (
m/z = 18), 2.6 × 10
−10 A for HO/NH
3 (
m/z = 17), 1.8 × 10
−10 A for CO
2 (
m/z = 44), 1.6 × 10
−10 A for NH (
m/z =15), and 2.8 × 10
−11 A for NO (
m/z = 30). The choice of such values of
m/z for the mass spectra determination was based on known (from the literature [
32,
33]) element composition of collagen, including C, H, O, and N. As was underlined in a previous article [
34], during heating, many chemical reactions can take place in collagen: dehydration, denaturation, and decomposition. All recorded MS graphs (especially for
m/z = 18,
m/z = 17, and
m/z = 15) had sequences of multiple peaks. Thus, the recorded MS graphs reflect a combination of complex processes occurring in the organic part of fish scale powder enriched with inorganic components when heating. It should be noted that the appearance of the TG curve of fish scale powder enriched with inorganic components is in good agreement with the TG curve for samples of bone materials with low organic contents in the investigation reflected in a previous article [
35].
A camera photo of powders after heat treatment at 400 °C, 700 °C, 900 °C, and 1000 °C of fish scale powder enriched with inorganic components is presented in
Figure 5.
Powders subjected to heat treatment at 400 °C, 700 °C, 900 °C, and 1000 °C had different colors (
Figure 5). Powder after heat treatment at 400 °C was colored orange-gray. Powder after heat treatment at 700 °C was colored dark gray. Powder after heat treatment at 900 °C was colored light gray, and powder after heat treatment at 1000 °C was colored white. The powder coloring can be explained by the presence of organic components that are thermally degraded to different extents. The greater the organic component degradation, the less coloring there is. The colors of powders heat-treated at 400 °C, 700 °C, 900 °C, and 1000 °C in the camera photo (
Figure 5) are in good agreement with the thermal analysis data presented as TG, DTG, and DTA curves (
Figure 4).
The XRD data of fish scale powder before heat treatment and powder after heat treatment at 400 °C are presented in
Figure 6. The XRD graph of fish scale powder enriched with inorganic components shows the evident presence of quasi-amorphous hydroxyapatite (PDF card #9-432). The XRD graph of powder after heat treatment at 400 °C is not much different from the XRD graph of the starting fish scale powder. In addition to broad reflexes of quasi-amorphous hydroxyapatite (PDF card #9-432), there are a couple of reflexes that can be attributed to magnesium whitlockite (Ca
2.82Mg
0.19(PO
4)
2 (PDF card #70-682) or Ca
18Mg
2H
2(PO
4)
14 (PDF card #70-2064)).
Micrographs of powders enriched with inorganic components prepared from the mixture of fish scales using high-speed grinding and vibration sieving before (a, b) and after heat treatment at 400 °C (c, d) are presented in
Figure 7. The dimensions of inorganic constituents of fish scale powders before and after heat treatment at 400 °C can hardly be estimated with high accuracy, but they are not bigger than 10 nm. The inorganic constituents of fish scales are in the collagen matrix before heat treatment, and they remain in the matrix of partially degraded collagen. As is shown in
Figure 7, the texture of powders heat-treated at 400 °C inherited the structure of the organic matrix. After heat treatment at 400 °C, particles of powder were still in the environment of the thermally degraded organic matrix with the dark-orange (400 °C) color, as shown in
Figure 5.
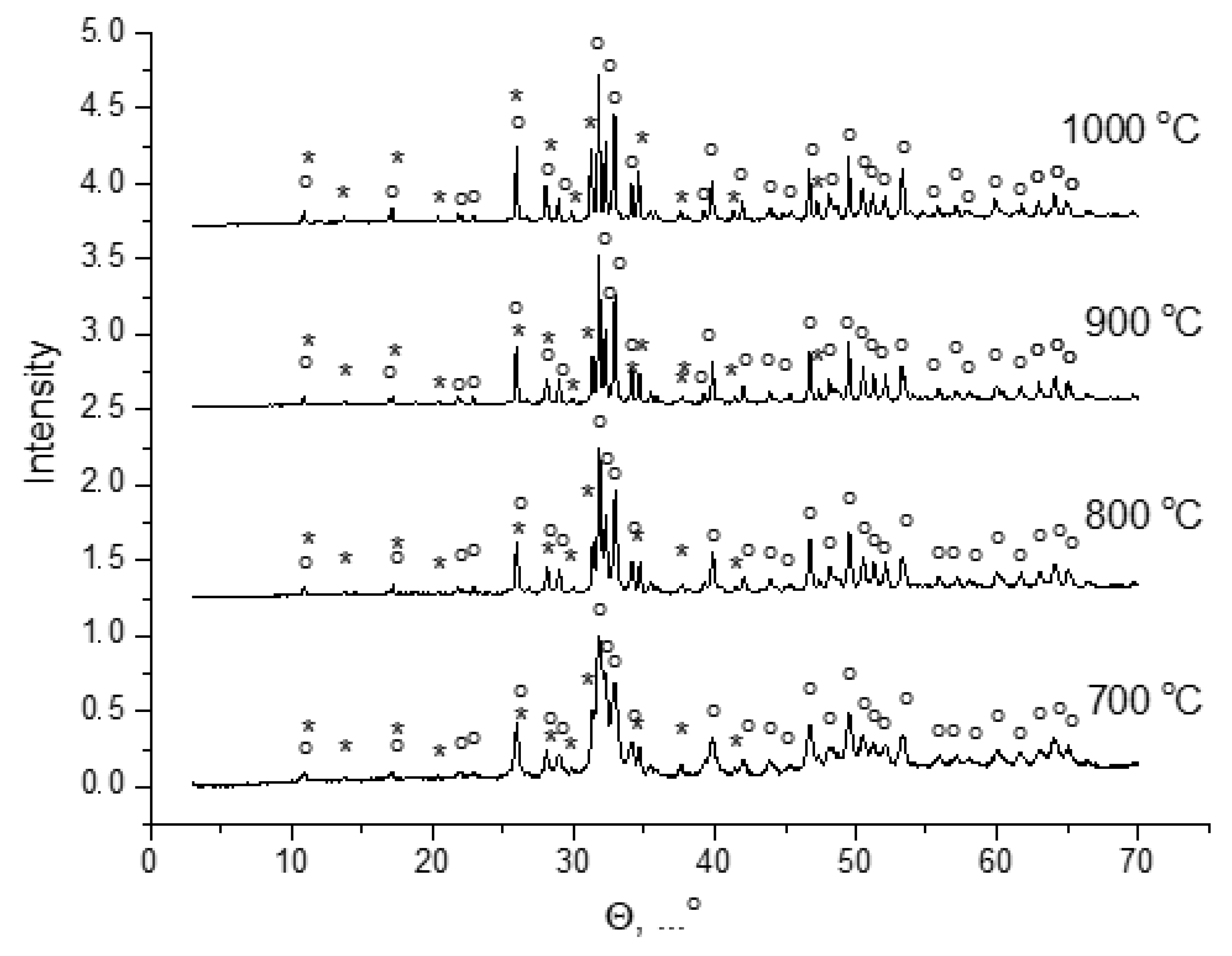
The phase composition of fish scale powders after heat treatment at 700–1000 °C (
Figure 8) consisted of hydroxyapatite (Ca
10(PO
4)
6(OH)
2 (PDF card #9-432)) and magnesium whitlockite (Ca
2,82Mg
0,19(PO
4)
2 (PDF card #70-682) or Ca
18Mg
2H
2(PO
4)
14 (PDF card #70-2064)) according to the ICDD database [
29]. According to the Match! software database, the phase composition of fish scale powder after heat treatment at 1000 °C can be described as containing: hydroxyapatite (Ca
10(PO
4)
6(OH)
2 (card 96-900-2215)) or hydroxyapatite-dental (Ca
4.7H
0.46Mg
0.05Na
0.1O
12.51P
1.61 (card 96-900-2220)) and magnesium whitlockite (Ca
10.115Mg
0.385(PO
4)
7 (card 96-901-2137) or Ca
9.5Mg(PO
4)
7 (card 96-901-2138)). According to Match!, the phase composition was determined to contain 71.8–73.4 wt.% hydroxyapatite and 26.6–28.2 wt.% magnesium whitlockite.
Figure 6 and
Figure 8 show the expected phenomena of the transformation of quasi-amorphous inorganic constituents of fish scales into crystalline phases due to heat treatment. Similar processes have been observed before [
24,
25] when preparing hydroxyapatite from fish scales.
Chemical analysis data of fish scale powder enriched with inorganic components before (sample 1) and after (sample 2) heat treatment at 1000 °C are presented in
Table 2.
In all samples, potassium was determined in trace amounts. Sodium (Na), calcium (Ca), and magnesium (Mg) ions were found in the samples under investigation. Chemical analysis data allow supposing that calcium ions in fish scale hydroxyapatite and whitlockite can be substituted by the detected cations. Both sodium (Na) and magnesium (Mg) could be integrated into the calcium phosphate structures of scales during the fish life cycle in an aqueous medium. Using sodium salts (NaCl and NaHCO
3) at stages of fish scale purification could also be a reason for the presence of sodium (Na) both in fish scale powder enriched with inorganic components and in fish scale powder after heat treatment at 1000 °C. The lower content of sodium in fish scale powder after heat treatment at 1000 °C may be explained, for example, by the possibility of NaCl evacuating from sample 2 at temperatures higher than its melting point (801 °C) [
36].
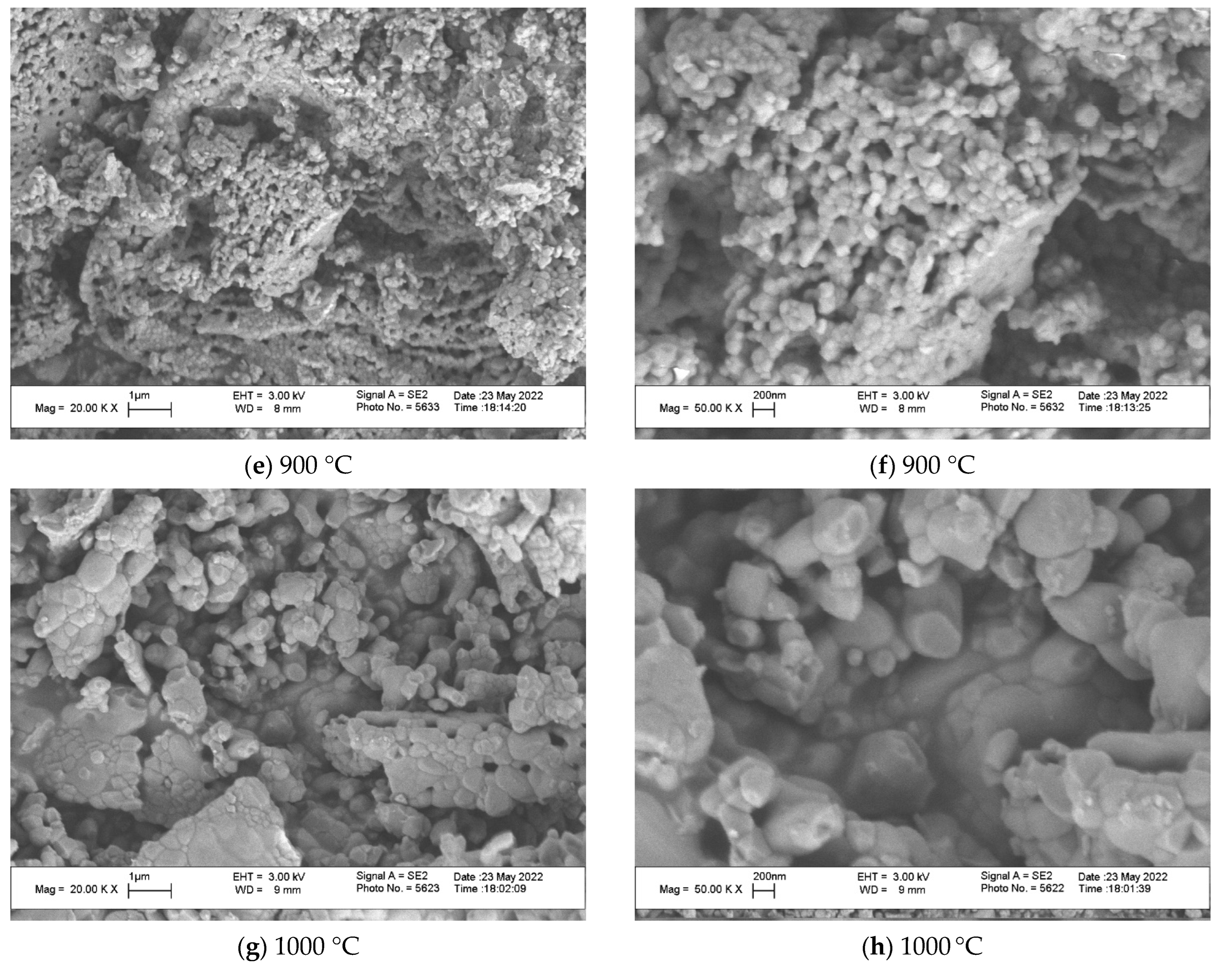
Micrographs of powders prepared from the mixture of fish scales using high-speed grinding and vibration sieving after heat treatment at 700 °C (a, b), 800 °C (c, d), 900 °C (e, f), and 1000 °C (g, h) using magnifications of ×20,000 (a, c, e, g, i) and ×50,000 (b, d, f, h, j) are presented in
Figure 9. After heat treatment at 400 °C and 700 °C, particles of powder are still in the environment of the thermally degraded organic matrix with the dark-orange (400 °C) or dark-gray (700 °C) color, as shown in
Figure 5. The arrangement of inorganic particles after heat treatment is determined by their places in the collagen matrix before heat treatment. The dimensions of inorganic particles after heat treatment at 400 °C (a, b) and 700 °C (c, d) were less than 50 nm. Due to the very small dimensions of inorganic crystals naturally formed in the organic (collagen) matrix, one can see signs of sintering in the SEM photos of powders heat-treated at 800–1000 °C. Particles of inorganic powders prepared from fish scale powder via heat treatment at 800–1000 °C consisted of sintered grains. The dimensions of grains in these particles after treatment at 800 °C were less than 100 nm; after heat treatment at 900 °C, they were 50–200 nm, and after heat treatment at 1000 °C, they were 100–1000 nm.
After heat treatment at 700 °C, particles of the powder are still in the environment of the thermally degraded organic matrix with the dark-gray (700 °C) color, as shown in
Figure 5. The arrangement of inorganic particles after heat treatment at this temperature was determined by their places in the collagen matrix before heat treatment. The dimensions of inorganic particles after heat treatment at 700 °C (
Figure 9a,b) were less than 50 nm. The black color of powder after heat treatment at 700 °C (
Figure 5) is presumably attributed to the presence of amorphous carbon, which could form from constituents of organic nature. Amorphous carbon on the surface of calcium phosphate particles, playing a role as a physical barrier [
37], may prevent possible noticeable grain growth at 700 °C.
One can see signs of sintering in the SEM photos of powders heat-treated at 800–1000 °C (
Figure 9c–h). The very small dimensions of inorganic crystals naturally formed in the organic (collagen) matrix provided very high sintering activity of inorganic powders prepared from fish scale powders due to the huge specific surface area and highly defected quasi-amorphous crystal structure. Particles of inorganic powders prepared from fish scale powder via heat treatment at 800–1000 °C consisted of sintered grains. The dimensions of grains after treatment at 800 °C were less than 100 nm (
Figure 9c,d); after heat treatment at 900 °C, they were 50–200 nm (
Figure 9e,f). The dimensions of sintered fragments in the inorganic powder after heat treatment at 1000 °C can be estimated at 1–3 μm (
Figure 9g). After heat treatment at 1000 °C, one can see a bimodal character in grains’ dimensions. There are regions in the microstructure with 100–200 nm and 500–1000 nm grains (
Figure 9g,h). Sintering of particles in aggregates took place due to diffusion at all temperatures chosen for heat treatment. The higher the heat treatment temperature, the more intensive the diffusion sintering. The eutectic point in the Ca
3(PO
4)
2-Mg
3(PO
4)
2 system is at 1120 °C [
38,
39]. The content of sodium (Na) in powders is too low to induce melt formation in sufficient quantity to intensify sintering. Therefore, it is hardly possible to imagine that the sintering of particles in aggregates took place due to liquid phase sintering at the temperatures used. It is possible to infer that the grain growth of particles in powders under investigation took place via a solid-state sintering mechanism. Bigger grains in sintered fragments of inorganic powders can grow at 1000 °C, probably due to the higher mobility of Mg ions with smaller dimensions [
40].