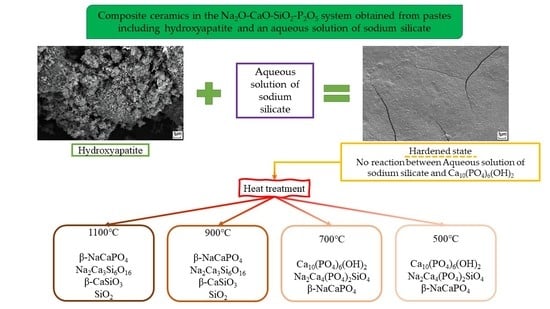
Composite Ceramics in the Na2O–CaO–SiO2–P2O5 System Obtained from Pastes including Hydroxyapatite and an Aqueous Solution of Sodium Silicate
Abstract
1. Introduction
2. Materials and Methods
2.1. Paste Preparation in the Na2O–CaO–SiO2–P2O5 System
2.2. Preparation of Pre-Ceramic Samples in the Na2O–CaO–SiO2–P2O5 System
2.3. Preparation of Ceramic Samples in the Na2O–CaO–SiO2–P2O5 System
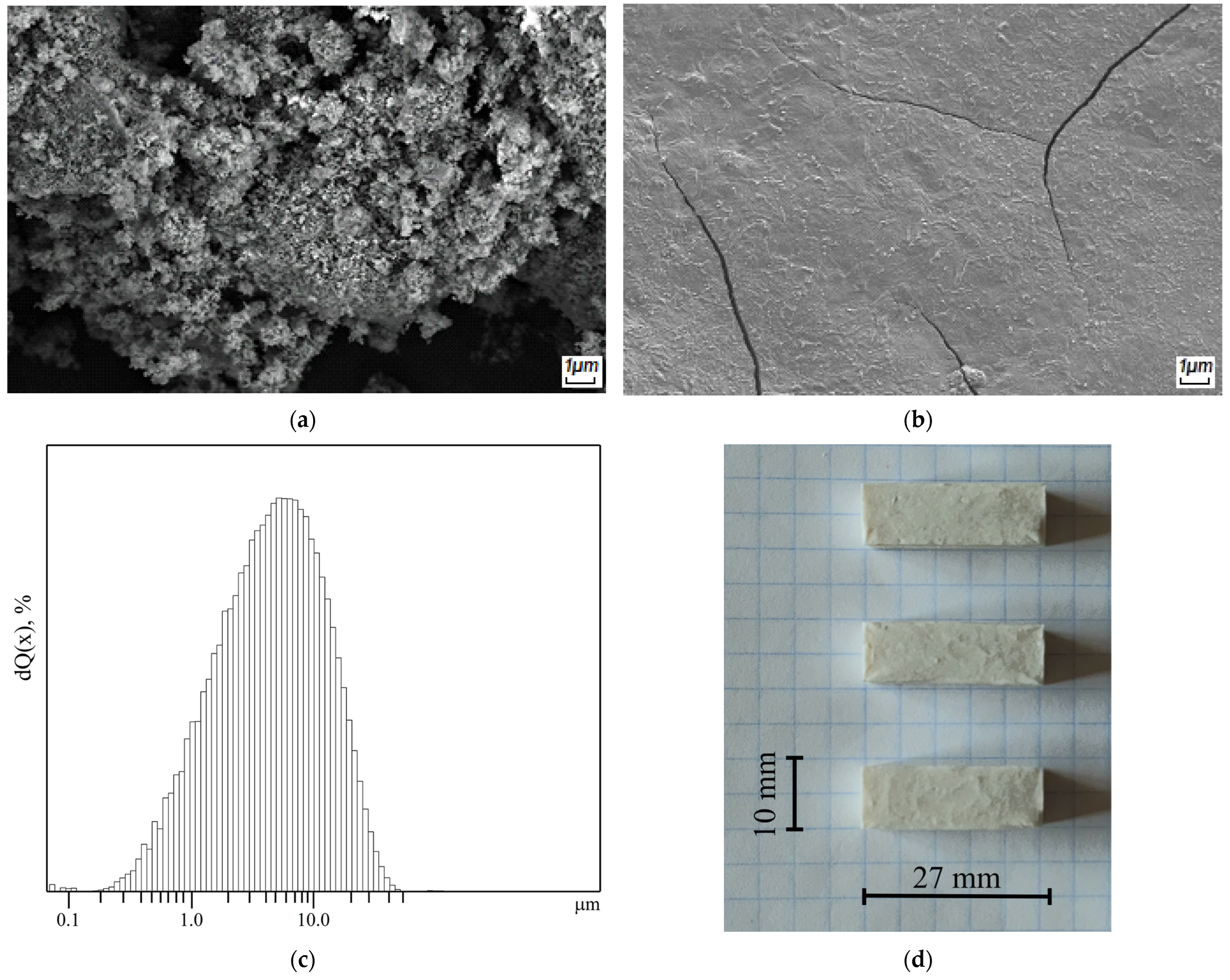
2.4. Morphological and Structural Characterization
2.4.1. Structural Characterization
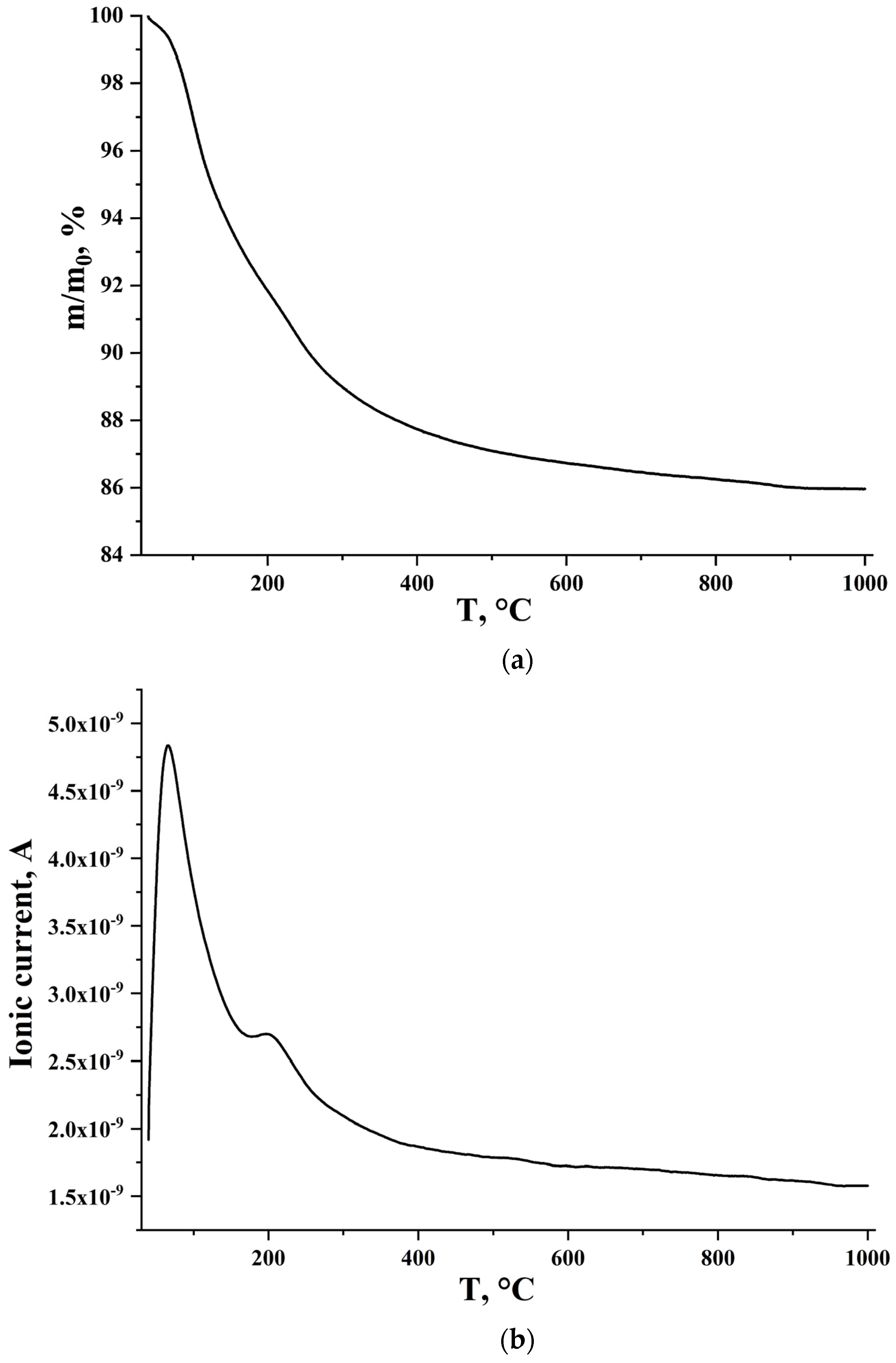
2.4.2. Thermogravimetric and Mass Spectrometric Analysis
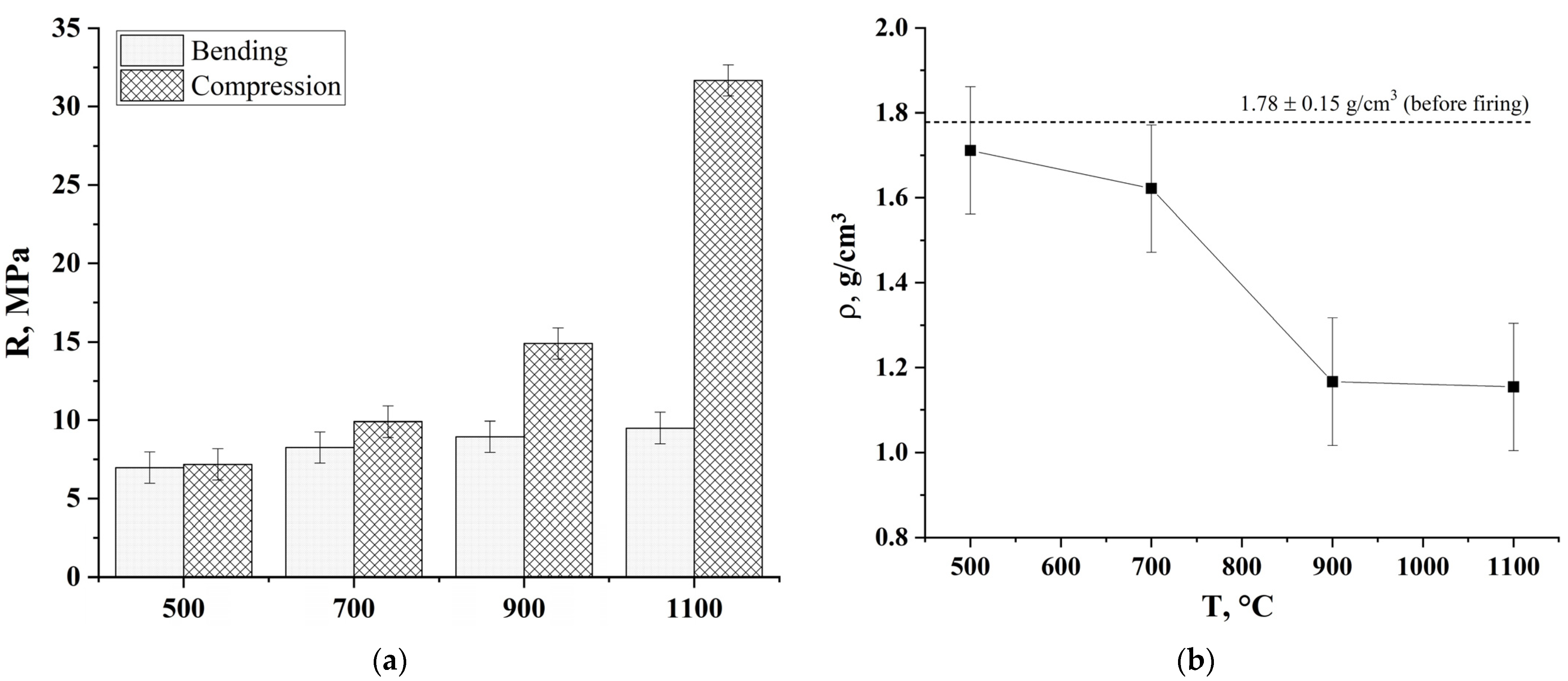
2.4.3. Determination of the Strength Properties
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aganesov, A.G.; KHeilo, A.L.; Mikaelian, K.P.; Galian, T.N.; Postnikov, Y.V. Long-Term Results of Treatment of Patients with Fractures of Cervical Spine Vertebrare with the Use of Coral-Based Bone Substitute Material BONEMEDIK-S. Bull. Natl. Med. Surg. Center. N.I. Pirogov. 2015, 10, 41–44. [Google Scholar]
- Abdul Halim, N.A.; Hussein, M.Z.; Kandar, M.K. Nanomaterials-Upconverted Hydroxyapatite for Bone Tissue Engineering and a Platform for Drug Delivery. Int. J. Nanomed. 2021, 16, 6477–6496. [Google Scholar] [CrossRef]
- Ievlev, V.M.; Putlyaev, V.I.; Safronova, T.V.; Evdokimov, P.V. Additive Technologies for Making Highly Permeable Inorganic Materials with Tailored Morphological Architectonics for Medicine. Inorg. Mater. 2015, 51, 1297–1315. [Google Scholar] [CrossRef]
- Lemesheva, S.A.; Golovanova, O.A.; Turenkov, S.V. Study of the Composition of Human Bone Tissues. Chem. Sustain. Dev. 2009, 17, 327–332. [Google Scholar]
- Tretyakov, Y.D. Inorganic Chemistry: Chemistry of Intransitive Elements; Academy: Moscow, Russia, 2004; Volume 2. [Google Scholar]
- Putlyaev, V.I. Modern Bioceramic Materials. Soros Educ. J. 2004, 8, 44–50. [Google Scholar]
- Morgan, E.F.; Gerstenfeld, L.C. The Bone Organ System: Form and Function. In Marcus and Feldman’s Osteoporosis, 5th ed.; Dempster, D.W., Cauley, J.A., Bouxsein, M.L., Cosman, F., Eds.; Academic Press: New York, NY, USA, 2021; Volume 1, pp. 15–35. ISBN 9780128130735. [Google Scholar]
- Boanini, E.; Gazzano, M.; Bigi, A. Ionic Substitutions in Calcium Phosphates Synthesized at Low Temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Z.; Li, W.; Fan, Y.; Li, Z.; Wei, J. Hydroxyapatite Based Materials for Bone Tissue Engineering: A Brief and Comprehensive Introduction. Crystals 2021, 11, 149. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L. The Story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Genetic Design of Bioactive Glass. J. Eur. Ceram. Soc. 2009, 29, 1257–1265. [Google Scholar] [CrossRef]
- Hench, L.L. Chronology of Bioactive Glass Development and Clinical Applications. New J. Glass Ceram. 2013, 3, 67–73. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, V.; Pickrell, G.R.; Mauro, J.C.; Lin, Y.; Arya, S.K. Bioactive Glasses in Gene Regulation and Proliferation. In Biomedical, Therapeutic and Clinical Applications of Bioactive Glasses; Elsevier: Amsterdam, The Netherlands, 2019; pp. 175–200. [Google Scholar]
- Hu, S.; Chang, J.; Liu, M.; Ning, C. Study on Antibacterial Effect of 45S5 Bioglass®. J. Mater. Sci. Mater. Med. 2008, 20, 281–286. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Nandi, S.K.; Kundu, B.; Datta, S.; De, D.K.; Roy, S.K.; Basu, D. In Vivo Response of Porous Hydroxyapatite and β-Tricalcium Phosphate Prepared by Aqueous Solution Combustion Method and Comparison with Bioglass Scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86B, 217–227. [Google Scholar] [CrossRef]
- Kansal, I.; Reddy, A.; Muñoz, F.; Choi, S.J.; Kim, H.W.; Tulyaganov, D.U.; Ferreira, J.M.F. Structure, Biodegradation Behavior and Cytotoxicity of Alkali-Containing Alkaline-Earth Phosphosilicate Glasses. Mater. Sci. Eng. C 2014, 44, 159–165. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Gazzarri, M.; Chiellini, F.; Cannillo, V. A New Hydroxyapatite-Based Biocomposite for Bone Replacement. Mater. Sci. Eng. C 2013, 33, 1091–1101. [Google Scholar] [CrossRef]
- Demirkiran, H.; Mohandas, A.; Dohi, M.; Fuentes, A.; Nguyen, K.; Aswath, P. Bioactivity and Mineralization of Hydroxyapatite with Bioglass as Sintering Aid and Bioceramics with Na3Ca6(PO4)5 and Ca5(PO4)2SiO4 in a Silicate Matrix. Mater. Sci. Eng. C 2010, 30, 263–272. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Cannillo, V. Hydroxyapatite and Tricalcium Phosphate Composites with Bioactive Glass as Second Phase: State of the Art and Current Applications. J. Biomed. Mater. Res. Part A 2016, 104, 1030–1056. [Google Scholar] [CrossRef]
- Goller, G.; Demirkiran, H.; Oktar, F.N.; Demirkesen, E. Processing and Characterization of Bioglass Reinforced Hydroxyapatite Composites. Ceram. Int. 2003, 29, 721–724. [Google Scholar] [CrossRef]
- Rizwan, M.; Hamdi, M.; Basirun, W.J. Bioglass® 45S5-Based Composites for Bone Tissue Engineering and Functional Applications. J. Biomed. Mater. Res. Part A 2017, 105, 3197–3223. [Google Scholar] [CrossRef]
- Rizwan, M.; Hamdi, M.; Basirun, W.J.; Kondoh, K.; Umeda, J. Low Pressure Spark Plasma Sintered Hydroxyapatite and Bioglass® Composite Scaffolds for Bone Tissue Repair. Ceram. Int. 2018, 44, 23052–23062. [Google Scholar] [CrossRef]
- Meng, Y.; Qiang, W.; Pang, J. Fabrication and Microstructure of Laminated HAP–45S5 Bioglass Ceramics by Spark Plasma Sintering. Materials 2019, 12, 484. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Review of Bioactive Glass: From Hench to Hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- GOST 13078-81; Liquid Sodium Glass. Specifications (with Changes N 1, 2). Publishing House of Standards: Moscow, Russia, 1989; p. 14.
- Korneev, V.I.; Danilov, V.V. Soluble and Liquid Glass; St. Petersburg Stroy-izdat: St. Petersburg, Russia, 1996. [Google Scholar]
- Alimov, L.A.; Voronin, V.V. Constructional Materials; Academy: Moscow, Russia, 2012; ISBN 978-5-7695-8336-0. [Google Scholar]
- Takadama, H.; Kim, H.M.; Kokubo, T.; Nakamura, T. Mechanism of Biomineralization of Apatite on a Sodium Silicate Glass: TEM−EDX Study In Vitro. Chem. Mater. 2001, 13, 1108–1113. [Google Scholar] [CrossRef]
- Golovanova, O.A.; Zaits, A.V. Biomimetic Coating of a Titanium Substrate with Silicon-Substituted Hydroxyapatite. Inorg. Mater. 2018, 54, 1124–1130. [Google Scholar] [CrossRef]
- ICDD. PDF-4+ 2010 (Database); Kabekkodu, S., Ed.; International Centre for Diffraction Data: Newtown Square, PA, USA, 2010; Available online: https://www.icdd.com/pdf-2/ (accessed on 7 May 2022).
- Medvedev, E.F.; Komarevskaya, A.S. IR Spectroscopic Study of the Phase Composition for Sodium Silicate Synthesized in Aqueous Medium. Glass Ceram. 2007, 64, 7–11. [Google Scholar] [CrossRef]
- Greaves, G.N.; Sen, S. Inorganic Glasses, Glass-Forming Liquids and Amorphizing Solids. Adv. Phys. 2007, 56, 1–166. [Google Scholar] [CrossRef]
- COUSIN, O.; RIVENET, M.; BOIVIN, J.C.; ABRAHAM, F. A REFRACTORY BONDING PHASE FUNDAMENTAL STUDY IN THE Na2O–CaO–P2O5–SiO2 SYSTEM. Phosphorus Res. Bull. 1999, 10, 183–188. [Google Scholar] [CrossRef][Green Version]
- Tancred, D.C.; Carr, A.J.; McCormack, B.A.O. The Sintering and Mechanical Behavior of Hydroxyapatite with Bioglass Additions. J. Mater. Sci. Mater. Med. 2001, 12, 81–93. [Google Scholar] [CrossRef]
- Aguilar-Reyes, E.A.; Leon-Patino, C.A.; Jacinto-Diaz, B.; Lefebvre, L.P. Mechanical and Microstructural Characterization of 45S5 Bioglass® Scaffolds for Tissue Engineering. In Biomaterials Science: Processing, Properties and Applications II; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 237, pp. 1–10. [Google Scholar]
- Chitra, S.; Bargavi, P.; Durgalakshmi, D.; Rajashree, P.; Balakumar, S. Role of Sintering Temperature Dependent Crystallization of Bioactiveglasses on Erythrocyte and Cytocompatibility. Processing Appl. Ceram. 2019, 13, 12–23. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Chen, Q.; Lefebvre, L.; Gremillard, L.; Chevalier, J. Sintering, Crystallisation and Biodegradation Behaviour of Bioglass®-Derived Glass–Ceramics. Faraday Discuss. 2007, 136, 27. [Google Scholar] [CrossRef] [PubMed]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. A New Rhenanite (β-NaCaPO4) and Hydroxyapatite Biphasic Biomaterial for Skeletal Repair. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80B, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Safronova, T.V.; Putlyayev, V.I.; Knotko, A.V.; Filippov, Y.Y.; Klimashina, E.S.; Ryzhov, A.P.; Saidzhonov, B.M. Powder Mixtures Based on Calcium Hydroxyapatite and Sodium Salts. Inorg. Mater. Appl. Res. 2018, 9, 726–731. [Google Scholar] [CrossRef]
- Safronova, T.V. Inorganic Materials for Regenerative Medicine. Inorg. Mater. 2021, 57, 443–474. [Google Scholar] [CrossRef]
- Safronova, T.V.; Putlyaev, V.I.; Steklov, M.Y.; Tretyakov, Y.D. Method for Obtaining a Ceramic Biodegradable Material Based on Rhenanite 2009. Patent RU 2362538 C2, 2009. [Google Scholar]
- Vuong, B.X.; Thy, N.N. A Quick Sol-Gel Process to Elaborate a High Bioactive Ceramic Powder. J. Sci. Technol. 2015, 6, 100–103. [Google Scholar]
- de Almeida, M.S.; de Oliveira Fernandes, G.V.; de Oliveira, A.M.; Granjeiro, J.M. Calcium Silicate as a Graft Material for Bone Fractures: A Systematic Review. J. Int. Med. Res. 2018, 46, 2537–2548. [Google Scholar] [CrossRef]
- Núñez-Rodríguez, L.A.; Encinas-Romero, M.A.; Gómez-Álvarez, A.; Valenzuela-García, J.L.; Tiburcio-Munive, G.C. Evaluation of Bioactive Properties of α and β Wollastonite Bioceramics Soaked in a Simulated Body Fluid. J. Biomater. Nanobiotechnology 2018, 9, 263–276. [Google Scholar] [CrossRef]
- Chitra, S.; Bargavi, P.; Durgalakshmi, D.; Rajashree, P.; Balakumar, S. On the Investigation of Structural and Biological Properties of 45S5 Bioglass and β-Tricalcium Phosphate Nanostructured Materials. In Proceedings of the AIP Conference Proceedings, Hisar, India, 18–22 December 2018; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2115, p. 030242. [Google Scholar]
- Ayawanna, J.; Kingnoi, N.; Wanpen, T. Porous Glass-Ceramic Orbital Implants from Egg Shell. Microsc. Microanal. Res. J. Microsc. Soc. Thail. 2020, 33, 4–8. [Google Scholar] [CrossRef]
- Durgalakshmi, D.; Balakumar, S. Nano-Bioglass (NBG) for Bone Regeneration Applications-Preparation and Its Characterization. In Proceedings of the AIP Conference Proceedings, Bombay, India, 3–7 December 2012; American Institute of PhysicsAIP: Hauppauge, NY, USA, 2013; Volume 1512, pp. 122–123. [Google Scholar]
- Carlisle, E.M. Silicon: A Requirement in Bone Formation Independent of Vitamin D1. Calcif. Tissue Int. 1981, 33, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F. Synthesis of SiO2 System via Sol–Gel Process: Biocompatibility Tests with a Fibroblast Strain and Release Kinetics. J. Biomed. Mater. Res. Part A 2014, 102, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Gallicchio, M.; Pacifico, S. Influence of the Polymer Amount on Bioactivity and Biocompatibility of SiO2/PEG Hybrid Materials Synthesized by Sol–Gel Technique. Mater. Sci. Eng. C 2015, 48, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Vapirov, V.V.; Feoktistov, V.M.; Venskovich, A.A.; Vapirova, N.V. On Silicon’s Behavior and Its Biological Role In Nature. Sci. Notes Petrozavodsk. State Univ. 2017, 2, 95–102. [Google Scholar]
- Carlisle, E.M. A Relationship between Silicon and Calcium in Bone Formation. Fed. Proc. 1970, 29, 565. [Google Scholar]
- Carlisle, E.M. Silicon: An Essential Element for the Chick. Science 1972, 178, 619–621. [Google Scholar] [CrossRef]
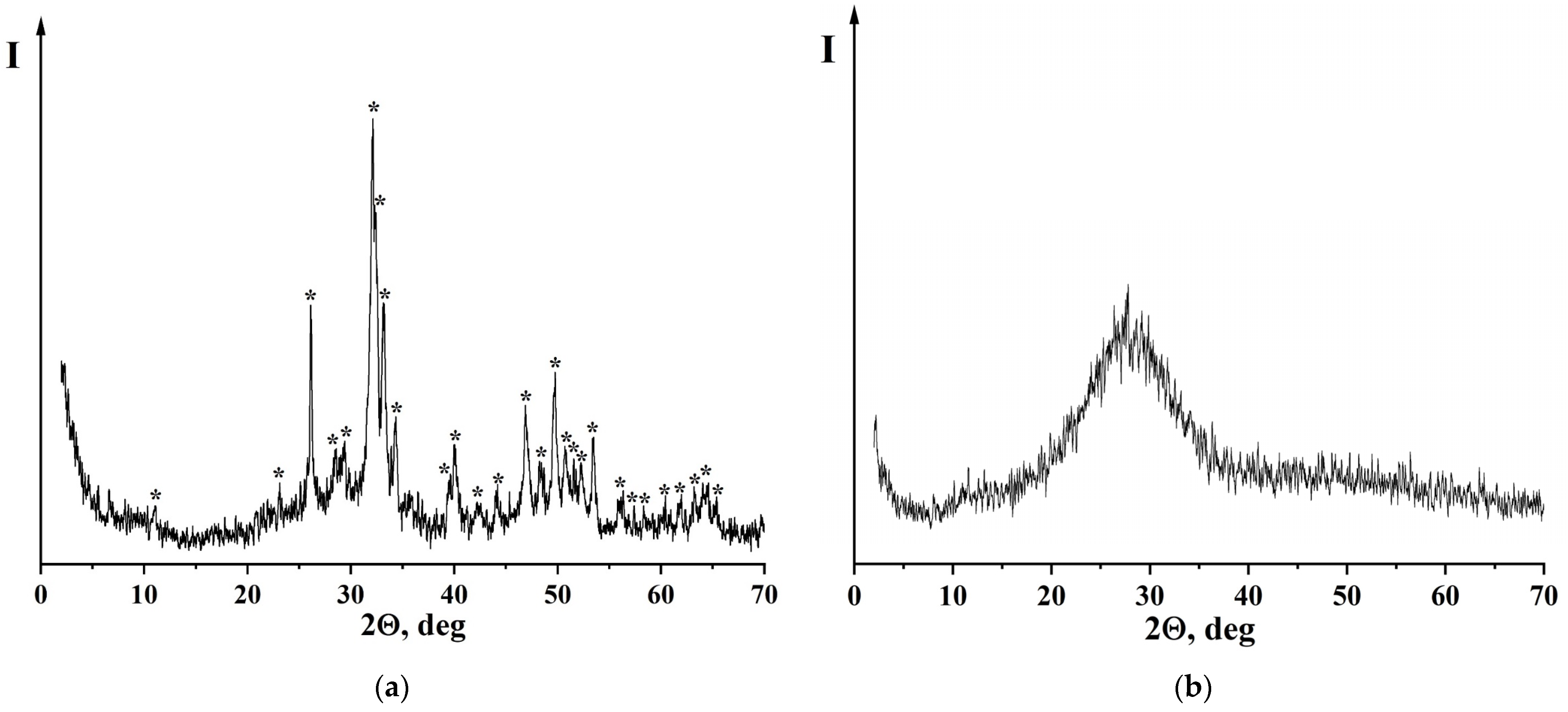
 —Na2Ca4(PO4)2SiO4, PDF [32-1053]; n—Na2Ca3Si6O16, PDF [23-671]; m—CaSiO3, PDF [27-88]; s—SiO2, PDF [29-85].
—Na2Ca4(PO4)2SiO4, PDF [32-1053]; n—Na2Ca3Si6O16, PDF [23-671]; m—CaSiO3, PDF [27-88]; s—SiO2, PDF [29-85].
 —Na2Ca4(PO4)2SiO4, PDF [32-1053]; n—Na2Ca3Si6O16, PDF [23-671]; m—CaSiO3, PDF [27-88]; s—SiO2, PDF [29-85].
—Na2Ca4(PO4)2SiO4, PDF [32-1053]; n—Na2Ca3Si6O16, PDF [23-671]; m—CaSiO3, PDF [27-88]; s—SiO2, PDF [29-85].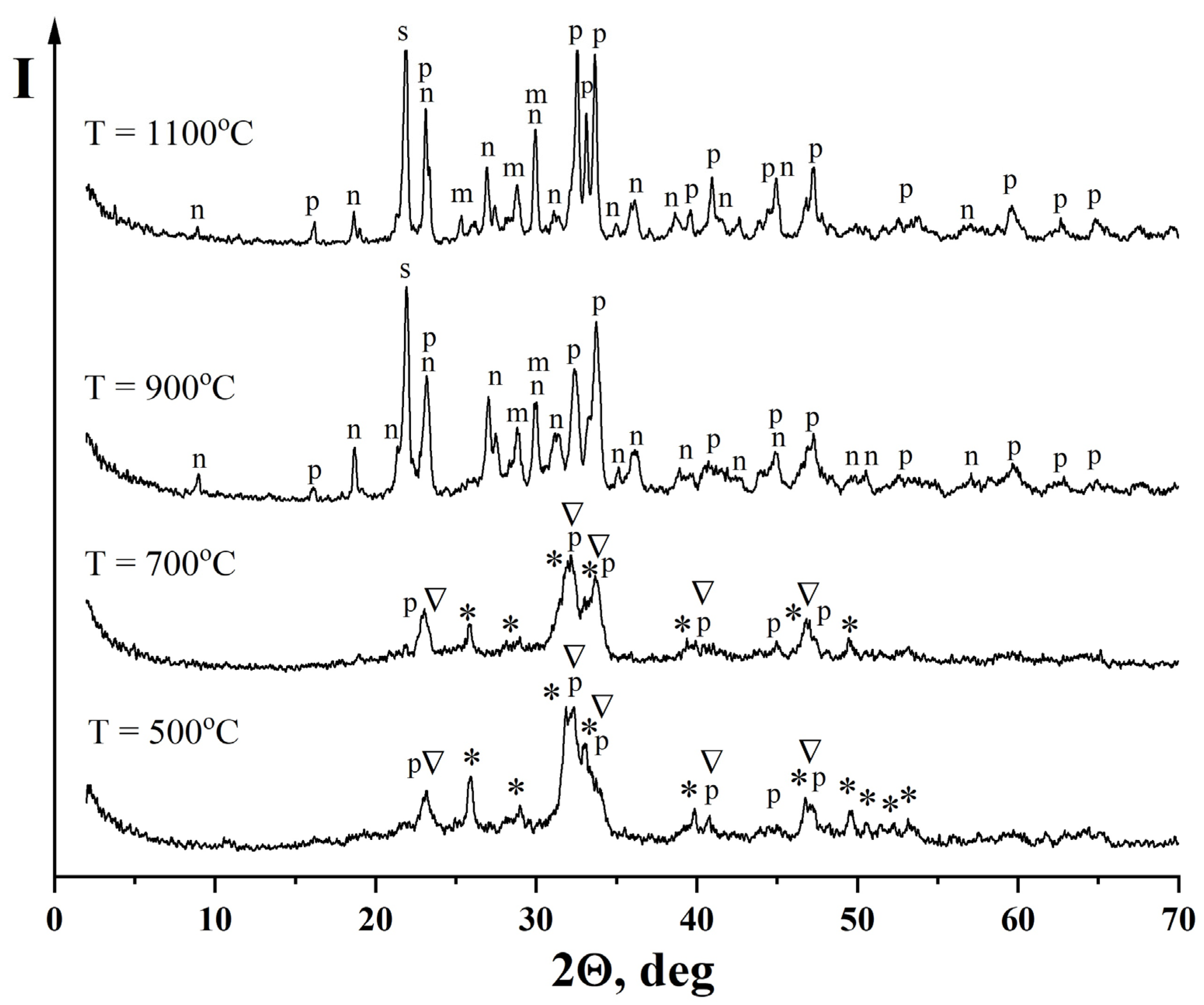

| Pre-Ceramic | 500 °C | 700 °C | 900 °C | 1100 °C |
|---|---|---|---|---|
| Ca10(PO4)6(OH)2 Non-identified phase of hydrated Na2O·2.87SiO2 | Ca10(PO4)6(OH)2 Na2Ca4(PO4)2SiO4 β-NaCaPO4 | Ca10(PO4)6(OH)2 Na2Ca4(PO4)2SiO4 β-NaCaPO4 | β-NaCaPO4 Na2Ca3Si6O16 β-CaSiO3 SiO2 | β-NaCaPO4 Na2Ca3Si6O16 β-CaSiO3 SiO2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaimonov, M.; Safronova, T.; Shatalova, T.; Filippov, Y.; Tikhomirova, I.; Sergeev, N. Composite Ceramics in the Na2O–CaO–SiO2–P2O5 System Obtained from Pastes including Hydroxyapatite and an Aqueous Solution of Sodium Silicate. Ceramics 2022, 5, 550-561. https://doi.org/10.3390/ceramics5030041
Kaimonov M, Safronova T, Shatalova T, Filippov Y, Tikhomirova I, Sergeev N. Composite Ceramics in the Na2O–CaO–SiO2–P2O5 System Obtained from Pastes including Hydroxyapatite and an Aqueous Solution of Sodium Silicate. Ceramics. 2022; 5(3):550-561. https://doi.org/10.3390/ceramics5030041
Chicago/Turabian StyleKaimonov, Maksim, Tatiana Safronova, Tatiana Shatalova, Yaroslav Filippov, Irina Tikhomirova, and Nikollay Sergeev. 2022. "Composite Ceramics in the Na2O–CaO–SiO2–P2O5 System Obtained from Pastes including Hydroxyapatite and an Aqueous Solution of Sodium Silicate" Ceramics 5, no. 3: 550-561. https://doi.org/10.3390/ceramics5030041
APA StyleKaimonov, M., Safronova, T., Shatalova, T., Filippov, Y., Tikhomirova, I., & Sergeev, N. (2022). Composite Ceramics in the Na2O–CaO–SiO2–P2O5 System Obtained from Pastes including Hydroxyapatite and an Aqueous Solution of Sodium Silicate. Ceramics, 5(3), 550-561. https://doi.org/10.3390/ceramics5030041