Fabrication of Basalt Matrix Composite Material by Pressureless Aluminum Melt Infiltration in Air Atmosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization
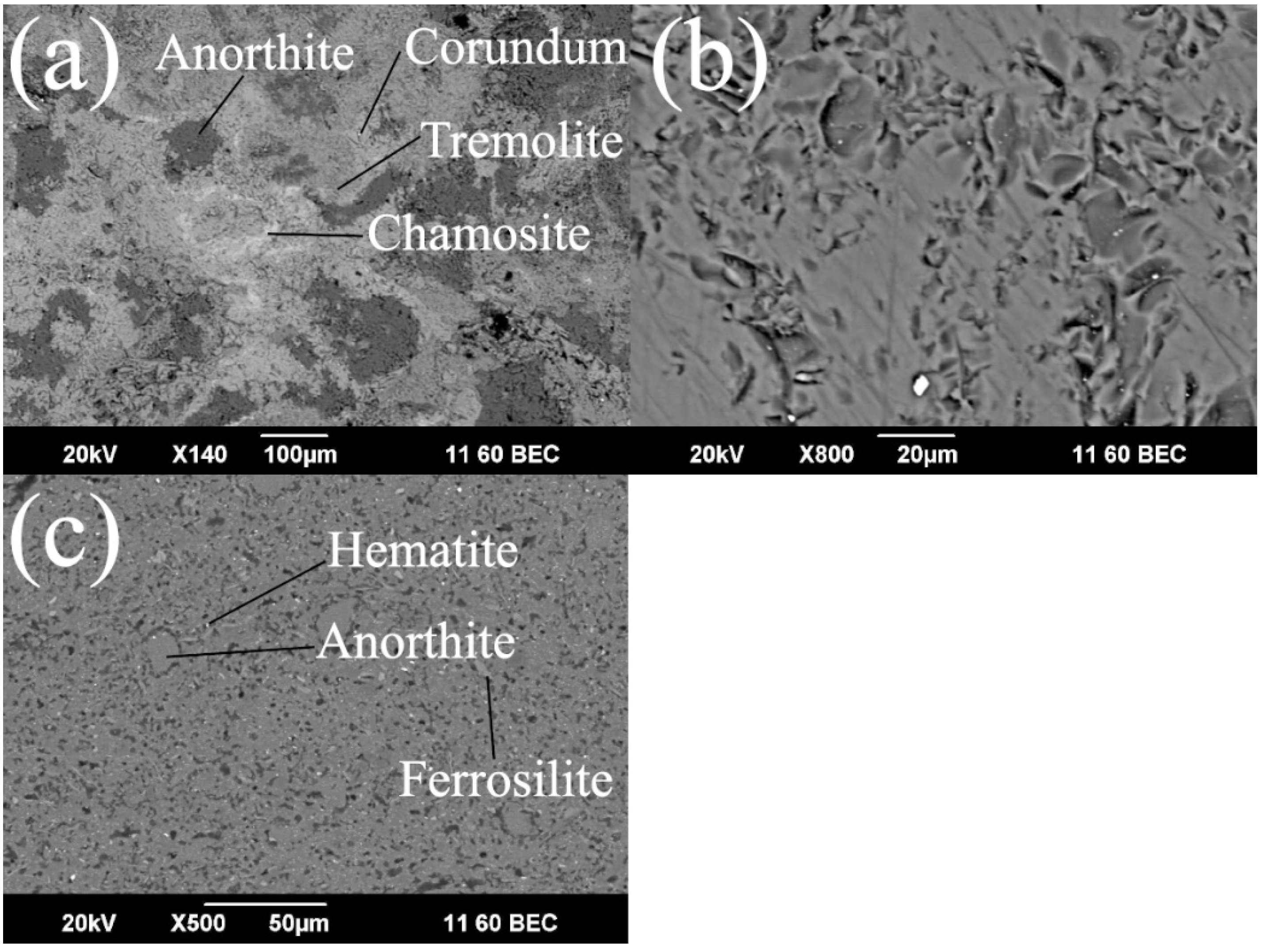
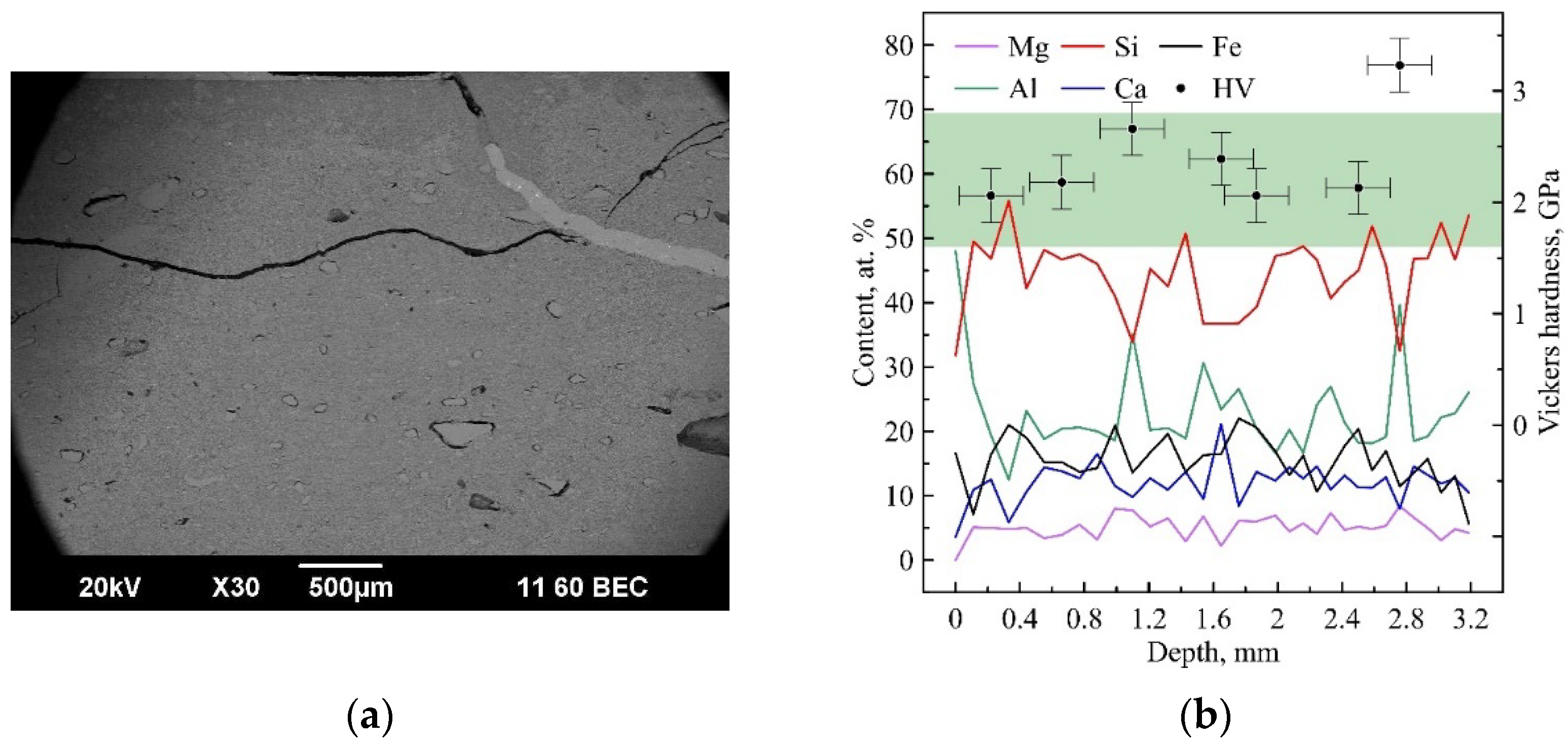
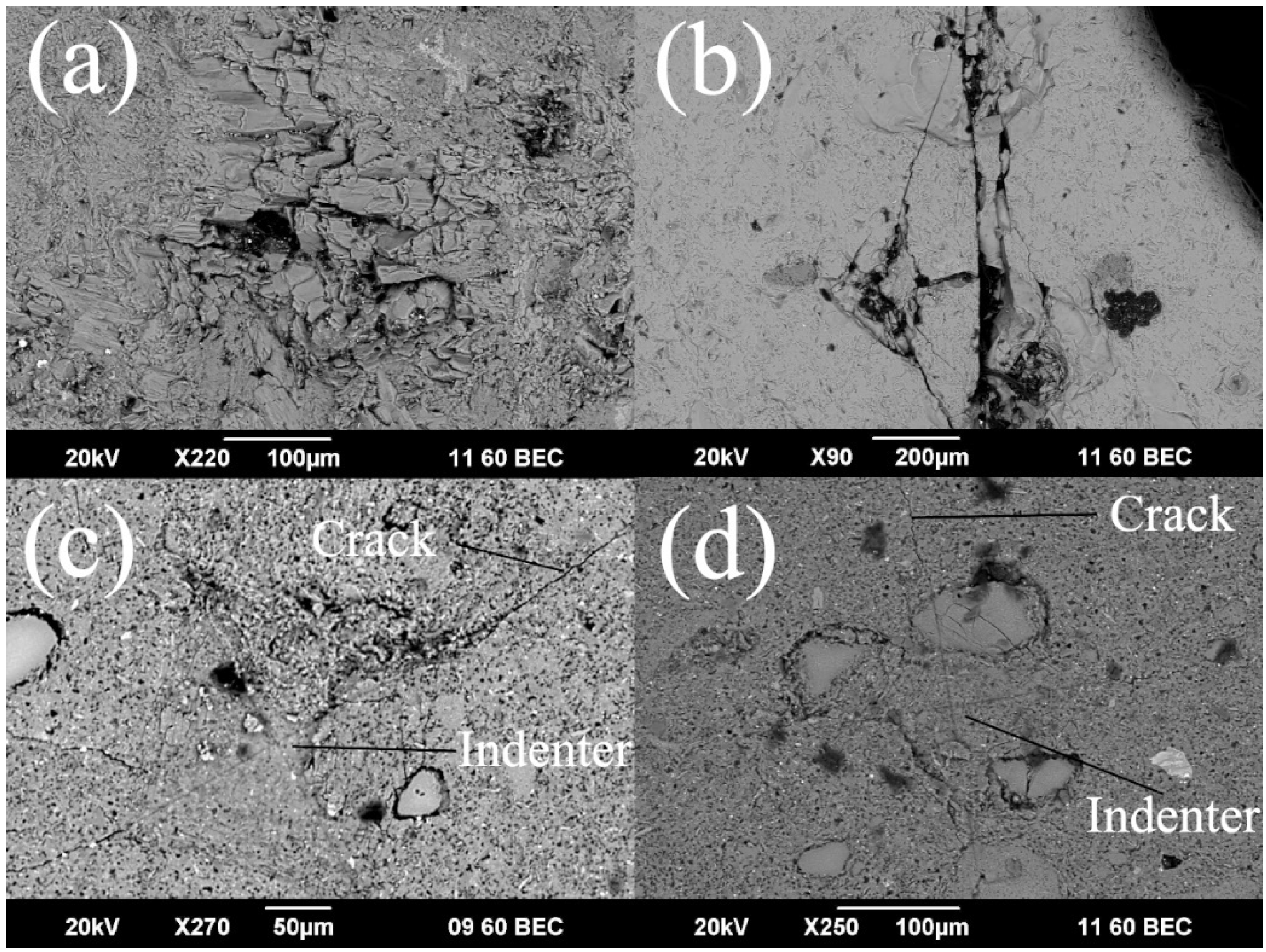
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foster, J. Ceramic Applications for Wear Protection of Pipe Lines and Cyclones. Key Eng. Mater. 1996, 122–124, 247–278. [Google Scholar] [CrossRef]
- Borawski, A. Common methods in analysing the tribological properties of brake pads and discs—A review. Acta Mech. Autom. 2019, 13, 189–199. [Google Scholar] [CrossRef]
- Irawan, A.P.; Fitriyana, D.F.; Tezara, C.; Siregar, J.P.; Laksmidewi, D.; Baskara, G.D.; Abdullah, M.Z.; Junid, R.; Hadi, A.E.; Hamdan, M.H.M.; et al. Overview of the Important Factors Influencing the Performance of Eco-Friendly Brake Pads. Polymers 2022, 14, 1180. [Google Scholar] [CrossRef] [PubMed]
- khodaei, M.; yaghobizadeh, O.; Alhosseini, S.H.N.; esmaeeli, S.; Mousavi, S.R. The effect of oxide, carbide, nitride and boride additives on properties of pressureless sintered SiC: A review. J. Eur. Ceram. Soc. 2019, 39, 2215–2231. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Wang, Q.; Miao, K.; Liang, X.; Lu, Z.; Li, D. Fabrication of 3D-SiC/aluminum alloy interpenetrating composites by DIW and pressureless infiltration. Ceram. Int. 2021, 47, 24340–24347. [Google Scholar] [CrossRef]
- Pech-Canul, M.I.; Katz, R.N.; Makhlouf, M.M. Optimum Parameters for Wetting Silicon Carbide by Aluminum Alloys. Metall. Mater. Trans. A 2000, 31, 565–573. [Google Scholar] [CrossRef]
- Nakae, H.; Hiramoto, Y. Spontaneous Infiltration of Al Melts into SiC Preform. Int. J. Met. 2011, 5, 23–28. [Google Scholar] [CrossRef]
- Zahedi, A.M.; Javadpour, J.; Rezaie, H.R.; Mazaheri, M. The effect of processing conditions on the microstructure and impact behavior of melt infiltrated Al/SiCp composites. Ceram. Int. 2011, 37, 3335–3341. [Google Scholar] [CrossRef]
- Lde Lima, F.; Zorzi, J.E.; Cruz, R.C.D. Basaltic glass-ceramic: A short review. Bol. Soc. Esp. Ceram. Vidr. 2020, 61, 2–12. [Google Scholar] [CrossRef]
- Pavlovic, M.; Dojcinovic, M.; Prokic-Cvetkovic, R.; Andric, L.; Ceganjac, Z.; Trumbulovic, L. Cavitation wear of basalt-based glass ceramic. Materials 2019, 12, 1552. [Google Scholar] [CrossRef]
- de Lima, L.F.; Perottoni, C.A.; Zorzi, J.E.; Cruz, R.C.D. Effect of iron on the microstructure of basalt glass-ceramics obtained by the petrurgic method. Int. J. Appl. Ceram. Technol. 2021, 18, 1950–1959. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Wu, Z. Effect of Fe2O3 Concentration on the Properties of Basalt Glasses. J. Nat. Fibers 2022, 19, 575–585. [Google Scholar] [CrossRef]
- Senol, Y.; Özkan, O.T.; Günay, V. Crystallization Kinetics of Basalt Glass. Ceram. Int. 1996, 22, 417481. [Google Scholar]
- Ercenk, E.; Guven, B.; Yilmaz, S. Crystallization kinetics of machinable glass ceramics produced from volcanic basalt rock. J. Non-Cryst. Solids 2018, 498, 262–271. [Google Scholar] [CrossRef]
- Ercenk, E.; Basaran, T.; Yilmaz, S. The effect of chromite ore addition on crystallization kinetics of basalt based machinable glass-ceramics. Ceram. Int. 2021, 47, 16902–16917. [Google Scholar] [CrossRef]
- Pavkov, V.; Bakic, G.; Maksimovic, V.; Cvijovic-Alagic, I.; Prekajski-Ðordjevic, M.; Bucevac, D.; Matovic, B. High-density ceramics obtained by andesite basalt sintering. Process. Appl. Ceram. 2022, 16, 143–152. [Google Scholar] [CrossRef]
- Fomichev, S.V.; Dergacheva, N.P.; Stevlevskii, A.V.; Krenev, V.A. Production of ceramic materials by the sintering of ground basalt. Theor. Found. Chem. Eng. 2011, 45, 526–529. [Google Scholar] [CrossRef]
- Grasso, S.; Biesuz, M.; Zoli, L.; Taveri, G.; Duff, A.I.; Ke, D.; Jiang, A.; Reece, M.J. A review of cold sintering processes. Adv. Appl. Ceram. 2020, 119, 115–143. [Google Scholar] [CrossRef]
- Mostafa, M.S.; Afify, N.; Gaber, A.; Zaid, E.F.A. Investigation of thermal properties of some basalt samples in Egypt. J. Therm. Anal. Calorim. 2004, 75, 179–188. [Google Scholar] [CrossRef]
- Robertson, E.C.; Peck, D.L. Thermal conductivity of vesicular basalt from Hawaii. J. Geophys. Res. 1974, 79, 4875–4888. [Google Scholar] [CrossRef]
- Wahid, S.M.S. Automotive brake wear: A review. Environ. Sci. Pollut. Res. 2018, 25, 174–180. [Google Scholar] [CrossRef]
- Borawski, A. Conventional and unconventional materials used in the production of brake pads—Review. Sci. Eng. Compos. Mater. 2020, 27, 374–396. [Google Scholar] [CrossRef]
- Kumar, V.V.; Kumaran, S.S. Friction material composite: Types of brake friction material formulations and effects of various ingredients on brake performance—A review. Mater. Res. Express 2019, 6, 082005. [Google Scholar] [CrossRef]
- Shishkin, R.; Yuferov, Y. Fabrication of SiC matrix composite material by pressureless aluminum melt infiltration in air atmosphere. Compos. Commun. 2022, 32, 101176. [Google Scholar] [CrossRef]
- Haeri, M.; Haeri, M. ImageJ Plugin for Analysis of Porous Scaffolds used in Tissue Engineering. J. Open Res. Softw. 2015, 3, e1. [Google Scholar] [CrossRef]
- Grove, C.; Jerram, D.A. JPOR: An ImageJ macro to quantify total optical porosity from blue-stained thin sections. Comput. Geosci. 2011, 37, 1850–1859. [Google Scholar] [CrossRef]
- Evans, A.G.; Charles, E.A. Fracture Toughness Determinations by Indentation. J. Am. Ceram. Soc. 1976, 59, 371–372. [Google Scholar] [CrossRef]
- Cuppoletti, J. Nanocomposites with Unique Properties and Applications in Medicine and Industry; InTech: London, UK, 2011. [Google Scholar] [CrossRef]
- Shishkin, R.A. Investigation of Thermal Greases with Hybrid Fillers and Its Operational Bench Test. J. Electron. Mater. 2022, 51, 1189–1201. [Google Scholar] [CrossRef]
- Schlichting, K.W.; Padture, N.P.; Klemens, P.G. Thermal conductivity of dense and porous yttria-stabilized zirconia. J. Mater. Sci. 2001, 36, 3003–3010. [Google Scholar] [CrossRef]
- Shishkin, R.A.; Yuferov, Y.V.; Karagergi, R.P.; Schak, A.V. Microstructural and mechanical properties of pressureless sintered high-wear-resistant SiC composite materials. J. Korean Ceram. Soc. 2022. In press. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Fe-O (Aluminum-iron-oxygen). J. Phase Equilibria Diffus. 2010, 31, 367. [Google Scholar] [CrossRef][Green Version]
- Dubey, P.; Kaurav, N. Structure Processing Properties Relationships in Stoichiometric and Nonstoichiometric Oxides; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Ashby, M.F. Materials Selection in Mechanical Design; Butterworth-Heinemann: Oxford, UK, 1999. [Google Scholar]
- Zeb, A.; Abid, M.; Zeb, M.A.; Qureshi, M.O.; Younas, U.; Batool, I. Measurement and prediction of thermal conductivity of volcanic basalt rocks from Warsak area. Adv. Mater. Sci. Eng. 2020, 2020, 4756806. [Google Scholar] [CrossRef]
- Shi, J.; Chen, Z.; Shao, S.; Zheng, J. Experimental and numerical study on effective thermal conductivity of novel form-stable basalt fiber composite concrete with PCMs for thermal storage. Appl. Therm. Eng. 2014, 66, 156–161. [Google Scholar] [CrossRef]
- Kim, S.H.; Heo, Y.J.; Park, M.; Min, B.G.; Rhee, K.Y.; Park, S.J. Effect of hydrophilic graphite flake on thermal conductivity and fracture toughness of basalt fibers/epoxy composites. Compos. Part B Eng. 2018, 153, 9–16. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkin, R.A.; Yuferov, Y.V.; Polyvoda, D.O. Fabrication of Basalt Matrix Composite Material by Pressureless Aluminum Melt Infiltration in Air Atmosphere. Ceramics 2022, 5, 780-788. https://doi.org/10.3390/ceramics5040056
Shishkin RA, Yuferov YV, Polyvoda DO. Fabrication of Basalt Matrix Composite Material by Pressureless Aluminum Melt Infiltration in Air Atmosphere. Ceramics. 2022; 5(4):780-788. https://doi.org/10.3390/ceramics5040056
Chicago/Turabian StyleShishkin, Roman A., Yuliy V. Yuferov, and Dmitriy O. Polyvoda. 2022. "Fabrication of Basalt Matrix Composite Material by Pressureless Aluminum Melt Infiltration in Air Atmosphere" Ceramics 5, no. 4: 780-788. https://doi.org/10.3390/ceramics5040056
APA StyleShishkin, R. A., Yuferov, Y. V., & Polyvoda, D. O. (2022). Fabrication of Basalt Matrix Composite Material by Pressureless Aluminum Melt Infiltration in Air Atmosphere. Ceramics, 5(4), 780-788. https://doi.org/10.3390/ceramics5040056






