Influence of the Preparation Method on the Physico-Chemical and Sorption Properties of Montmorillonite
Abstract
:1. Introduction
- Hydrothermal treatment of hydrogels of the appropriate composition at temperatures of 300–350 °C in a neutral environment (pH = 7)
- Hydrothermal treatment of a mixture of acetates of the corresponding metals and SiO2 at a temperature of 200–250 °C in a slightly acidic medium (pH = 4–6)
- Hydrothermal treatment of a mixture of the corresponding oxides at a temperature of 300–350 °C in an alkaline medium, pH = 9–10.
2. Materials and Methods
2.1. Reagents
2.2. Synthesis
2.3. Characterization
3. Results and Discussion
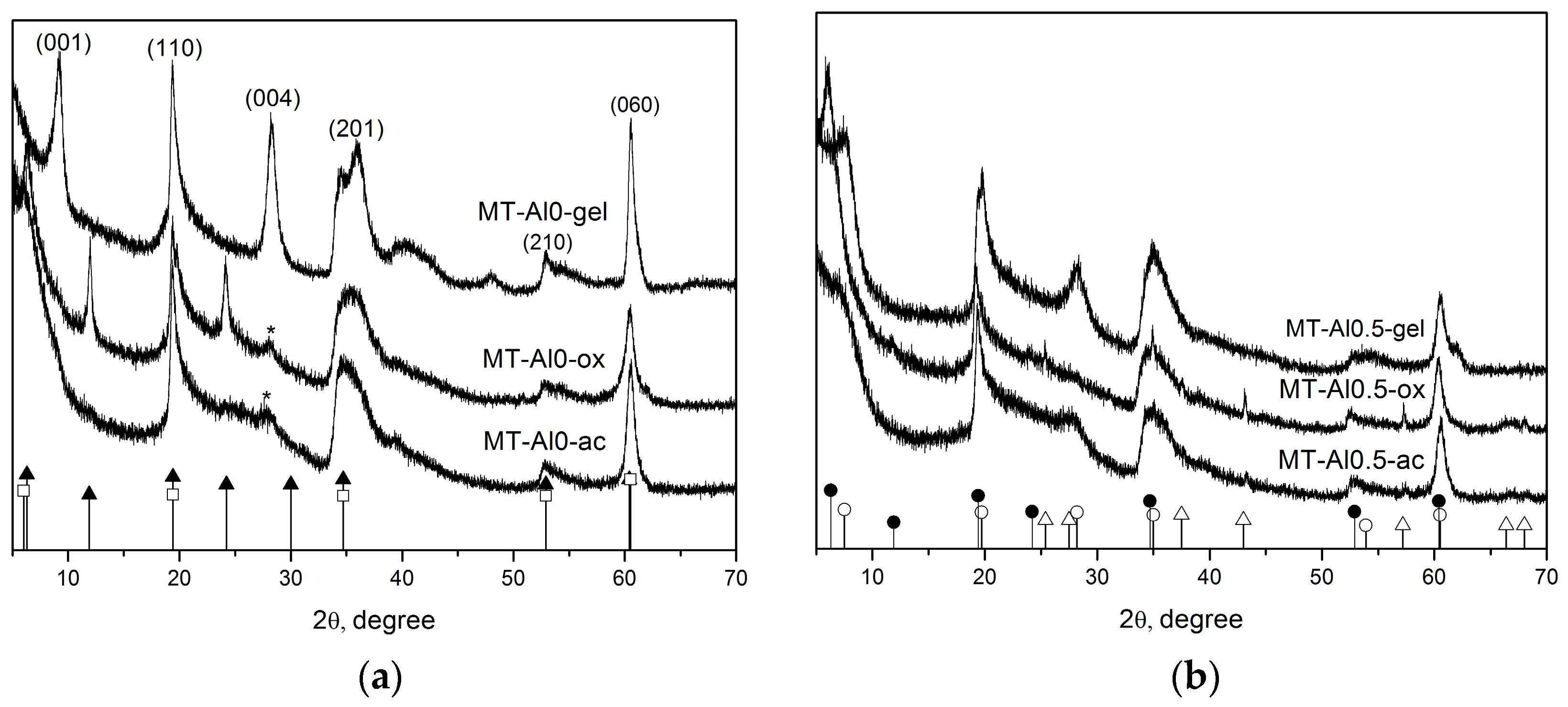
3.1. Phase and Chemical Composition of the Samples
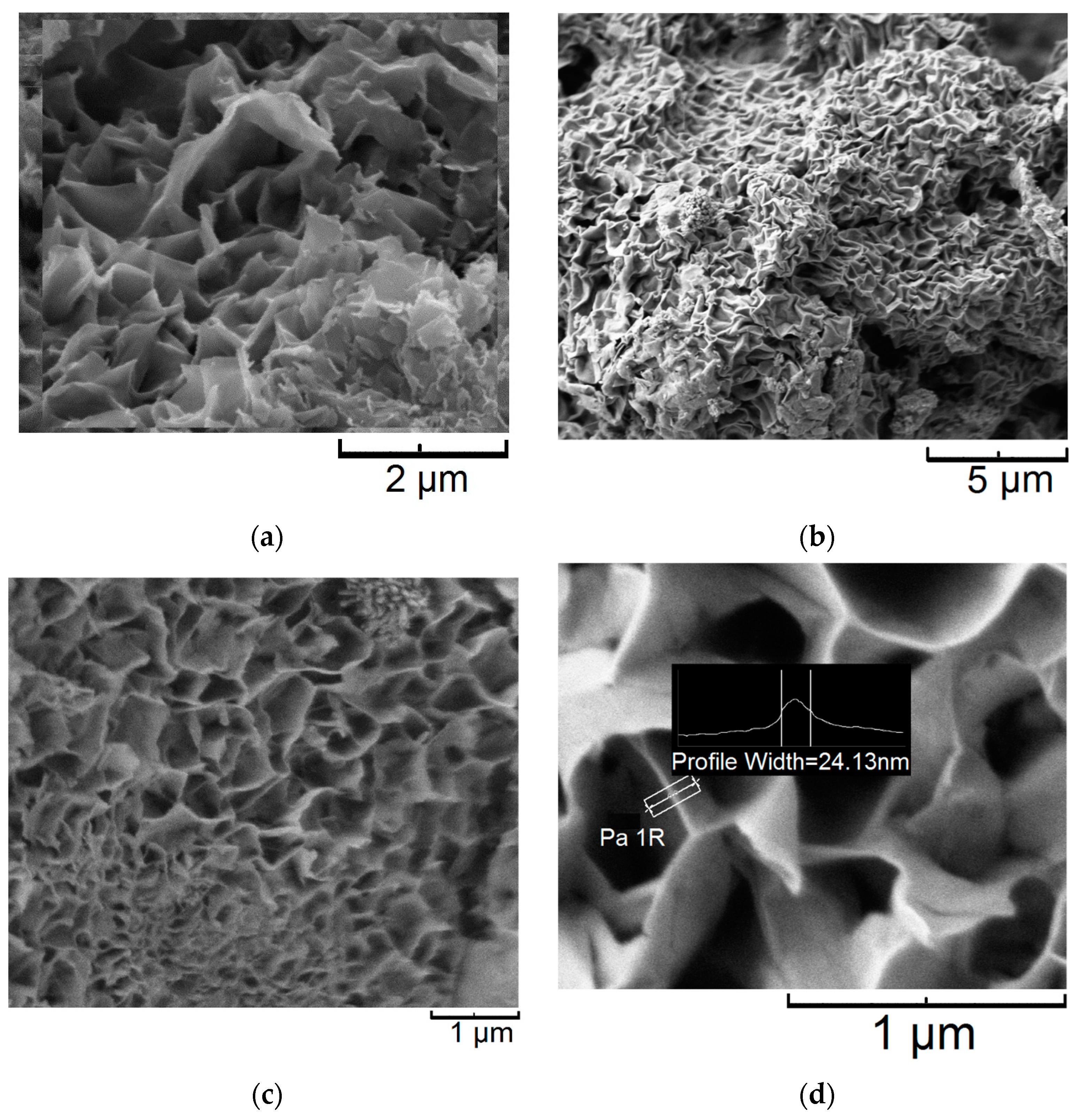
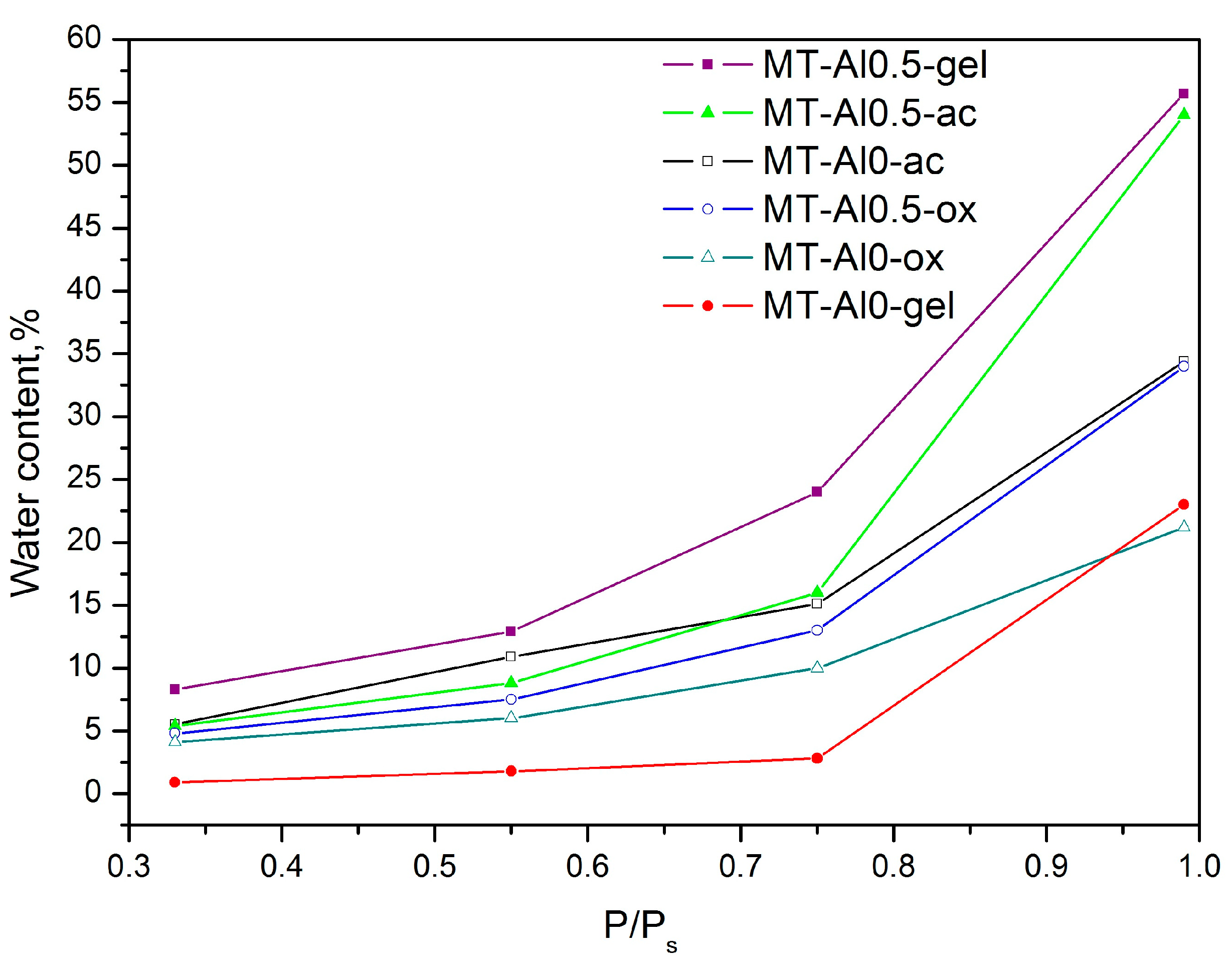
3.2. Porous Textural Characteristics of the Samples
3.3. Desiccant Capacity of the Samples
3.4. Distribution of Active Sites on the Surface
3.5. Methylene Blue Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hearon, S.E.; Orr, A.A.; Moyer, H.; Wang, M.; Tamamis, P.; Phillips, T.D. Montmorillonite clay-based sorbents decrease the bioavailability of per- and polyfluoroalkyl substances (PFAS) from soil and their translocation to plants. Environ. Res. 2022, 205, 112433. [Google Scholar] [CrossRef] [PubMed]
- França, D.B.; Oliveira, L.S.; Filho, F.G.N.; Filho, E.C.S.; Osajima, J.A.; Jaber, M.; Fonseca, M.G. The versatility of montmorillonite in water remediation using adsorption: Current studies and challenges in drug removal. J. Environ. Chem. Eng. 2022, 10, 107341. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Cooper, C.; Ouki, S. Comparison of modified montmorillonite adsorbents: Part I: Preparation, characterization and phenol adsorption. Chemosphere 2002, 47, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Iroh, J.O.; Rajagopolan, R.; Aykanat, A.; Vaia, R. Optimizing the Synthesis and Thermal Properties of Conducting Polymer–Montmorillonite Clay Nanocomposites. Energies 2022, 15, 1291. [Google Scholar] [CrossRef]
- Kumar, B.S.; Dhakshinamoorthy, A.; Pitchumani, K. K10 montmorillonite clays as environmentally benign catalysts for organic reactions. Catal. Sci. Technol. 2014, 4, 2378–2396. [Google Scholar] [CrossRef]
- Takabatake, M.; Motokura, K. Montmorillonite-based heterogeneous catalysts for efficient organic reactions. Nano Express 2022, 3, 2–13. [Google Scholar] [CrossRef]
- Park, J.-H.; Shin, H.-J.; Kim, M.H.; Kim, J.-S.; Kang, N.; Lee, J.-Y.; Kim, K.-T.; Lee, J.I.; Kim, D.-D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef]
- Okada, A.; Kawasumi, M.; Usuki, A.; Kojima, Y.; Kurauchi, T.; Kamigaito, O. Nylon 6—Clay hybrid. MRS Online Proc. Libr. 1989, 171, 45–50. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. Polymer-layered silicate nanocomposites: An overview. Macromol. Mater. Eng. 2006, 291, 1449–1476. [Google Scholar] [CrossRef]
- Abollino, O.; Aceto, M.; Malandrino, M.; Sarzanini, C.; Mentasti, E. Adsorption of heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Res. 2003, 37, 1619–1627. [Google Scholar] [CrossRef]
- Rytwo, G.; Nir, S.; Margulies, L. A model for adsorption of divalent organic cations to montmorillonite. J. Colloid Interface Sci. 1996, 181, 551–560. [Google Scholar] [CrossRef]
- Tang, G.; Jia, C.; Wang, G.; Yu, P.; Jiang, X. Adsorption mechanism of bacteria onto a Na-montmorillonite surface with organic and inorganic calcium. bioRxiv 2020. [Google Scholar] [CrossRef]
- Olopade, B.K.; Oranusi, S.U.; Nwinyi, O.C.; Lawal, I.A.; Gbashi, S.; Njobeh, P.B. Decontamination of T-2 toxin in maize by modified montmorillonite clay. Toxins 2019, 11, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, N.J.; Xue, K.S.; Lin, S.; Marroquin-Cardona, A.; Brown, K.A.; Elmore, S.E.; Tang, L.; Romoser, A.; Gelderblom, W.C.A.; Wang, J.-S.; et al. Calcium montmorillonite clay reduces AFB1 and FB1 biomarkers in rats exposed to single and co-exposures of aflatoxin and fumonisin. J. Appl. Toxicol. 2014, 34, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Xu, J.; Sun, Z.; Zheng, S. Surface functionalization of montmorillonite with chitosan and the role of surface properties on its adsorptive performance: A comparative study on mycotoxins adsorption. Langmuir 2020, 36, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P. Montmorillonite-catalyzed formation of RNA oligomers: The possible role of catalysis in the origin of life. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2006, 361, 1777–1786; discussion 1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jheeta, S.; Joshi, P.C. Prebiotic RNA synthesis by montmorillonite catalysis. Life 2014, 4, 318–330. [Google Scholar] [CrossRef]
- Aldersley, M.F.; Joshi, P.C.; Price, J.D.; Ferris, J.P. The role of montmorillonite in its catalysis of RNA synthesis. Appl. Clay Sci. 2011, 54, 1–14. [Google Scholar] [CrossRef]
- Yamada, H.; Nakazawa, H.; Yoshioka, K.; Fujita, T. Smectites in the montmorillonite-beidellite series. Clay Miner. 1991, 26, 359–369. [Google Scholar] [CrossRef]
- Carrado, K.A.; Csencsits, R.; Thiyagarajan, P.; Seifert, S.; Macha, S.M.; Harwood, J.S. Crystallization and textural porosity of synthetic clay minerals. J. Mater. Chem. 2002, 12, 3228–3237. [Google Scholar] [CrossRef]
- Shao, H.; Pinnavaia, T.J. Synthesis and properties of nanoparticle forms saponite clay, cancrinite zeolite and phase mixtures thereof. Microporous Mesoporous Mater. 2010, 133, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloprogge, J.T. Synthesis of smectites and porous pillared clay catalysts: A review. J. Porous Mater. 1998, 5, 5–41. [Google Scholar] [CrossRef]
- Golubeva, O.Y.; Ul’yanova, N.Y.; Kostyreva, T.G.; Drozdova, I.A.; Mokeev, M.V. Synthetic nanoclays with the structure of montmorillonite: Preparation, structure, and physico-chemical properties. Glass Phys. Chem. 2013, 39, 533–539. [Google Scholar] [CrossRef]
- Harder, H. The role of magnesium in the formation of smectite minerals. Chem. Geol. 1972, 10, 31–39. [Google Scholar] [CrossRef]
- Reinholdt, M.; Miehé-Brendlé, J.; Delmotte, L.; Tuilier, M.-H.; le Dred, R.; Cortès, R.; Flank, A.-M. Fluorine route synthesis of montmorillonites containing Mg or Zn and characterization by XRD, thermal analysis, MAS NMR, and EXAFS spectroscopy. Eur. J. Inorg. Chem. 2001, 2001, 2831–2841. [Google Scholar] [CrossRef]
- Reinholdt, M.; Miehe-Brendle, J.; Delmotte, L.; Le Dred, R.; Tuilier, M.H. Synthesis and characterization of montmorillonite-type phyllosilicates in a fluoride medium. Clay Miner. 2005, 40, 177–190. [Google Scholar] [CrossRef]
- Golubeva, O.Y. Features of the Hydrothermal Synthesis of Montmorillonite in an Acidic Medium. Glas. Phys. Chem. 2018, 44, 616–619. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Bardakhanov, S.P.; Vasiljeva, I.V.; Kuksanov, N.K.; Mjakin, S.V. Surface functionality features of nanosized silica obtained by electron beam evaporation at ambient pressure. Adv. Mater. Sci. Eng. 2010, 2010, 241695. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, D.; Bhattacharyya, K.G. Adsorption of methylene blue on kaolinite. Appl. Clay Sci. 2002, 20, 295–300. [Google Scholar] [CrossRef]
- MacEwan, D.M. Identification of the montmorillonite group of minerals by X-rays. Nature 1944, 154, 577–578. [Google Scholar] [CrossRef]
- Golubeva, O.Y. Effect of synthesis conditions on hydrothermal crystallization, textural characteristics and morphology of aluminum-magnesium montmorillonite. Microporous Mesoporous Mater. 2016, 224, 271–276. [Google Scholar] [CrossRef]
- Golubeva, O.Y.; Korytkova, E.N.; Gusarov, V.V. Hydrothermal Synthesis of Magnesium Silicate Montmorillonite for Polymer-Clay Nanocomposites. Russ. J. Appl. Chem. 2005, 78, 26–32. [Google Scholar] [CrossRef]
- Cases, J.M.; Berend, I.; Besson, G.; Francois, M.; Uriot, J.P.; Thomas, F.; Poirier, J.E. Mechanism of adsorption and desorption of water vapor by homoionic montmorillonite. 1. The sodium-exchanged form. Langmuir 1992, 8, 2730–2739. [Google Scholar] [CrossRef]
- Chai, Y.; Dai, W.; Wu, G.; Guan, N.; Li, L. Confinement in a zeolite and zeolite catalysis. Acc. Chem. Res. 2021, 54, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sievers, C.; Hong, S.B.; Prins, R.; van Bokhoven, J.A. Catalytic activity of Brønsted acid sites in zeolites: Intrinsic activity, rate-limiting step, and influence of the local structure of the acid sites. J. Catal. 2006, 244, 163–168. [Google Scholar] [CrossRef]
- Bonacci, S.; Iriti, G.; Mancuso, S.; Novelli, P.; Paonessa, R.; Tallarico, S.; Nardi, M. Montmorillonite K10: An efficient organo-heterogeneous catalyst for synthesis of benzimidazole derivatives. Catalysts 2020, 10, 845. [Google Scholar] [CrossRef]
- Golubeva, O.Y.; Pavlova, S.V. Adsorption of methylene blue from aqueous solutions by synthetic montmorillonites of different compositions. Glas. Phys. Chem. 2016, 42, 207–213. [Google Scholar] [CrossRef]
- Alikina, Y.A.; Kalashnikova, T.A.; Golubeva, O.Y. Sorption capacity of synthetic aluminosilicates of the kaolinite group of various morphology. Glas. Phys. Chem. 2021, 47, 42–48. [Google Scholar] [CrossRef]
- Chaari, I.; Fakhfakh, E.; Medhioub, M.; Jamoussi, F. Comparative study on adsorption of cationic and anionic dyes by smectite rich natural clays. J. Mol. Struct. 2019, 1179, 672–677. [Google Scholar] [CrossRef]
- Nogueira, F.G.; Lopes, J.H.; Silva, A.C.; Gonçalves, M.; Anastácio, A.S.; Sapag, K.; Oliveira, L.C. Reactive adsorption of methylene blue on montmorillonite via an ESI-MS study. Appl. Clay Sci. 2009, 43, 190–195. [Google Scholar] [CrossRef]
- Chen, G.; Pan, J.; Han, B.; Yan, H. Adsorption of methylene blue on montmorillonite. J. Dispers. Sci. Technol. 1999, 20, 1179–1187. [Google Scholar] [CrossRef]
- Krishna Kumar, A.S.; Warchol, J.; Matusik, J.; Tseng, W.L.; Rajesh, N.; Bajda, T. Heavy metal and organic dye removal via a hybrid porous hexagonal boron nitride-based magnetic aerogel. NPJ Clean Water 2022, 5, 24. [Google Scholar] [CrossRef]
- Markelov, D.A.; Nitsak, O.V.; Gerashchenko, I.I. Comparative study of the adsorption activity of medicinal sorbents. Pharm. Chem. J. 2008, 42, 405–408. [Google Scholar] [CrossRef]
- Rahimi, M.; Vadi, M.J. Langmuir, Freundlich and Temkin Adsorption Isotherms of Propranolol on Multi-Wall Carbon Nanotube. Mod. Drug Discov. Drug Deliv. Res. 2014, 1, 2348–3776. [Google Scholar]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O.J.I.J.C. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Zhang, Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019, 277, 646–648. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Golubeva, O.Y.; Alikina, Y.A.; Brazovskaya, E.Y.; Vasilenko, N.M. Adsorption Properties and Hemolytic Activity of Porous Aluminosilicates in a Simulated Body Fluid. ChemEngineering 2022, 6, 78. [Google Scholar] [CrossRef]
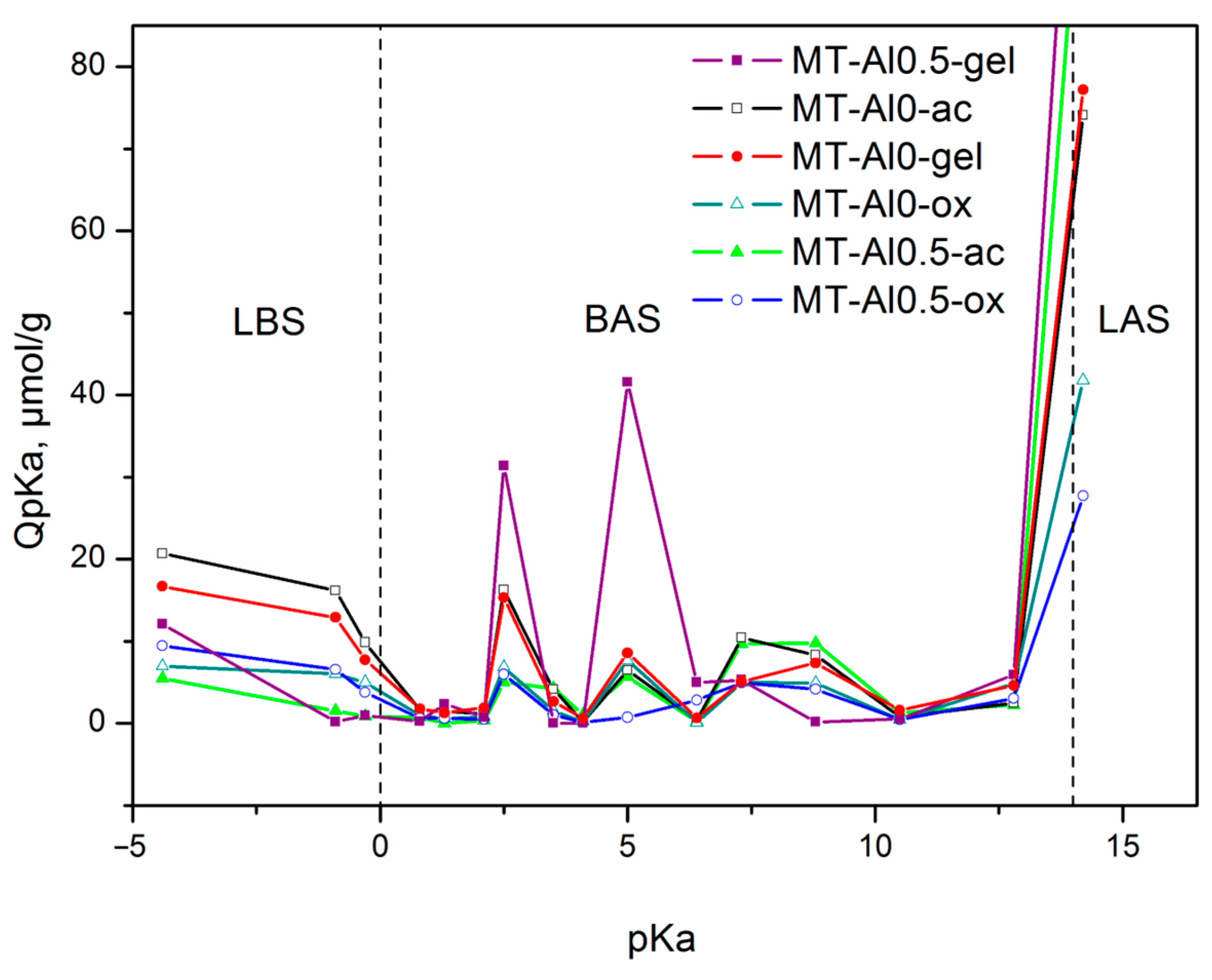
| Synthesis Method Designation | Sample Designation | Samples (Composition by Synthesis) | Synthesis Conditions | |||
|---|---|---|---|---|---|---|
| Reagents | pH | T, °C | Processing Time, Days | |||
| 1 | MT-Al0-ac | Mg3Si4O10(OH)2 nH2O | Mg(COOCH3)2 4H2O, NaCOOCH3 Al2O3, SiO2 | 4–5 | 220 | 4–5 |
| MT-Al0.5-ac | Na1.5Al0.5Mg1.5Si4O10(OH)2 H2O | |||||
| 2 | MT-Al0 gel | Mg3Si4O10(OH)2 nH2O | Dried hydrogels of the appropriate composition | 7 | 350 | 3–4 |
| MT-Al0.5 gel | Na1.5Al0.5Mg1.5Si4O10(OH)2 H2O | |||||
| 3 | MT-Al0-ox | Mg3Si4O10(OH)2 nH2O | MgO, SiO2, Al2O3 NaNO3 | 7–9 | 350 | 3–4 |
| MT-Al0.5-ox | Na1.5Al0.5Mg1.5Si4O10(OH)2 H2O | |||||
| Sample | Chemical Composition, wt.% | ||||
|---|---|---|---|---|---|
| SiO2 | Al2O3 | MgO | Na2O | Loss on Ignition, % | |
| MT-Al0-ac | 59.1 | - | 21.0 | 0.04 | 13.1 |
| MT-Al0 gel | 61.2 | - | 32.2 | 0.02 | 6.6 |
| MT-Al0-ox | 52.6 | - | 27.3 | 0.13 | 12.9 |
| MT-Al0.5-ac | 52.7 | 7.5 | 11.7 | 4.47 | 17.4 |
| MT-Al05 gel | 58.1 | 13.8 | 13.1 | 2.76 | 8.9 |
| MT-Al0.5-ox | 57.3 | 10.8 | 16.2 | 0.20 | 11.0 |
| Sample | SSA a, m2/g | n | ζ-Potential, mV |
|---|---|---|---|
| MT-Al0 gel | 188 ± 15 | 4 | −11.8 ± 1.9 |
| MT-Al0-ac | 134 ± 7 | 6 | −10.3 ± 0.6 |
| MT-Al0-ox | 84.7 ± 12 | 10 | −18.6 ± 2.5 |
| MT-Al0.5 gel | 184 ± 15 | 4 | −25.4 ± 0.8 |
| MT-Al0.5-ac | 89.3 ± 11 | 9.5 | −22.5 ± 2.2 |
| MT-Al0.5-ox | 67.4 ± 8 | 13 | −17.2 ± 2.6 |
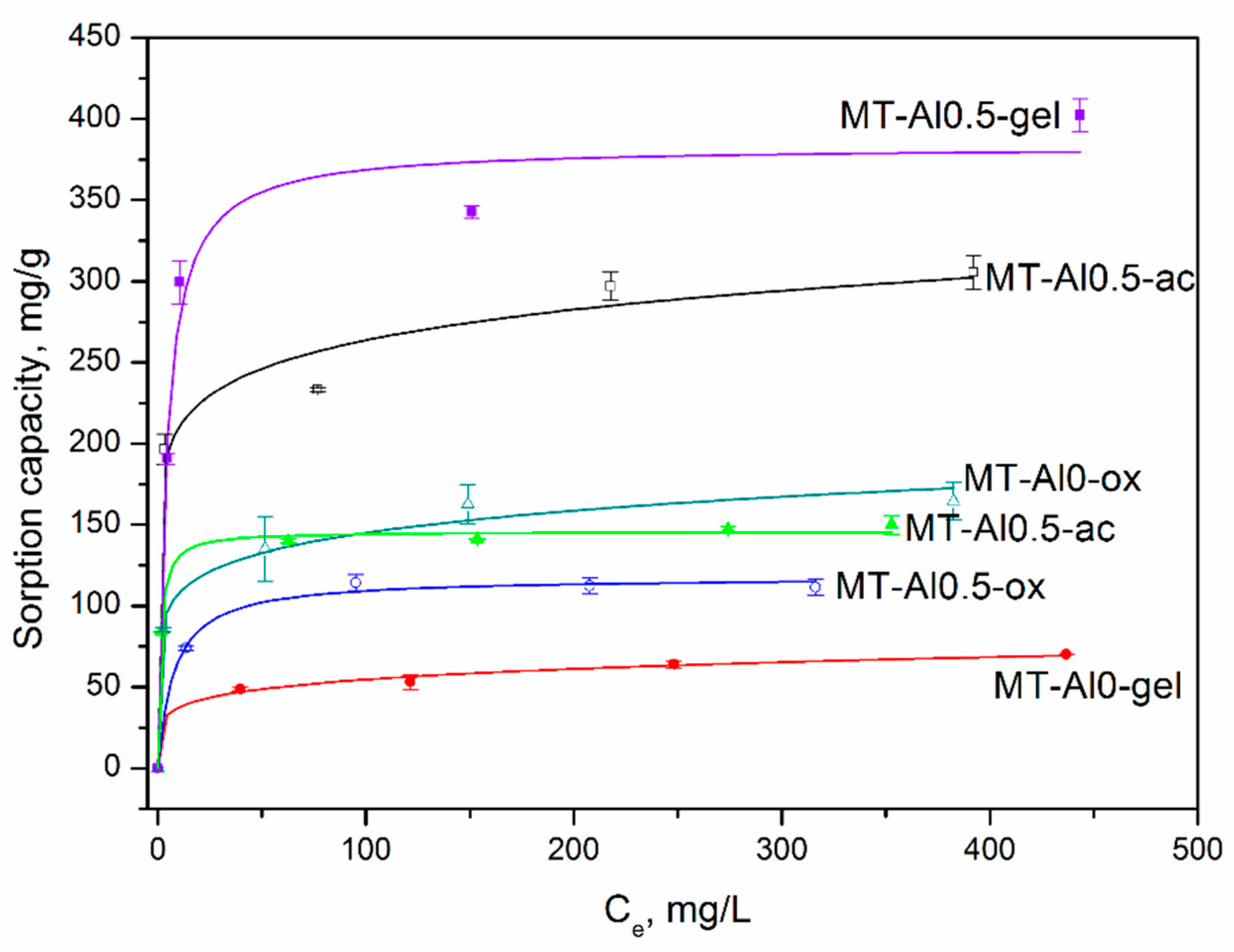
| Samples Morphology | qexp | Langmuir Equation | Freundlich Equation | Dubinin-Radushkevich | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qm | KL, | R2 | n | KF | R2 | qm | β | E | R2 | ||
| Al0 gel | 70 ± 1 | 69 ± 4 | (5.1 ± 0.2) × 10−2 | 0.97 | 6.1 ± 0.8 | 25 ± 3 | 0.99 | 63 ± 2 | 7.2 ± 0.1 | 0.30 ± 0.06 | 0.6 |
| Al0-ox | 164 ± 11 | 158 ± 7 | 0.45 ± 0.1 | 0.97 | 7.7 ± 1.1 | 80 ± 7 | 0.98 | 202 ± 15 | (1.1 ±0.2) × 10−3 | 21 ± 1 | 0.97 |
| Al0-ac | 305 ± 10 | 282 ± 18 | 0.7 ± 0.3 | 0.94 | 10 ± 2 | 166 ± 17 | 0.98 | 411 ± 34 | (5.0 ± 0.1) × 10−3 | 10 ± 2 | 0.97 |
| Al0.5 gel | 402 ± 10 | 383 ± 20 | (2.5 ± 0.7) × 10−2 | 0.97 | 8 ± 2 | 190 ± 29 | 0.95 | 459 ± 22 | (5.0 ± 0.1) × 10−3 | 10 ± 1 | 0.95 |
| Al0.5-ox | 111.5 | 117 ± 3 | (1.3 ± 0.2) × 10−2 | 0.99 | 8 ± 3 | 58 ± 12 | 0.97 | 154 ± 9 | 6.7 ± 0.2 | 0.30 ± 0.02 | 0.99 |
| Al0.5-ac | 150 ± 5 | 145 ± 28 | (8.4 ± 0.8) × 10−2 | 0.99 | 9 ± 1 | 84 ± 6 | 0.98 | 182 ± 33 | 9.6 ± 0.5 | 0.20 ± 0.04 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golubeva, O.Y.; Brazovskaya, E.Y.; Alikina, Y.A. Influence of the Preparation Method on the Physico-Chemical and Sorption Properties of Montmorillonite. Ceramics 2023, 6, 922-934. https://doi.org/10.3390/ceramics6020054
Golubeva OY, Brazovskaya EY, Alikina YA. Influence of the Preparation Method on the Physico-Chemical and Sorption Properties of Montmorillonite. Ceramics. 2023; 6(2):922-934. https://doi.org/10.3390/ceramics6020054
Chicago/Turabian StyleGolubeva, Olga Yu., Elena Yu. Brazovskaya, and Yulia A. Alikina. 2023. "Influence of the Preparation Method on the Physico-Chemical and Sorption Properties of Montmorillonite" Ceramics 6, no. 2: 922-934. https://doi.org/10.3390/ceramics6020054
APA StyleGolubeva, O. Y., Brazovskaya, E. Y., & Alikina, Y. A. (2023). Influence of the Preparation Method on the Physico-Chemical and Sorption Properties of Montmorillonite. Ceramics, 6(2), 922-934. https://doi.org/10.3390/ceramics6020054