Abstract
Over the past decades, Na0.5Bi0.5TiO3 (NBT)-based ceramics have received increasing attention in energy storage applications due to their high power density and relatively large maximum polarization. However, their high remnant polarization (Pr) and low breakdown field strength are detrimental for their practical applications. In this paper, a new solid solution (1−x)Na0.5Bi0.5TiO3–xCaHfO3 (x = 0.04, 0.08, 0.12, 0.16) was constructed by introducing CaHfO3 into NBT, and thus was prepared using a conventional solid-state reaction. With the addition of CaHfO3, the disorder of the structure increased, A-site vacancies formed, and thus oxygen vacancies were suppressed due to the replacement of the Na+ by Ca2+, resulting in the enhanced relaxation behavior and the reduced Pr, the refined grain, and improved breakdown strength. Furthermore, an optimal recoverable energy storage density (Wrec) of 1.2 J/cm3 was achieved in 0.92Na0.5Bi0.5TiO3–0.08CaHfO3 ceramics under the breakdown strength of 140 kV/cm, which is mainly attributed to the resultant defect of Na+ vacancy.
1. Introduction
Due to their high power density and quick charge/discharge rate, dielectric capacitors have drawn more attention in recent decades in the field of energy and electricity, and electronic circuit systems [1,2]. Among them, ceramic-based dielectric materials have high breakdown field strength (Eb), low dielectric loss (tan(δ)), excellent fatigue resistance, and superior thermal stability, making them suitable in a variety of industries, including in oil exploration, aerospace, and military applications [3,4,5]. Usually, dielectric ceramic materials with large maximum polarization (Pmax), small remnant polarization (Pr) and high Eb are perfect for creating energy storage capacitors, and energy storage performance can be evacuated by using the polarization-electric field (P-E) loop according to the subsequent Equations (1)–(3).
where E, Wtot, Wrec, Wloss, and η denote the applied electric field, total energy storage density, recoverable energy storage density, energy loss density, and energy storage efficiency, respectively.
Although Pb-based ceramics exhibit excellent energy storage performance due to their larger Pmax and higher Eb, the resultant heavy metal Pb due to the volatilization during high-temperature preparation process and the subsequent scrap of products all pose risks to the ecological environment and people’s health [6,7]. Therefore, designing and researching lead-free materials are considered a feasible solution to solve this problem. In numerous lead-free dielectric ceramics, Na0.5Bi0.5TiO3 (NBT) based ceramics have attracted much attention on account of high Curie temperature (Tc) (~320 °C) and large saturation polarization strength (Ps, 45 µC/cm2) [8]. However, the energy storage properties of NBT ceramics were dissatisfied because of the high Pr (~38 µC/cm2). In addition, the elements Bi and Na are easily volatile during the process of high-temperature sintering, which may lead to the generation of cationic vacancies and oxygen vacancies, and thus the Eb reduced [9,10]. As a consequence, many researchers have focused on the settlement of these issues [11,12,13,14,15,16,17,18,19,20,21]. Thereinto, Yan et al. [12] prepared 0.75Bi(0.5+x)Na(0.5−x)TiO3–0.25SrTiO3 ceramics by defect engineering and suppressed the formation of oxygen vacancies by adjusting the non-stoichiometric ratio of the A-site, as well as refined the grains size to increase Eb significantly. Eventually, the outstanding Wrec (~5.63 J/cm3) and the relatively high η (~94%) were achieved under 569 kV/cm as x = 0.08. The 0.9(Na0.4Bi0.4Ba0.06Sr0.14Ti(1−x)TaxO3)–0.1NaNbO3 ceramics designed and prepared by Jiang et al. [13] effectively suppressed oxygen vacancies and increased the Eb through defect engineering and finally realized a relative high Wrec (~3.12 J/cm3) and excellent η (~87.68%). By replacing Bi3+ and Na+ at A-site with La3+ to increase the concentration of cation vacancies and decrease oxygen vacancies, the 0.85NBT–0.15CaTiO3–0.75La ceramics showed the improved energy storage performance with Wrec of ~2.15 J/cm3 and η of ~81% [16]. Apparently, the defect engineering modulation is an effective way to improve the energy storage performance of NBT-based ceramics.
In this study, by incorporating CaHfO3 into NBT ceramics from a defect engineering perspective, a binary solid solution Na0.5Bi0.5TiO3–CaHfO3 was designed. On the one hand, the introduction of Ca2+ at A-site may induce the cation vacancies and thus significantly suppress oxygen vacancies, along with Hf4+ replacing Ti4+ in the B-site with a relatively strong bond strength of Hf–O, ultimately all improving the Eb. On the other hand, due to the incorporation of non-equivalent cations as Na+, Ca2+, and Bi3+, the creation of a local random field may break the long-range order ferroelectric domain and facilitate the formation of polar nanoregions (PNRs), which contributed to the decrease of Pr [22,23,24]. On the basis of the above analysis, (1−x)Na0.5Bi0.5TiO3–xCaHfO3 (NBT–xCaHfO3, x = 0.04, 0.08, 0.12, 0.16) solid solution was prepared using the conventional solid state method, and the phase structure, microstructure, dielectric properties and energy storage characteristics were investigated in detail. The ceramics designed and fabricated using this strategy are expected to be used in the field of pulsed power capacitors.
2. Experimental Procedures
(1−x)Na0.5Bi0.5TiO3–xCaHfO3 (NBT−xCaHfO3, x = 0.04, 0.08, 0.12, 0.16) ceramics were prepared using a conventional solid phase method [25,26]. The starting materials including Na2CO3 (analytical reagent (AR), ≥ 99.8%), Bi2O3 (AR, ≥ 99.9%), TiO2 (chemically pure, ≥ 98.0%), CaCO3 (AR, ≥ 99.0%), and HfO2 (AR, ≥ 98.0%), which were all from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, were weighed according to the stoichiometric ratio of their components. The weighed powders were ball milled for 8 h by using the alcohol as medium. After that, the mixture was dried in an oven before calcination in a muffle furnace at 850 °C for 2 h. Following that, the powder was ball milled for another 6 h, and then was dried and sieved. The sieved powder was granulated with 6% polyvinyl alcohol (PVA) and then pressed into disks with a diameter of 13 mm under a pressure of 300 MPa. Finally, the disks were heated to 550 °C at the speed of 3 °C/min for 3 h to remove the PVA, and then sintered at 1100–1140 °C for 2 h at the rate of 5 °C/min to obtain the final ceramic samples.
X-ray diffraction (XRD, D8 Advanced, Bruker AXS Ltd., Karlsruhe, Germany) was used to analyze the crystal structure of the ceramic samples with diffraction angle 2θ in the range of 20–80°, and the accurate crystal structure of the ceramic materials were obtained using Rietveld full diffraction patterns fitting. The microstructures of the ceramic samples were observed using field emission scanning electron microscopy (SEM, S-4800, Hitachi, Japan). As for electrical testing, the ceramic samples were polished into a thickness of ~1 mm, covered with silver paste on the top and bottom surfaces, and then were fired at 850 °C for 10 min to form the electrodes. The LCR impedance meter (E4294A, Agilent, Santa Clara, USA) was used to measure the dielectric properties in the temperature range of 25–400 °C with a frequency in the range of 1 kHz to 1 MHz. In addition, the P-E curves of ceramic samples under different electric field strength were tested using a ferroelectric analyzer (TF2000, AixACCT, Aachen, Germany).
3. Results and Discussions
The XRD patterns of the NBT–xCaHfO3 (x = 0.04, 0.08, 0.12, 0.16) ceramics sintered at the optimum temperature are shown in Figure 1a. As can be seen from this Figure, the X-ray diffraction patterns of all ceramic component exhibited a single perovskite structure, and no second phases were detected, which indicated that CaHfO3 was completely diffused into the NBT lattice and formed a perfect solid solution. Figure 1b,c present the magnified plots of the diffraction peaks near 40.2° and 46.8° to further investigate the structural evolution of the ceramics. It can be seen that a significant splitting presented in both peaks near 40.2° and 46.8°, confirming that all ceramics were in the coexistence of the rhombohedral phase (R3c) and the tetragonal phase (P4bm) at room temperature [27]. The two split diffraction peaks weakened and merged gradually when CaHfO3 content increased, which demonstrated that the coexistence of R3c and P4bm phases had evolved into a pseudo-cubic phase [28,29]. In addition, as CaHfO3 doping content increased, the diffraction peaks were first shifted to higher angles (in particular, x = 0.12 is the most prominent), confirming the decreased cell volumes owing to the smaller radius Ca2+ (1.34 Å) replacing the larger radius Na+ (1.39 Å). However, due to the limited solid solubility of the A-site, when CaHfO3 doping content exceeds the threshold, the Ti4+ (0.605 Å) with a smaller radius at the B-site could be replaced by Ca2+ (1.34 Å) [30], and at the same time, more Hf4+ (0.71 Å) with a larger radius enters the B-site to replace Ti4+, resulting in lattice volume expansion and diffraction peaks shifting towards small angles. In addition, the bulk density of NBT–xCaHfO3 (x = 0.04, 0.08, 0.12, 0.16) ceramics sintered at different temperatures was plotted in Figure 1d. The bulk density of NBT–xCaHfO3 increased initially with increasing sintering temperatures and then decreased after reaching the maximum value. It should be noted that the sintering temperature of the maximum bulk density was 1120 °C for all the different compositions.
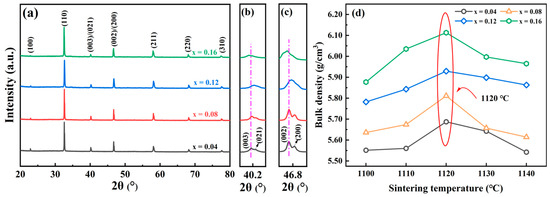
Figure 1.
(a) XRD pattern of NBT–xCaHfO3 (x = 0.04, 0.08, 0.12, 0.16) ceramics. The magnified view at 2θ (b) near 40.2° and (c) near 46.8°. (d) The bulk density of NBT–xCaHfO3 ceramics sintered at different temperatures.
In order to further analyze the effect of CaHfO3 doping on the phase structure and lattice parameters of the NBT–xCaHfO3 ceramics, Figure 2 presents the Rietveld refinement of the full-scan XRD diffraction patterns based on the R3c and P4bm phase, and the corresponding phase proportion, lattice parameters, and reliability factors are shown in Table 1. The reliability factors of weighted patterns (Rwp) and the goodness-of-fit indicator (χ2) of all component ceramics are within the confidence interval, confirming that the results of XRD refinement are relatively reliable. Clearly, the fraction of the P4bm phase first increased and then decreased with the increase in CaHfO3 content, as x = 0.08 reached the maximum. Usually, P4bm as the weakly polar phase could be converted into a polar ferroelectric state under an electric field and return to the original weakly polar state when the electric field was removed, hence the enhancement proportion of the P4bm phase could contribute to the reduction in Pr and thus promote the energy storage properties [31,32]. It can be assumed that the NBT–0.08CaHfO3 ceramics may have optimal energy storage performance accompanied with relatively low Pr.
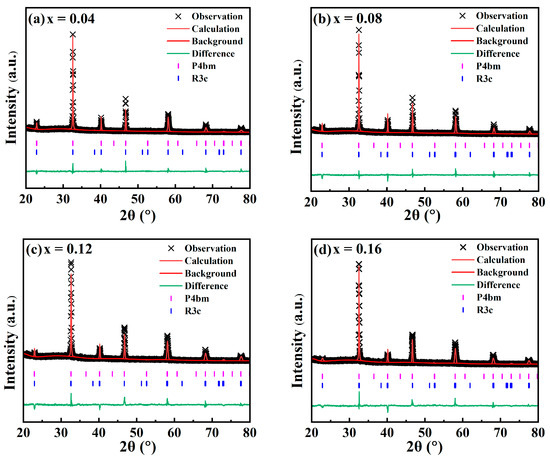
Figure 2.
Rietveld Refined XRD Patterns of NBT–xCaHfO3 ceramics: (a) x = 0.4, (b) x = 0.08, (c) x = 0.12, and (d) x = 0.16.

Table 1.
The refinement results from full-diffraction pattern fitting of NBT–xCaHfO3 ceramics.
Figure 3 shows the natural surface micrographs of NBT–xCaHfO3 ceramics. All samples present clear grain morphology and a relatively dense microstructure. In order to further investigate the effect of CaHfO3 doping on the microscopic morphology of NBT ceramics, the average grain (AG) sizes of each component ceramics were analyzed, as shown in Figure 4. The average grain size of the ceramics decreased from 3.14 μm for x = 0.04 to 1.35 μm for x = 0.12, verifying that the addition of CaHfO3 could refine grain size to a certain extent. Since Ca2+ tended to displace Na+ at A-site due to the similar ionic radii (RCa2+ = 1.34 Å, RNa+ = 1.39 Å), and thus produced cation defects with positive charges, denoted as
[32,33], and Na+ vacancies (expressed as V′Na) for charge compensation and for maintaining electrical neutrality. Consequently, the oxygen vacancy (), created by loss of oxygen from the crystal lattice at low oxygen partial pressure during sintering at high temperatures had to be inhabited [13,34,35], which further suppressed the conductivity and refined the grain size as well as beneficial to the enhancement of the Eb [36,37]. Furthermore, with the CaHfO3 increasing, more V′Na favor the mass transfer in ceramics during sintering [13,38], and as a consequence, the grain size increased up to 1.40 μm when x = 0.16. The equation for the defect is as follows (Equations (4) and (5)), in which the Kröger–Vink notation was employed.
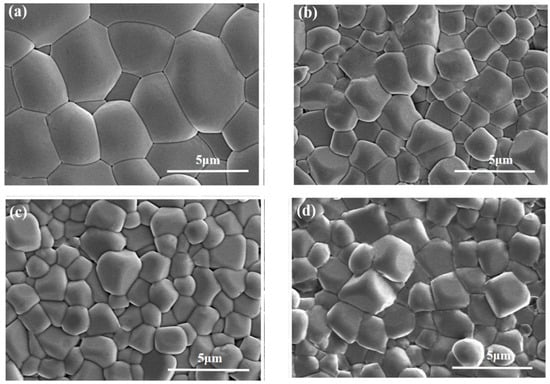
Figure 3.
SEM images of NBT–xCaHfO3 ceramics: (a) x = 0.4, (b) x = 0.08, (c) x = 0.12, and (d) x = 0.16.
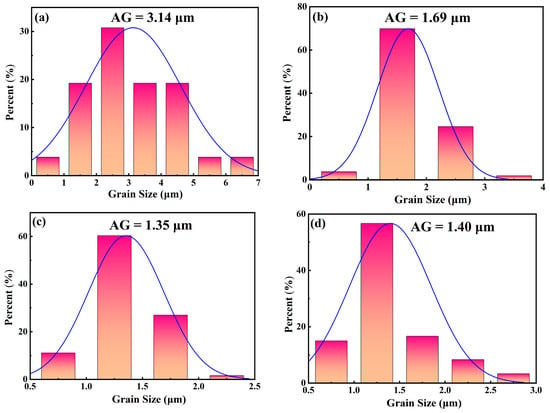
Figure 4.
Grain size distribution diagram of NBT–xCaHfO3 ceramics: (a) x = 0.4, (b) x = 0.08, (c) x = 0.12, (d) x = 0.16.
Figure 5 shows the variation curves of dielectric constant (εr) and dielectric loss (tan(δ)) under different frequency for NBT–xCaHfO3 ceramics. The εr of all ceramics presents a decreasing trend to a different extent with the frequency increased, which may be due to the inability of defect dipoles to respond at high frequencies [39,40]. Besides that, when the CaHfO3 content increased, εr at the frequency of 10 kHz decreased from 811 for the composition of x = 0.04 to 606 (x = 0.08) and then increased sharply to 1066 (x = 0.12), accomplished by the minimum dielectric loss (tan(δ) less than 0.022) emerging in the composition of x = 0.08 in a wide range of frequencies from 1k Hz to 1 MHz, implying that the appropriate amount of CaHfO3 can reduce the concentration of oxygen vacancies and decrease the dielectric loss, which is conducive to the enhancement of the breakdown field strength. Figure 6 illustrates the temperature-dependent dielectric properties of NBT–xCaHfO3 (x = 0.04, 0. 08, 0.12, and 0.16) ceramics at various frequencies. The dielectric anomaly peak (Tm) clearly observed refers to the ferro-paraelectric phase transition [41,42]. It is noteworthy that with the increase of CaHfO3 content, the dielectric peak became flatter and broader, accompanied by the Tm shifting to the lower temperature, which may be caused by the diminished coupling effect of [TiO6] octahedron and weakened structure stability, since A-site vacancies (V′Na) formed as excessively Ca2+ doped [43,44]. In the meantime, as the frequency increased from 1 kHz to 1 MHz, εr decreased steadily and Tm shifted to the higher temperature, manifesting a relaxed behavior to a certain extent [45]. To further analyze the effect of CaHfO3 content on the degree of relaxation, the diffuseness parameter γ between 1 (normal ferroelectric) and 2 (ideal relaxer) was calculated using the modified Curie–Weiss equation (Equation (6)) [46,47,48]:
where C represents the Curie–Weiss constant and εm is the dielectric constant corresponding to Tm. The γ obtained by fitting the curves between ln(T−Tm) and ln((1/εr)−(1/εm)) at 1 kHz, as shown in Figure 7, increased from 1.44 (x = 0.04) to 1.63 (x = 0.08) and then decreased to 1.57 (x = 0.12) and 1.54 (x = 0.16), hinting that the optimal relaxation characteristics can be obtained in the composition of x = 0.08. The optimizing relaxation performance may be related to the increased ionic disorder in the microstructure, where the non-equivalent substitution of Ca2+ for Na+ and Bi3+ broke the long-range ferroelectric domains and facilitated the formation of polar nanoregions [49,50,51].
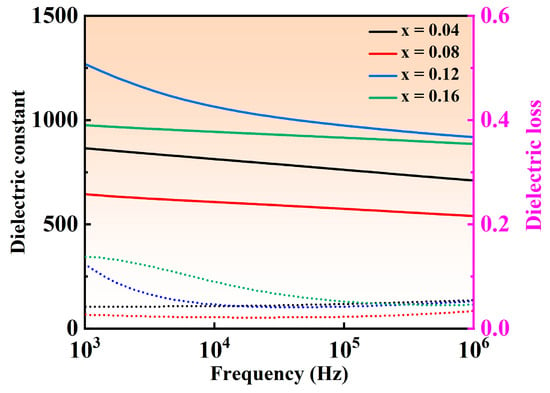
Figure 5.
Frequency-dependent dielectric properties of NBT–xCaHfO3 (x = 0.04, 0.08, 0.12, 0.16) ceramics at room temperature. Solid lines signify dielectric constant and dashed lines denote dielectric loss.
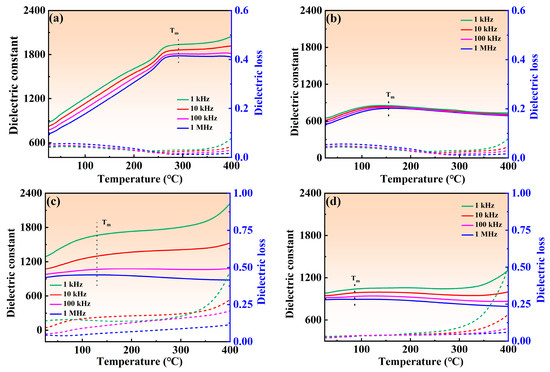
Figure 6.
Temperature-dependent dielectric properties of NBT–xCaHfO3 ceramics under various frequencies: (a) x = 0.04, (b) x = 0.08, (c) x = 0.12, and (d) x = 0.16. Solid lines signify dielectric constant and dashed lines denote dielectric loss.
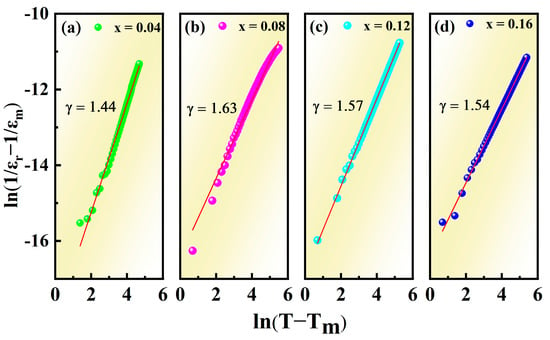
Figure 7.
ln((1/εr)−(1/εm)) as a function of ln(T−Tm) for NBT–xCaHfO3 ceramics: (a) x = 0.04, (b) x = 0.08, (c) x = 0.12, and (d) x = 0.16. (solid lines—fitting to Equation (6)).
Figure 8 displays the P-E hysteresis loops of NBT–xCaHfO3 ceramics under the electric fields of 120 kV/cm. Evidently, as CaHfO3 content increases, the P-E loops become slimmer, especially for the NBT–0.08CaHfO3 ceramics, which is in agreement with the result of diffuseness parameter γ. The slender P-E loops can be mainly ascribed to the broken long-range ferroelectric domain as the non-equivalent substitution of Na+ and Bi3+ by Ca2+. Thus, the formed polar microregions suppressed the early polarization saturation of the ceramics and reduced the Pr, which facilitated the achievement of excellent energy storage performance [24,52]. In addition, Figure 9 displays the P-E hysteresis loops of NBT–xCaHfO3 ceramics under various electric fields. Apparently, P-E loops of all composition turn more slender with increasing applied electric filed, among which Pmax and ∆P (∆P = Pmax−Pr) of the composition with x = 0.08 increases significantly, while Pr increases slightly. Ultimately, under an electric fields of 140 kV/cm, Pmax and ∆P reach their maximums, as displayed in the inset of Figure 9b.
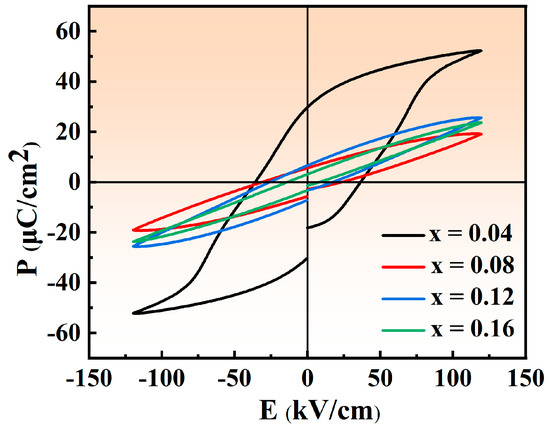
Figure 8.
P-E curves of NBT–xCaHfO3 ceramic samples under the electric field of 120 kV/cm.
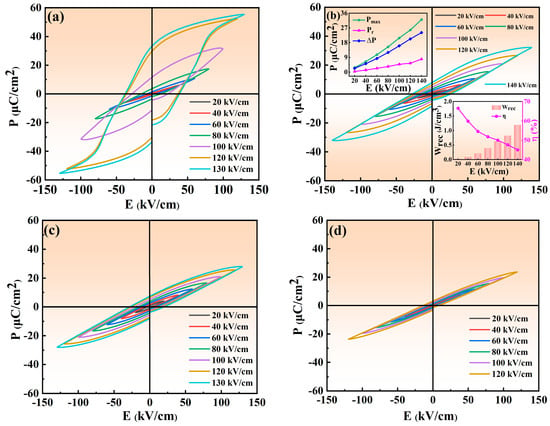
Figure 9.
P-E curves of NBT–xCaHfO3 ceramic samples under different electric field: (a) x = 0.04, (b) x = 0.08, (c) x = 0.12, and (d) x = 0.16.
The improvement of Wrec requires not only high Pmax and low Pr, but also high applied field and, subsequently, the P-E hysteresis loops of NBT–xCaHfO3 ceramics at 20 Hz under critical electric fields are displayed in Figure 10a. Compared to NBT–xCaHfO3 ceramics with x = 0.04, 0.12, and 0.16, the NBT–0.08CaHfO3 ceramics demonstrated the maximum Eb value of 140 kV/cm because of the dense microstructure and relatively small grain size (in Figure 3). The variation of Wrec, η and Pmax, Pr, and ∆P for each ceramic under a critical electric field are shown in Figure 10b,c, and the relevant parameters are listed in Table 2. Overall, with the increase in the CaHfO3 content, the Pmax decreased from 55.39 μC/cm2 (x = 0.04) to 23.66 μC/cm2 (x = 0.16) together with Pr maintaining in a relatively low value. Finally, the ∆P attained its maximum for NBT–0.08CaHfO3 ceramics, achieving the optimizing energy storage performance with Wrec of 1.2 J/cm3 and η of 45%. Also, the energy storage performance and polarization properties of NBT–0.08CaHfO3 ceramics under various electric fields from 20 kV/cm to 140 kV/cm are demonstrated in the inset of Figure 9b. Apparently, ∆P enhanced approximately linearly from 2.33 μC/cm2 to 24.12 μC/cm2, with the increase in applied electric field as a result of the significant increase of Pmax along with a slight increase of Pr. The comparatively large ΔP and Eb facilitated excellent energy storage properties. Hence, the Wrec of 1.20 J/cm3 was highlighted in 0.92NBT–0.08CaHfO3 ceramics under the maximum electric field of 140 kV/cm, indicating certain potential in energy storage application.
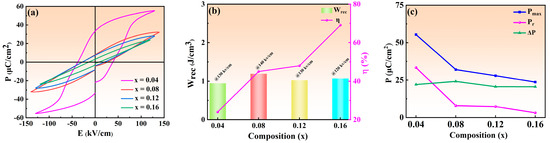
Figure 10.
(a) P-E curves, (b) Wrec and η, (c) Pmax, Pr, and ∆P of NBT–xCaHfO3 ceramic samples under critical electric field.

Table 2.
Pmax, Pr, Wrec, and η of NBT–xCaHfO3 ceramics under the critical electric field.
4. Conclusions
NBT–xCaHfO3 (x = 0.04, 0.08, 0.12, 0.16) ceramics were prepared using a conventional solid phase method. The introduction of Ca2+ substituting for Na+ and Bi3+ at the A-site induced the cation vacancies and then suppressed the concentration of oxygen vacancies as well as refined the grain size, associated with Hf 4+ replacing Ti4+ in the B-site with a relatively strong bond strength of Hf–O, eventually fulfilling the improvement of the Eb. Besides that, a local random field created by the incorporation of non-equivalent cations as Na+, Ca2+, Bi3+, and cation vacancies, as well as bond strength of Hf–O, broke the long-range order ferroelectric domain and facilitated the formation of PNRs, resulting in an enhancement of relaxation properties and a decrease in Pr. Combined with enhanced relaxation behavior and increased Eb, an optimizing Wrec of 1.2 J/cm3 was achieved in NBT–0.08CaHfO3 ceramics under the Eb of 140 kV/cm. All of these demonstrated that the NBT–0.08CaHfO3 ceramics have an attractive potential in the field of energy storage ceramics.
Author Contributions
Conceptualization, Z.L.; methodology, J.Z.; formal analysis, Z.L. and D.L.; investigation, X.W., D.L. and X.Z.; resources, Y.N.; data curation, X.Z. and Y.N.; writing—original draft, J.Z.; writing—review and editing, Z.L. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities, CHD (No. 300102314904), the Natural Science Foundation of Shaanxi province, China (No. 2021JM-172) and the National Natural Science Foundations of China (Grant No. 52278427).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, Z.; Du, H.; Jin, L.; Poelman, D. High-Performance Lead-Free Bulk Ceramics for Electrical Energy Storage Applications: Design Strategies and Challenges. J. Mater. Chem. A 2021, 9, 18026–18085. [Google Scholar] [CrossRef]
- Palneedi, H.; Peddigari, M.; Hwang, G.; Jeong, D.; Ryu, J. High-Performance Dielectric Ceramic Films for Energy Storage Capacitors: Progress and Outlook. Adv. Funct. Mater. 2018, 28, 1803665. [Google Scholar] [CrossRef]
- Yuan, Q.; Chen, M.; Zhan, S.; Li, Y.; Lin, Y.; Yang, H. Ceramic-Based Dielectrics for Electrostatic Energy Storage Applications: Fundamental Aspects, Recent Progress, and Remaining Challenges. Chem. Eng. J. 2022, 446, 136315. [Google Scholar]
- Hu, Q.; Tian, Y.; Zhu, Q.; Bian, J.; Jin, L.; Du, H.; Alikin, D.O.; Shur, V.Y.; Feng, Y.; Xu, Z.; et al. Achieve Ultrahigh Energy Storage Performance in BaTiO3–Bi(Mg1/2Ti1/2)O3 Relaxor Ferroelectric Ceramics via Nano-Scale Polarization Mismatch and Reconstruction. Nano Energy 2020, 67, 104264. [Google Scholar]
- Muhammad, R.; Iqbal, Y.; Reaney, I.M. BaTiO3–Bi(Mg2/3Nb1/3)O3 Ceramics for High-Temperature Capacitor Applications. J. Am. Ceram. Soc. 2016, 99, 2089–2095. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Yang, T.; Zhang, S. Ultrahigh Energy-Storage Density in Antiferroelectric Ceramics with Field-Induced Multiphase Transitions. Adv. Funct. Mater. 2019, 29, 1807321. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Q.; Liu, X.; Zheng, Q.; Jiang, N.; Yang, Y.; Kwok, K.W.; Xu, C.; Lin, D. A High-Tolerance BNT-Based Ceramic with Excellent Energy Storage Properties and Fatigue/Frequency/Thermal Stability. Ceram. Int. 2019, 45, 23233–23240. [Google Scholar]
- Wang, G.; Lu, Z.; Li, Y.; Li, L.; Ji, H.; Feteira, A.; Zhou, D.; Wang, D.; Zhang, S.; Reaney, I.M. Electroceramics for High-Energy Density Capacitors: Current Status and Future Perspectives. Chem. Rev. 2021, 121, 6124–6172. [Google Scholar] [CrossRef] [PubMed]
- Höfling, M.; Steiner, S.; Hoang, A.-P.; Seo, I.-T.; Frömling, T. Optimizing the Defect Chemistry of Na1/2Bi1/2TiO3 -Based Materials: Paving the Way for Excellent High Temperature Capacitors. J. Mater. Chem. C 2018, 6, 4769–4776. [Google Scholar] [CrossRef]
- Carter, J.; Aksel, E.; Iamsasri, T.; Forrester, J.S.; Chen, J.; Jones, J.L. Structure and Ferroelectricity of Nonstoichiometric (Na0.5Bi0.5)TiO3. Appl. Phys. Lett. 2014, 104, 112904. [Google Scholar] [CrossRef]
- Yang, H.; Cai, Z.; Zhu, C.; Feng, P.; Wang, X. Ultra-High Energy Storage Performance in BNT-Based Ferroelectric Ceramics with Simultaneously Enhanced Polarization and Breakdown Strength. ACS Sustain. Chem. Eng. 2022, 10, 9176–9183. [Google Scholar] [CrossRef]
- Yan, F.; Huang, K.; Jiang, T.; Zhou, X.; Shi, Y.; Ge, G.; Shen, B.; Zhai, J. Significantly Enhanced Energy Storage Density and Efficiency of BNT-Based Perovskite Ceramics via A-Site Defect Engineering. Energy Storage Mat. 2020, 30, 392–400. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, H.; Cao, L.; Yang, Z.; Yuan, Y.; Li, E. Enhanced Breakdown Strength and Energy Storage Density of Lead-Free Bi0.5Na0.5TiO3-Based Ceramic by Reducing the Oxygen Vacancy Concentration. Chem. Eng. J. 2021, 414, 128921. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, R.; Huang, Y.; Hu, Q.; Alikin, D.O.; Shur, V.Y.; Gao, J.; Wei, X.; Zhang, L.; Liu, G.; et al. Enhanced Antiferroelectric-like Relaxor Ferroelectric Characteristic Boosting Energy Storage Performance of (Bi0.5Na0.5)TiO3-Based Ceramics via Defect Engineering. J. Mat. 2022, 8, 527–536. [Google Scholar] [CrossRef]
- Liu, G.; Dong, J.; Zhang, L.; Yu, L.; Wei, F.; Li, Y.; Gao, J.; Hu, J.; Yan, Y.; Li, Q.; et al. Na0.25Sr0.5Bi0.25TiO3 Relaxor Ferroelectric Ceramic with Greatly Enhanced Electric Storage Property by a B-Site Ion Doping. Ceram. Int. 2020, 46, 11680–11688. [Google Scholar] [CrossRef]
- Yu, L.; Dong, J.; Tang, M.; Liu, Y.; Wu, F.; Yan, Y.; Liu, G.; Song, C. Enhanced Electrical Energy Storage Performance of Pb-Free A-Site La3+-Doped 0.85Na0.5Bi0.5TiO3-0.15CaTiO3 Ceramics. Ceram. Int. 2020, 46, 28173–28182. [Google Scholar] [CrossRef]
- Li, T.; Chen, P.; Si, R.; Li, F.; Guo, Y.; Wang, C. High Energy Storage Density and Efficiency with Excellent Temperature and Frequency Stabilities under Low Operating Field Achieved in Ag0.91Sm0.03NbO3-Modified Na0.5Bi0.5TiO3-BaTiO3 Ceramics. J. Mater. Sci. Mater. Electron. 2020, 31, 16928–16937. [Google Scholar] [CrossRef]
- Liu, X.; Rao, R.; Shi, J.; He, J.; Zhao, Y.; Liu, J.; Du, H. Effect of Oxygen Vacancy and A-Site-Deficiency on the Dielectric Performance of BNT-BT-BST Relaxors. J. Alloys Compd. 2021, 875, 159999. [Google Scholar] [CrossRef]
- Chen, Z.; Pu, Y.; Ning, Y.; Hui, Y.; Wu, C.; Zhang, L.; Zhang, X.; Wang, B. Prominent Energy Storage Density and Efficiency of Na0.5Bi0.5TiO3 -based Ceramics via Multiscale Amelioration Strategy. J. Am. Ceram. Soc. 2024, 107, 2360–2370. [Google Scholar] [CrossRef]
- Zhao, H.; Cao, W.; Liang, C.; Wang, C.; Wang, C. High Energy-Storage Performance in X9R-Type Na0.5Bi0.5TiO3-Based Lead-Free Ceramics. Ceram. Int. 2023, 49, 15939–15946. [Google Scholar] [CrossRef]
- Li, T.; Jiang, X.; Li, J.; Xie, A.; Fu, J.; Zuo, R. Ultrahigh Energy-Storage Performances in Lead-Free Na0.5Bi0.5TiO3 -Based Relaxor Antiferroelectric Ceramics through a Synergistic Design Strategy. ACS Appl. Mater. Interfaces 2022, 14, 22263–22269. [Google Scholar] [CrossRef] [PubMed]
- Roukos, R.; Zaiter, N.; Chaumont, D. Relaxor Behaviour and Phase Transition of Perovskite Ferroelectrics-Type Complex Oxides (1–x)Na0.5Bi0.5TiO3–xCaTiO3 System. J. Adv. Ceram. 2018, 7, 124–142. [Google Scholar] [CrossRef]
- Luo, C.; Feng, Q.; Luo, N.; Yuan, C.; Zhou, C.; Wei, Y.; Fujita, T.; Xu, J.; Chen, G. Effect of Ca2+/Hf4+ Modification at A/B Sites on Energy-Storage Density of Bi0.47Na0.47Ba0.06TiO3 Ceramics. Chem. Eng. J. 2021, 420, 129861. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Zhao, R.; Tang, X.; Zhang, S.; Zhang, Y.; Hu, J.; Shen, Z.; Jiang, Y.; Guo, X. High Energy Storage Density Obtained by Bi(Ni0.5Hf0.5)O3-Modified NBT-Based Ceramic at a Low Electric Field. Ceram. Int. 2024, 50, 5276–5284. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Wang, C.; Wang, Z.; Lei, N.; Zheng, L.; Long, D.; Wei, X.; Zhang, J.; Wang, Z.; et al. Phase Structure, Dielectric and Energy Storage Properties of Na0.5Bi0.5TiO3-BaTiO3 Ceramics with Bi(Mg2/3Nb1/3)O3 Modification. Ceram. Int. 2023, 49, 38735–38742. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, Y.; Chen, M.; Wei, T.; Keipper, W.; Shi, R.; Guo, X.; Li, R.; Peng, X. High Energy-Storage Density under Low Electric Fields and Improved Optical Transparency in Novel Sodium Bismuth Titanate-Based Lead-Free Ceramics. J. Eur. Ceram. Soc. 2020, 40, 71–77. [Google Scholar] [CrossRef]
- Yang, F.; Bao, S.; Zhai, Y.; Zhang, Y.; Su, Z.; Liu, J.; Zhai, J.; Pan, Z. Enhanced Energy-Storage Performance and Thermal Stability in Bi0.5Na0.5TiO3-Based Ceramics through Defect Engineering and Composition Design. Mater. Today Chem. 2021, 22, 100583. [Google Scholar] [CrossRef]
- Qiao, X.; Wu, D.; Zhang, F.; Niu, M.; Chen, B.; Zhao, X.; Liang, P.; Wei, L.; Chao, X.; Yang, Z. Enhanced Energy Density and Thermal Stability in Relaxor Ferroelectric Bi0.5Na0.5TiO3-Sr0.7Bi0.2TiO3 Ceramics. J. Eur. Ceram. Soc. 2019, 39, 4778–4784. [Google Scholar] [CrossRef]
- Yang, L.; Kong, X.; Cheng, Z.; Zhang, S. Ultra-High Energy Storage Performance with Mitigated Polarization Saturation in Lead-Free Relaxors. J. Mater. Chem. A 2019, 7, 8573–8580. [Google Scholar] [CrossRef]
- Zhao, P.; Tang, B.; Si, F.; Yang, C.; Li, H.; Zhang, S. Novel Ca Doped Sr0.7Bi0.2TiO3 Lead-Free Relaxor Ferroelectrics with High Energy Density and Efficiency. J. Eur. Ceram. Soc. 2020, 40, 1938–1946. [Google Scholar] [CrossRef]
- Han, J.; Yin, J.; Wu, J. BNT-based Ferroelectric Ceramics: Electrical Properties Modification by Ta2O5 Oxide Addition. J. Am. Ceram. Soc. 2019, 103, 412–422. [Google Scholar] [CrossRef]
- Fan, J.; He, G.; Cao, Z.; Cao, Y.; Long, Z.; Hu, Z. Ultrahigh Energy-Storage Density of a Lead-Free 0.85Bi0.5Na0.5TiO3 –0.15Ca(Nb0.5Al0.5)O3 Ceramic under Low Electric Fields. Inorg. Chem. Front. 2023, 10, 1561–1573. [Google Scholar] [CrossRef]
- Xie, A.; Fu, J.; Zuo, R.; Zhou, C.; Qiao, Z.; Li, T.; Zhang, S. NaNbO3-CaTiO3 Lead-Free Relaxor Antiferroelectric Ceramics Featuring Giant Energy Density, High Energy Efficiency and Power Density. Chem. Eng. J. 2022, 429, 132534. [Google Scholar] [CrossRef]
- Prasertpalichat, S.; Cann, D.P. Hardening in Non-Stoichiometric (1−x)Bi0.5Na0.5TiO3–xBaTiO3 Lead-Free Piezoelectric Ceramics. J. Mater. Sci. 2016, 51, 476–486. [Google Scholar] [CrossRef]
- Kumar, N.; Ansell, T.Y.; Cann, D.P. Role of Point Defects in Bipolar Fatigue Behavior of Bi(Mg1/2Ti1/2)O3 Modified (Bi1/2K1/2)TiO3-(Bi1/2Na1/2)TiO3 Relaxor Ceramics. J. Appl. Phys. 2014, 115, 154104. [Google Scholar] [CrossRef]
- Li, M.; Pietrowski, M.J.; De Souza, R.A.; Zhang, H.; Reaney, I.M.; Cook, S.N.; Kilner, J.A.; Sinclair, D.C. A Family of Oxide Ion Conductors Based on the Ferroelectric Perovskite Na0.5Bi0.5TiO3. Nat. Mater. 2014, 13, 31–35. [Google Scholar] [CrossRef]
- Malik, R.A.; Hussain, A.; Maqbool, A.; Zaman, A.; Song, T.K.; Kim, W.-J.; Kim, M.-H. Giant Strain, Thermally-Stable High Energy Storage Properties and Structural Evolution of Bi-Based Lead-Free Piezoceramics. J. Alloys Compd. 2016, 682, 302–310. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, Y.; Shi, P.; Kang, R.; Kang, F.; Qiao, W.; Zhao, J.; Wang, Z.; Yuan, Y.; Lou, X. Ultrahigh Energy Storage Density in (Bi0.5Na0.5)0.65Sr0.35TiO3-Based Lead-Free Relaxor Ceramics with Excellent Temperature Stability. Nano Energy 2022, 98, 107276. [Google Scholar] [CrossRef]
- Ding, J.; Pan, Z.; Chen, P.; Hu, D.; Yang, F.; Li, P.; Liu, J.; Zhai, J. Enhanced Energy Storage Capability of (1-x)Na0.5Bi0.5TiO3-xSr0.7Bi0.2TiO3 Free-Lead Relaxor Ferroelectric Thin Films. Ceram. Int. 2020, 46, 14816–14821. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, X.; Zhang, L. Enhanced Energy-Storage Performances of Bi2O3–Li2O Added (1−x)(Na0.5Bi0.5)TiO3–xBaTiO3 Thick Films. Ceram. Int. 2014, 40, 8847–8851. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, H.; Li, X.; Zeng, F.; Wu, K.; Zheng, Q.; Fan, G.; Lin, D. Enhanced Energy Density and Discharged Efficiency of Lead-Free Relaxor (1−x)[(Bi0.5Na0.5)0.94Ba0.06]0.98La0.02TiO3-xKNb0.6Ta0.4O3 Ceramic Capacitors. Chem. Eng. J. 2020, 394, 124879. [Google Scholar] [CrossRef]
- Huang, J.; Qi, H.; Gao, Y.; Xie, A.; Zhang, Y.; Li, Y.; Wang, S.; Zuo, R. Expanded Linear Polarization Response and Excellent Energy-Storage Properties in (Bi0.5Na0.5)TiO3-KNbO3 Relaxor Antiferroelectrics with Medium Permittivity. Chem. Eng. J. 2020, 398, 125639. [Google Scholar] [CrossRef]
- Li, D.; Lin, Y.; Zhang, M.; Yang, H. Achieved Ultrahigh Energy Storage Properties and Outstanding Charge–Discharge Performances in (Na0.5Bi0.5)0.7Sr0.3TiO3-Based Ceramics by Introducing a Linear Additive. Chem. Eng. J. 2020, 392, 123729. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Wang, F.; Xu, Q.; Chen, Y.; Xie, L.; Tan, Z.; Xing, J.; Zhu, J. Enhancement of Energy Storage and Hardness of (Na0.5Bi0.5)0.7Sr0.3TiO3-Based Relaxor Ferroelectrics via Introducing Ba(Mg1/3Nb2/3)O3. Chem. Eng. J. 2022, 431, 133441. [Google Scholar] [CrossRef]
- Luo, C.; Wei, Y.; Feng, Q.; Wang, M.; Luo, N.; Yuan, C.; Zhou, C.; Fujita, T.; Xu, J. Significantly Enhanced Energy-Storage Properties of Bi0.47Na0.47Ba0.06TiO3-CaHfO3 Ceramics by Introducing Sr0.7Bi0.2TiO3 for Pulse Capacitor Application. Chem. Eng. J. 2022, 429, 132165. [Google Scholar] [CrossRef]
- Yan, F.; Zhou, X.; He, X.; Bai, H.; Wu, S.; Shen, B.; Zhai, J. Superior Energy Storage Properties and Excellent Stability Achieved in Environment-Friendly Ferroelectrics via Composition Design Strategy. Nano Energy 2020, 75, 105012. [Google Scholar] [CrossRef]
- Jiang, Z.; Yuan, Y.; Yang, H.; Li, E.; Zhang, S. Relaxor Regulation and Improvement of Breakdown Strength for Bi0.5Na0.5TiO3-Based Ceramics by Co-Doping with Ca and Nb. Ceram. Int. 2022, 48, 9702–9709. [Google Scholar] [CrossRef]
- Meng, K.; Li, W.; Tang, X.; Liu, Q.; Jiang, Y. The Defect Related Energy-Storage Properties of A-Site off-Stoichiometry Ferroelectric Ceramic. Appl. Phys. A 2021, 127, 337. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Jing, R.; Hu, Q.; Alikin, D.O.; Shur, V.Y.; Islam, S.S.; Du, H.; Wei, X.; Feng, G.; et al. Thermal Stability of Dielectric and Energy Storage Performances of Ca-Substituted BNTZ Ferroelectric Ceramics. Ceram. Int. 2021, 47, 6298–6309. [Google Scholar] [CrossRef]
- Krogstad, M.J.; Gehring, P.M.; Rosenkranz, S.; Osborn, R.; Ye, F.; Liu, Y.; Ruff, J.P.C.; Chen, W.; Wozniak, J.M.; Luo, H.; et al. The Relation of Local Order to Material Properties in Relaxor Ferroelectrics. Nat. Mat. 2018, 17, 718–724. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, R.; Zhang, L.; Mao, P.; Sun, Q.; Kang, F.; Wang, J. Remarkably Enhanced Energy-Storage Density and Excellent Thermal Stability under Low Electric Fields of (Na0.5Bi0.5)TiO3-Based Ceramics via Composition Optimization Strategy. J. Eur. Ceram. Soc. 2021, 41, 1917–1924. [Google Scholar] [CrossRef]
- Li, X.; Dong, X.; Wang, F.; Tan, Z.; Zhang, Q.; Chen, H.; Xi, J.; Xing, J.; Zhou, H.; Zhu, J. Realizing Excellent Energy Storage Properties in Na0.5Bi0.5TiO3-Based Lead-Free Relaxor Ferroelectrics. J. Eur. Ceram. Soc. 2022, 42, 2221–2229. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).