Advances in Corrosion of High-Temperature Materials: Interfacial Migration and Alloy Design Strategies
Abstract
1. Introduction
2. The Big Question
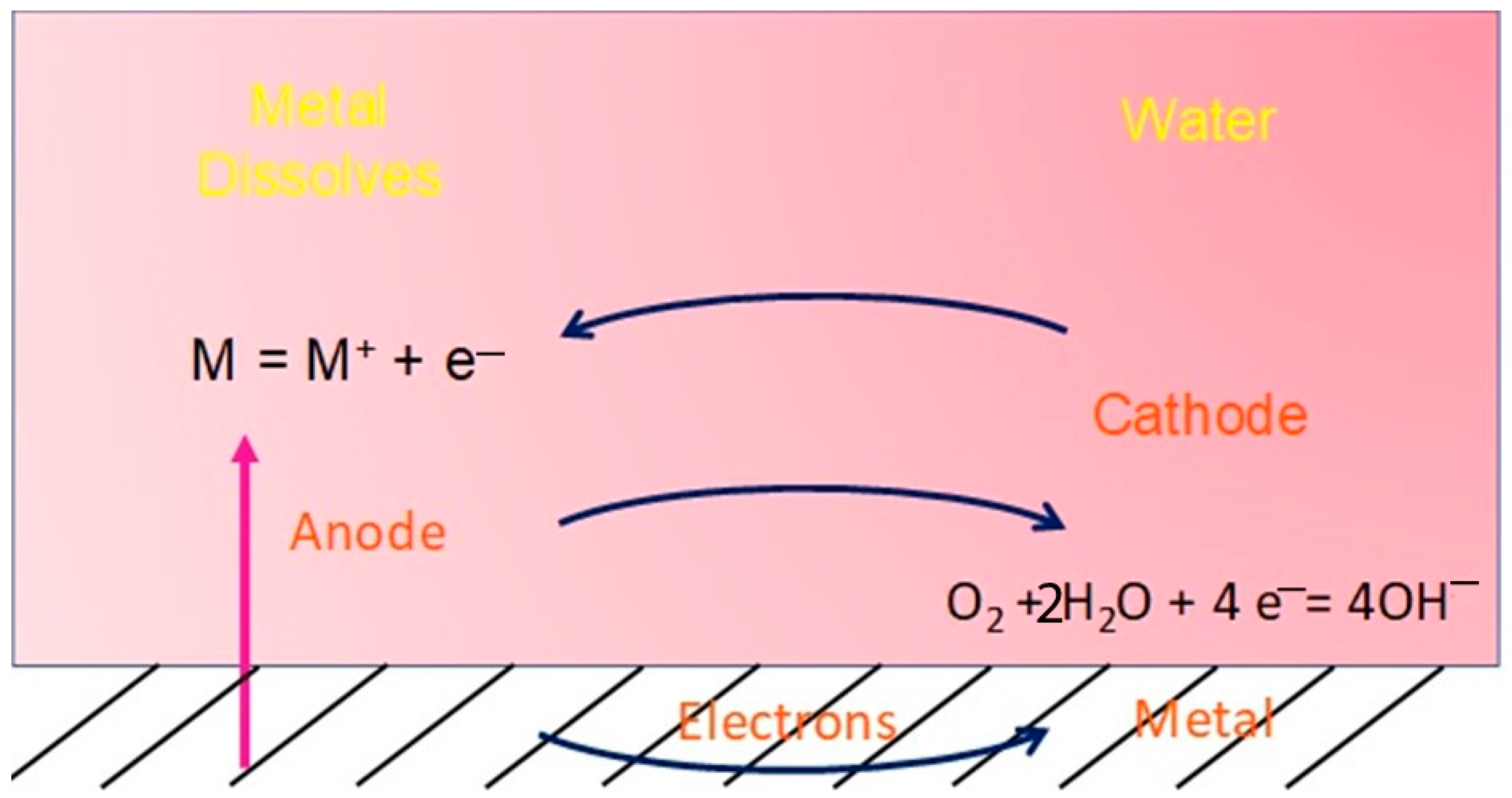
3. Corrosion Mechanism
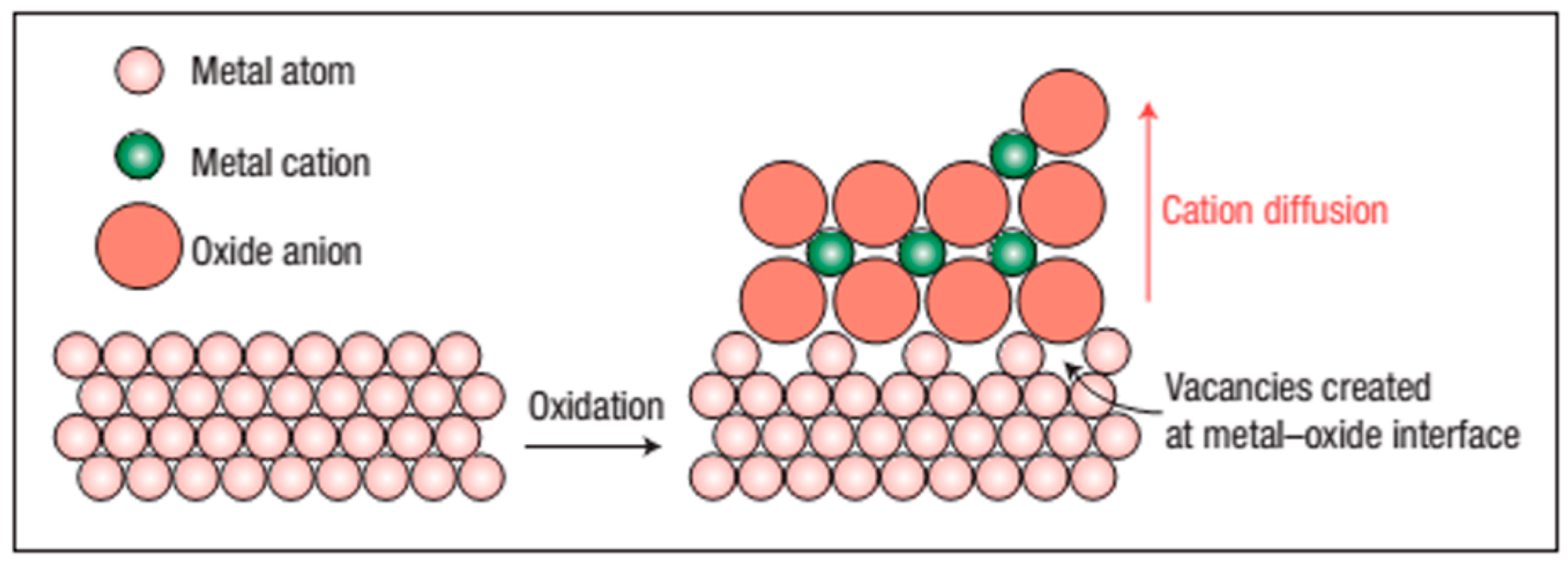
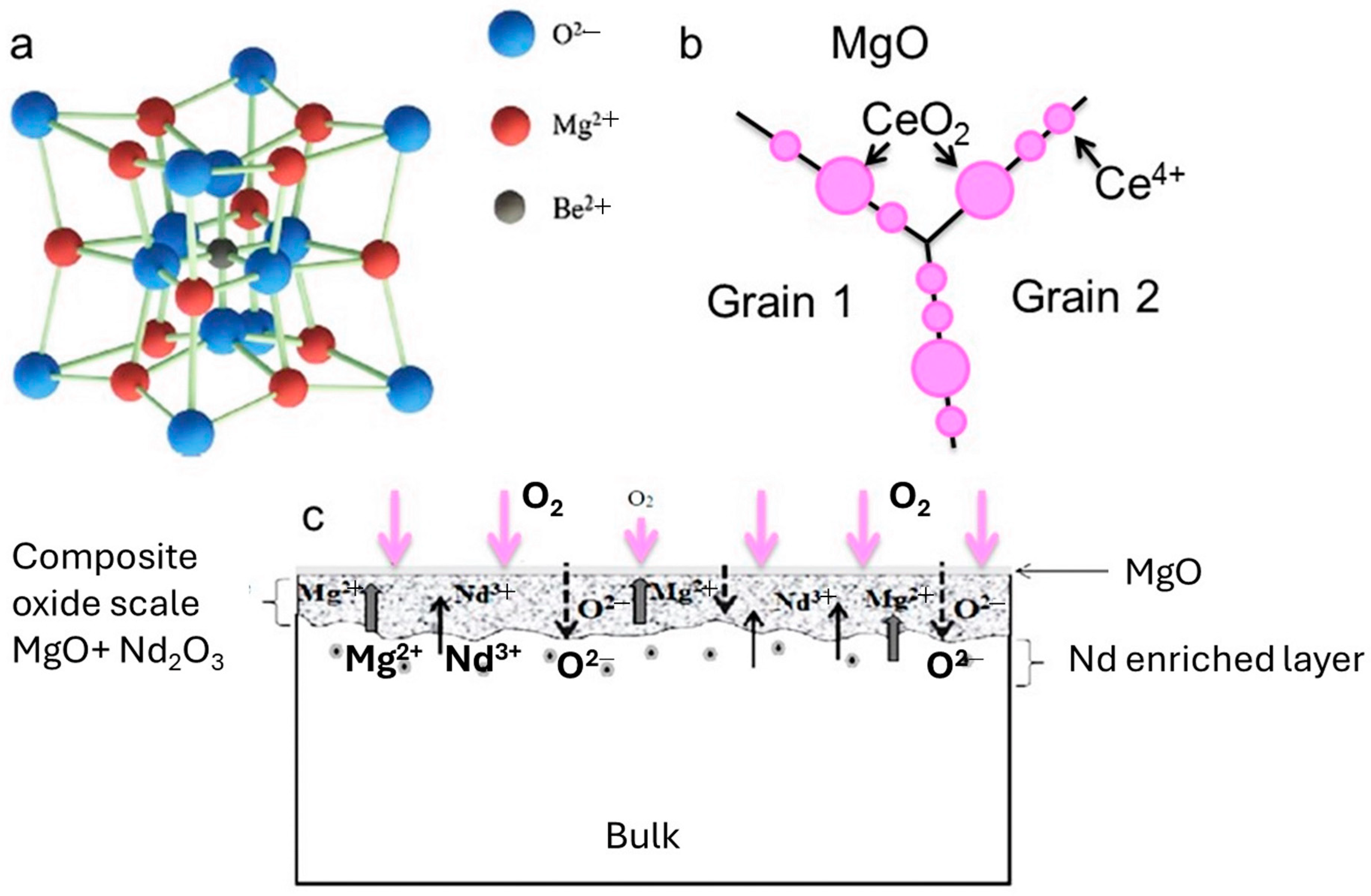
4. Why Does Metal Diffuse Outwards and Oxygen Inward in the Alloy?
5. Quantum Approach in Understanding the Electron Transfer at Passive Interfaces
6. Metallic Additions in Alloys to Scale up the Corrosion Resistance

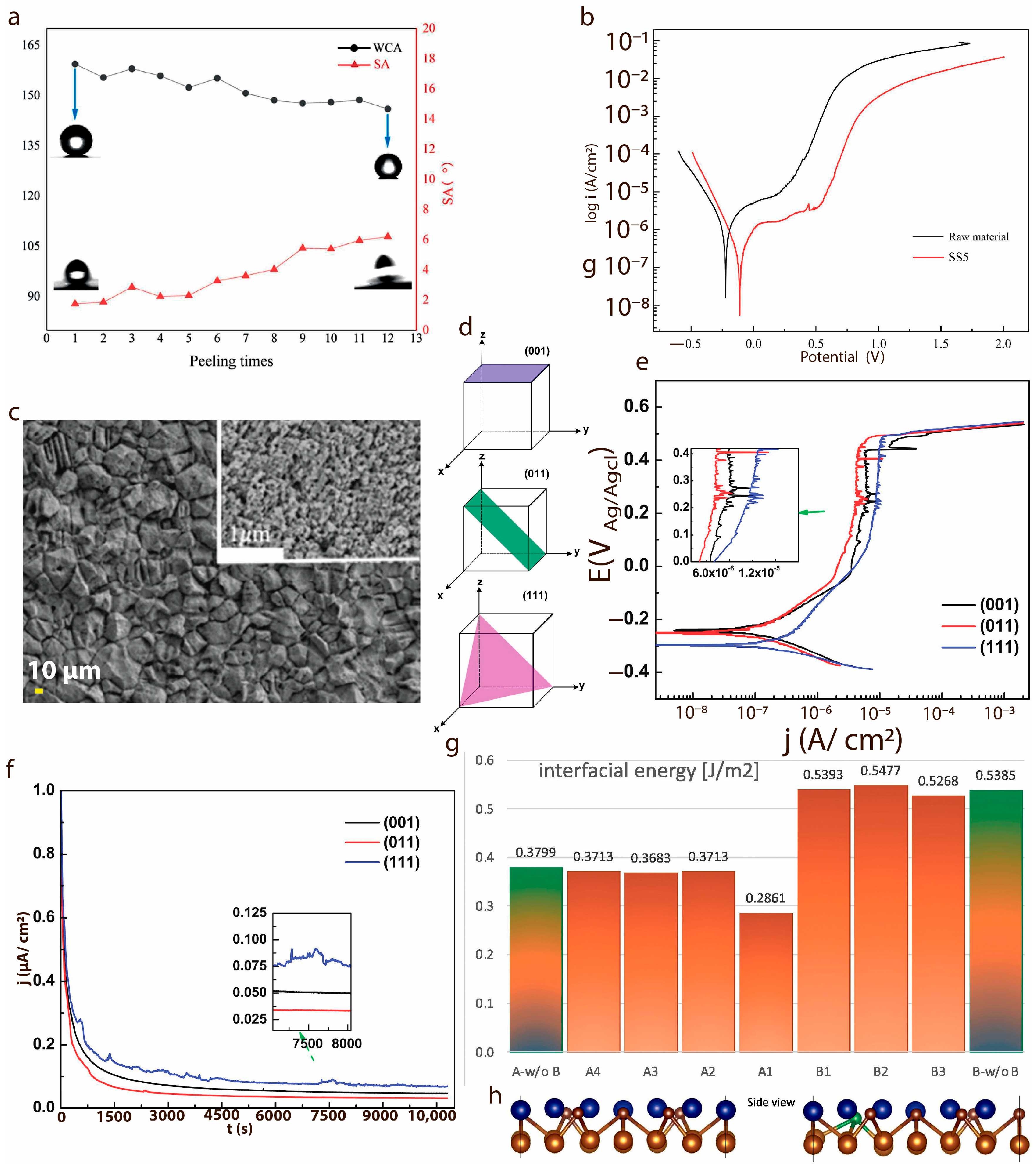
7. Role of Texture Control and Surface Energy in Corrosion Inhibition
8. Atomistic Study of Corrosion
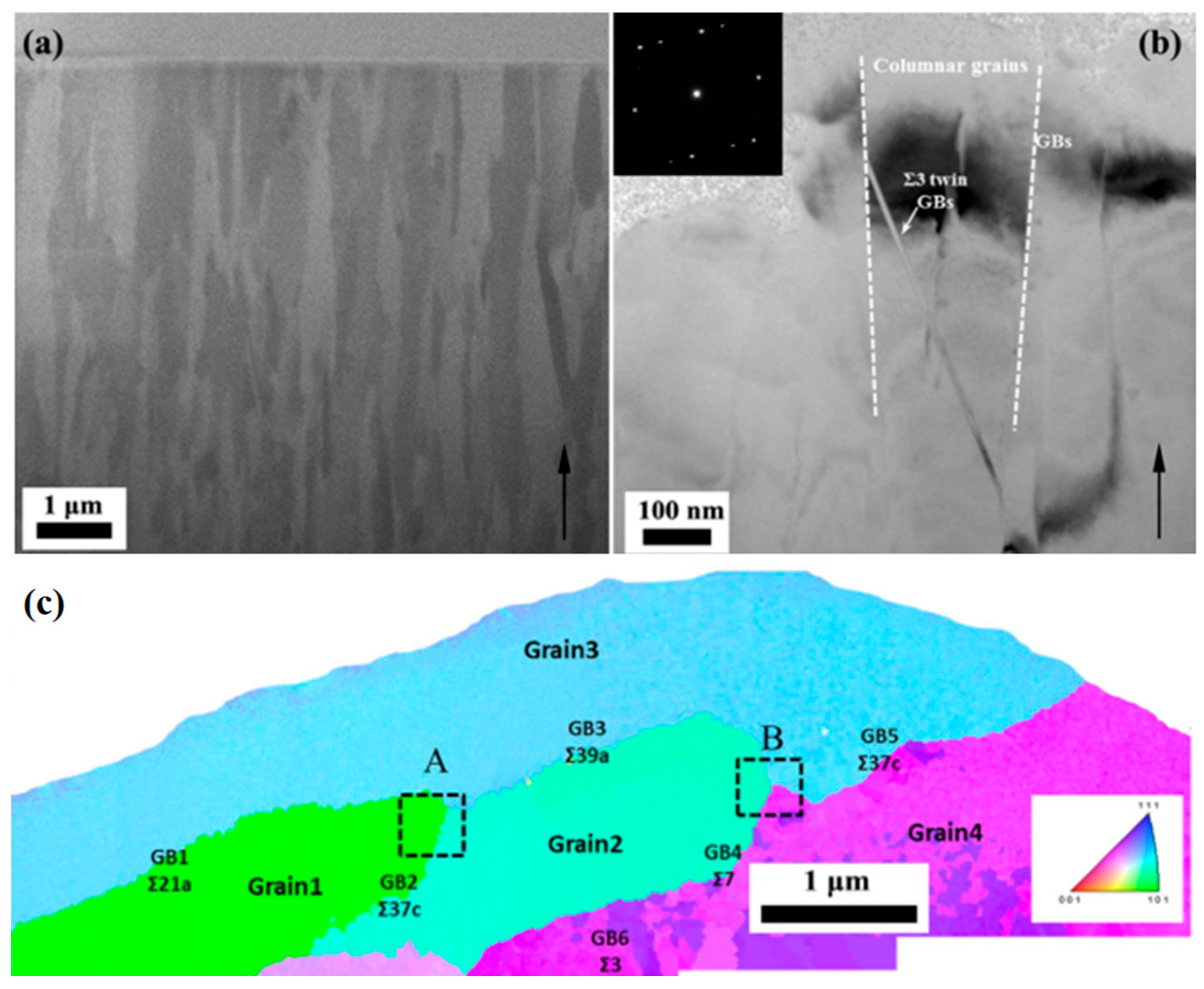
9. Corrosion and Sensitization Control by Grain Boundary Engineering
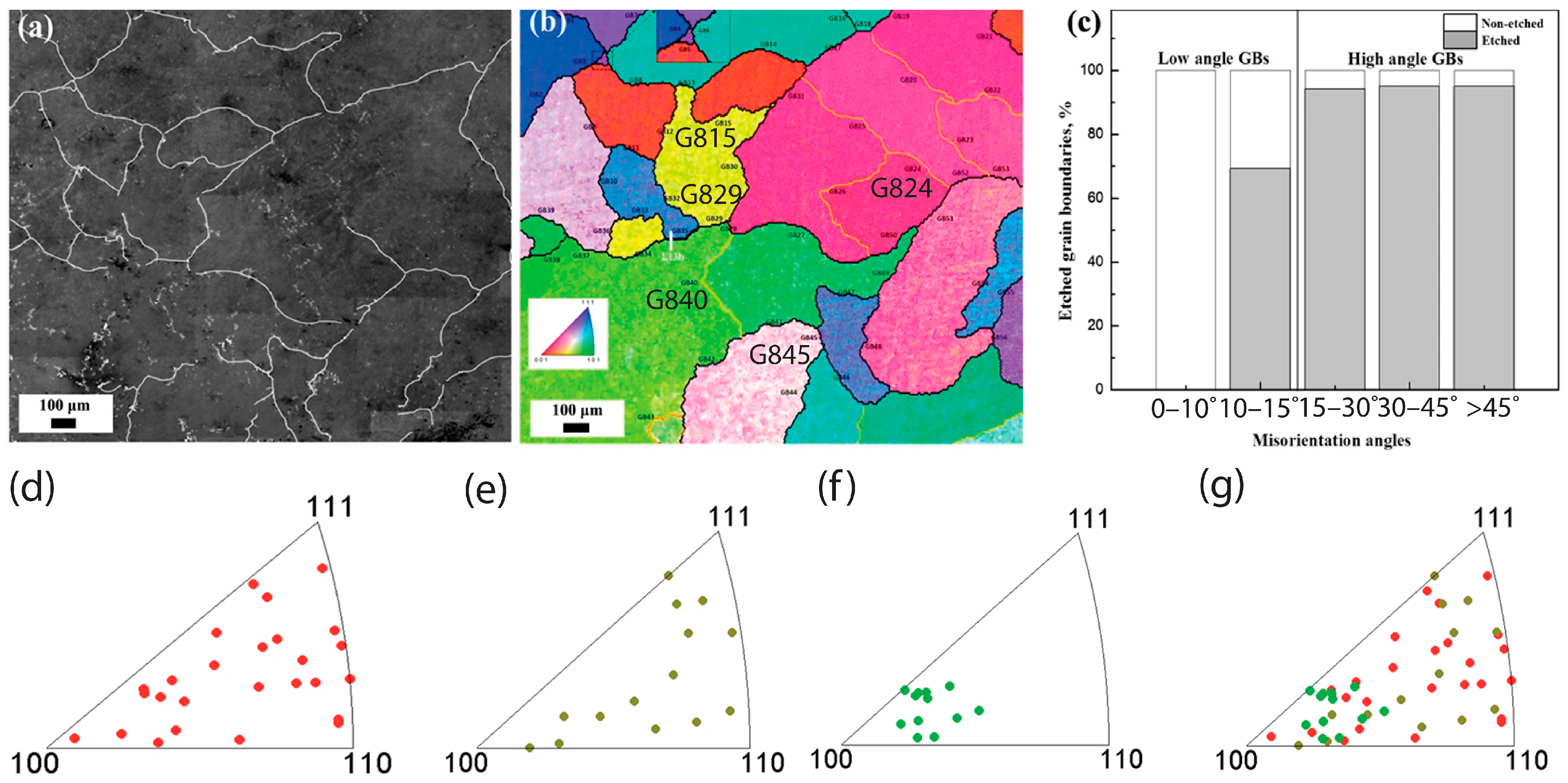
10. Effect of Grain Misorientation
Effect of GB Plane Orientations
11. The Emergence of New Material as a Guard Against Corrosion
12. Alloy Design Strategy in a Nutshell
13. Current Challenges in High-Temperature Corrosion and Material Design
14. Market Potentials of Protective Systems Used in High-Temperature Materials
15. Diverse Ideas in Research Do Wonder in Science
16. Outlook and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ranganathan, S. Alloyed pleasures: Multimetallic cocktails. Curr. Sci. 2003, 85, 1404–1406. [Google Scholar]
- Ruzette, A.-V.; Leibler, L. Block copolymers in tomorrow’s plastics. Nat. Mater. 2005, 4, 19. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.-X.; Kaub, T.; Martens, R.L.; Thompson, G.B. Grain Boundary Specific Segregation in Nanocrystalline Fe (Cr). Sci. Rep. 2016, 6, 34642. [Google Scholar] [CrossRef]
- Fukuda, M.; Sato, H. System evaluation for high temperature ultra supercritical steam power plants. Nihon Kikai Gakkai Ronbunshu B Hen/Trans. Jpn. Soc. Mech. Eng. Part B 2006, 72, 2570–2577. [Google Scholar] [CrossRef]
- Ahn, Y.; Bae, S.J.; Kim, M.; Cho, S.K.; Baik, S.; Lee, J.I.; Cha, J.E. Review of supercritical CO2 power cycle technology and current status of research and development. Nucl. Eng. Technol. 2015, 47, 647–661. [Google Scholar] [CrossRef]
- Singh, A.N.; Moitra, A.; Bhaskar, P.; Sasikala, G.; Dasgupta, A.; Bhaduri, A.K. Study of Aging-Induced Degradation of Fracture Resistance of Alloy 617 Toward High-Temperature Applications. Metall. Mater. Trans. A 2017, 48, 3269–3278. [Google Scholar] [CrossRef]
- Mohan, G.; Venkataraman, M.B.; Coventry, J. Sensible energy storage options for concentrating solar power plants operating above 600 °C. Renew. Sust. Energ. Rev. 2019, 107, 319–337. [Google Scholar] [CrossRef]
- Walczak, M.; Pineda, F.; Fernández, Á.G.; Mata-Torres, C.; Escobar, R.A. Materials corrosion for thermal energy storage systems in concentrated solar power plants. Renew. Sust. Energ. Rev. 2018, 86, 22–44. [Google Scholar] [CrossRef]
- Ryan, M.P.; Williams, D.E.; Chater, R.J.; Hutton, B.M.; McPhail, D.S. Why stainless steel corrodes. Nature 2002, 415, 770–774. [Google Scholar] [CrossRef]
- Kahraman, N.; Gulenc, B.; Findik, F. Corrosion and mechanical-microstructural aspects of dissimilar joints of Ti–6Al–4V and Al plates. Int. J. Impact Eng. 2007, 34, 1423–1432. [Google Scholar] [CrossRef]
- Degerbeck, J.; Gille, I. Crevice corrosion—A new crevice former. Corros. Sci. 1979, 19, 1113–1115. [Google Scholar] [CrossRef]
- Mazario, E.; Venegas, R.; Herrasti, P.; Alonso, M.C.; Recio, F.J. Pitting corrosion and stress corrosion cracking study in high strength steels in alkaline media. J. Solid State Electrochem. 2016, 20, 1223–1227. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Chen, Z.-Y.; Luo, H.-F.; Teng, Z.; Zhao, Y.-L.; Ling, Z.-C. Corrosion resistances of metallic materials in environments containing chloride ions: A review. Trans. Nonferrous Met. Soc. China 2022, 32, 377–410. [Google Scholar] [CrossRef]
- Eliaz, N.; Shemesh, G.; Latanision, R.M. Hot corrosion in gas turbine components. Eng. Fail. Anal. 2002, 9, 31–43. [Google Scholar] [CrossRef]
- Zeng, L.; Shuang, S.; Guo, X.P.; Zhang, G.A. Erosion-corrosion of stainless steel at different locations of a 90° elbow. Corros. Sci. 2016, 111, 72–83. [Google Scholar] [CrossRef]
- Ige, O.O.; Umoru, L.E. Effects of shear stress on the erosion-corrosion behaviour of X-65 carbon steel: A combined mass-loss and profilometry study. Tribol. Int. 2016, 94, 155–164. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.A.; Guo, X.P. Erosion–corrosion at different locations of X65 carbon steel elbow. Corros. Sci. 2014, 85, 318–330. [Google Scholar] [CrossRef]
- Gleeson, B. Still plenty to explore. Nat. Mater. 2018, 17, 574–576. [Google Scholar] [CrossRef]
- Ryan, M. Peering below the surface. Nat. Mater. 2004, 3, 663–664. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Barnoush, A.; Johnsen, R. Materials and corrosion trends in offshore and subsea oil and gas production. npj Mater. Degrad. 2017, 1, 2. [Google Scholar] [CrossRef]
- McMillan, P.F. New materials from high-pressure experiments. Nat. Mater. 2002, 1, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.D.; Tossey, B.M. High temperature oxidation of corrosion resistant alloys from machine learning. npj Mater. Degrad. 2021, 5, 38. [Google Scholar] [CrossRef]
- Oparaodu, K.O.; Okpokwasili, G.C. Comparison of Percentage Weight Loss and Corrosion Rate Trends in Different Metal Coupons from two Soil Environments. Int. J. Environ. Bioremediat. Biodegrad. 2014, 2, 243–249. [Google Scholar]
- Chigondo, M.; Chigondo, F. Recent Natural Corrosion Inhibitors for Mild Steel: An Overview. J. Chem. 2016, 2016, 6208937. [Google Scholar] [CrossRef]
- Muhammad, S. Corrosion protection with eco-friendly inhibitors. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 043001. [Google Scholar]
- Randle, V. Grain boundary engineering: An overview after 25 years. Mater. Sci. Technol. 2010, 26, 253–261. [Google Scholar] [CrossRef]
- Randle, V. The Measurement of Grain Boundary Geometry; Institute of Physics Pub.: Bristol, UK, 1993. [Google Scholar]
- Randle, V. The coincidence site lattice and the ‘sigma enigma’. Mater. Charact. 2001, 47, 411–416. [Google Scholar] [CrossRef]
- Watanabe, T. Grain boundary engineering: Historical perspective and future prospects. J. Mater. Sci. 2011, 46, 4095–4115. [Google Scholar] [CrossRef]
- Shimada, M.; Kokawa, H.; Wang, Z.J.; Sato, Y.S.; Karibe, I. Optimization of grain boundary character distribution for intergranular corrosion resistant 304 stainless steel by twin-induced grain boundary engineering. Acta Mater. 2002, 50, 2331–2341. [Google Scholar] [CrossRef]
- Lehockey, E.M.; Limoges, D.; Palumbo, G.; Sklarchuk, J.; Tomantschger, K.; Vincze, A. On improving the corrosion and growth resistance of positive Pb-acid battery grids by grain boundary engineering. J. Power Sources 1999, 78, 79–83. [Google Scholar] [CrossRef]
- Gabb, T.P.; Telesman, J.; Garg, A.; Lin, P.; Provenzano, V.; Heard, R.; Miller, H.M. Grain Boundary Engineering the Mechanical Properties of Allvac 718Plus™ Superalloy. In Proceedings of the 7th International Symposium on Superalloy 718 and Derivatives, Pittsburgh, PA, USA, 10–13 October 2010; pp. 254–269. [Google Scholar]
- Moore, W.C. Aqua regia: Preliminary paper. J. Am. Chem. Soc. 1911, 33, 1091–1099. [Google Scholar] [CrossRef][Green Version]
- Karen, P. Oxidation State, A Long-Standing Issue. Angew. Chem. (Int. Ed. Engl.) 2015, 54, 4716–4726. [Google Scholar] [CrossRef] [PubMed]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, E.E.; Buchanan, R.A. Fundamentals of Electrochemical Corrosion; ASM international: Almere, The Netherlands, 2000. [Google Scholar]
- Cao, G. Nanostructures and Nanomaterials: Synthesis, Properties and Applications; Imperial College Press: London, UK, 2004. [Google Scholar]
- Jinich, A.; Rappoport, D.; Dunn, I.; Sanchez-Lengeling, B.; Olivares-Amaya, R.; Noor, E.; Even, A.B.; Aspuru-Guzik, A. Quantum Chemical Approach to Estimating the Thermodynamics of Metabolic Reactions. Sci. Rep. 2014, 4, 7022. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Clarke, D.R. Spalling failure of α-alumina films grown by oxidation: I. Dependence on cooling rate and metal thickness. Mater. Sci. Eng. A 2000, 278, 142–150. [Google Scholar] [CrossRef]
- Lin, C.; Li, Y. Interface stress evolution considering the combined creep–plastic behavior in thermal barrier coatings. Mater. Design 2016, 89, 245–254. [Google Scholar] [CrossRef]
- Knipe, K.; Manero Ii, A.; Siddiqui, S.F.; Meid, C.; Wischek, J.; Okasinski, J.; Almer, J.; Karlsson, A.M.; Bartsch, M.; Raghavan, S. Strain response of thermal barrier coatings captured under extreme engine environments through synchrotron X-ray diffraction. Nat. Commun. 2014, 5, 4559. [Google Scholar] [CrossRef]
- Clarke, D.R.; Phillpot, S.R. Thermal barrier coating materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Kamrunnahar, M.; Bao, J.; Macdonald, D.D. Challenges in the theory of electron transfer at passive interfaces. Corros. Sci. 2005, 47, 3111–3139. [Google Scholar] [CrossRef]
- Gurney, R. The quantum mechanics of electrolysis. Proc. R. Soc. Lond. A 1931, 134, 137–154. [Google Scholar]
- Burstein, G.T. A hundred years of Tafel’s Equation: 1905–2005. Corros. Sci. 2005, 47, 2858–2870. [Google Scholar] [CrossRef]
- Liu, R.L.; Hurley, M.F.; Kvryan, A.; Williams, G.; Scully, J.R.; Birbilis, N. Controlling the corrosion and cathodic activation of magnesium via microalloying additions of Ge. Sci. Rep. 2016, 6, 28747. [Google Scholar] [CrossRef]
- Bai, P.; Bazant, M.Z. Charge transfer kinetics at the solid–solid interface in porous electrodes. Nat. Commun. 2014, 5, 3585. [Google Scholar] [CrossRef] [PubMed]
- Tafel, J. Über die Polarisation bei kathodischer Wasserstoffentwicklung. Z. Phys. Chem. 1905, 50, 641–712. [Google Scholar] [CrossRef]
- Gabe, D. The centenary of Tafel’s equation. Trans. IMF 2005, 83, 121–124. [Google Scholar] [CrossRef]
- Marcus, R.A.; Sutin, N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta (BBA)—Rev. Bioenerg. 1985, 811, 265–322. [Google Scholar] [CrossRef]
- Marcus, R.A. On the Theory of Oxidation‐Reduction Reactions Involving Electron Transfer. I. J. Chem. Phys. 1956, 24, 966–978. [Google Scholar] [CrossRef]
- Marcus, R.A. On the theory of chemiluminescent electron-transfer reactions. J. Chem. Phys. 1965, 43, 2654–2657. [Google Scholar] [CrossRef]
- Marcus, R.A. Chemical and electrochemical electron-transfer theory. Annu. Rev. Phys. Chem. 1964, 15, 155–196. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Ulstrup, J. Theory of electron transfer at electrified interfaces. Electrochim. Acta 2000, 45, 2339–2361. [Google Scholar] [CrossRef]
- Schmickler, W. A general model for electron-transfer reactions via electronic intermediate states. J. Electroanal. Chem. Interfacial Electrochem. 1982, 137, 189–198. [Google Scholar] [CrossRef]
- Esaki, L. Discovery of the tunnel diode. IEEE Trans. Electron Devices 1976, 23, 644–647. [Google Scholar] [CrossRef]
- Mizuta, H.; Tanoue, T. The Physics and Applications of Resonant Tunnelling Diodes; Cambridge University Press: Cambridge, UK, 2006; Volume 2. [Google Scholar]
- Schmickler, W.; Ulstrup, J. A theory of electron transfer reactions at film-covered metal electrodes. Chem. Phys. 1977, 19, 217–232. [Google Scholar] [CrossRef]
- Oksa, M.; Metsäjoki, J.; Kärki, J. Thermal Spray Coatings for High-Temperature Corrosion Protection in Biomass Co-Fired Boilers. J. Therm. Spray Technol. 2015, 24, 194–205. [Google Scholar] [CrossRef]
- Kang, Y.; Leng, X.; Zhao, L.; Bai, B.; Wang, X.; Chen, H. Review on the Corrosion Behaviour of Nickel-Based Alloys in Supercritical Carbon Dioxide under High Temperature and Pressure. Crystals 2023, 13, 725. [Google Scholar] [CrossRef]
- Cho, F.-Y.; Tung, S.-W.; Ouyang, F.-Y. High temperature oxidation behavior of high entropy alloy Al4Co3Cr25Cu10Fe25Ni33 in oxygen-containing atmospheres. Mater. Chem. Phys. 2022, 278, 125678. [Google Scholar] [CrossRef]
- Zhang, X.; Corrêa da Silva, C.; Liu, C.; Prabhakar, M.; Rohwerder, M. Selective oxidation of ternary Fe-Mn-Si alloys during annealing process. Corros. Sci. 2020, 174, 108859. [Google Scholar] [CrossRef]
- Li, L.; Guo, X. Microstructure and oxidation resistance of Ti modified Si-Mo coating on Nb-Si based ultrahigh temperature alloy. Corros. Sci. 2023, 220, 111293. [Google Scholar] [CrossRef]
- Reddy, G.M.S.; Prasad, C.D.; Shetty, G.; Ramesh, M.R.; Rao, T.N.; Patil, P. High-temperature oxidation behavior of plasma-sprayed NiCrAlY/TiO2 and NiCrAlY/Cr2O3/YSZ coatings on titanium alloy. Weld. World 2022, 66, 1069–1079. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Mukherjee, S.; Singh, S.; Singh, H.; Grewal, H.S. Exceptionally high cavitation erosion and corrosion resistance of a high entropy alloy. Ultrason. Sonochem. 2018, 41, 252–260. [Google Scholar] [CrossRef]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Birbilis, N. Corrosion of high entropy alloys. npj Mater. Degrad. 2017, 1, 15. [Google Scholar] [CrossRef]
- Singh, A.N.; Islam, M.; Meena, A.; Faizan, M.; Han, D.; Bathula, C.; Hajibabaei, A.; Anand, R.; Nam, K.-W. Unleashing the Potential of Sodium-Ion Batteries: Current State and Future Directions for Sustainable Energy Storage. Adv. Funct. Mater. 2023, 33, 2304617. [Google Scholar] [CrossRef]
- Xie, X.; Li, N.; Liu, W.; Huang, S.; He, X.; Yu, Q.; Xiong, H.; Wang, E.; Hou, X. Research Progress of Refractory High Entropy Alloys: A Review. Chin. J. Mech. Eng. 2022, 35, 142. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-entropy alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Nascimento, C.B.; Donatus, U.; Ríos, C.T.; Oliveira, M.C.L.d.; Antunes, R.A. A review on corrosion of high entropy alloys: Exploring the interplay between corrosion properties, alloy composition, passive film stability and materials selection. Mater. Res. 2022, 25, e20210442. [Google Scholar] [CrossRef]
- Godlewska, E.M.; Mitoraj-Królikowska, M.; Czerski, J.; Jawańska, M.; Gein, S.; Hecht, U. Corrosion of Al (Co) CrFeNi high-entropy alloys. Front. Mater. 2020, 7, 566336. [Google Scholar] [CrossRef]
- Liu, R.; Sun, H.; Jiang, X.; Liu, X.; Yan, W. Study of microstructure and corrosion resistance of FeCrAl-Gd alloys. Mater. Chem. Phys. 2023, 297, 127384. [Google Scholar] [CrossRef]
- Rodriguez, A.A.; Tylczak, J.H.; Gao, M.C.; Jablonski, P.D.; Detrois, M.; Ziomek-Moroz, M.; Hawk, J.A. Effect of Molybdenum on the Corrosion Behavior of High-Entropy Alloys CoCrFeNi2 and CoCrFeNi2Mo0. 25 under Sodium Chloride Aqueous Conditions. Adv. Mater. Sci. Eng. 2018, 2018, 3016304. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, S.; Wang, Z.Y.; Zhang, C.H.; Chen, H.T.; Chen, J. New studies on wear and corrosion behavior of laser cladding FeNiCoCrMox high entropy alloy coating: The role of Mo. Int. J. Refract. Met. Hard Mater. 2022, 102, 105721. [Google Scholar] [CrossRef]
- Mishra, A.; Shoesmith, D. The activation/depassivation of nickel–chromium–molybdenum alloys: An oxyanion or a pH effect—Part II. Electrochim. Acta 2013, 102, 328–335. [Google Scholar] [CrossRef]
- Hayes, J.; Gray, J.; Szmodis, A.; Orme, C. Influence of chromium and molybdenum on the corrosion of nickel-based alloys. Corrosion 2006, 62, 491–500. [Google Scholar] [CrossRef]
- Singh, A.N.; Kim, M.-H.; Meena, A.; Wi, T.-U.; Lee, H.-W.; Kim, K.S. Na/Al Codoped Layered Cathode with Defects as Bifunctional Electrocatalyst for High-Performance Li-Ion Battery and Oxygen Evolution Reaction. Small 2021, 17, 2005605. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Hajibabaei, A.; Kim, D.Y.; Singh, A.N.; Yun, J.; Myung, C.W.; Kim, K.S. Al-Doping Driven Suppression of Capacity and Voltage Fadings in 4d-Element Containing Li-Ion-Battery Cathode Materials: Machine Learning and Density Functional Theory. Adv. Energy Mater. 2022, 12, 2201497. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Li, Q.; Jin, C.; Hua, K.; Wang, H. The determining role of Al addition on tribology properties and oxidation behavior at elevated temperatures of TiZrHfNb refractory high-entropy alloy. Mater. Charact. 2022, 189, 111921. [Google Scholar] [CrossRef]
- Lin, C.-M.; Juan, C.-C.; Chang, C.-H.; Tsai, C.-W.; Yeh, J.-W. Effect of Al addition on mechanical properties and microstructure of refractory AlxHfNbTaTiZr alloys. J. Alloys Compd. 2015, 624, 100–107. [Google Scholar] [CrossRef]
- Hong, M.-S.; Park, I.-J.; Kim, J.-G. Alloying effect of copper concentration on the localized corrosion of aluminum alloy for heat exchanger tube. Met. Mater. Int. 2017, 23, 708–714. [Google Scholar] [CrossRef]
- Seyeux, A.; Frankel, G.; Missert, N.; Unocic, K.; Klein, L.; Galtayries, A.; Marcus, P. ToF-SIMS imaging study of the early stages of corrosion in Al-Cu thin films. J. Electrochem. Soc. 2011, 158, C165–C171. [Google Scholar] [CrossRef]
- Larsen, M.H.; Walmsley, J.C.; Lunder, O.; Mathiesen, R.H.; Nisancioglu, K. Intergranular Corrosion of Copper-Containing AA6x xx AlMgSi Aluminum Alloys. J. Electrochem. Soc. 2008, 155, C550–C556. [Google Scholar] [CrossRef]
- Yan, H.Y.; Vorontsov, V.A.; Dye, D. Effect of alloying on the oxidation behaviour of Co–Al–W superalloys. Corros. Sci. 2014, 83, 382–395. [Google Scholar] [CrossRef]
- Giovanardi, C.; Hammer, L.; Heinz, K. Ultrathin cobalt oxide films on Ir (100) − (1 × 1). Phys. Rev. B 2006, 74, 125429. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Selloni, A. Electronic structure and bonding properties of cobalt oxide in the spinel structure. Phys. Rev. B 2011, 83, 245204. [Google Scholar] [CrossRef]
- Tan, H.B.; Ke, K.; Ma, B.G.; Xiao, J. Effect and Solid Solution Mechanism of Co2O3 during C3S Formation. Open Mater. Sci. J. 2011, 5, 118–122. [Google Scholar] [CrossRef]
- Doležal, T.D.; Valverde, N.A.; Yuwono, J.A.; Kemnitz, R.A. Mo-Re-W alloys for high temperature applications: Phase stability, elasticity, and thermal property insights via multi-cell Monte Carlo and machine learning. Mater. Des. 2024, 244, 113226. [Google Scholar] [CrossRef]
- Dwivedi, D.; Lepková, K.; Becker, T. Carbon steel corrosion: A review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. [Google Scholar] [CrossRef]
- Kangazian, J.; Shamanian, M. Micro-texture and corrosion behavior of dissimilar joints of UNS S32750 stainless steel/UNS N08825 Ni-based superalloy. Mater. Charact. 2019, 155, 109802. [Google Scholar] [CrossRef]
- Bateni, M.R.; Szpunar, J.A.; Wang, X.; Li, D.Y. The effect of wear and corrosion on internal crystalline texture of carbon steel and stainless steel. Wear 2005, 259, 400–404. [Google Scholar] [CrossRef]
- Fushimi, K.; Miyamoto, K.; Konno, H. Anisotropic corrosion of iron in pH 1 sulphuric acid. Electrochim. Acta 2010, 55, 7322–7327. [Google Scholar] [CrossRef]
- Kumar, B.R.; Singh, R.; Mahato, B.; De, P.; Bandyopadhyay, N.; Bhattacharya, D. Effect of texture on corrosion behavior of AISI 304L stainless steel. Mater. Charact. 2005, 54, 141–147. [Google Scholar] [CrossRef]
- Park, S.-A.; Kim, J.; He, Y.; Shin, K.; Yoon, J. Comparative study on the corrosion behavior of the cold rolled and hot rolled low-alloy steels containing copper and antimony in flue gas desulfurization environment. Phys. Met. Metallogr. 2014, 115, 1285–1294. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, W.; Zhu, S.; Chen, G.; Wang, L.; Su, Y. Preparation of super-hydrophobic surface with micro-nano layered structure on 316 stainless steel by one-step wet chemical method. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130291. [Google Scholar] [CrossRef]
- Wang, L.; Xiao, X.; Liu, E.; Yu, S.; Yin, X.; Wang, J.; Zhu, G.; Li, Q.; Li, J. Fabrication of superhydrophobic needle-like Ca-P coating with anti-fouling and anti-corrosion properties on AZ31 magnesium alloy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126568. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, J.; Lei, Y.; Wang, Y.; Zhou, G.; Xu, C.; Rao, Y.; Wang, K. Preparation of intricate nanostructures on 304 stainless steel surface by SiO2-assisted HF etching for high superhydrophobicity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124287. [Google Scholar] [CrossRef]
- Pan, Q.; Cao, Y.; Xue, W.; Zhu, D.; Liu, W. Picosecond Laser-Textured Stainless Steel Superhydrophobic Surface with an Antibacterial Adhesion Property. Langmuir 2019, 35, 11414–11421. [Google Scholar] [CrossRef] [PubMed]
- Emelyanenko, K.A.; Emelyanenko, A.M.; Boinovich, L.B. Laser Obtained Superhydrophobic State for Stainless Steel Corrosion Protection: A Review. Coatings 2023, 13, 194. [Google Scholar] [CrossRef]
- Wang, H.; Zhuang, J.; Qi, H.; Yu, J.; Guo, Z.; Ma, Y. Laser-chemical treated superhydrophobic surface as a barrier to marine atmospheric corrosion. Surf. Coat. Technol. 2020, 401, 126255. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid fabrication of large-area, corrosion-resistant superhydrophobic Mg alloy surfaces. ACS Appl. Mater. Inter. 2011, 3, 4404–4414. [Google Scholar] [CrossRef]
- Liu, Y.X.; Lei, X.W.; Hao, L.Y.; Han, S.X.; Yang, R.N.; Wang, N. The anisotropy electrochemical corrosion behavior of Ni-based single crystal superalloy on different crystal planes: An investigation from the film growth aspect. Appl. Surf. Sci. 2022, 576, 151785. [Google Scholar] [CrossRef]
- Zhang, L.; Ojo, O. Crystallographic orientation dependence of corrosion behavior of a single crystal nickel-based alloy. Metall. Mater. Trans. A 2018, 49, 295–304. [Google Scholar] [CrossRef]
- Yu, J.; Glazoff, M.V.; Capolungo, L.; Gao, M.C.; Ilevbare, G.O. Boron substitution induced FCC Fe/Cr23C6 interfacial strengthening: An ab initio study. Comput. Mater. Sci. 2023, 228, 112370. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V. Atomic level characterization in corrosion studies. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20160414. [Google Scholar] [CrossRef]
- Kokalj, A. Molecular modeling of organic corrosion inhibitors: Calculations, pitfalls, and conceptualization of molecule–surface bonding. Corros. Sci. 2021, 193, 109650. [Google Scholar] [CrossRef]
- Mendonça, G.L.F.; Costa, S.N.; Freire, V.N.; Casciano, P.N.S.; Correia, A.N.; Lima-Neto, P.d. Understanding the corrosion inhibition of carbon steel and copper in sulphuric acid medium by amino acids using electrochemical techniques allied to molecular modelling methods. Corros. Sci. 2017, 115, 41–55. [Google Scholar] [CrossRef]
- Zuili, D.; Maurice, V.; Marcus, P. Surface structure of nickel in acid solution studied by in situ scanning tunneling microscopy. J. Electrochem. Soc. 2000, 147, 1393–1400. [Google Scholar] [CrossRef]
- Magnussen, O.M.; Scherer, J.; Ocko, B.M.; Behm, R.J. In Situ X-ray Scattering Study of the Passive Film on Ni(111) in Sulfuric Acid Solution. J. Phys. Chem. B 2000, 104, 1222–1226. [Google Scholar] [CrossRef]
- Scherer, J.; Ocko, B.; Magnussen, O. Structure, dissolution, and passivation of Ni (111) electrodes in sulfuric acid solution: An in situ STM, X-ray scattering, and electrochemical study. Electrochim. Acta 2003, 48, 1169–1191. [Google Scholar] [CrossRef]
- Maurice, V.; Talah, H.; Marcus, P. A scanning tunneling microscopy study of the structure of thin oxide films grown on Ni (111) single crystal surfaces by anodic polarization in acid electrolyte. Surf. Sci. 1994, 304, 98–108. [Google Scholar] [CrossRef]
- Bouzoubaa, A.; Diawara, B.; Maurice, V.; Minot, C.; Marcus, P. Ab initio study of the interaction of chlorides with defect-free hydroxylated NiO surfaces. Corros. Sci. 2009, 51, 941–948. [Google Scholar] [CrossRef]
- Bouzoubaa, A.; Diawara, B.; Maurice, V.; Minot, C.; Marcus, P. Ab initio modelling of localized corrosion: Study of the role of surface steps in the interaction of chlorides with passivated nickel surfaces. Corros. Sci. 2009, 51, 2174–2182. [Google Scholar] [CrossRef]
- Maurice, V.; Klein, L.; Marcus, P. Atomic structure of metastable pits formed on nickel. Electrochem. Solid-State Lett. 2001, 4, B1–B3. [Google Scholar] [CrossRef]
- Seyeux, A.; Maurice, V.; Klein, L.; Marcus, P. In situ STM study of the effect of chloride on passive film on nickel in alkaline solution. J. Electrochem. Soc. 2006, 153, B453–B463. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.-H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Maurice, V.; Nakamura, T.; Klein, L.; Marcus, P. Initial stages of localised corrosion by pitting of passivated nickel surfaces studied by STM and AFM. In Local Probe Techniques for Corrosion Research; Elsevier: Amsterdam, The Netherlands, 2007; pp. 71–83. [Google Scholar]
- Ball, P. A sense of dislocations. Nat. Mater. 2015, 14, 9688. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.H.; Li, C.X.; Han, P.D. Synergetic effects of impurities and alloying element Cr on oxidation and dissolution corrosion of Ni (111) surfaces: A DFT study. Chin. J. Phys. 2019, 61, 1–7. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsurekawa, S.; Zhao, X.; Zuo, L. The Coming of Grain Boundary Engineering in the 21st Century. In Microstructure and Texture in Steels: And Other Materials; Haldar, A., Suwas, S., Bhattacharjee, D., Eds.; Springer: London, UK, 2009; pp. 43–82. [Google Scholar]
- Watanabe, T. An approach to grain-boundary design for strong and ductile polycrystals. Res. Mech. 1984, 11, 47–84. [Google Scholar]
- Randle, V.; Brown, A. Development of grain misorientation texture, in terms of coincident site lattice structures, as a function of thermomechanical treatments. Philos. Mag. A 1989, 59, 1075–1089. [Google Scholar] [CrossRef]
- Cheng, Y.; Jin, Z.H.; Zhang, Y.W.; Gao, H. On intrinsic brittleness and ductility of intergranular fracture along symmetrical tilt grain boundaries in copper. Acta Mater. 2010, 58, 2293–2299. [Google Scholar] [CrossRef]
- Hossein Nedjad, S.; Nili Ahmadabadi, M.; Furuhara, T. Correlation between the intergranular brittleness and precipitation reactions during isothermal aging of an Fe–Ni–Mn maraging steel. Mater. Sci. Eng. A 2008, 490, 105–112. [Google Scholar] [CrossRef]
- Yan, J.; Heckman, N.M.; Velasco, L.; Hodge, A.M. Improve sensitization and corrosion resistance of an Al-Mg alloy by optimization of grain boundaries. Sci. Rep. 2016, 6, 26870. [Google Scholar] [CrossRef]
- Yamaura, S.-I.; Tsurekawa, S.; Watanabe, T. The Control of Oxidation-Induced Intergranular Embrittlement by Grain Boundary Engineering in Rapidly Solidified Ni-Fe Alloy Ribbons. Mater. Trans. 2003, 44, 1494–1502. [Google Scholar] [CrossRef]
- Kobayashi, S.; Maruyama, T.; Tsurekawa, S.; Watanabe, T. Grain boundary engineering based on fractal analysis for control of segregation-induced intergranular brittle fracture in polycrystalline nickel. Acta Mater. 2012, 60, 6200–6212. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsurekawa, S.; Watanabe, T. A new approach to grain boundary engineering for nanocrystalline materials. Beilstein J. Nanotechnol. 2016, 7, 1829. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsurekawa, S.; Fujii, H.; Kanno, T. The control of texture and grain boundary microstructure by magnetic annealing. Mater. Sci. Forum 2005, 495–497, 1151–1158. [Google Scholar] [CrossRef]
- Bettayeb, M.; Maurice, V.; Klein, L.H.; Lapeire, L.; Verbeken, K.; Marcus, P. Nanoscale Intergranular Corrosion and Relation with Grain Boundary Character as Studied In Situ on Copper. J. Electrochem. Soc. 2018, 165, C835–C841. [Google Scholar] [CrossRef]
- Yang, F.-Q.; Xue, H.; Zhao, L.-Y.; Fang, X.-R.; Zhang, H.-B. Effects of Crystal Orientation and Grain Boundary Inclination on Stress Distribution in Bicrystal Interface of Austenite Stainless Steel 316L. Adv. Mater. Sci. Eng. 2019, 2019, 2468487. [Google Scholar] [CrossRef]
- Ralston, K.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 075005-075005-13. [Google Scholar] [CrossRef]
- Polmear, I. Light Alloys: Metallurgy of the Light Metals; Edward Arnold: London, UK; New York, NY, USA, 1989; p. 288. [Google Scholar]
- Shahabi-Navid, M.; Esmaily, M.; Svensson, J.-E.; Halvarsson, M.; Nyborg, L.; Cao, Y.; Johansson, L.-G. NaCl-induced atmospheric corrosion of the MgAl alloy AM50-the influence of CO2. J. Electrochem. Soc. 2014, 161, C277–C287. [Google Scholar] [CrossRef]
- Birbilis, N.; Buchheit, R. Electrochemical characteristics of intermetallic phases in aluminum alloys an experimental survey and discussion. J. Electrochem. Soc. 2005, 152, B140–B151. [Google Scholar] [CrossRef]
- Jones, R.; Baer, D.; Danielson, M.; Vetrano, J. Role of Mg in the stress corrosion cracking of an Al-Mg alloy. Metall. Mater. Trans. A. 2001, 32, 1699–1711. [Google Scholar] [CrossRef]
- Zhang, R.; Gupta, R.; Davies, C.; Hodge, A.; Tort, M.; Xia, K.; Birbilis, N. The influence of grain size and grain orientation on sensitization in AA5083. Corrosion 2015, 72, 160–168. [Google Scholar]
- Choi, D.-H.; Ahn, B.-W.; Quesnel, D.J.; Jung, S.-B. Behavior of β phase (Al3Mg2) in AA 5083 during friction stir welding. Intermetallics 2013, 35, 120–127. [Google Scholar] [CrossRef]
- Walczak, M.S.; Morales-Gil, P.; Belashehr, T.; Kousar, K.; Lozada, P.A.; Lindsay, R. Determining the Chemical Composition of Corrosion Inhibitor/Metal Interfaces with XPS: Minimizing Post Immersion Oxidation. J. Vis. Exp. JoVE 2017, 15, 55163. [Google Scholar] [CrossRef]
- Wang, Y.; Gupta, R.; Sukiman, N.; Zhang, R.; Davies, C.; Birbilis, N. Influence of alloyed Nd content on the corrosion of an Al–5Mg alloy. Corros. Sci. 2013, 73, 181–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Zhao, Y.; Li, Z.; Xing, Y.; Zhou, K. Effect of thermo-mechanical treatments on corrosion behavior of Cu-15Ni-8Sn alloy in 3.5 wt% NaCl solution. Mater. Chem. Phys. 2017, 199, 54–66. [Google Scholar] [CrossRef]
- Tan, L.; Allen, T.R. Effect of thermomechanical treatment on the corrosion of AA5083. Corros. Sci. 2010, 52, 548–554. [Google Scholar] [CrossRef]
- Oguocha, I.; Adigun, O.; Yannacopoulos, S. Effect of sensitization heat treatment on properties of Al–Mg alloy AA5083-H116. J. Mater. Sci. 2008, 43, 4208–4214. [Google Scholar] [CrossRef]
- Shi, P.; Hu, R.; Zhang, T.; Yuan, L.; Li, J. Grain boundary character distribution and its effect on corrosion of Ni–23Cr–16Mo superalloy. Mater. Sci. Technol. 2017, 33, 84–91. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsurekawa, S. The control of brittleness and development of desirable mechanical properties in polycrystalline systems by grain boundary engineering. Acta Mater. 1999, 47, 4171–4185. [Google Scholar] [CrossRef]
- Hickman, J.; Mishin, Y. Extra variable in grain boundary description. Phys. Rev. Mater. 2017, 1, 010601. [Google Scholar] [CrossRef]
- Homer, E.R.; Patala, S.; Priedeman, J.L. Grain boundary plane orientation fundamental zones and structure-property relationships. Sci. Rep. 2015, 5, 15476. [Google Scholar] [CrossRef]
- Davenport, A.J.; Yuan, Y.; Ambat, R.; Connolly, B.J.; Strangwood, M.; Afseth, A.; Scamans, G.M. Intergranular corrosion and stress corrosion cracking of sensitised AA5182. Mater. Sci. Forum 2006, 519–521, 641–646. [Google Scholar] [CrossRef]
- Kaigorodova, L. The effect of grain-boundary structure formation on β-precipitation in aged Al-Mg alloys. Mater. Sci. Forum 1999, 294–296, 477–480. [Google Scholar] [CrossRef]
- D’Antuono, D.S.; Gaies, J.; Golumbfskie, W.; Taheri, M. Grain boundary misorientation dependence of β phase precipitation in an Al–Mg alloy. Scr. Mater. 2014, 76, 81–84. [Google Scholar] [CrossRef]
- Brandon, D. The structure of high-angle grain boundaries. Acta Metall. 1966, 14, 1479–1484. [Google Scholar] [CrossRef]
- Zhao, Y.; Polyakov, M.N.; Mecklenburg, M.; Kassner, M.E.; Hodge, A.M. The role of grain boundary plane orientation in the β phase precipitation of an Al–Mg alloy. Scr. Mater. 2014, 89, 49–52. [Google Scholar] [CrossRef]
- Zhao, Y. The Role of Nanotwins and Grain Boundary Plane in the Thermal, Corrosion, and Sensitization Behavior of Nanometals. Ph.D. Thesis, University of Southern California, Los Angeles, CA, USA, 2014. [Google Scholar]
- Klostermann, J. The concept of the habit plane and the phenomenological theories of the martensite transformation. J. Less Common Met. 1972, 28, 75–94. [Google Scholar] [CrossRef]
- Baur, A.P.; Cayron, C.; Logé, R.E. {225}(γ) habit planes in martensitic steels: From the PTMC to a continuous model. Sci. Rep. 2017, 7, 40938. [Google Scholar] [CrossRef]
- Rusakov, G.M.; Lobanov, M.L.; Redikultsev, A.A.; Kagan, I.V. Retention of the Twinning Σ3 Misorientation in the Process of Lattice Transformation during Cold Rolling of a Fe3 Pct Si Single Crystal. Metall. Mater. Trans. A 2011, 42, 1435–1438. [Google Scholar] [CrossRef]
- Wood, R.M.; Entwisle, A.R. Martensite habit planes in Fe-Mn-C alloys. Met. Sci. 1976, 10, 72–76. [Google Scholar] [CrossRef]
- Dai, C.; Balogh, L.; Yao, Z.; Daymond, M.R. The habit plane of <a>-type dislocation loops in α-zirconium: An atomistic study. Philos. Mag. 2017, 97, 944–956. [Google Scholar] [CrossRef]
- Cayron, C. Continuous atomic displacements and lattice distortions during martensitic transformations in fcc-bcc-hcp systems. arXiv 2015, arXiv:1502.07152. [Google Scholar]
- Zhang, Y.-W. Mechanical properties: Nanotwins only. Nat. Nano 2012, 7, 551–552. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, W.; Qiu, D.; Zhang, M.-X.; Pan, F. Grain refinement of Ca addition in a twin-roll-cast Mg–3Al–1Zn alloy. Mater. Chem. Phys. 2012, 133, 611–616. [Google Scholar] [CrossRef]
- Jeong, J.; Im, J.; Song, K.; Kwon, M.; Kim, S.K.; Kang, Y.-B.; Oh, S.H. Transmission electron microscopy and thermodynamic studies of CaO-added AZ31 Mg alloys. Acta Mater. 2013, 61, 3267–3277. [Google Scholar] [CrossRef]
- Prasad, A.; Shi, Z.; Atrens, A. Influence of Al and Y on the ignition and flammability of Mg alloys. Corros. Sci. 2012, 55, 153–163. [Google Scholar] [CrossRef]
- Czerwinski, F. Overcoming Barriers. Adv. Mater. Process. 2014, 28, 28–31. [Google Scholar]
- Czerwinski, F. Controlling the ignition and flammability of magnesium for aerospace applications. Corros. Sci. 2014, 86, 1–16. [Google Scholar] [CrossRef]
- Medved, J.; Mrvar, P.; Vončina, M. Oxidation resistance of cast magnesium alloys. Oxid. Met. 2009, 71, 257–270. [Google Scholar] [CrossRef]
- Tan, Q.; Atrens, A.; Mo, N.; Zhang, M.-X. Oxidation of magnesium alloys at elevated temperatures in air: A review. Corros. Sci. 2016, 112, 734–759. [Google Scholar] [CrossRef]
- Czerwinski, F. Oxidation characteristics of magnesium alloys. JOM 2012, 64, 1477–1483. [Google Scholar] [CrossRef]
- Zhao, W.; Sun, Y.; Li, H.; Liang, C. The effects of some elements on the igniting temperature of magnesium alloys. Mater. Sci. Eng. B 2006, 127, 105–107. [Google Scholar]
- Czerwinski, F. The early stage oxidation and evaporation of Mg–9% Al–1% Zn alloy. Corros. Sci. 2004, 46, 377–386. [Google Scholar] [CrossRef]
- Tan, Q.; Mo, N.; Jiang, B.; Pan, F.; Atrens, A.; Zhang, M.-X. Oxidation resistance of Mg–9Al–1Zn alloys micro-alloyed with Be. Scr. Mater. 2016, 115, 38–41. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Q.; Lü, Y.; Ding, W.; Zhu, Y.; Zhai, C.; Lu, C.; Xu, X. Behavior of surface oxidation on molten Mg–9Al–0.5 Zn–0.3 Be alloy. Mater. Sci. Eng., A 2001, 301, 154–161. [Google Scholar] [CrossRef]
- Inoue, S.-I.; Yamasaki, M.; Kawamura, Y. Formation of an incombustible oxide film on a molten Mg-Al-Ca alloy. Corros. Sci. 2017, 122, 118–122. [Google Scholar] [CrossRef]
- Aydin, D.S.; Bayindir, Z.; Hoseini, M.; Pekguleryuz, M.O. The high temperature oxidation and ignition behavior of Mg–Nd alloys part I: The oxidation of dilute alloys. J. Alloys Compd. 2013, 569, 35–44. [Google Scholar] [CrossRef]
- Cheng, X.; Fan, L.; Liu, L.; Du, K.; Wang, D. Effect of doping aluminum and yttrium on high-temperature oxidation behavior of Ni-11Fe-10Cu alloy. J. Rare Earth 2016, 34, 1139–1147. [Google Scholar] [CrossRef]
- Liu, X.; Shan, D.; Song, Y.; Han, E.-H. Influence of yttrium element on the corrosion behaviors of Mg–Y binary magnesium alloy. J. Magnes. Alloys 2017, 5, 26–34. [Google Scholar] [CrossRef]
- Fan, Q.; Yu, H.; Wang, T.; Liu, Y. Microstructure and Oxidation Resistance of a Si Doped Platinum Modified Aluminide Coating Deposited on a Single Crystal Superalloy. Coatings 2018, 8, 264. [Google Scholar] [CrossRef]
- Fan, Q.; Peng, X.; Yu, H.; Jiang, S.; Gong, J.; Sun, C. The isothermal and cyclic oxidation behaviour of two Co modified aluminide coatings at high temperature. Corros. Sci. 2014, 84, 42–53. [Google Scholar] [CrossRef]
- Azarmehr, S.A.; Shirvani, K.; Schütze, M.; Galetz, M. Microstructural evolution of silicon-platinum modified aluminide coatings on superalloy GTD-111. Surf. Coat. Technol. 2017, 321, 455–463. [Google Scholar] [CrossRef]
- Bautista, A.; Velasco, F.; Campos, M.; Torralba, J.M. Low-temperature and high-temperature corrosion behaviour of powder metallurgical duplex stainless steels. Mater. Sci. Forum 2003, 426, 4367–4372. [Google Scholar] [CrossRef]
- Lin, X.; Liang, X.; Li, Y.; He, K.; Du, P.; Zhang, R.; Chen, P. Microstructural understanding of general and localized corrosion of Fe13Cr4Al2Mo1.2Nb alloy in high temperature aerated water and superheated steam. Corros. Sci. 2022, 203, 110341. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Yu, X.; Tang, D. Role of Cr in the high-temperature oxidation behavior of CrxMnFeNi high-entropy alloys at 800 °C in air. Corros. Sci. 2022, 200, 110211. [Google Scholar] [CrossRef]
- Singh, A.N.; Moitra, A.; Bhaskar, P.; Sasikala, G.; Dasgupta, A.; Bhaduri, A.K. Effect of thermal aging on microstructure, hardness, tensile and impact properties of Alloy 617. Mater. Sci. Eng. A 2018, 710, 47–56. [Google Scholar] [CrossRef]
- Singh, A.N.; Moitra, A.; Bhaskar, P.; Dasgupta, A.; Sasikala, G.; Bhaduri, A.K. A Study of Tensile Flow and Work-Hardening Behavior of Alloy 617. J. Mater. Eng. Perform. 2018, 27, 3812–3823. [Google Scholar] [CrossRef]
- Haider, S.B.; Heon, E.; Neveau, M.; Chen, P.; Houston, A.; Rios, O.; Lass, E.A. Castable eutectic Ni–Ce high temperature alloys strengthened by γ/γʹ microstructure. J. Mater. Res. Technol. 2024, 28, 3943–3950. [Google Scholar] [CrossRef]
- Evans, D.; Chen, J.; Bokas, G.; Chen, W.; Hautier, G.; Sun, W. Visualizing temperature-dependent phase stability in high entropy alloys. npj Comput. Mater. 2021, 7, 151. [Google Scholar] [CrossRef]
- Fu, T.; Shen, F.; Zhang, Y.; Yu, L.; Cui, K.; Wang, J.; Zhang, X. Oxidation Protection of High-Temperature Coatings on the Surface of Mo-Based Alloys—A Review. Coatings 2022, 12, 141. [Google Scholar] [CrossRef]
- Narita, T.; Kato, Y.; Narita, T.; Ara, M. Advances in Diffusion Barrier Coatings for High-Temperature Applications. High Temp. Corros. Mater. 2023, 100, 399–411. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Wu, J.; Wang, C.; Hou, Z.; Tang, Y.; Liu, Z.; Ouyang, X. Improvement of corrosion resistance of Ni-based alloy by adding 5 wt.% rhenium. Mater. Today Commun. 2023, 35, 106387. [Google Scholar] [CrossRef]
- Mortazavi, N.; Geers, C.; Esmaily, M.; Babic, V.; Sattari, M.; Lindgren, K.; Malmberg, P.; Jönsson, B.; Halvarsson, M.; Svensson, J.E.; et al. Interplay of water and reactive elements in oxidation of alumina-forming alloys. Nat. Mater. 2018, 17, 610–617. [Google Scholar] [CrossRef]
- Eswarappa Prameela, S.; Pollock, T.M.; Raabe, D.; Meyers, M.A.; Aitkaliyeva, A.; Chintersingh, K.-L.; Cordero, Z.C.; Graham-Brady, L. Materials for extreme environments. Nat. Rev. Mater. 2023, 8, 81–88. [Google Scholar] [CrossRef]
- Corrosion Protective Coatings Market Size, Share, and Trends 2024 to 2033; Persistence Market Research: London, UK, 2024.
- Kania, H. Corrosion and Anticorrosion of Alloys/Metals: The Important Global Issue. Coatings 2023, 13, 216. [Google Scholar] [CrossRef]
- Singh, R.; Mandal, G.; Bhagat, A.; Misra, S. Special Issue on Corrosion and Coating Technology. Trans. Indian Inst. Met. 2024, 77, 1247–1248. [Google Scholar] [CrossRef]
- Xie, J.; Ma, X.; Kuang, W. Improving the corrosion resistance of FeCrAl alloy in high temperature hydrogenated water through gaseous pre-oxidation. Corros. Commun. 2024, 15, 36–41. [Google Scholar] [CrossRef]
- Tang, C.; Große, M.; Ulrich, S.; Klimenkov, M.; Jäntsch, U.; Seifert, H.J.; Stüber, M.; Steinbrück, M. High-temperature oxidation and hydrothermal corrosion of textured Cr2AlC-based coatings on zirconium alloy fuel cladding. Surf. Coat. Technol. 2021, 419, 127263. [Google Scholar] [CrossRef]
- Li, M.; Henein, H.; Zhou, C.; Liu, J. Towards high-entropy alloys with high-temperature corrosion resistance and structural stability. J. Mater. Sci. Technol. 2024, 174, 133–144. [Google Scholar] [CrossRef]
- Khantisopon, K.; Singh, S.; Jitputti, J.; Berndt, C.C.; Ang, A.S.M. High Temperature Corrosion Resistant and Anti-slagging Coatings for Boilers: A Review. High Temp. Corros. Mater. 2024, 101, 1–55. [Google Scholar] [CrossRef]
- Gao, Y.; Chong, K.; Liu, C.; Cao, Y.; Wu, D.; Zou, Y. Electrochemical corrosion and high temperature hot corrosion behavior of NbTaTiV and CrNbTaTiV high entropy alloy. J. Mater. Res. Technol. 2024, 28, 216–234. [Google Scholar] [CrossRef]
- Gunduz, K.O.; Visibile, A.; Sattari, M.; Fedorova, I.; Saleem, S.; Stiller, K.; Halvarsson, M.; Froitzheim, J. The effect of additive manufacturing on the initial High temperature oxidation properties of RE-containing FeCrAl alloys. Corros. Sci. 2021, 188, 109553. [Google Scholar] [CrossRef]
- Wenga, T.; Macdonald, D.D.; Ma, W. Multi-scale computational study of high-temperature corrosion and the design of corrosion-resistant alloys. Prog. Mater. Sci. 2024, 148, 101359. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Maghraby, Y.R.; Ibrahim, A.H.; Shouier, K.R.; Alturki, A.M.; El-Shabasy, R.M. Role of green chemistry in sustainable corrosion inhibition: A review on recent developments. Mater. Today Sustain. 2022, 20, 100242. [Google Scholar] [CrossRef]

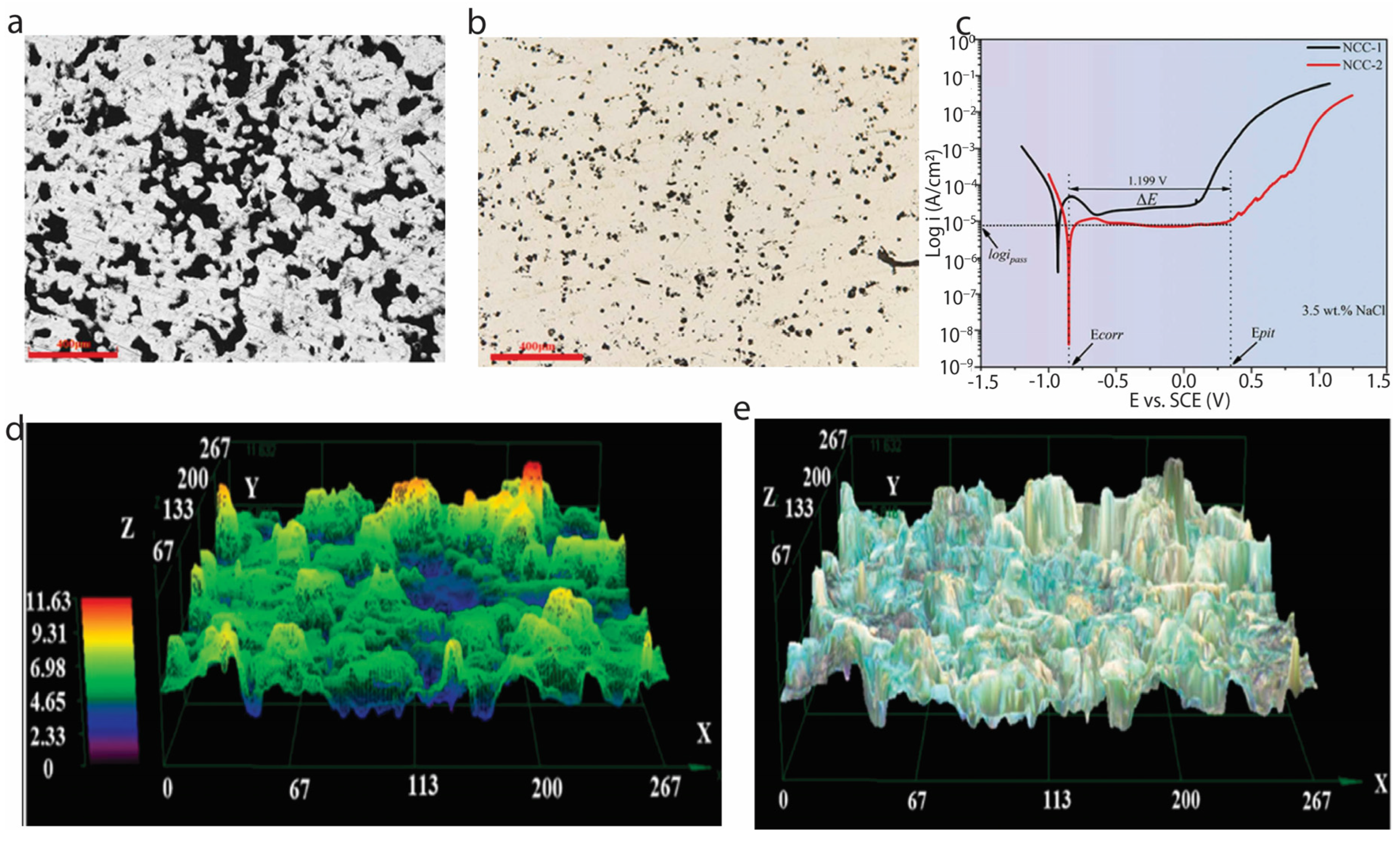
| Alloy | Treatment Method | Hydrophobic Agent | Contact Angle | Corrosion Inhibition Efficiency (CIE), % | Coating Corrosion Current (Ic), A/cm2 | Bare Steel Corrosion Current (I₀), A/cm2 | Key Observations | Ref. |
|---|---|---|---|---|---|---|---|---|
| 316SS | H2O2, HF etching + PFOS modification | PFOS | 161.78 | 83.5 | 3.965 × 10−7 | 2.4 × 10−6 | Achieved high corrosion resistance and maintained hydrophobicity for 3 months in ambient. | [95] |
| AISI 420SS | 515 nm pulsed laser + stearic acid | Stearic acid | 163 | Initial corrosion tolerance; reduced after 30 days in 0.5 M NaCl. | [98] | |||
| 1095 Carbon steel | 1064 nm laser with 20 ns pulse | Perfluorooctylsilane | 160 | 94.6 | 2.9 × 10−8 | 5.4 × 10−7 | Achieved superhydrophobicity with high corrosion resistance. | [100] |
| Base Material | Alloying Element(s) | Corrosion Resistance Improvement | Mechanism | Ref. |
|---|---|---|---|---|
| Mg alloy | Be | Increased ignition temperature to 1033 K; enhanced oxidation resistance | Formation of stable BeO; reduction in inclusion impurities; Be2⁺ segregation inhibits Mg2⁺ migration | [169,170,171,172,173] |
| Mg alloy | Ce, Nd | Improved oxidation resistance and passive film stability | Formation of CeO2/Nd2O3 layers at GBs; inhibition of Mg2⁺ cation migration | [168,175] |
| Mg-Y alloys | Y (1–5 wt.%) | Corrosion resistance increased to 6945 Ω·cm2; reduced current density (4.01 µA·cm2) | Absence of Mg24Y5 phase; suppression of structural impurities | [176,177] |
| Ni-based superalloys | Si (in PtSiAl coating) | Enhanced oxidation resistance at 1373 K for 320 h | Formation of silicides; retardation of voids and phase transitions; stabilization of Al2O3 layer | [178,179,180] |
| Metallic Addition | Alloy Type | Environment | Outcome | Ref. |
|---|---|---|---|---|
| Cr | Ni-based | High-temperature oxidations | Formation of protective Cr2O3 layer; enhances oxidation resistance. | [73] |
| Al | Ni-based | High-temperature | Formation of Al2O3 oxide layer, providing a dense protective barrier. | [6] |
| Mo | CoCrFeNi HEA | 3.5% NaCl solution | Formation of stable Mo-oxides; reduces pitting. | [74] |
| Cu | Al alloy | Acid rain (200 ppm Cl−) | Formation of Al2Cu phase; with high cathodic current density. | [81] |
| W | High-temperature alloy | High-temperature alloy | Selective oxidation; improves mechanical stability. | [88] |
| Co | Co-Al-W alloy | High-temperature air | Formation of Co3O4 and Al2O3 for dual-layer protection. | [87] |
| Y | Mg-Y alloy | 3.5% NaCl solution | Formation of stable Y oxides; prevents Mg2+ migration. | [176,177] |
| Si | Ni-based superalloy (PtSiAl) | High-temperature air | Formation of silicides; controls phase formation. | [179] |
| Be | My alloys | High-temperature oxidizing environments | Forms BeO, which stabilizes MgO layer, and reduces oxidation rate and internal stress, enhancing oxidation resistance. | [170] |
| Re | Ni-based superalloys | NaCl solution | Forms passivation layer, and enhances corrosion resistance by reducing current density and promoting impedance. | [190] |
| Ti | Ni-based superalloys | High-temperature air | Contributes to passive oxide layer formation, enhancing oxidation resistance at elevated temperatures. | [64] |
| Ce | Ni alloys | High-temperature oxidizing environments | Acts as a deoxidizing agent. | [186] |
| Nd | Mg alloys | High-temperature oxidizing environments | Forms Nd2O3 layer, enhancing corrosion resistance by stabilizing the passive film. | [175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.N.; Swain, S.K.; Meena, A.; Islam, M.; Nam, K.-W. Advances in Corrosion of High-Temperature Materials: Interfacial Migration and Alloy Design Strategies. Ceramics 2024, 7, 1928-1963. https://doi.org/10.3390/ceramics7040121
Singh AN, Swain SK, Meena A, Islam M, Nam K-W. Advances in Corrosion of High-Temperature Materials: Interfacial Migration and Alloy Design Strategies. Ceramics. 2024; 7(4):1928-1963. https://doi.org/10.3390/ceramics7040121
Chicago/Turabian StyleSingh, Aditya Narayan, Shashwat Kumar Swain, Abhishek Meena, Mobinul Islam, and Kyung-Wan Nam. 2024. "Advances in Corrosion of High-Temperature Materials: Interfacial Migration and Alloy Design Strategies" Ceramics 7, no. 4: 1928-1963. https://doi.org/10.3390/ceramics7040121
APA StyleSingh, A. N., Swain, S. K., Meena, A., Islam, M., & Nam, K.-W. (2024). Advances in Corrosion of High-Temperature Materials: Interfacial Migration and Alloy Design Strategies. Ceramics, 7(4), 1928-1963. https://doi.org/10.3390/ceramics7040121









