Long Term Post-Fire Vegetation Dynamics in North-East Mediterranean Ecosystems. The Case of Mount Athos Greece
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. In Situ Sampling and Data Collection
2.3. Plant Community Identification
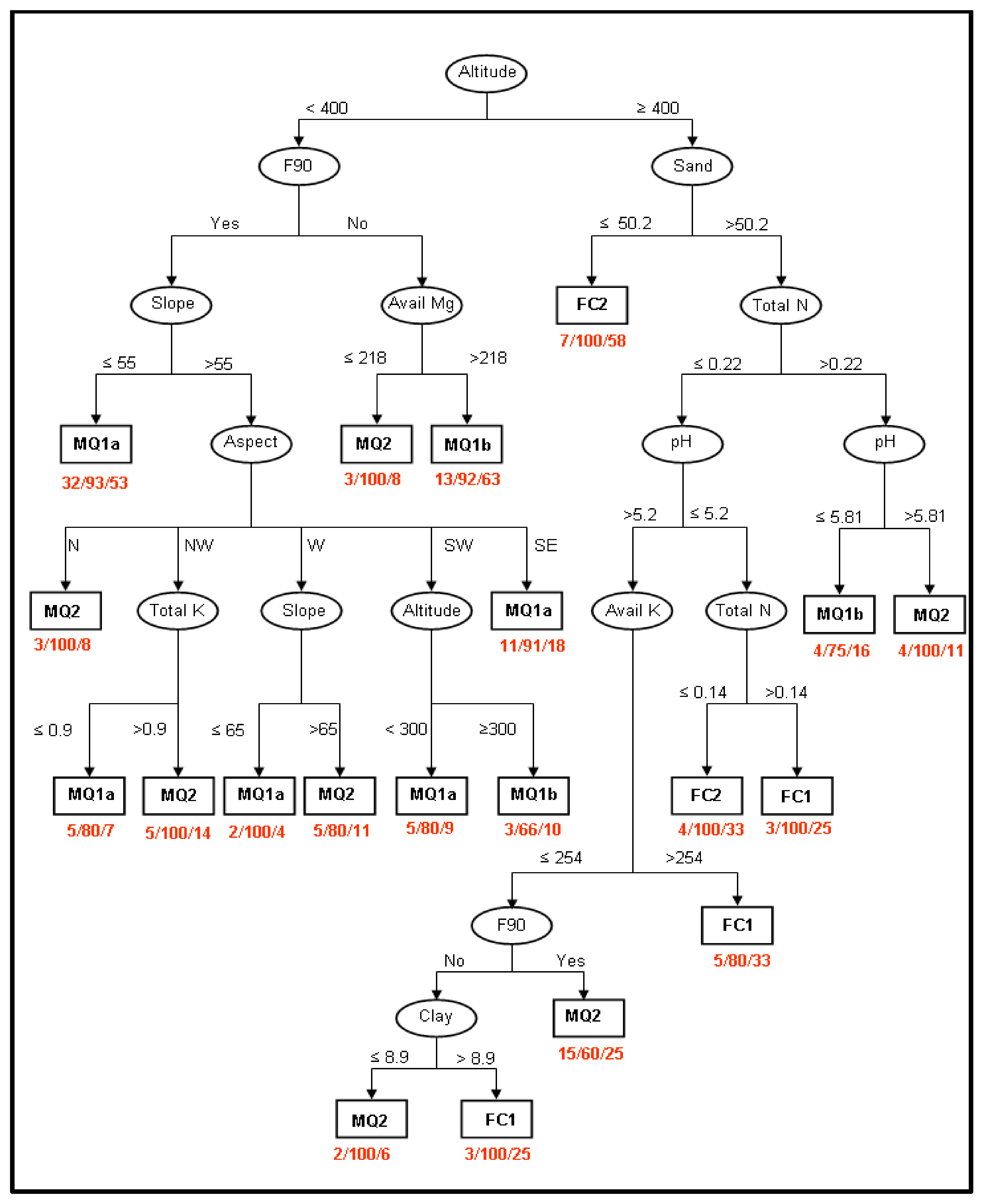
2.4. Identification of the Factors Determining Community Distribution-Hypothesis Generation
2.5. Remote Sensing Data
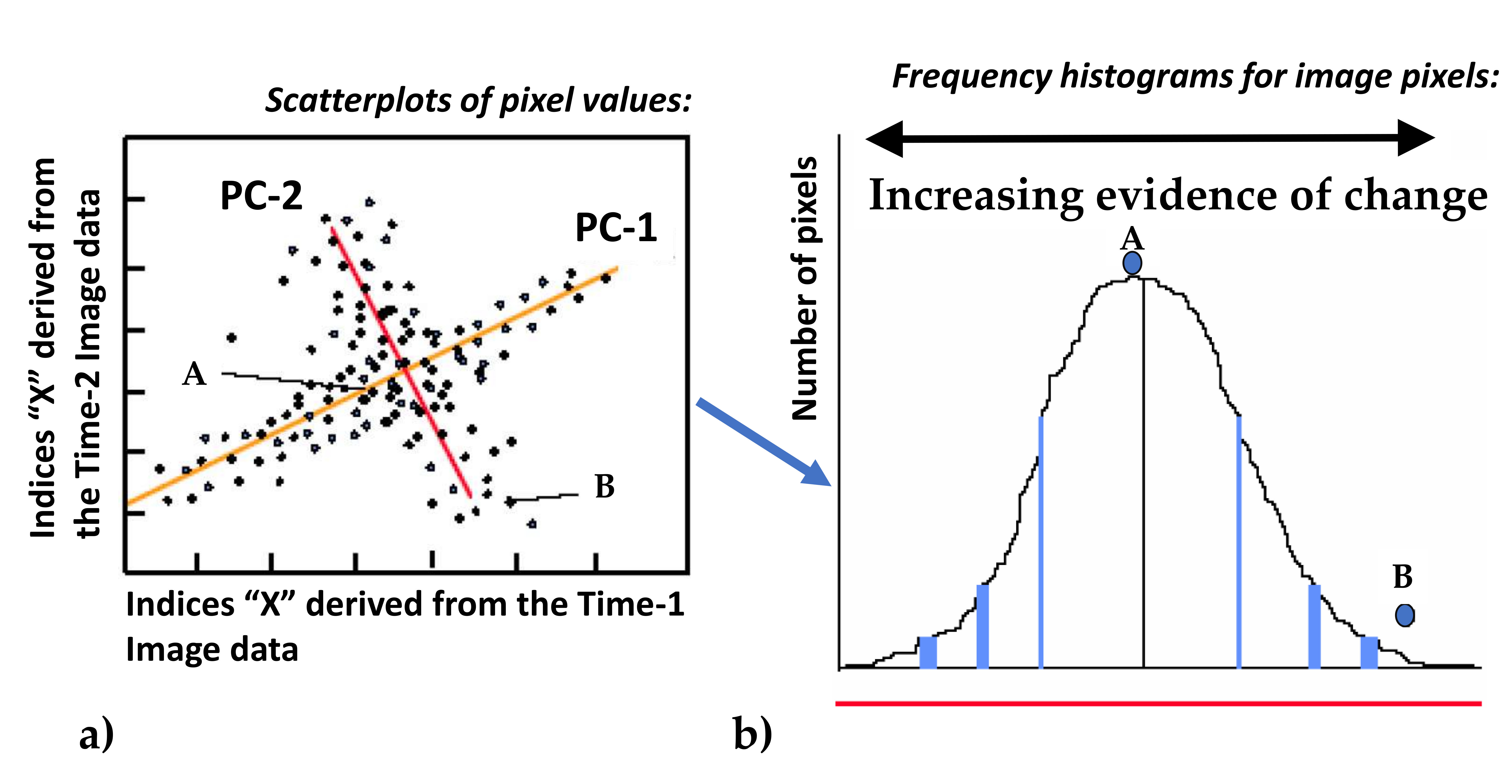
2.6. Remote Sensing Methods
- NIR = Reflective Near infrared band
- R = Red band.
3. Results
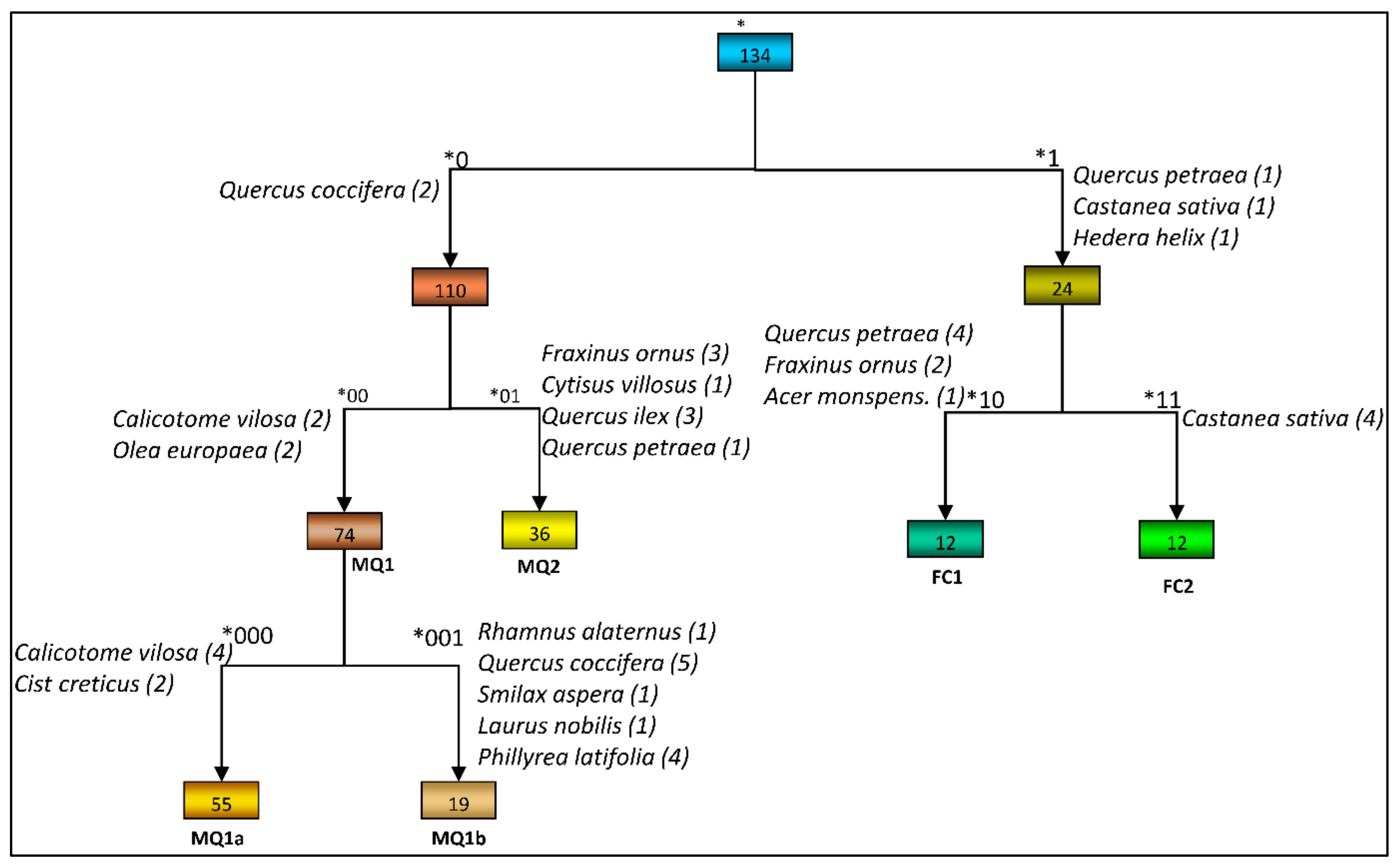
3.1. Plant Community Identification
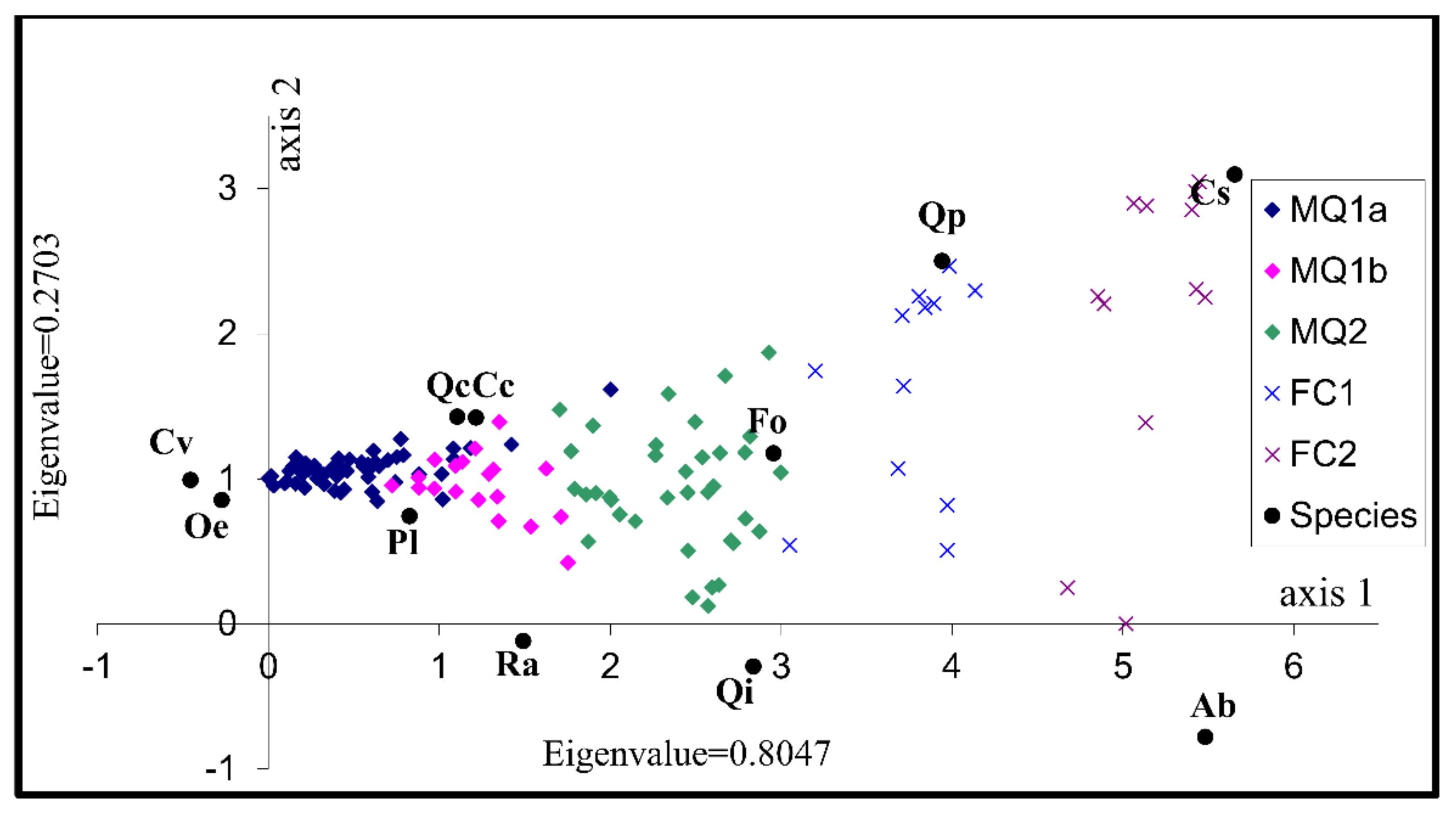
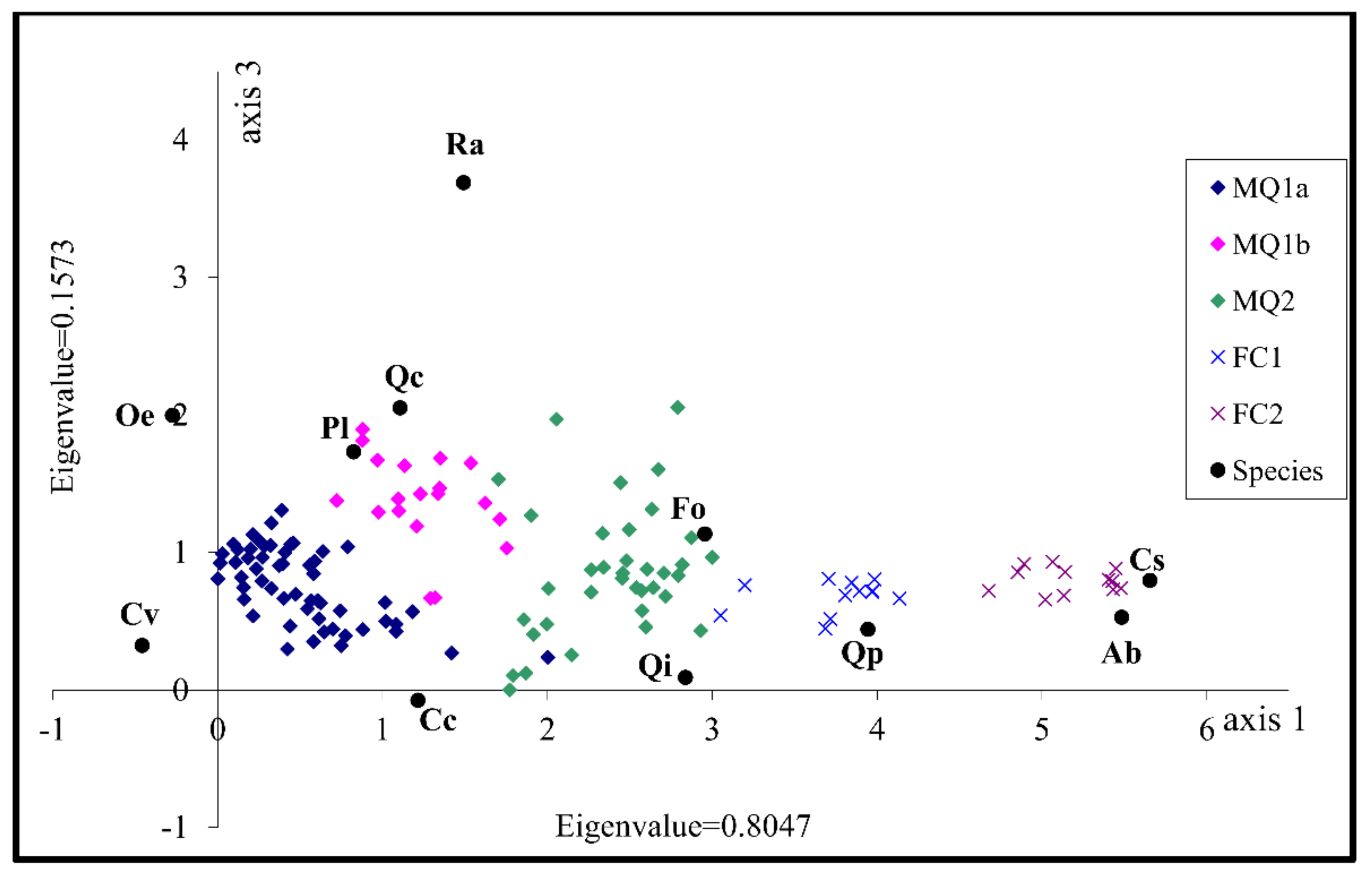
3.2. Identification of the Factors Determining Community Distribution-Hypothesis Generation
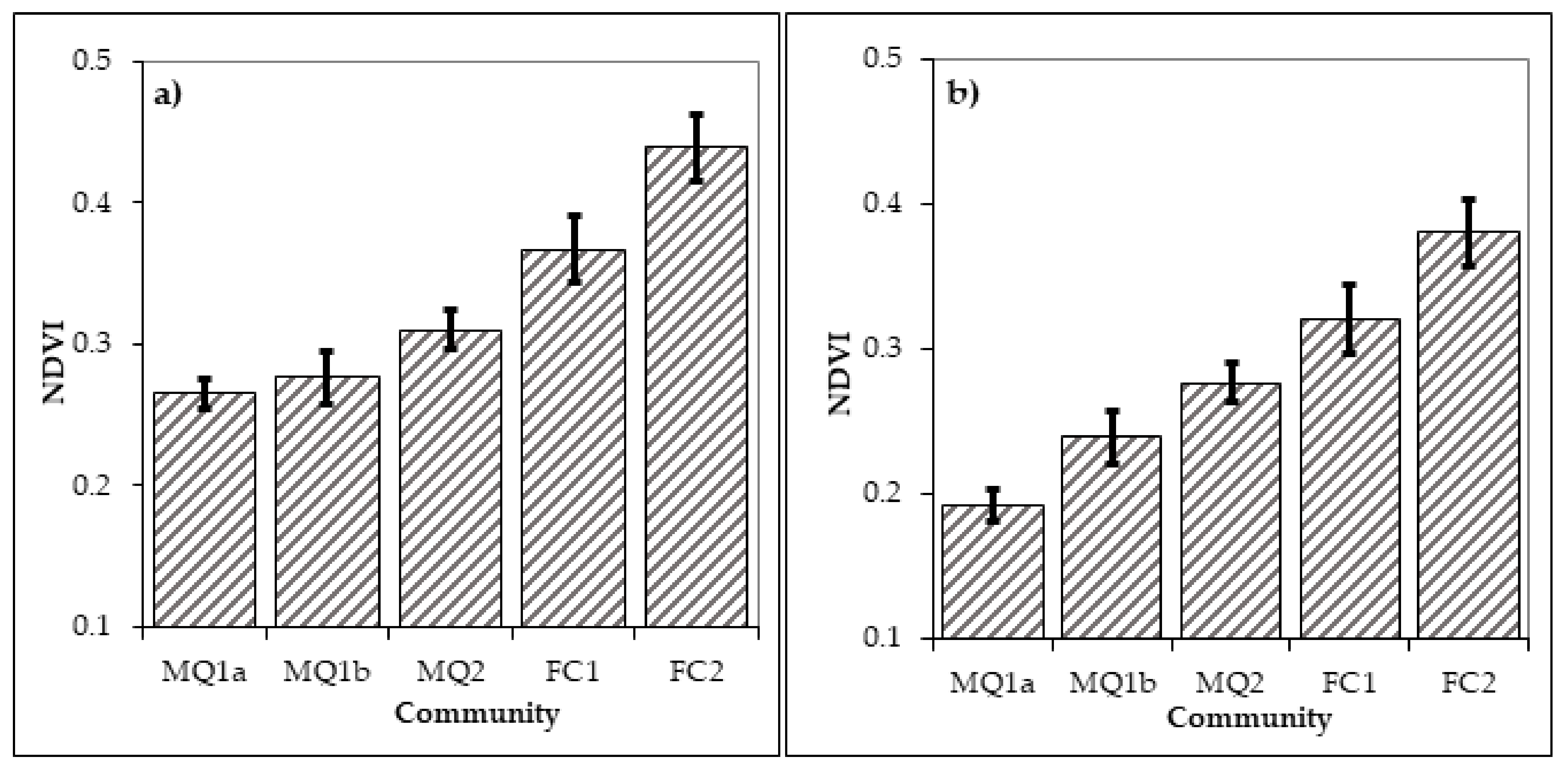
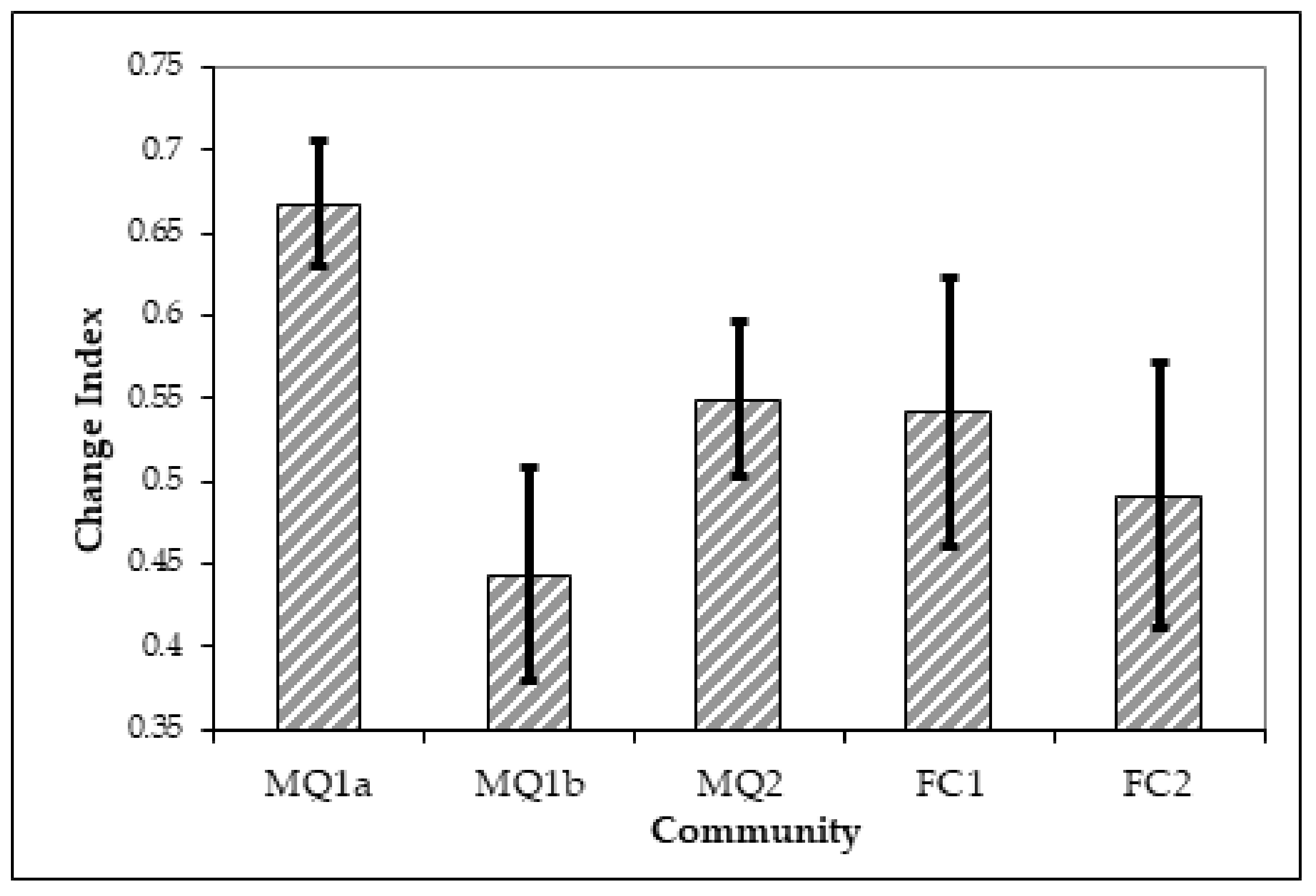
3.3. Distribution of Plant Communities and Sub-Communities before and after the Fire of 1990
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davis, A.M. Wetland succession, fire and the pollen record: A midwestern example. Am. Midl. Nat. 1979, 102, 86–94. [Google Scholar] [CrossRef]
- Trabaud, L. Fire and the survival traits of plants. In The Role of Fire in Ecological Systems; Trabaud, L., Ed.; SPB Academic Publishing: The Hague, The Netherlands, 1987; pp. 65–89. [Google Scholar]
- Trabaud, L. Postfire Plant Community Dynamics in the Mediterranean Basin. In The Role of Fire in Mediterranean-Type Ecosystems; Moreno, J.M., Oechel, W.C., Eds.; Ecological Studies; Springer: New York, NY, USA, 1994; Volume 107, pp. 1–15. [Google Scholar]
- Whelan, R.J. The Ecology of Fire; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Edwards, D. Fire Regimes in the Biomes of South Africa. In Ecological Effects of Fire in South African Ecosystems; Booysen, P.D.V., Tainton, N.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 19–37. [Google Scholar]
- Kruger, F.J.; Bigalke, R.C. Fire in Fynbos. In Ecological Effects of Fire in South African Ecosystems; Booysen, P.D.V., Tainton, N.M., Eds.; Ecological Studies; Springer: Heidelberg, Germany, 1984; Volume 48, pp. 67–114. [Google Scholar]
- Trabaud, L.V.; Christensen, N.L.; Gill, A.M. Historical biogeography of fire in temperate and mediterranean ecosystems. In Fire in the Environment: Its Ecological and Atmospheric Importance; Grutzen, P.J., Goldamer, J.G., Eds.; John Wiley: New York, NY, USA, 1993; pp. 277–295. [Google Scholar]
- Van der Werf, G.R.; Randerson, J.T.; Giglio, L.; Collatz, G.J.; Mu, M.; Kasibhatla, P.S.; Morton, D.C.; DeFries, R.S.; Jin, Y.; van Leeuwen, T.T. Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009). Atmos. Chem. Phys. 2010, 10, 11707–11735. [Google Scholar] [CrossRef] [Green Version]
- Bowman, D.M.J.S.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; Defries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth System. Science 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, S.; Kaplan, J.O. Sensitivity of global wildfire occurrences to various factors in the context of global change. Atmos. Environ. 2015, 121, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Naveh, Z. Fire in the Mediterranean—A landscape ecological perspespective. In Fire in Ecosystems Dunamics; Goldammer, J.G., Jenkins, M.J., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1990; pp. 1–20. [Google Scholar]
- Naveh, Z. The evolutionary significance of fire in the Mediterranean region. Vegetatio 1975, 29, 199–208. [Google Scholar] [CrossRef]
- Naveh, Z. The role of fire as an evolutionary and ecological factor on the landscapes and vegetation of Mt. Carmel. J. Mediterr. Ecol. 1999, 1, 11–25. [Google Scholar]
- Xofis, P.; Konstantinidis, P.; Papadopoulos, I.; Tsiourlis, G. Integrating Remote Sensing Methods and Fire Simulation Models to Estimate Fire Hazard in a South-East Mediterranean Protected Area. Fire 2020, 3, 31. [Google Scholar] [CrossRef]
- Xofis, P.; Tsiourlis, G.; Konstantinidis, P. A Fire Danger Index for the early detection of areas vulnerable to wildfires in the Eastern Mediterranean region. Euro-Mediterr. J. Environ. Integr. 2020, 5, 32. [Google Scholar] [CrossRef]
- Suc, J.P. Origin and evolution of the Mediterranean vegetation and climate in Europe. Nature 1984, 307, 429–432. [Google Scholar] [CrossRef]
- Hanes, T.L. Succession after fire in the chaparral of southern California. Ecol. Monogr. 1971, 41, 27–52. [Google Scholar] [CrossRef]
- Kazanis, D.; Arianoutsou, M. Post-fire succession in Pinus halepensis Mill. forests: Plant diversity. In Proceedings of the 7th Scientific Conference of the Hellenic Botanical Society, Alexandroupolis, Greece, 1–4 October 1998; pp. 169–172. [Google Scholar]
- Trabaud, L. Fire resistance of Quercus coccifera L. garrigue. In Fire in Ecosystem Dynamics. Mediterranean and Northern Perspectives; Goldamer, J.G., Jenkins, M.J., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1990; pp. 21–32. [Google Scholar]
- Trabaud, L.; Lepart, J. Changes in the floristic composition of a Quercus coccifera L. garrigue in relation to different fire regimes. Vegetatio 1981, 46, 105–116. [Google Scholar] [CrossRef]
- Arianoutsou, M. Aspects of demography in post-fire Mediterranean plant communities. In Landscape Disturbance and Biodiversity in Mediterranean-Type Ecosystems; Rundel, P.W., Montenegro, G., Jaksic, F.M., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 1998; Volume 136, pp. 273–295. [Google Scholar]
- Bond, W.J.; Midgley, J.J. Ecology of resprouting in woody plants: The persistence niche. Trends Ecol. Evol. 2001, 16, 45–51. [Google Scholar] [CrossRef]
- Bond, W.; van Wilgen, B.W. Fire and Plants; Chapman & Hall: London, UK, 1996. [Google Scholar]
- Pausas, J.G.; Verdu, M. Plant persistence traits in fire-prone ecosystems of the Mediterranean basin: A phylogenetic approach. Oikos 2005, 109, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Pausas, J.G.; Keeley, J.E.; Verdu, M. Inferring differential evolutionary processes of plant persistence traits in Northern Hemisphere Mediterranean fire-prone ecosystems. J. Ecol. 2006, 94, 31–39. [Google Scholar] [CrossRef]
- Gunster, A. Aerial seed banks in the central Namib: Distribution of serotinus plants in relation to climate and habitat. J. Biogeogr. 1992, 19, 563–572. [Google Scholar] [CrossRef]
- Bellingham, P.J.; Tanner, E.V.J.; Healey, J.R. Sprouting of trees in Jamaican montane forests, after hurricane. J. Ecol. 1994, 82, 747–758. [Google Scholar] [CrossRef]
- Trabaud, L. Fire regimes and phytomass growth dynamics in a Quercus coccifera garrigue. J. Veg. Sci. 1991, 2, 307–314. [Google Scholar] [CrossRef]
- Trabaud, L.; Lepart, J. Diversity and stability in garrigue ecosystems after fire. Vegetatio 1980, 43, 49–57. [Google Scholar] [CrossRef]
- Malanson, G.P.; Trabaud, L. Vigour of post-fire resprouting by Quercus coccifera L. J. Ecol. 1988, 76, 351–365. [Google Scholar] [CrossRef]
- Rego, F.; Pereira, J.; Trabaud, L. Modeling community dynamics of a Quercus coccifera L. garrigue in relation to fire using Markov-Chains. Ecol. Model. 1993, 66, 251–260. [Google Scholar] [CrossRef]
- Delitti, W.; Ferran, A.; Trabaud, L.; Vallejo, V.R. Effects of fire recurrence in Quercus coccifera L. shrublands of the Valencia Region (Spain): I. plant composition and productivity. Plant Ecol. 2005, 177, 57–70. [Google Scholar] [CrossRef]
- Lloret, F.; Vila, M. Clearing of vegetation in Mediterranean garrigue: Response after a wildfire. For. Ecol. Manag. 1997, 93, 227–234. [Google Scholar] [CrossRef]
- Ferran, A.; Delitti, W.; Vallejo, V.R. Effects of fire recurrence in Quercus coccifera L. shrublands of the Valencia Region (Spain): II. plant and soil nutrients. Plant Ecol. 2005, 177, 71–83. [Google Scholar] [CrossRef]
- Pausas, J.G.; Carbo, E.; Caturla, R.; Gil, J.M.; Vallejo, R. Post-fire regeneration patterns in the eastern Iberian Peninsula. Acta Oecol. 1999, 20, 499–508. [Google Scholar] [CrossRef]
- Clemente, A.S.; Rego, F.C.; Correia, O.A. Demographic patterns and productivity of post-fire regeneration in Portuguese Mediterranean maquis. Int. J. Wildland Fire 1996, 6, 5–12. [Google Scholar] [CrossRef]
- Arianoutsou-Faraggitaki, M. Post-fire successional recovery of a phryganic (East Mediterranean) ecosystem. Acta Oecol. 1984, 5, 387–394. [Google Scholar]
- Arianoutsou-Faraggitaki, M.; Margaris, N.S. Producers and the fire cycle in a phryganic ecosystem. In Components of Productivity of Mediterranean-Climate Regions—Basic and Applied; Margaris, N.S., Mooney, H.A., Eds.; Dr. W. Junk Publishers: The Hague, The Netherlands; Boston, MA, USA; London, UK, 1981; pp. 181–190. [Google Scholar]
- Arianoutsou-Faraggitaki, M.; Margaris, N.S. Phryganic (East Mediterranean) ecosystems and fire. Ecol. Mediterr. 1982, 8, 473–480. [Google Scholar] [CrossRef]
- Papanastasis, V.P. Effects of season and frequency of burning on a phryganic rangeland in Greece. J. Range Manag. 1980, 33, 251–255. [Google Scholar] [CrossRef]
- Henkin, Z.; Selingman, N.; Noy-Meir, I.; Kafkafi, U. Secondary succession after fire in a Mediterranean dwarf-shrub community. J. Veg. Sci. 1999, 10, 503–514. [Google Scholar] [CrossRef]
- Seligman, N.; Henkin, Z. Regeneration of a dominant Mediterranean dwarf-shrub after fire. J. Veg. Sci. 2000, 11, 893–902. [Google Scholar] [CrossRef]
- Tsitsoni, T. Conditions determining the natural regeneration after wildfires in the Pinus halepensis (Miller, 1768) forests of Kassandra Peninsula (North Greece). For. Ecol. Manag. 1997, 92, 199–208. [Google Scholar] [CrossRef]
- Daskalakou, E.N.; Thanos, C.A. Aleppo pine (Pinus halepensis) postfire regeneration: The role of canopy and soil seed banks. Int. J. Wildland Fire 1996, 6, 59–66. [Google Scholar] [CrossRef]
- Kazanis, D.; Arianoutsou, M. Contribution of legumes in the post-fire succession of Pinus halepensis forests in Attica, Greece. In Proceedings of the Hellenic Botanical Society-5th Scientific Conference, Delphi, Greece, 21–23 October 1994; pp. 259–264. [Google Scholar]
- Thanos, C.A.; Skordilis, A.; Daskalakou, E.N. Comparative ecophysiology of the postfire regeneration in the Mediterranean pines Pinus halepensis and Pinus brutia. In Proceedings of the Hellenic Botanical Society-5th Scientific Conference, Delphi, Greece, 21–23 October 1994; pp. 183–186. [Google Scholar]
- Tsitsoni, T.; Ganatsas, P.; Zagas, T.; Tsakaldimi, M. Dynamics of postfire regeneration of Pinus brutia Ten. in an artificial forest ecosystem of northern Greece. Plant Ecol. 2004, 171, 165–174. [Google Scholar] [CrossRef]
- Zagas, T.; Ganatsas, P.; Tsitsoni, T.; Tsakaldimi, M. Post-fire regeneration of Pinus halepensis Mill. stands in the Sithonia peninsula, northern Greece. Plant Ecol. 2004, 171, 91–99. [Google Scholar] [CrossRef]
- Rackham, O. The Holly Mountain. Plant Talk 2002, 27, 19–23. [Google Scholar]
- Makrogiannis, T.; Flokas, A. The analysis of climatic parameters in the major area of Agion Oros. In Mount Athos. Nature-Worship-Art; Dafis, S., Tsigaridas, E.N., Fountoulis, I.M., Eds.; Shape & Art: Thessaloniki, Greece, 2001; Volume 1, pp. 83–92. [Google Scholar]
- Tzanopoulos, J. Modeling Spatial Variation of the Vegetation of a Typical North-East Mediterranean Island. Ph.D. Thesis, Imperial College at Wye University of London, Wye, UK, 2002. [Google Scholar]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; John Willey and Sons: New York, NY, USA, 1974. [Google Scholar]
- Westhoff, V.; van der Maarel, E. The Braun-Blanquet approach. In Classification of Plant Communities; Whittaker, R.H., Ed.; Dr. W. Junk Publishers: The Hague, The Netherlands, 1978; pp. 287–399. [Google Scholar]
- Van der Maarel, E. Transformation of cover-abundance values in Phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar]
- Arampatzis, T.I. Shrubs and Trees of Greece; Technological Education Institute of Kavala: Drama, Greece, 1998; Volume 1. [Google Scholar]
- Arampatzis, T.I. Shrubs and Trees of Greece; Technological Education Institute of Kavala: Drama, Greece, 2001; Volume 2. [Google Scholar]
- Allen, S.E. Chemical Analysis of Ecological Materials; Blackwell Scientific Publication: Oxford, UK, 1989. [Google Scholar]
- Agricultural Development and Advisory Service. The Analysis of Agricultural Materials; Ministry of Agriculture Fisheries and Food: London, UK, 1986.
- McRae, S.G. Practical Pedology-Studying Soils in the Field; Ellis Horwood Ltd.: Chichester, UK, 1988. [Google Scholar]
- Hill, M.O. TWINSPAN—A Fortran Program for Arranging Multivariate Data in an Ordered Two Way Table by Classification of the Individuals and the Atributes; Cornell University Press: Ithaca, NY, USA, 1979. [Google Scholar]
- Hill, M.O.; Bunce, R.G.H.; Shaw, M.W. Indicator Species Analysis, a divisive polythetic method of classification, and its application to a survey of native pinewoods in Scotland. J. Ecol. 1975, 63, 597–613. [Google Scholar] [CrossRef]
- Gauch, H.G.; Whittaker, R.H. Hierarchical classification of community data. J. Ecol. 1981, 69, 537–557. [Google Scholar] [CrossRef]
- Kent, M.; Coker, P. Vegetation Description and Analysis. A Practical Aproach; Belhaven Press: London, UK, 1992; p. 363. [Google Scholar]
- Rodwell, J.S. British Plant Communities. Woodlands and Scrub; Volume 1, Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Hill, M.O.; Gauch, H.G. Detrended Correspondence Analysis: An inmproved ordination technique. Vegetatio 1980, 42, 47–58. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Ordination. In Data Analysis in Community and Landscape Ecology; Jongman, R.H.G., ter Braak, C.J.F., van Tongeren, O.F.R., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 91–173. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. Canoco for Windows; Centre for Biometry Wgeningen: Wageningen, The Netherlands, 1997. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4); Canoco: Ithaka, NY, USA, 1998. [Google Scholar]
- Witten, I.H.; Frank, E. Data Mining: Practical Machine Learning Tools and Techniques with Java Implementation; Morgan Kaufmann Publishers: San Francisco, CA, USA, 2000. [Google Scholar]
- Breiman, J.D.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; CRC Press: Wadsworth, OH, USA, 1984. [Google Scholar]
- De’ath, G.; Fabricious, K.E. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- Miller, J.; Franklin, J. Modeling the distribution of four vegetation alliances using generalised linear models and classification trees with spatial dependence. Ecol. Model. 2002, 157, 227–247. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.C. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Banskota, A.; Kayastha, N.; Falkowski, M.J.; Wulder, M.A.; Froese, R.E.; White, J.C. Forest Monitoring Using Landsat Time Series Data: A Review. Can. J. Remote Sens. 2014, 40, 362–384. [Google Scholar] [CrossRef]
- Bright, B.C.; Hudak, A.T.; Kennedy, R.E.; Braaten, J.D.; Khalyani, A.H. Examining post-fire vegetation recovery with Landsat time series analysis in three western North American forest types. Fire Ecol. 2019, 15, 8. [Google Scholar] [CrossRef] [Green Version]
- Kibler, C.L.; Parkinson, A.-M.L.; Peterson, S.H.; Roberts, D.A.; D’Antonio, C.M.; Meerdink, S.K.; Sweeney, S.H. Monitoring Post-Fire Recovery of Chaparral and Conifer Species Using Field Surveys and Landsat Time Series. Remote Sens. 2019, 11, 2963. [Google Scholar] [CrossRef] [Green Version]
- Meneses, B.M. Vegetation Recovery Patterns in Burned Areas Assessed with Landsat 8 OLI Imagery and Environmental Biophysical Data. Fire 2021, 4, 76. [Google Scholar] [CrossRef]
- Morresi, M.; Vitali, A.; Urbinati, C.; Garbarino, M. Forest Spectral Recovery and Regeneration Dynamics in Stand-Replacing Wildfires of Central Apennines Derived from Landsat Time Series. Remote Sens. 2019, 11, 308. [Google Scholar] [CrossRef] [Green Version]
- Vogelmann, J.E.; Gallant, A.L.; Shi, H.; Zhu, Z. Perspectives on monitoring gradual change across the continuity of Landsat sensors using time-series data. Remote Sens. Environ. 2016, 185, 258–270. [Google Scholar] [CrossRef] [Green Version]
- Viedma, O.; Melia, J.; Segarra, D.; Garcia-Haro, J. Modeling rates of ecosystem recovery after fires by using Landstat TM Data. Remote Sens. Environ. 1997, 61, 383–398. [Google Scholar] [CrossRef]
- Volkova, L.; Adinugroho, W.C.C.; Krisnawati, H.; Imanuddin, R.; Weston, C.J.J. Loss and Recovery of Carbon in Repeatedly Burned Degraded Peatlands of Kalimantan, Indonesia. Fire 2021, 4, 64. [Google Scholar] [CrossRef]
- Storey, E.A.; Stow, D.A.; O’Leary, J.F. Assessing postfire recovery of chamise chaparral using multi-temporal spectral vegetation index trajectories derived from Landsat imagery. Remote Sens. Environ. 2016, 183, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Abram, N.K.; MacMillan, D.C.; Xofis, P.; Ancrenaz, M.; Tzanopoulos, J.; Ong, R.; Goossens, B.; Koh, L.P.; Del Valle, C.; Peter, L.; et al. Identifying Where REDD+ Financially Out-Competes Oil Palm in Floodplain Landscapes Using a Fine-Scale Approach. PLoS ONE 2016, 11, e0156481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenna, P.; Phinn, S.; Erskine, D.P. Fire severity and vegetation recovery on mine site Rehabilitation Using World-View-3 Imagery. Fire 2018, 1, 22. [Google Scholar] [CrossRef] [Green Version]
- Chavez, P.S.; Kwarteng, A.Y. Extracting spectral contrast in Landsat Thematic Mapper image data using selective principal component analysis. Photogramm. Eng. Remote Sens. 1989, 55, 229–348. [Google Scholar]
- Weiers, S.; Bock, M.; Wissen, M.; Rossner, G. Mapping and indicator approaches for the assessment of habitats at different scales using remote sensing and GIS methods. Landsc. Urban Plan. 2004, 67, 43–65. [Google Scholar] [CrossRef]
- Kleinod, K.; Wissen, M.; Bock, M. Detecting vegetation changes in a wetland area in Northern Germany using earth observation and geodata. J. Nat. Conserv. 2005, 13, 115–125. [Google Scholar] [CrossRef]
- Gauch, H.G. Multivariate Analysis in Community Ecology; Cambridge University Press: New York, NY, USA, 1982. [Google Scholar]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Dafis, S.A.; Kailidis, D.; Smyris, P.; Markalas, S.; Zagas, T.; Siamidis, F.; Poroizidis, K. Study for the Ecological Management of the Area of the H. M. of Simonos Petra; Holly Monastery of Simonos Petra: Mount Athos, Greece, 1992. [Google Scholar]
- Lopez-Soria, L.; Castell, C. Comparative genet survival after fire in woody Mediterranean species. Oecologia 1992, 91, 493–499. [Google Scholar] [CrossRef]
- Cruz, A.; Perez, B.; Quintana, J.R.; Moreno, J.M. Resprouting in the Mediterranean-type shrub Erica australis affected by soil resource availability. J. Veg. Sci. 2002, 13, 641–650. [Google Scholar]
- Cruz, A.; Moreno, J.M. Plant stored reserves do not drive resprouting of the lignotuberous shrub Erica australis. New Phytol. 2003, 157, 251–261. [Google Scholar] [CrossRef]
- Castel, C.; Terradas, J. Effects of water and nutrient availability on water relations, gas exchange and growth rate of mature plants and resprouts of Arbutus unedo L. Ann. Bot. 1994, 73, 595–602. [Google Scholar] [CrossRef]
- Castel, C.; Terradas, J.; Tenhunen, J.D. Water relations, gas exchange and growth of resprouts and mature plant shoots of Arbutus unedo L. and Quercus ilex L. Oecologia 1994, 98, 201–211. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pfaff, A.H.; Safford, H.D. Fire suppression impacts on postfire recovery of Sierra Nevada chaparral shrublands. Int. J. Wildland Fire 2005, 14, 255–265. [Google Scholar] [CrossRef] [Green Version]
- De Groot, W.J.; Wein, R. Effects of fire severity and season of burn on Betula glandulosa growth dynamics. Int. J. Wildland Fire 2004, 13, 287–295. [Google Scholar] [CrossRef]
- Moreno, J.M.; Oechel, W.C. Fire intensity as a determinant factor of postfire plant recovery in Southern California chaparral. In The Role of Fire in Mediterranean—Type Ecosystems; Moreno, J.M., Oechel, W.C., Eds.; Springer: New York, NY, USA, 1994; pp. 26–45. [Google Scholar]
- Moreno, J.M.; Oechel, W.C. Fire Intensity and Herbivory Effects on Postfire Resprouting of Adenostoma-Fasciculatum in Southern California Chaparral. Oecologia 1991, 85, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.M.; Oechel, W.C. Demography of Adenostoma-Fasciculatum after Fires of Different Intensities in Southern California Chaparral. Oecologia 1993, 96, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Lopez-Soria, L. Resprouting of Erica multiflora after experimental fire treatments. J. Veg. Sci. 1993, 4, 367–374. [Google Scholar] [CrossRef]
- Drewa, P.B. Effects of fire season and intensity on Prosopis glandulosa Torr. var. glandulosa. Int. J. Wildland Fire 2003, 12, 147–157. [Google Scholar] [CrossRef]
- Keeley, J.E. Seed-germination patterns in fire-prone Mediterranean climate regions. In Ecology and Biogeography of Mediterranean Ecosystems in Chile, California and Australia; Arroyo, M.T.K., Zedler, P.H., Fox, M.D., Eds.; Springer: New York, NY, USA, 1995; pp. 239–273. [Google Scholar]
- Gratani, L.; Amadori, M. Post-fire resprouting of shruby species in Mediterranean maquis. Vegetatio 1991, 96, 137–143. [Google Scholar] [CrossRef]
- Broncano, M.J.; Retana, J. Topography and forest composition affecting the variability in fire severity and post-fire regeneration occurring after a large fire in the Mediterranean basin. Int. J. Wildland Fire 2004, 13, 209–216. [Google Scholar] [CrossRef]
- Terradas, J. Holm oak and Holm oak forests: An introduction. In Ecology of Mediterranean Evergreen Oak Forests; Rodà, F., Retana, J., Gracia, C.A., Bellot, J., Eds.; Springer: Berlin, Germany, 1999; pp. 1–14. [Google Scholar]
- Espelta, J.M.; Sabatι, S.; Retana, J. Resprouting dynamics. In Ecology of Mediterranean Evergreen Oak Forests; Rodà, F., Retana, J., Gracia, C.A., Bellot, J., Eds.; Springer: Berlin, Germany, 1999; pp. 61–73. [Google Scholar]
- Luis-Calabuig, E.; Tarrega, R.; Calvo, L.; Marcos, E.; Valbuena, L. History of landscape changes in northwest Spain according to land use and management. In Life and Environment in the Mediterranean; Trabaud, L., Ed.; WIT Press: Southampton, UK; Boston, MA, USA, 2000. [Google Scholar]
- Tarrega, R.; Calvo, L.; Trabaud, L. Effect of high temperatures on seed germination of two woody Leguminosae. Vegetatio 1992, 102, 139–147. [Google Scholar] [CrossRef]
- Wuthrich, C.; Schaub, D.; Weber, M.; Marxer, P.; Conedera, M. Soil respiration and soil microbial biomass after fire in a sweet chestnut forest in southern Switzerland. Catena 2002, 48, 201–215. [Google Scholar] [CrossRef]
- Providoli, I.; Elsenbeer, H.; Conedera, M. Post-fire management and splash erosion in a chestnut coppice in southern Switzerland. For. Ecol. Manag. 2002, 162, 219–229. [Google Scholar] [CrossRef]
- Cheney, N.P. Fire Behaviour. In Fire and the Australian Biota; Gill, A.M., Groves, R.H., Noble, I.R., Eds.; Australian Academy of Science: Canberra, Australia, 1981; pp. 151–175. [Google Scholar]
- Fox, M.D.; Fox, B.J. The role of fire in the scleromorphic forests and shrublands of eastern Australia. In The Role of Fire in Ecological Systems; Trabaud, L., Ed.; SPB Academic Publishing: The Hague, The Netherlands, 1987; pp. 23–48. [Google Scholar]
- Foulkes, J.A.; Prior, L.D.; Leonard, S.W.J.; Bowman, D.M.J.S. Demographic Effects of Severe Fire in Montane Shrubland on Tasmania’s Central Plateau. Fire 2021, 4, 32. [Google Scholar] [CrossRef]



| Bands (Wavelength μm)-Spatial Resolution | ||
|---|---|---|
| Landsat 4-TM | Landsat 7-ETM | Landsat 8-OLI |
| B1-Coastal/Aerosol (0.435–0.451)–30 m | ||
| B1-Blue (0.45–0.52)–30 m | B1-Blue (0.441–0.514)–30 m | B2-Blue (0.452–0.512)–30 m |
| B2-Green (0.52–0.60)–30 m | B2-Green (0.519–0.601)–30 m | B3-Green (0.533–0.590)–30 m |
| B3- Red (0.63–0.69)–30 m | B3- Red (0.631–0.692)–30 m | B4- Red (0.636–0.673)–30 m |
| B4-NIR (0.76–0.90)–30 m | B4-NIR (0.772–0.898)–30 m | B5-NIR (0.851–0.879)–30 m |
| B5-SWIR 1 (1.55–1.75)—30 m | B5-SWIR 1 (1.547–1.749)–30 m | B6-SWIR 1 (1.566–1.651)–30 m |
| B7-SWIR 2 (2.08–2.35)–30 m | B7-SWIR 2 (2.064–2.345)–30 m | B7-SWIR 2 (2.107–2.294)–30 m |
| B6-TIR (10.40–12.50)–120 m | B6-TIR (10.31–12.36)–60 m | B10-TIR 1 (10.60–11.19)–30 m |
| B11-TIR 2 (11.50–12.51)–30 m | ||
| B9-Cirrus (1.363–1.384)–30 m | ||
| B8-Pan (0.515–0.896)–15 m | B8-Pan (0.503–0.676)–15 m | |
| Species | Group *000 n = 55 | Group *001 n = 19 | Group *00 n = 55 + 19 = 74 | Group *01 n = 36 | Group *10 n = 12 | Group *11 n = 12 | Total n = 134 |
|---|---|---|---|---|---|---|---|
| Quercus coccifera | V (2–5) | V (2–6) | V (2–6) | V (1–6) | III (1) | V | |
| Phillyrea latifolia | V (1–5) | V (1–6) | V (1–6) | V (1–4) | V | ||
| Ruscus aculeatus | IV (1–2) | V (1–2) | IV (1–2) | V (1–2) | V (1–2) | V (1–2) | V |
| Olea europaea | V (1–5) | IV (1–3) | V (1–5) | IV | |||
| Fraxinus ornus | III (1–2) | IV (1–2) | III (1–2) | V (1–5) | V (1–3) | III (1–2) | IV |
| Cistus creticus | V (1–4) | V (1–3) | V (1–4) | IV (1–4) | III (1–2) | IV | |
| Arbutus unedo. | IV (1–3) | IV (1–3) | IV (1–3) | IV (1–3) | IV | ||
| Asparagus acutifolious | V (1–2) | V (1–2) | V (1–2) | V (1–2) | IV | ||
| Calicotome villosa | V (2–6) | IV (1–4) | V (1–6) | III | |||
| Quercus ilex | IV (1–5) | V (1–6) | V (1–6) | III (1–3) | III | ||
| Erica arborea | III (1–4) | IV (1–3) | III (1–4) | III (1–3) | III | ||
| Spartium junceum | V (1–6) | IV (1–5) | V (1–6) | III (1–6) | III | ||
| Pistacia terebinthus | III (1–2) | IV (1–2) | IV (1–2) | III | |||
| Cytisus villosus | V (1–5) | V (1–3) | V (1–5) | III | |||
| Laurus nobilis | III (1–3) | IV (1–5) | II | ||||
| Rhamnus alaternus L. | IV (1–3) | II | |||||
| Osyris alba L. | III (1–2) | III (1–3) | III (1–3) | II | |||
| Smilax aspera | IV (1–5) | III (1–5) | II | ||||
| Lonicera implexa Aiton. | III (1) | III (1) | II | ||||
| Quercus petraea | III (1–6) | V (2–6) | V (1–3) | II | |||
| Sorbus domestica L. | III (1) | I | |||||
| Rubus sp L. | III (1) | III (1) | I | ||||
| Hedera helix L. | IV (1) | V (1–2) | I | ||||
| Acer monspessulanum L. | IV (1–2) | I | |||||
| Abies borisii–regis | III (1–2) | IV (1–6) | I | ||||
| Sorbus torminalis (L.) Crantz | III (1) | I | |||||
| Castanea sativa | IV (2–3) | V (3–6) | I | ||||
| Ruscus hypoglossum | IV (1–2) | I | |||||
| Ilex aquifolium L. | III (1–2) | III (1–2) | I | ||||
| Quercus frainetto | III (2–6) | I | |||||
| Genista tinctoria L. | V (1–3) | III (1–3) | I | ||||
| Rosa sp L. | III (1) | I | |||||
| Comm. Codenames 1 | MQ1a | MQ1b | MQ1 | MQ2 | FC1 | FC2 |
| Axes | 1 | 2 | 3 | 4 | Total Inertia |
|---|---|---|---|---|---|
| Eigenvalues: | 0.805 | 0.270 | 0.157 | 0.121 | 3.865 |
| Cumulative percentage variance of species data: | 20.8 | 27.8 | 31.9 | 35.0 | |
| Sum of all eigenvalues | 3.865 |
| Species | MQ1a | MQ1b | MQ2 | FC1 | FC2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn | SD | Md | Mn | SD | Md | Mn | SD | Md | Mn | SD | Md | Mn | SD | Md | |
| Quercus coccifera | 19.0 | 7.5 | 20.5 | 41.0 | 17.6 | 37.0 | 13.9 | 16.1 | 9.2 | 1.5 | 3.5 | 0.3 | 0.1 | 0.4 | 0.0 |
| Phillyrea latifolia | 12.7 | 8.2 | 12.4 | 25.4 | 17.4 | 30.9 | 6.9 | 6.3 | 5.4 | 0.7 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ruscus aculeatus | 0.9 | 1.2 | 0.6 | 2.7 | 1.6 | 2.6 | 3.7 | 2.5 | 4.3 | 2.9 | 1.7 | 3.5 | 1.6 | 1.6 | 1.4 |
| Olea europaea | 7.1 | 7.9 | 4.3 | 7.1 | 7.0 | 6.5 | 0.2 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Fraxinus ornus | 1.6 | 2.8 | 0.0 | 3.8 | 3.8 | 2.6 | 18.4 | 9.6 | 20.5 | 14.9 | 7.1 | 14.3 | 1.4 | 2.8 | 0.0 |
| Cistus creticus | 10.0 | 8.4 | 9.2 | 3.4 | 3.7 | 1.4 | 6.6 | 7.6 | 3.5 | 0.9 | 1.8 | 0.3 | 0.0 | 0.0 | 0.0 |
| Arbutus unedo | 2.2 | 3.2 | 0.6 | 2.4 | 3.5 | 1.4 | 4.6 | 5.6 | 2.0 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Asparagus acutifolious | 1.4 | 1.3 | 0.6 | 1.8 | 1.3 | 1.4 | 2.0 | 1.9 | 1.4 | 0.4 | 0.7 | 0.1 | 0.0 | 0.0 | 0.0 |
| Calicotome villosa | 48.0 | 16.0 | 51.0 | 5.0 | 7.5 | 1.4 | 0.8 | 3.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Quercus ilex | 1.1 | 2.4 | 0.0 | 7.5 | 10.8 | 2.6 | 29.8 | 22.0 | 28.2 | 16.6 | 22.2 | 6.8 | 1.4 | 3.5 | 0.1 |
| Erica arborea | 2.6 | 4.9 | 0.0 | 4.0 | 5.5 | 1.4 | 2.0 | 4.0 | 0.3 | 0.3 | 0.8 | 0.0 | 0.0 | 0.0 | 0.0 |
| Spartium junceum | 10.9 | 13.3 | 4.3 | 6.8 | 11.9 | 1.4 | 11.2 | 15.9 | 1.4 | 0.4 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Pistacia terebinthus | 0.8 | 1.5 | 0.1 | 1.3 | 1.4 | 0.6 | 0.3 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Cytisus villosus | 0.0 | 0.0 | 0.0 | 0.3 | 1.0 | 0.0 | 14.5 | 13.3 | 12.4 | 6.0 | 6.4 | 4.3 | 13.3 | 13.8 | 9.2 |
| Laurus nobilis | 0.3 | 1.2 | 0.0 | 1.8 | 3.4 | 0.1 | 7.5 | 10.6 | 1.4 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| Rhamnus alaternus | 0.0 | 0.1 | 0.0 | 2.1 | 3.8 | 0.6 | 0.5 | 1.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Osyris alba | 0.8 | 1.2 | 0.0 | 1.4 | 3.0 | 0.0 | 0.6 | 1.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Smilax aspera | 1.2 | 4.4 | 0.0 | 3.7 | 9.9 | 1.4 | 7.3 | 13.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Euphorbia characias | 0.6 | 0.8 | 0.0 | 0.3 | 0.5 | 0.0 | 0.3 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Lonicera implexa | 0.3 | 0.9 | 0.0 | 0.3 | 0.4 | 0.0 | 0.5 | 0.6 | 0.6 | 0.2 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Quercus petraea | 0.5 | 3.4 | 0.0 | 0.2 | 1.0 | 0.0 | 7.9 | 13.0 | 2.6 | 53.4 | 25.5 | 58.9 | 7.1 | 5.2 | 7.9 |
| Sorbus domestica | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 3.5 | 0.0 | 1.1 | 3.6 | 0.0 | 0.2 | 0.3 | 0.0 |
| Rubus sp | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 1.6 | 0.0 | 1.2 | 1.4 | 1.0 | 0.4 | 0.7 | 0.0 |
| Hedera helix | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 0.0 | 0.8 | 1.0 | 0.6 | 2.9 | 1.9 | 2.6 |
| Acer monspessulanum | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 | 1.9 | 0.0 | 1.3 | 1.6 | 0.6 | 0.0 | 0.0 | 0.0 |
| Abies borisii-regis | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 1.0 | 1.7 | 0.0 | 15.9 | 20.1 | 3.5 |
| Sorbus torminalis | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.4 | 0.5 | 0.0 | 0.1 | 0.2 | 0.0 |
| Castanea sativa | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 7.0 | 5.4 | 7.9 | 74.9 | 31.5 | 97.3 |
| Ruscus hypoglossum | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.3 | 0.0 | 1.0 | 1.3 | 0.6 |
| Ilex aquifolium | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 2.6 | 0.7 | 1.4 | 2.6 | 0.6 |
| Quercus frainetto | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 7.9 | 18.5 | 0.0 | 13.3 | 19.9 | 0.0 |
| Genista trinctoria | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.5 | 0.0 | 4.3 | 6.8 | 1.4 | 2.1 | 3.8 | 0.3 |
| Rosa sp | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.3 | 0.0 | 0.1 | 0.2 | 0.0 |
| 1989 | 2001 | |||||||
|---|---|---|---|---|---|---|---|---|
| Community | MQ1a | MQ1b | MQ2 | FC1 | MQ1a | MQ1b | MQ2 | FC1 |
| MQ1b | 0.84 ns | <0.001 *** | ||||||
| MQ2 | <0.001 *** | 0.032 * | <0.001 *** | 0.012 * | ||||
| FC1 | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | 0.009 ** | ||
| FC2 | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | 0.003 ** |
| Community | MQ1a | MQ1b | MQ2 | FC1 |
|---|---|---|---|---|
| MQ1b | <0.001 *** | |||
| MQ2 | <0.001 *** | 0.066 ns | ||
| FC1 | <0.043 * | 0.324 ns | 0.999 ns | |
| FC2 | <0.001 *** | 0.893 ns | 0.737 ns | 0.904 ns |
| 2011 | 2021 | |||||||
|---|---|---|---|---|---|---|---|---|
| Community | MQ1a | MQ1b | MQ2 | FC1 | MQ1a | MQ1b | MQ2 | FC1 |
| MQ1b | 1 ns | 0.907 ns | ||||||
| MQ2 | <0.001 *** | 0.020 * | <0.001 *** | 0.001 ** | ||||
| FC1 | <0.001 *** | <0.001 *** | 0.009 ** | <0.001 *** | <0.001 *** | 0.029 * | ||
| FC2 | <0.001 *** | <0.001 *** | <0.001 *** | 0.010 ** | <0.001 *** | <0.001 *** | <0.001 *** | 0.741 ns |
| Country | Mean Annual Precipitation (mm) | Mean Annual Temperature (°C) |
|---|---|---|
| France (Trabaud, 1990) | 800–1000 | - |
| France (Trabaud & Lepart, 1981) | 1100 | 14.4 |
| France (Trabaud, 1991) | 919 | 13.2 |
| France (Malanson & Trabaud, 1988) | 980 | - |
| Spain (Delitti et al., 2005) | 600 | 14 |
| Spain (Lloret & Vila, 1997) | 591 | - |
| Portugal (Clemente et al., 1996) | 1080 | - |
| Current Study | 470 | 15.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xofis, P.; Buckley, P.G.; Takos, I.; Mitchley, J. Long Term Post-Fire Vegetation Dynamics in North-East Mediterranean Ecosystems. The Case of Mount Athos Greece. Fire 2021, 4, 92. https://doi.org/10.3390/fire4040092
Xofis P, Buckley PG, Takos I, Mitchley J. Long Term Post-Fire Vegetation Dynamics in North-East Mediterranean Ecosystems. The Case of Mount Athos Greece. Fire. 2021; 4(4):92. https://doi.org/10.3390/fire4040092
Chicago/Turabian StyleXofis, Panteleimon, Peter G. Buckley, Ioannis Takos, and Jonathan Mitchley. 2021. "Long Term Post-Fire Vegetation Dynamics in North-East Mediterranean Ecosystems. The Case of Mount Athos Greece" Fire 4, no. 4: 92. https://doi.org/10.3390/fire4040092
APA StyleXofis, P., Buckley, P. G., Takos, I., & Mitchley, J. (2021). Long Term Post-Fire Vegetation Dynamics in North-East Mediterranean Ecosystems. The Case of Mount Athos Greece. Fire, 4(4), 92. https://doi.org/10.3390/fire4040092







