The Fire and Fodder Reversal Phenomenon: Vertebrate Herbivore Activity in Burned and Unburned Tasmanian Ecosystems
Abstract
:1. Introduction
- Do native and exotic herbivores occupy different post-fire environments?
- Are patterns of herbivore activity consistent across vegetation communities and fire attributes?
- What are the attributes (biophysical, biological, and climatic) that are associated with herbivore scat deposition after fire?
2. Study Area
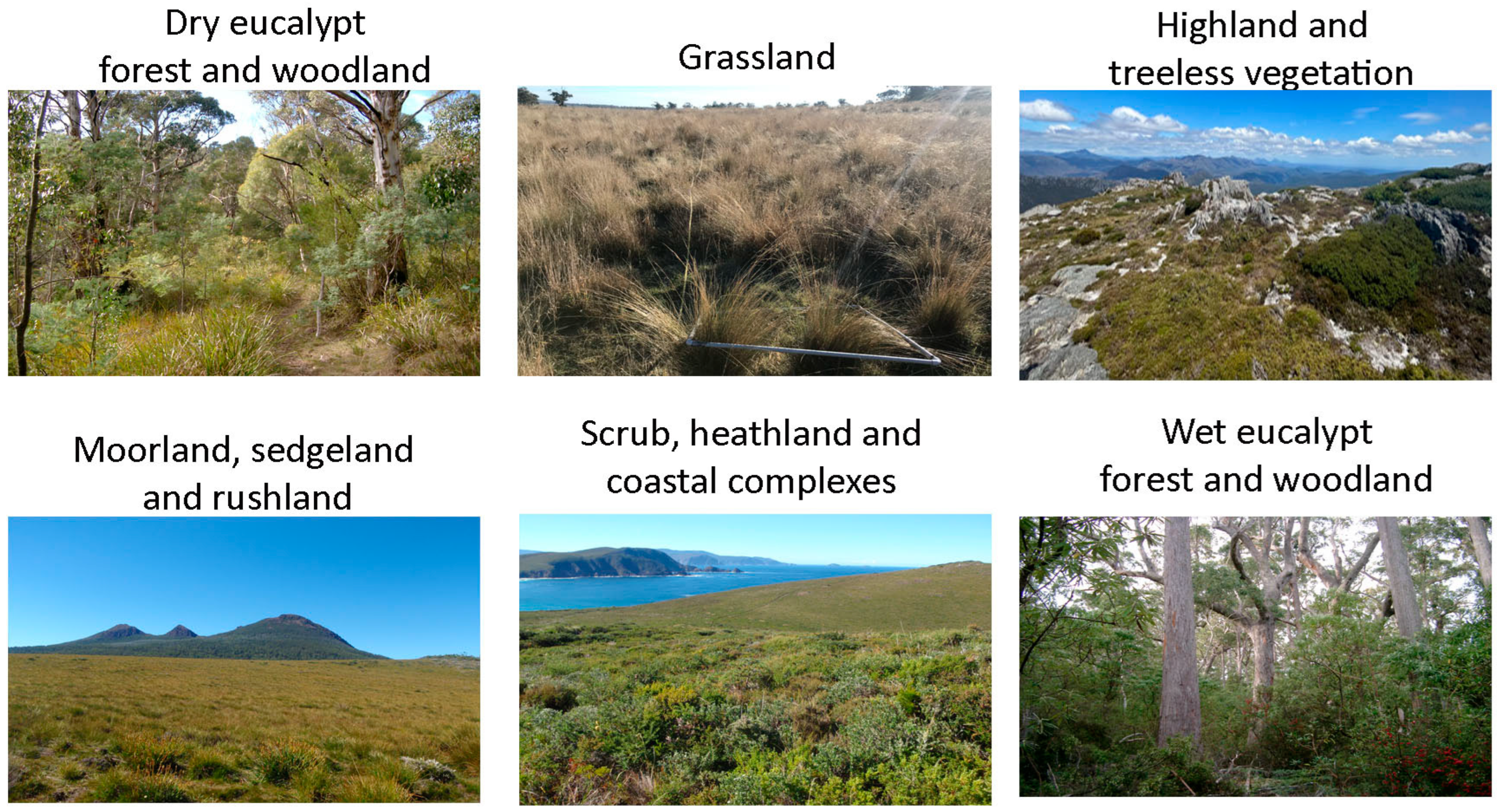
- Dry eucalypt forests-flora has many scleromorphic plants and an open understorey. Bushfire is critical to the regeneration of scleromorphic plants, such as those in the genus Eucalyptus [24].
- Highland treeless vegetation–This ecosystem is typically dominated by scleromorphic shrubs that are not well-adapted to fire [25].
- Grasslands-in Tasmania, grasslands are typically dominated by native tussock grasses and have low forb and shrub cover. Both fire and grazing can be important in preventing invasion of woody plants [26].
- Moorlands, sedgelands, and rushlands-These ecosystems dominated by sedges, rushes and other hard graminoids predominantly occur in areas with high rainfall and low fertility. Fire can burn in any season after as little as 1–2 rain-free days [27].
- Scrub, heathland, and coastal complexes occurring near the coast, this vegetation grouping has little else in common, with heath and scrub being scleromorphic vegetation occurring largely on infertile soils, with the latter replacing the former in the absence of fire [28], and the latter being the vegetation within the fertile saltspray zone, variously dominated by large-leaved shrubs and sand-binding grasses [29].
- Wet eucalypt forests are characterised by understoreys dominated by rainforest trees, broad-leaved small trees or by tree ferns [30]. They occur in areas of high rainfall with reliable summer rain. Ignition is infrequent and undesirable due to accumulation of large fuel loads and the potential for catastrophic loss of biota.
Native and Introduced Vertebrate Herbivores of Tasmania
3. Materials and Methods
3.1. Site Selection
3.2. Sampling Strategy
3.3. Statistical Analyses
4. Results
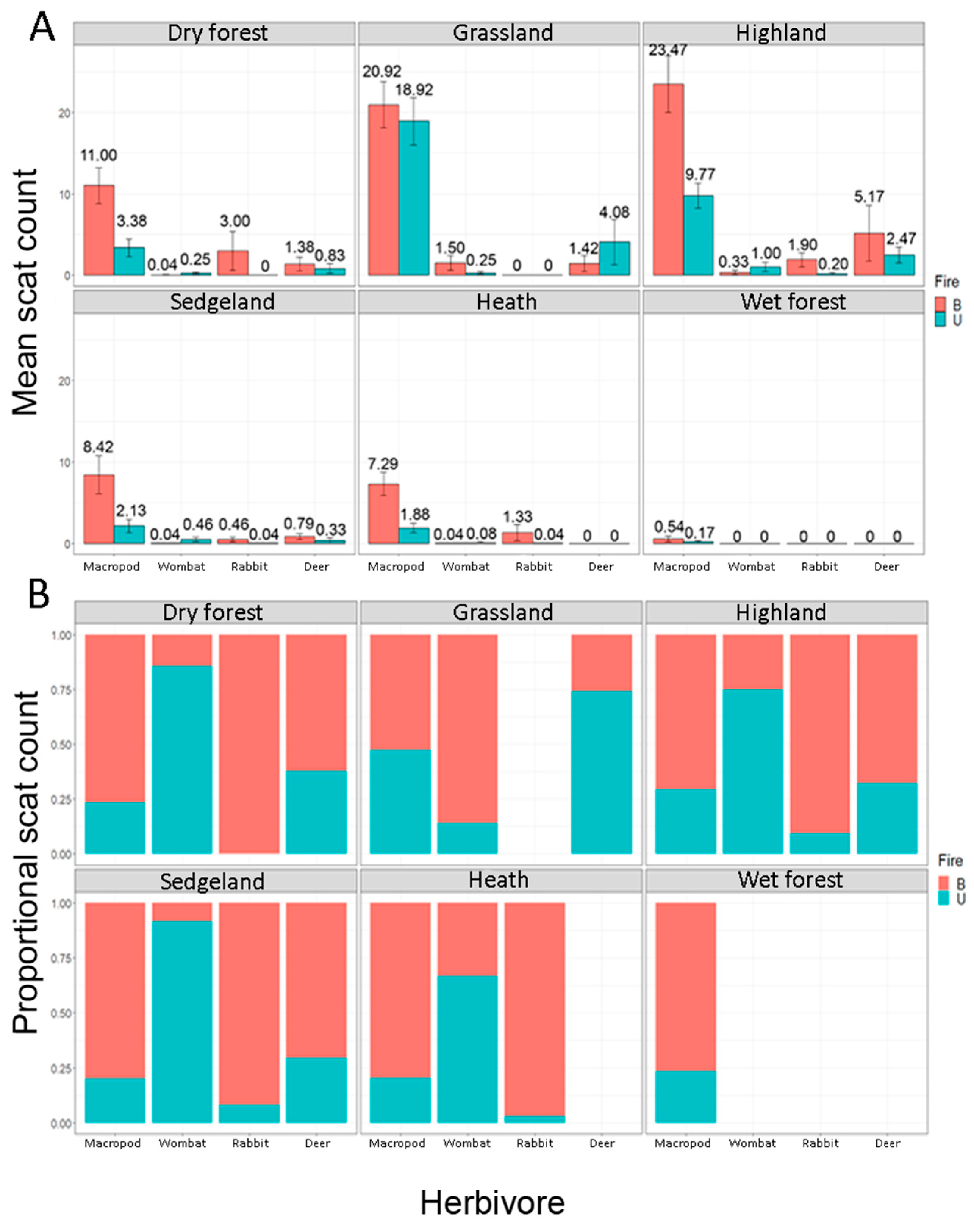
4.1. The Main Effect–Fire, Vegetation and Herbivores
4.2. Fire Attributes as Predictors for Herbivore Activity
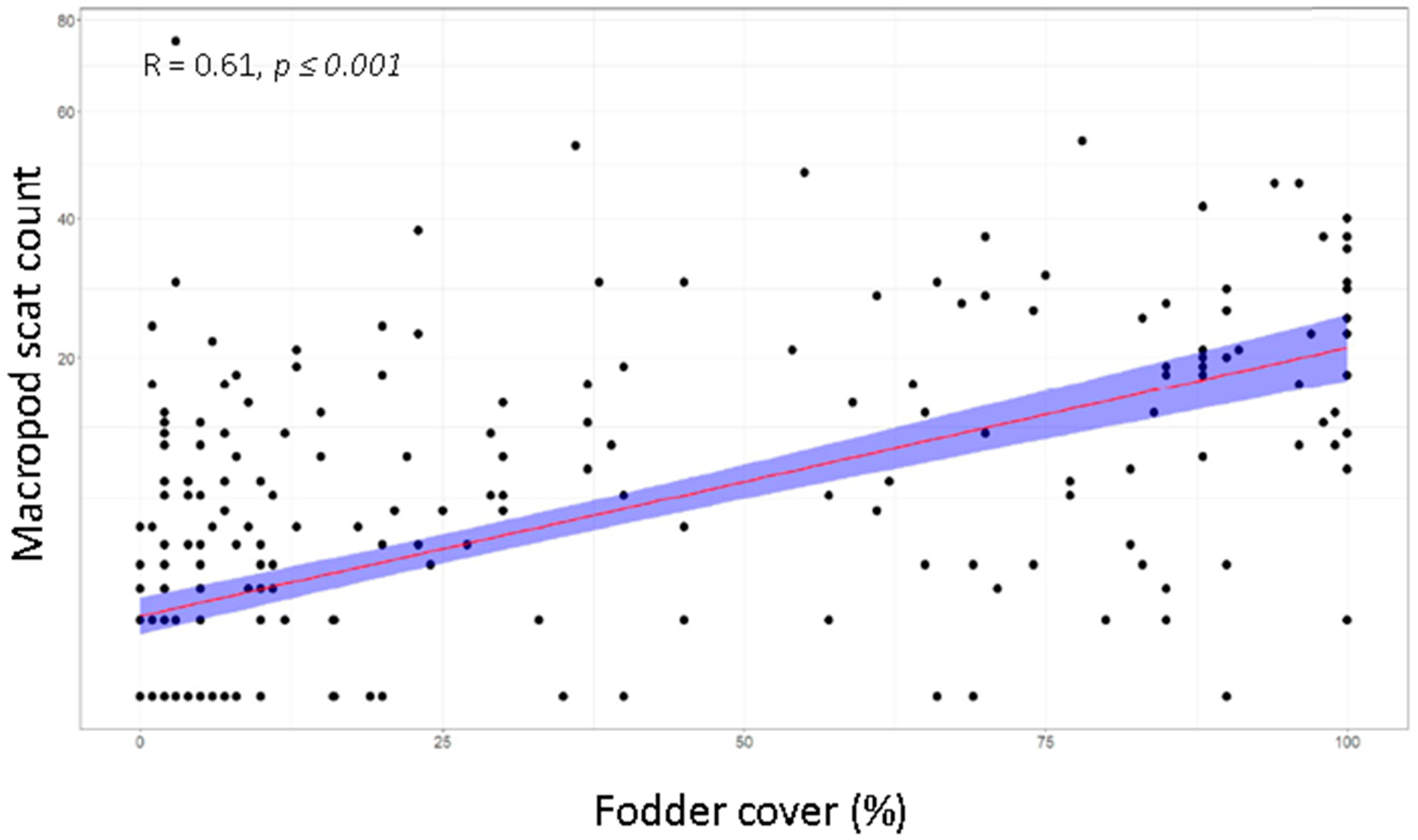
4.3. Vegetation Attributes as Predictors for Herbivore Activity
4.4. Biophysical Contributions to Observed Vegetation Patterns
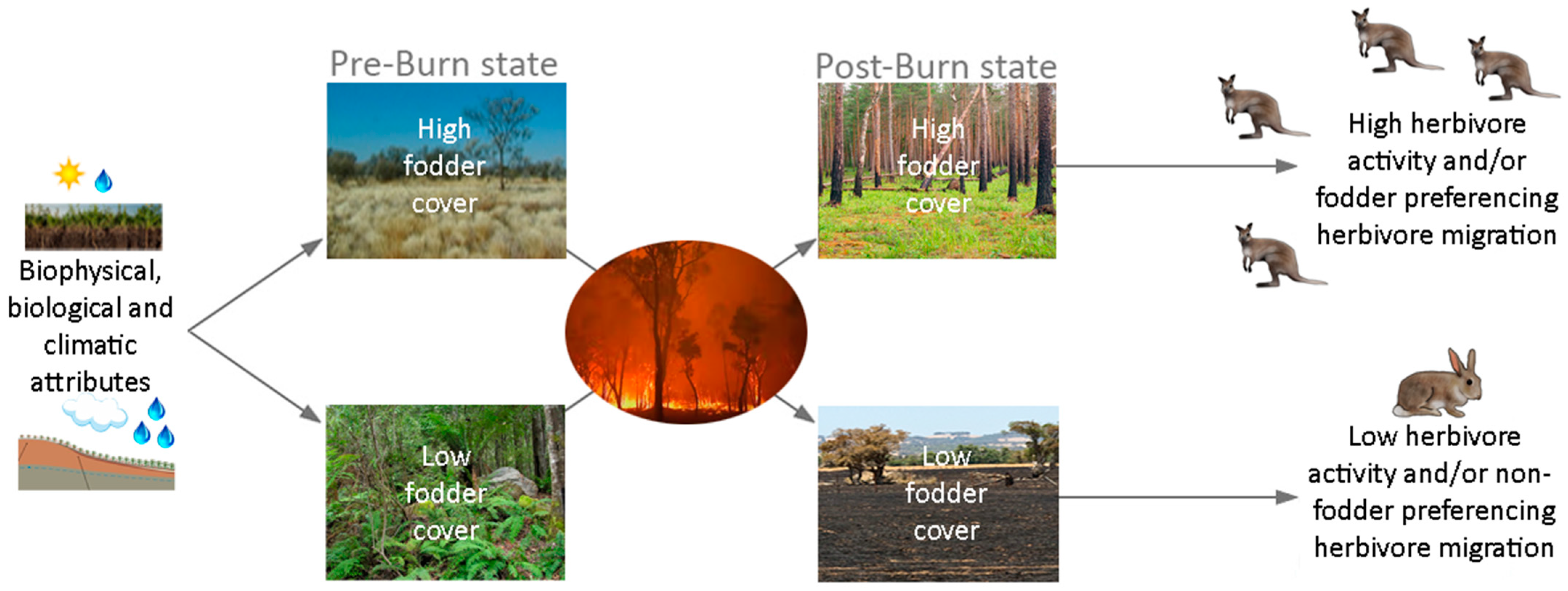
4.5. The Fire and Fodder Reversal Phenomenon
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Archibald, S.; Hempson, G.P. Competing Consumers: Contrasting the Patterns and Impacts of Fire and Mammalian Herbivory in Africa. Philos. Trans. R. Soc. B 2016, 371, 20150309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bond, W.; Keeley, J. Fire as a Global ‘Herbivore’: The Ecology and Evolution of Flammable Ecosystems. Trends Ecol. Evol. 2005, 20, 387–394. [Google Scholar] [CrossRef]
- Cooke, B.D. The Effects of Rabbit Grazing on Regeneration of Sheoaks, Allocasuarina Verticilliata and Saltwater Ti-Trees, Melaleuca Halmaturorum, in the Coorong National Park, South Australia. Austral Ecol. 1988, 13, 11–20. [Google Scholar] [CrossRef]
- Bird, P.; Mutze, G.; Peacock, D.; Jennings, S. Damage Caused by Low-Density Exotic Herbivore Populations: The Impact of Introduced European Rabbits on Marsupial Herbivores and Allocasuarina and Bursaria Seedling Survival in Australian Coastal Shrubland. Biol. Invasions 2012, 14, 743–755. [Google Scholar] [CrossRef]
- Kirkpatrick, J.B.; Marsden-Smedley, J.B.; Leonard, S.W.J. Influence of Grazing and Vegetation Type on Post-Fire Flammability: Grazing, Vegetation, Post-Fire Flammability. J. Appl. Ecol. 2011, 48, 642–649. [Google Scholar] [CrossRef]
- Leonard, S.; Kirkpatrick, J.; Marsden-Smedley, J. Variation in the Effects of Vertebrate Grazing on Fire Potential between Grassland Structural Types: Grazing Effects on Fire Potential. J. Appl. Ecol. 2010, 47, 876–883. [Google Scholar] [CrossRef]
- Archibald, S.; Bond, W.J. Grazer Movements: Spatial and Temporal Responses to Burning in a Tall-Grass African Savanna. Int. J. Wildland Fire 2004, 13, 377. [Google Scholar] [CrossRef]
- Sprent, J.A.; McArthur, C. Diet and diet selection of two species in the macropodid browser–grazer continuum—Do they eat what they ‘should’? Aust. J. Zool. 2002, 50, 183–192. [Google Scholar] [CrossRef]
- Le Mar, K.; Mcarthur, C. Comparison of Habitat Selection by Two Sympatric Macropods, Thylogale Billardierii and Macropus Rufogriseus Rufogriseus, in a Patchy Eucalypt-Forestry Environment. Austral Ecol. 2005, 30, 674–683. [Google Scholar] [CrossRef]
- Wood, D. Estimating Rabbit Density by Counting Dung Pellets. Wildl. Res. 1988, 15, 665. [Google Scholar] [CrossRef]
- Foster, C.N.; Barton, P.S.; Sato, C.F.; MacGregor, C.I.; Lindenmayer, D.B. Synergistic Interactions between Fire and Browsing Drive Plant Diversity in a Forest Understorey. J. Veg. Sci. 2015, 26, 1112–1123. [Google Scholar] [CrossRef]
- Neff, D.J. The Pellet-Group Count Technique for Big Game Trend, Census, and Distribution: A Review. J. Wildl. Manag. 1968, 32, 597. [Google Scholar] [CrossRef]
- Rhodes, A.C.; St. Clair, S.B. Measures of Browse Damage and Indexes of Ungulate Abundance to Quantify Their Impacts on Aspen Forest Regeneration. Ecol. Indic. 2018, 89, 648–655. [Google Scholar] [CrossRef]
- Burkepile, D.E.; Thompson, D.I.; Fynn, R.W.S.; Koerner, S.E.; Eby, S.; Govender, N.; Hagenah, N.; Lemoine, N.P.; Matchett, K.J.; Wilcox, K.R.; et al. Fire Frequency Drives Habitat Selection by a Diverse Herbivore Guild Impacting Top-down Control of Plant Communities in an African Savanna. Oikos 2016, 125, 1636–1646. [Google Scholar] [CrossRef]
- Jachmann, H. Evaluation of Four Survey Methods for Estimating Elephant Densities. Afr. J. Ecol. 1991, 29, 188–195. [Google Scholar] [CrossRef]
- Archibald, S.; Bond, W.J.; Stock, W.D.; Fairbanks, D.H.K. Shaping the landscape: Fire–grazer interactions in an African Savanna. Ecol. Appl. 2005, 15, 96–109. [Google Scholar] [CrossRef]
- Sankaran, M.; Ratnam, J.; Hanan, N. Woody Cover in African Savannas: The Role of Resources, Fire and Herbivory. Glob. Ecol. Biogeogr. 2008, 17, 236–245. [Google Scholar] [CrossRef]
- Anderson, T.M.; Ritchie, M.E.; Mayemba, E.; Eby, S.; Grace, J.B.; McNaughton, S.J. Forage Nutritive Quality in the Serengeti Ecosystem: The Roles of Fire and Herbivory. Am. Nat. 2007, 170, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Burkepile, D.E.; Burns, C.E.; Tambling, C.J.; Amendola, E.; Buis, G.M.; Govender, N.; Nelson, V.; Thompson, D.I.; Zinn, A.D.; Smith, M.D. Habitat Selection by Large Herbivores in a Southern African Savanna: The Relative Roles of Bottom-up and Top-down Forces. Ecosphere 2013, 4, art139. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, J.E.; Archibald, S.; Govender, N.; Pollard, D.; Luhdo, Z.; Parr, C.L. Ecological Engineering through Fire-Herbivory Feedbacks Drives the Formation of Savanna Grazing Lawns. J. Appl. Ecol. 2018, 55, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Yoganand, K.; Owen-Smith, N. Restricted habitat use by an African savanna herbivore through the seasonal cycle: Key resources concept expanded. Ecography 2014, 37, 969–982. [Google Scholar] [CrossRef]
- Grose, M.R.; Barnes-Keoghan, I.; Corney, S.P.; White, C.J.; Holz, G.K.; Bennett, J.B.; Gaynor, S.M.; Bindoff, N.L. Climate Futures for Tasmania: General Climate Impacts; Antarctic Climate and Ecosystems Cooperative Research Centre: Hobart, Australia, 2010; ISBN 978-1-921197-05-5. [Google Scholar]
- Complete National Parks and Reserves Listings. Parks and Wildlife Service. Available online: https://parks.tas.gov.au/about-us/managing-our-parks-and-reserves/reserve-listing (accessed on 11 January 2021).
- Jackson, W.D. Fire, air, water and earth—An elemental ecology of Tasmania. Proc. Ecol. Soc. Aust. 1968, 3, 9–16. [Google Scholar]
- Kirkpatrick, J.B.; Bridle, K.L. Natural and cultural histories of fire differ between Tasmanian and mainland alpine vegetation. Aust. J. Bot. 2013, 65, 465–474. [Google Scholar] [CrossRef]
- Kirkpatrick, J.B.; Jenkinson, I. Effects of Increasing Fire Frequency on Conservation Values in Eucalyptus Grassy Woodland in the Process of Invasion by Allocasuarina Verticillata. Fire 2022, 5, 31. [Google Scholar] [CrossRef]
- Balmer, J. Buttongrass moorland vegetation. In Tasmanian Native Bush; A Management Handbook; Kirkpatrick, J.B., Ed.; Tasmanian Environment Centre Inc.: Hobart, Australia, 1991. [Google Scholar]
- Bergman, T.; Kirkpatrick, J.B. Transition from heathland to scrub in south-eastern Tasmania—Extent of change since the 1970s, floristic depletion and management implications. Biodivers. Conserv. 2015, 24, 213–228. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, J.B.; Harris, S. The Conservation of Tasmanian Dry Coastal Vascular Plant Communities; National Parks and Wildlife Service: Hobart, Australia, 1995; p. 129. [Google Scholar]
- Kirkpatrick, J.B. Conservation of rainforest in Tasmania. In Anon (ed.) Tasmania Rainforests: What Future? Wilderness Society: Hobart, Australia, 1988; pp. 47–56. [Google Scholar]
- Wiggins, N.L.; Bowman, D.M.J.S. Macropod Habitat Use and Response to Management Interventions in an Agricultural—Forest Mosaic in North-Eastern Tasmania as Inferred by Scat Surveys. Wildl. Res. 2011, 38, 103. [Google Scholar] [CrossRef]
- Scurr, G.; Kirkpatrick, J.B.; Daniels, G.D.; Mcquillan, P.B. Biotic Resistance to Chrysanthemoides Monilifera ssp. Monilifera in Tasmania: HERBIVORES AND BONESEED. Austral Ecol. 2008, 33, 941–950. [Google Scholar] [CrossRef]
- Hazeldine, A.; Kirkpatrick, J.B. Practical and Theoretical Implications of a Browsing Cascade in Tasmanian Forest and Woodland. Aust. J. Bot. 2015, 63, 435. [Google Scholar] [CrossRef]
- Johnson, C.; Jarman, P.; Southwell, C.J. Macropod Studies at Wallaby Creek. 5. Patterns of Defecation by Eastern Gray Kangaroos and Red-Necked Wallabies. Wildl. Res. 1987, 14, 133–138. [Google Scholar] [CrossRef]
- Hume, I.D. Marsupial Nutrition; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Evans, M.; Green, B.; Newgrain, K. The Field Energetics and Water Fluxes of Free-Living Wombats (Marsupialia: Vombatidae). Oecologia 2003, 137, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Guy, T.R.; Kirkpatrick, J.B. Environmental Associations and Effects of Disturbances by Common Wombats in Alpine Tasmania. Austral Ecol. 2021, 46, 1392–1403. [Google Scholar] [CrossRef]
- Sharp, A.C. Comparative Finite Element Analysis of the Cranial Performance of Four Herbivorous Marsupials: Cranial Performance in Marsupial Herbivores. J. Morphol. 2015, 276, 1230–1243. [Google Scholar] [CrossRef]
- Chapman, N.G.; Chapman, D.I. The distribution of fallow deer: A worldwide review. Mammal Rev. 1980, 10, 61–138. [Google Scholar] [CrossRef]
- Cunningham, C.X.; Perry, G.L.W.; Bowman, D.M.J.S.; Forsyth, D.M.; Driessen, M.M.; Appleby, M.; Brook, B.W.; Hocking, G.; Buettel, J.C.; French, B.J.; et al. Dynamics and predicted distribution of an irrupting ‘sleeper’ population: Fallow deer in Tasmania. Biol. Invasions 2022, 24, 1131–1147. [Google Scholar] [CrossRef]
- Chapman, N.G.; Chapman, D.I. Fallow Deer: Their History, Distribution and Biology. In Coch-y-Bonddu Books; Dalton: Machynlleth, UK, 1997. [Google Scholar]
- Locke, S. The Distribution and Abundance of Fallow Deer in the Central Plateau Conservation Area and Adjacent Areas in Tasmania. In Nature Conservation Report 07/02; Department of Primary Industries and Water: Hobart, Australia, 2007. [Google Scholar]
- Kay, S. Factors Affecting Severity of Deer Browsing Damage within Coppiced Woodlands in the South of England. Biol. Conserv. 1993, 63, 217–222. [Google Scholar] [CrossRef]
- Mouissie, A.M.; Van Der Veen, C.E.J.; Ciska Veen, G.F.; Van Diggelen, R. Ecological Correlates of Seed Survival after Ingestion by Fallow Deer. Funct Ecol. 2005, 19, 284–290. [Google Scholar] [CrossRef]
- Gill, R.M.A. The Impact of Deer on Woodlands: The Effects of Browsing and Seed Dispersal on Vegetation Structure and Composition. Forestry 2001, 74, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Košnář, A.; Rajnyšová, R. Assessment of a daily defecation rate of fallow deer upon a closed population study. Acta Univ. Agric. Silvic. Mendel. Brun. 2012, 60, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Davis, N.E.; Coulson, G.; Forsyth, D.M. Diets of Native and Introduced Mammalian Herbivores in Shrub-Encroached Grassy Woodland, South-Eastern Australia. Wildl. Res. 2008, 35, 684. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Myers, C.A. The Impact of Warrens of the European Rabbit (Oryctolagus cuniculus L.) on Soil and Ecological Processes in a Semi-Arid Australian Woodland. J. Arid Environ. 2001, 47, 325–337. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.K.; Parer, I.; Coman, B.J.; Burley, J.; Braysher, M.L. Managing Vertebrate Pests: Rabbits. In Bureau of Resource Sciences/CSIRO Division of Wildlife and Ecology; Australian Government Publishing Service: Canberra, Australia, 1995. [Google Scholar]
- Hirakawa, H. Coprophagy in Leporids and Other Mammalian Herbivores. Mammal Rev. 2001, 31, 61–80. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Casado, J.; Ström, H.; Hilström, L.; Prada, L.M.; Carpio, A.J.; Tortosa, F.S. Assessment of the Suitability of Latrine Counts as an Indirect Method by Which to Estimate the Abundance of European Rabbit Populations at High and Low Abundance. Eur. J. Wildl. Res. 2020, 66, 10. [Google Scholar] [CrossRef]
- Land Information System Tasmania (LIST). Available online: https://maps.thelist.tas.gov.au/listmap/app/list/map (accessed on 11 January 2021).
- Walsh, A.M.; Wardlaw, T.J. Variation in mammal browsing damage between eucalypt plantations in Tasmania, and attempts to associate the variation with environmental features. Aust. For. 2011, 74, 197–204. [Google Scholar] [CrossRef]
- Land Tasmania. Available online: https://www.thelist.tas.gov.au/app/content/data/geo-meta-datarecord?detailRecordUID=b94d4388-995d-416a-9844-a39de2798bed (accessed on 11 January 2021).
- Department of Primary Industries, Parks, Water and Environment (DPIPWE). Available online: https://www.thelist.tas.gov.au/app/content/data/geo-meta-data-record?detailRecordUID=b5c7a079-14bc-4b3caf73-db7585d34cdd (accessed on 11 January 2021).
- Bulinski, J.; McArthur, C. Observer Error in Counts of Macropod Scats. Wildl. Res. 2000, 27, 277. [Google Scholar] [CrossRef]
- R Core Team (R). R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 13 March 2021).
- Minitab 16 Statistical Software (2010). Computer Software; Minitab, Inc.: State College, PA, USA. Available online: www.minitab.com (accessed on 24 September 2021).
- Kirkpatrick, J.B.; Marsden-Smedley, J.B.; Di Folco, M.-B.; Leonard, S.W.J. Influence of Grazing and Vegetation Type on Post-Fire Floristic and Lifeform Composition in Tasmania, Australia. Plant Ecol. 2016, 217, 57–69. [Google Scholar] [CrossRef]
- Jesser, P.; Queensland Land Protection. Deer (Family Cervidae) in Queensland; Land Protection, Department of Natural Resources and Mines: Brisbane, Australia, 2005. [Google Scholar]
- Silva, J.S.; Catry, F.X.; Moreira, F.; Lopes, T.; Forte, T.; Bugalho, M.N. Effects of Deer on the Post-Fire Recovery of a Mediterranean Plant Community in Central Portugal. J. For. Res. 2014, 19, 276–284. [Google Scholar] [CrossRef]
- Kie, J.G. Deer habitat use after prescribed burning in northern California. In Pacific Southwest Forest and Range Experiment Station, Forest Service; U.S. Department of Agriculture: Berkeley, CA, USA, 1984; Volume 3. [Google Scholar]
- Fellers, G.M. Abundance and impacts of fallow deer leks at point reyes national seashore. Calif. Fish Game 2007, 93, 149. [Google Scholar]
- Bennett, A. Sambar presence at Lake Mountain Yarra Ranges National Park and Mount Bullfight Nature Conservation Reserve alpine bogs. In A Report Prepared for Parks Victoria; The University of Melbourne: Melbourne, Australia, 2012. [Google Scholar]
- Ferreira, C.; Alves, P.C. Influence of Habitat Management on the Abundance and Diet of Wild Rabbit (Oryctolagus Cuniculus Algirus) Populations in Mediterranean Ecosystems. Eur. J. Wildl. Res. 2009, 55, 487–496. [Google Scholar] [CrossRef]
- Rollan, À.; Real, J. Effect of Wildfires and Post-Fire Forest Treatments on Rabbit Abundance. Eur. J. Wildl. Res. 2011, 57, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Leigh, J.H.; Wimbush, D.J.; Wood, D.H.; Holgate, M.D.; Slee, A.V.; Stanger, M.G.; Forrester, R.I. Effects of Rabbit Grazing and Fire on a Sub-Alpine Environment.I. Herbaceous and Shrubby Vegetation. Aust. J. Bot. 1987, 35, 433–464. [Google Scholar] [CrossRef]
- Green, K.; Davis, N.; Robinson, W. Diet of two fossorial herbivores in a seasonally snow-covered environment. Aust. Mammal. 2016, 39, 169–177. [Google Scholar] [CrossRef]
- Croft, J.D.; Fleming, P.J.S.; van de Ven, R. The impact of rabbits on a grazing system in eastern New South Wales. Aust. J. Exp. Agric. 2002, 42, 909–916. [Google Scholar] [CrossRef]
- Styger, J.K.; Kirkpatrick, J.B.; Marsden-Smedley, J.; Leonard, S.W.J. Fire incidence, but not fire size, affects macropod densities: Fire incidence affects macropod densities. Austral Ecol. 2010, 36, 679–686. [Google Scholar] [CrossRef]
- Grace, J.B.; Anderson, T.M.; Olff, H.; Scheiner, S.M. On the specification of structural equation models for ecological systems. Ecol. Monogr. 2010, 80, 67–87. [Google Scholar] [CrossRef] [Green Version]
- Mason, D.S.; Lashley, M.A. Spatial scale in prescribed fire regimes: An understudied aspect in conservation with examples from the southeastern United States. Fire Ecol. 2021, 17, 3. [Google Scholar] [CrossRef]
- Fox-Hughes, P.; Harris, R.; Lee, G.; Grose, M.; Bindoff, N. Future Fire Danger Climatology for Tasmania, Australia, Using a Dynamically Downscaled Regional Climate Model. Int. J. Wildland Fire 2014, 23, 309. [Google Scholar] [CrossRef]



| Fire Attributes | Dry Forest | Grassland | Highland | Sedgeland | Heath | Wet Forest |
|---|---|---|---|---|---|---|
| Number of burned sites/Number of different fire events | 4/4 | 2/2 | 5/1 | 4/4 | 4/4 | 4/2 |
| Average burn size (ha) | 14,881.1 ± 4427.4 (2–51,185.3) | 2.9 ± 0.8 (0.4–5.4) | 51,185.3 ± 0 (NA) | 14,807.5 ± 4406.3 (736.1–51,185.3) | 1689.5 ± 578.2 (67.0–6492.0) | 49,870.3 ± 5001.7 (8322.8–63,719.4) |
| Fire type/Time since fire (years) | Planned/1 Wildfire/1 Wildfire/2 Wildfire/2 | Planned/1 Planned/1 | Wildfire/2 | Planned/2 Planned/1 Wildfire/2 Wildfire/2 | Planned/1 Planned/3 Planned/3 Wildfire/2 | Planned/1 Wildfire/2 |
| Average scorch height (m) | 6.4 (0–14) | NA | 2.0 (0–2) | NA | 4.0 (0–4) | 8.9 (0–13) |
| Herbivore | Vegetation Community | Fire Status | Vegetation Community * Fire Status |
|---|---|---|---|
| Macropod * | df(5,264); F = 26.558; p ≤ 0.001 | df(1,264); F = 34.253; p ≤ 0.001 | df(5,264); F = 3.162; p = 0.00864 |
| Wombat * | df(5,264); F = 2.471; p = 0.0329 | df (1,264); F = 0.813; p = 0.3682 | df (5,264); F = 1.736; p = 0.1266 |
| Deer | df(5,264); F = 2.536; p = 0.0291 | df (1,264); F = 0.395; p = 0.5302 | df (5,264); F = 0.568; p = 0.7247 |
| Rabbit | df (5,264); F = 0.990; p = 0.4242 | df(1,264); F = 5.851; p = 0.0162 | df (5,264); F = 0.913; p = 0.4732 |
| Burned | Unburned | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry Forest | Grassland | Highland | Sedgeland | Heath | Wet Forest | Dry Forest | Grassland | Highland | Sedgeland | Heath | Wet Forest | |
| Macropod | 11.0 ± 2.20 (0–31) | 20.9 ± 2.84 (0–37) | 23.5 ± 3.49 (0–75) | 8.42 ± 2.34 (0–46) | 7.29 ± 1.46 (0–24) | 0.54 ± 0.33 (0–7) | 3.38 ± 1.09 (0–19) | 18.9 ± 2.93 (0–37) | 9.77 ± 1.56 (0–30) | 2.12 ± 0.82 (0–12) | 1.88 ± 0.55 (0–8) | 0.17 ± 0.17 (0–4) |
| Wombat | 0.04 ± 0.04 (0–1) | 1.50 ± 0.93 (0–11) | 0.33 ± 0.25 (0–7) | 0.04 ± 0.04 (0–1) | 0.04 ± 0.04 (0–1) | 0.25 ± 0.15 (0–3) | 0.25 ± 0.18 (0–2) | 1.00 ± 0.57 (0–15) | 0.05 ± 0.30 (0–6) | 0.08 ± 0.08 (0–2) | ||
| Deer | 1.38 ± 0.82 (0–15) | 1.42 ± 0.97 (0–10) | 5.17 ± 3.43 (0–101) | 0.79 ± 0.35 (0–5) | 0.83 ± 0.59 (0–12) | 4.08 ± 2.77 (0–27) | 2.47 ± 0.98 (0–26) | 0.33 ± 0.33 (0–8) | ||||
| Rabbit | 3.0 ± 2.39 (0–56) | 1.9 ± 0.874 (0–26) | 0.46 ± 0.27 (0–6) | 1.33 ± 0.97 (0–23) | 0.2 ± 0.14 (0–2) | 0.04 ± 0.04 (0–1) | 0.04 ± 0.04 (0–1) | |||||
| Vegetation Type | Macropod | Wombat | Deer | Rabbit | Fodder (%) |
|---|---|---|---|---|---|
| Dry eucalypt | 3.25 | 0.16 | 50.00 | 1.66 | 34.0 |
| Grassland | 1.11 | 6.00 | * | 0.35 | 95.0 |
| Highland | 2.40 | 0.33 | 9.50 | 2.09 | 30.0 |
| Sedgeland | 3.95 | 0.09 | 11.50 | 2.39 | 22.0 |
| Heath | 3.88 | 0.50 | 33.25 | * | 4.5 |
| Wet forest | 3.18 | * | * | * | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heaton, D.J.; McHenry, M.T.; Kirkpatrick, J.B. The Fire and Fodder Reversal Phenomenon: Vertebrate Herbivore Activity in Burned and Unburned Tasmanian Ecosystems. Fire 2022, 5, 111. https://doi.org/10.3390/fire5040111
Heaton DJ, McHenry MT, Kirkpatrick JB. The Fire and Fodder Reversal Phenomenon: Vertebrate Herbivore Activity in Burned and Unburned Tasmanian Ecosystems. Fire. 2022; 5(4):111. https://doi.org/10.3390/fire5040111
Chicago/Turabian StyleHeaton, David J., Melinda T. McHenry, and Jamie B. Kirkpatrick. 2022. "The Fire and Fodder Reversal Phenomenon: Vertebrate Herbivore Activity in Burned and Unburned Tasmanian Ecosystems" Fire 5, no. 4: 111. https://doi.org/10.3390/fire5040111
APA StyleHeaton, D. J., McHenry, M. T., & Kirkpatrick, J. B. (2022). The Fire and Fodder Reversal Phenomenon: Vertebrate Herbivore Activity in Burned and Unburned Tasmanian Ecosystems. Fire, 5(4), 111. https://doi.org/10.3390/fire5040111







