Research Progress of Gel Foam Extinguishing Agent in Coal Mines
Abstract
1. Introduction
2. Study on Coal Spontaneous Combustion and Prevention Technology
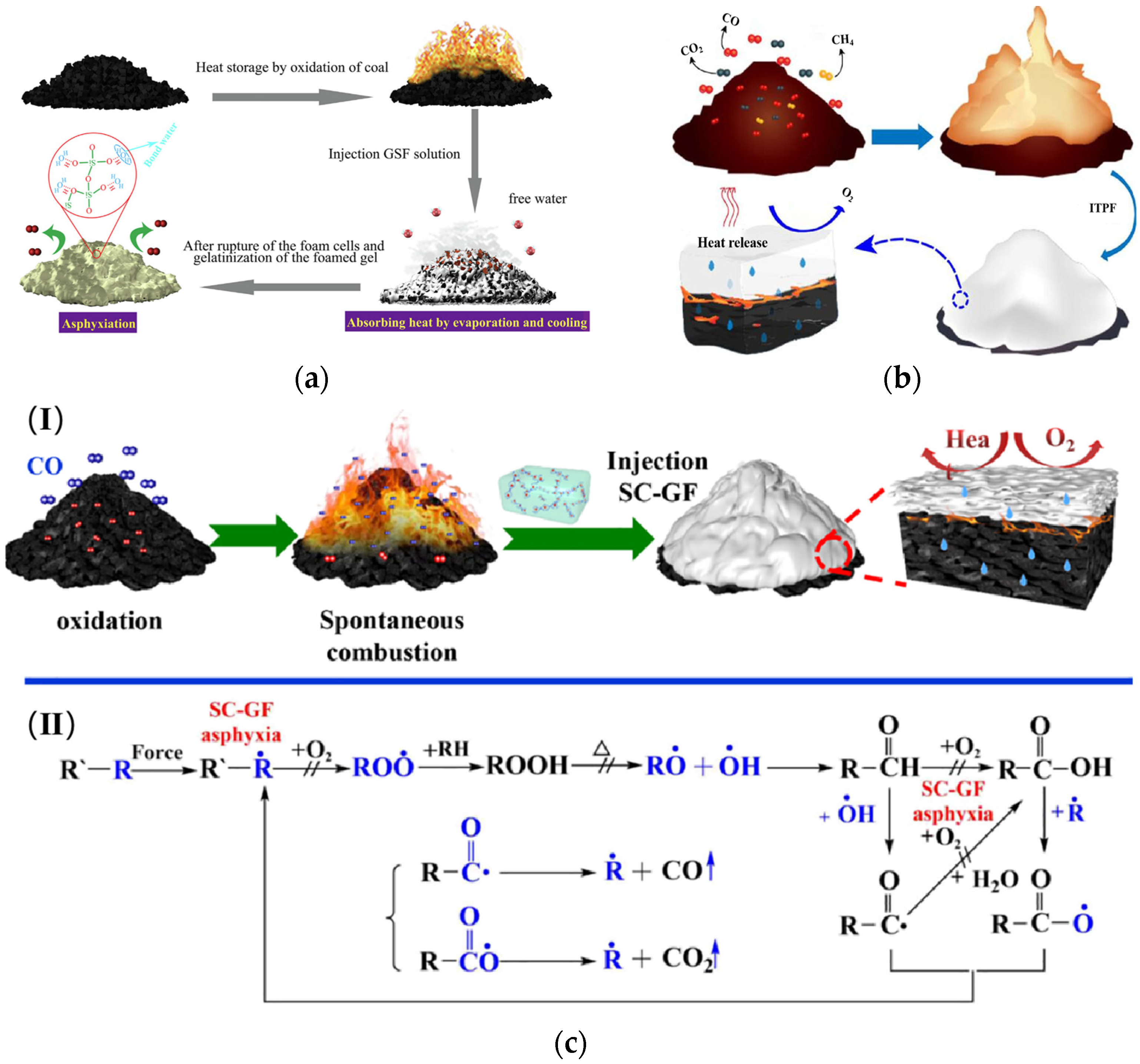
2.1. The Mechanism of Coal Spontaneous Combustion
2.2. Technologies of Prevention and Control of Coal Spontaneous Combustion
2.2.1. Grouting
2.2.2. Inert Gas
2.2.3. Inhibitor
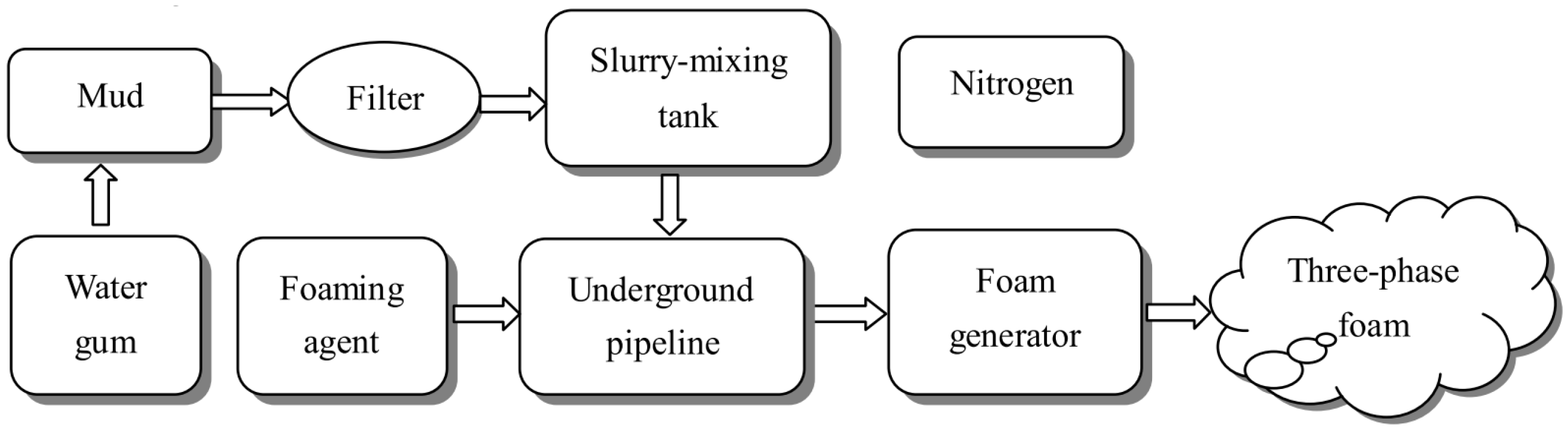
2.2.4. Foam
2.2.5. Gel
3. Structure and Principle of Gel Foam
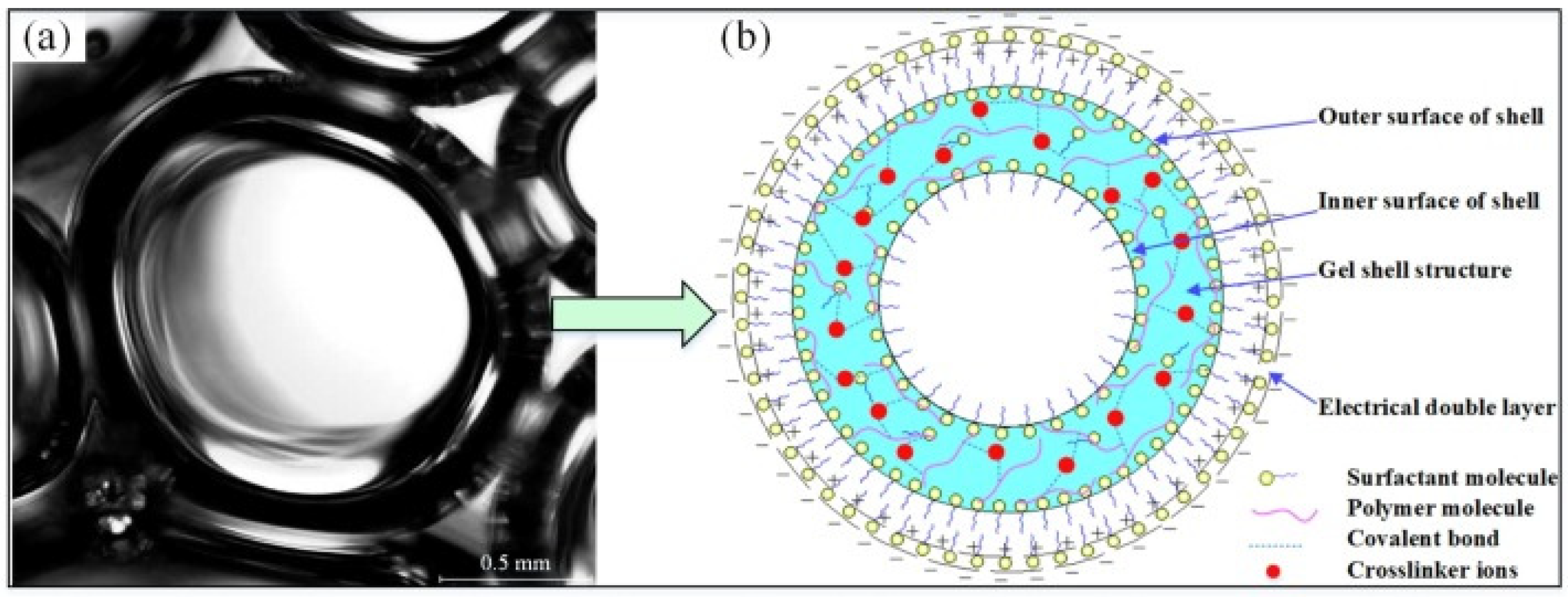
3.1. Structural Features of Gel Foam
3.2. Principle of Gel Foam Action
- (a)
- The cooling effect [57]
- (b)
- The plugging effect
- (c)
- The inhibition effect
4. Materials Research
4.1. Silicate Gel Foam
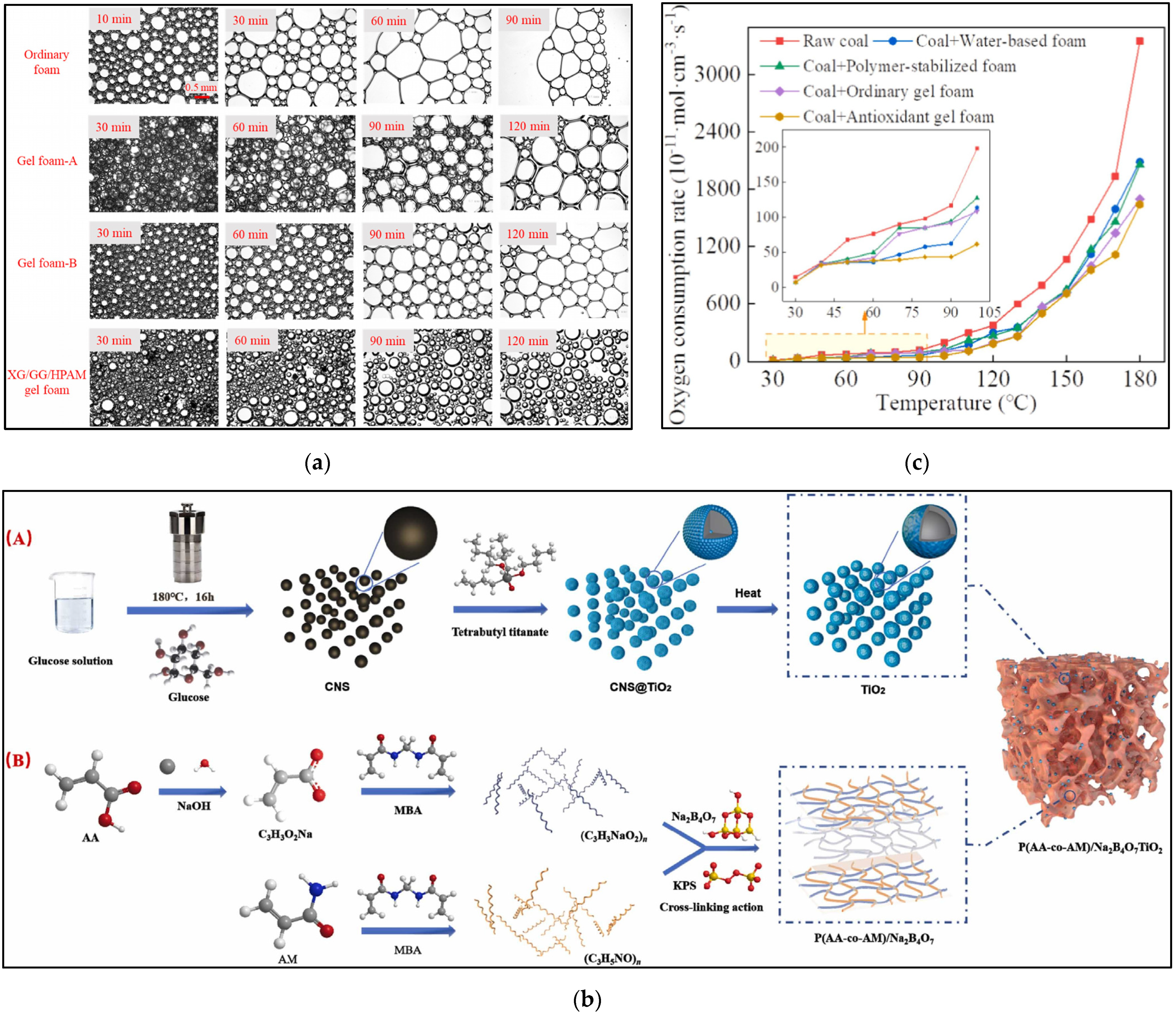
4.2. Acrylamide Copolymer Gel Foam
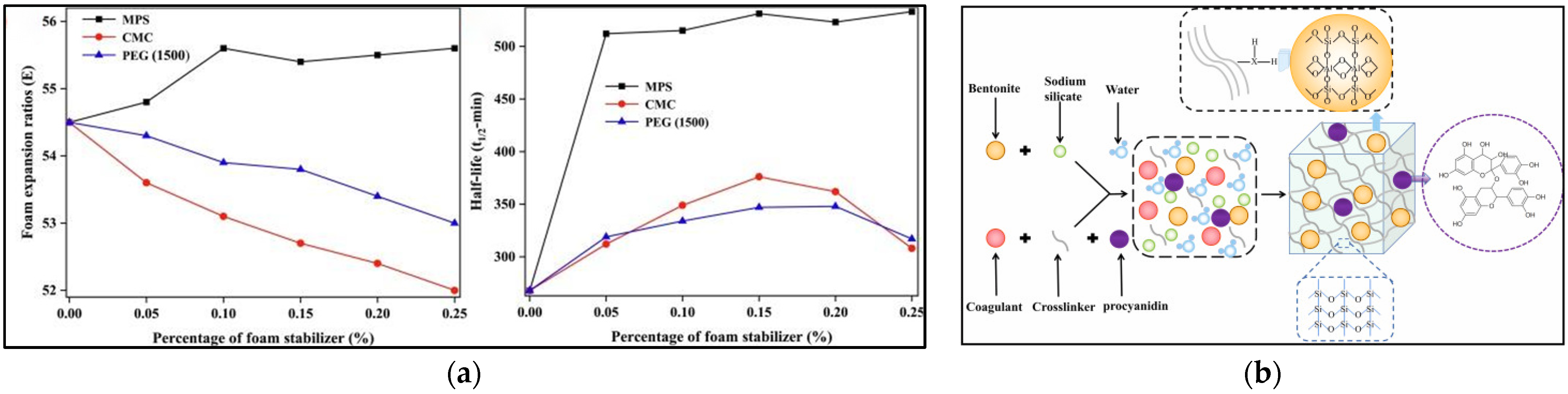
4.3. Natural Polymer Gel Foam
5. Performance Research
5.1. Rheological Properties
5.2. Stability Properties
5.3. Plugging Properties
5.4. Inhibition Properties
6. Prospects
- (1)
- Systematic configuration of gel foam materials
- (2)
- Further basic research on the macroscopic role and microscopic mechanism of gel foam
- (3)
- Research on multi-stage cooperative fire extinguishing technology
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Musa, S.D.; Zhonghua, T.; Ibrahim, A.O.; Habib, M. China’s energy status: A critical look at fossils and renewable options. Renew. Sustain. Energy Rev. 2018, 81, 2281–2290. [Google Scholar] [CrossRef]
- Onifade, M.; Genc, B.; Carpede, A. A new apparatus to establish the spontaneous combustion propensity of coals and coal-shales. Int. J. Min. Sci. Technol. 2018, 28, 649–655. [Google Scholar] [CrossRef]
- Kong, B.; Li, Z.; Yang, Y.; Liu, Z.; Yan, D. A review on the mechanism, risk evaluation, and prevention of coal spontaneous combustion in China. Environ. Sci. Pollut. Res. Int. 2017, 24, 23453–23470. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.W.; Xiao, Y.; Zhong, K.Q.; Shu, C.M.; Lü, H.F.; Deng, J.; Wu, S. Overview of commonly used materials for coal spontaneous combustion prevention. Fuel 2020, 275, 117981. [Google Scholar] [CrossRef]
- Yu, M.; Wang, L.; Li, H. Research status and development trend of fire-extinguishing materials in Chinese coal mines. Min. Saf. Environ. Prot. 2022, 49, 22–36. (In Chinese) [Google Scholar]
- Onifade, M.; Genc, B. A review of spontaneous combustion studies—South African context. Int. J. Min. Reclam. Environ. 2019, 33, 527–547. [Google Scholar] [CrossRef]
- Civeira, M.S.; Pinheiro, R.N.; Gredilla, A.; de Vallejuelo, S.F.O.; Oliveira, M.L.; Ramos, C.G.; Taffarel, S.R.; Kautzmann, R.M.; Madariaga, J.M.; Silva, L.F. The properties of the nano-minerals and hazardous elements: Potential environ-mental impacts of Brazilian coal waste fire. Sci. Total Environ. 2016, 544, 892–900. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, J.; Zhao, L. Investigation of the potential risk of coal fire to local en-vironment: A case study of Daquanhu coal fire, Xinjiang region. China. Sci. Total Environ. 2018, 640, 1478–1488. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, T.; Liang, Y.; Wang, Z. A review on numerical solutions to self-heating of coal stockpile: Mechanism, theoretical basis, and variable study. Fuel 2016, 182, 80–109. [Google Scholar] [CrossRef]
- Qin, B.T.; Zhong, X.X.; Wang, D.M.; Xin, H.H.; Shi, Q.L. Research progress of coal spontaneous combustion process characteristics and prevention technology. Coal Sci. Technol. 2021, 49, 66–99. (In Chinese) [Google Scholar]
- Wang, H.H.; Dlugogorski, B.Z.; Kennedy, E.M. Kinetic modeling of low-temperature oxidation of coal. Combust. Flame 2002, 131, 452–464. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, S.; Liang, Y.; Song, S.; Ren, T. Analytical prediction of coal spontaneous combustion tendency: Velocity range with high possibility of self-ignition. Fuel Process. Technol. 2017, 159, 38–47. [Google Scholar] [CrossRef]
- Ma, T.; Zhai, X.W.; Xiao, Y.; Bai, Y.E.; Shen, K.; Song, B.B.; Hao, L.; Ren, L.F.; Chen, X.K. Study on the influence of key active groups on gas products in spontaneous combustion of coal. Fuel 2023, 344, 128020. [Google Scholar] [CrossRef]
- Qi, X.; Wang, D.; Xin, H.; Qi, G. An In Situ Testing Method for Analyzing the Changes of Active Groups in Coal Oxidation at Low Temperatures. Spectrosc. Lett. 2014, 47, 495–503. [Google Scholar] [CrossRef]
- Qi, X.; Xue, H.; Xin, H.; Wei, C. Reaction pathways of hydroxyl groups during coal spontaneous combustion. Can. J. Chem. 2016, 94, 494–500. [Google Scholar] [CrossRef]
- Wang, D.M.; Xin, H.H.; Qi, X.Y.; Dou, G.L.; Zhong, X.X. Mechanism and relationships of elementary reactions in spontaneous combustion of coal: The coal oxidation kinetics theory and application. J. China Coal Soc. 2014, 39, 1667–1674. (In Chinese) [Google Scholar]
- Xi, Z.; Suo, L.; Xia, T. Characterization of elementary reactions inducing coal spontaneous combustion. Fuel 2024, 358, 130138. [Google Scholar] [CrossRef]
- Wei, A.; Li, Z.; Pan, S.; Yang, Y. Experimental study on free radical reaction of coal initiated by ultraviolet light. J. China Univ. Min. Technol. 2007, 36, 582–585. (In Chinese) [Google Scholar]
- Marinov, V.N. Self-ignition and mechanisms of interaction of coal with oxygen at low temperatures. Fuel 1977, 56, 158–164. [Google Scholar] [CrossRef]
- Clemens, A.H.; Matheson, T.W.; Rogers, D.E. Low temperature oxidation studies of dried New Zealand coals. Fuel 1991, 70, 215–221. [Google Scholar] [CrossRef]
- Ren, W.X.; Guo, Q.; Zuo, B.Z.; Wang, Z.F.; Fang, S.Q. Characteristics and mechanism of prevention and control of coal spontaneous combustion with foam-gel. J. China Coal Soc. 2015, 40 (Suppl. S2), 401–406. (In Chinese) [Google Scholar]
- Zhao, J.; Zhang, X. Experimental study of mine grout injection plastic fire prevention materials flow properties. J. China Coal Soc. 2015, 40, 383–388. (In Chinese) [Google Scholar]
- Zhai, X.; Wu, S.; Wang, K.; Drebenstedt, C.; Zhao, J. Environment influences and extinguish technology of spontaneous combustion of coal gangue heap of Baijigou coal mine in China. Energy Procedia 2017, 136, 66–72. [Google Scholar] [CrossRef]
- Wu, J.M.; Zhang, J.H.; Wu, Y.G. Detection on Spontaneous Combustion Area in Surface Mine and Study on Comprehensive Control Technology of Spontaneous Combustion Area. Coal Eng. 2012, 44, 53–56. [Google Scholar]
- Gao, G. The present state and prospects for nitrogen fire prevention and fire-elimination technology in coal mines of China. J. China Coal Soc. 1999, 24, 48–51. (In Chinese) [Google Scholar]
- Zhang, C.H.; Wang, J.; Zhang, Z.M. Liquid Carbon Dioxide Fire Extinguishing Equipments and Their Engendering Applications. Sci. Technol. Rev. 2013, 31, 44–48. (In Chinese) [Google Scholar]
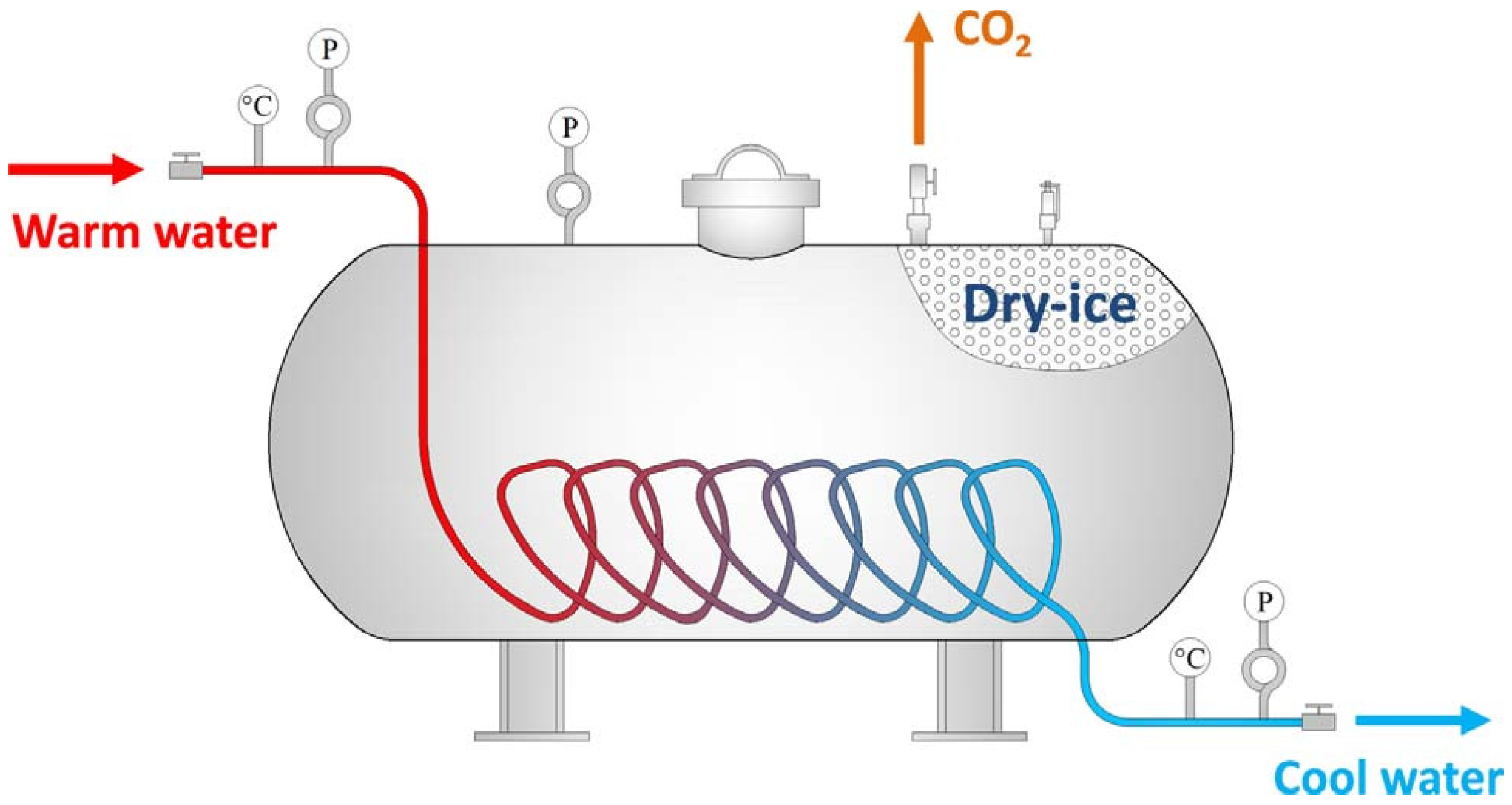
- Liu, W.; Qin, Y.; Yang, X.; Wang, W.; Chen, Y. Early extinguishment of spontaneous combustion of coal underground by using dry-ice’s rapid sublimation: A case study of application. Fuel 2018, 217, 544–552. [Google Scholar] [CrossRef]
- Chi, K.; Wang, J.; Ma, L.; Wang, J.; Zhou, C. Synergistic Inhibitory Effect of Free Radical Scavenger/Inorganic Salt Compound Inhibitor on Spontaneous Combustion of Coal. Combust. Sci. Technol. 2022, 194, 2146–2162. [Google Scholar] [CrossRef]
- Lu, W.; Guo, B.; Qi, G.; Cheng, W.; Yang, W. Experimental study on the effect of preinhibition temperature on the spontaneous combustion of coal based on an MgCl2 solution. Fuel 2020, 265, 117032. [Google Scholar] [CrossRef]
- Liu, W.Y.; Wen, H.; Xiao, Y.; Li, Y.H.; Yin, L.; Bai, G.Y. Inhibiting effects of layered double hydroxides containing the rare-earth element lanthanum on coal spontaneous combustion. Thermochim. Acta 2020, 687, 178573. [Google Scholar] [CrossRef]
- Pan, R.; Ma, J.; Fu, D.; Li, C.; Jia, H.; Zheng, L. Experimental study on the new environmental protection chemical composite inhibitor for the inhibition of coal spontaneous combustion. J. Therm. Anal. Calorim. 2020, 139, 37–45. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Gao, Y.; Zhang, Y.; Li, Z.; Wang, H.; Shi, X. Experimental study on the compound system of proanthocyanidin and polyethylene glycol to prevent coal spontaneous combustion. Fuel 2019, 254, 115610. [Google Scholar] [CrossRef]
- Zhong, X.; Qin, B.; Dou, G.; Xia, C.; Wang, F. A chelated calcium-procyanidine-attapulgite composite inhibitor for the suppression of coal oxidation. Fuel 2018, 217, 680–688. [Google Scholar] [CrossRef]
- Qin, B.; Dou, G.; Wang, Y.; Xin, H.; Ma, L.; Wang, D. A superabsorbent hydrogel–ascorbic acid composite inhibitor for the suppression of coal oxidation. Fuel 2017, 190, 129–135. [Google Scholar] [CrossRef]
- Wang, X.; Deng, H.; Deng, C.; Zhang, X.; Zhao, Q. Research on Selection of Coal Spontaneous Combustion Inhibitors and Spraying Process. China Saf. Sci. J. 2013, 23, 105–109. (In Chinese) [Google Scholar]
- Wang, D. A Novel Technology of Mine Fire Fighting-Three-phrase Foam. Saf. Coal Mines 2004, 35, 16–18. (In Chinese) [Google Scholar]
- Xie, Z.; Li, X.; Liu, M. Application of Three-phase Foam Technology for Spontaneous Combustion Prevention in Longdong Coal Mine. Procedia Eng. 2011, 26, 63–69. [Google Scholar] [CrossRef][Green Version]
- Ren, W.; Kang, Z.; Wang, D. Causes of Spontaneous Combustion of Coal and Its Prevention Technology in the Tunnel Fall of Ground of Extra-thick Coal Seam. Procedia Eng. 2011, 26, 717–724. [Google Scholar] [CrossRef]
- Qin, B.; Lu, Y.; Li, Y.; Wang, D. Aqueous three-phase foam supported by fly ash for coal spontaneous combustion prevention and control. Adv. Powder Technol. 2014, 25, 1527–1533. [Google Scholar] [CrossRef]
- Zhang, L.; Shu, S.; Bian, Y. Inhibition effect and mechanism of nano-aluminum hydroxide foam on coal spontaneous combustion. Thermochim. Acta 2022, 718, 179389. [Google Scholar]
- Zhou, C.; Tang, Y. A novel sodium carboxymethyl ellulose/aluminium citrate gel for extinguishing spontaneous fire in coal mines. Fire Mater. 2018, 42, 760–769. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, C.; Gao, Y.; Ji, Y.; Wang, H.; Zhang, Y.; Yang, R. RD of colloid components of composite material for fire prevention and extinguishing and an investigation of its performance. Process Saf. Environ. Prot. 2018, 113, 357–368. [Google Scholar] [CrossRef]
- Ren, X.; Hu, X.; Xue, D.; Li, Y.; Shao, Z.; Dong, H.; Cheng, W.; Zhao, Y.; Xin, L.; Lu, W. Novel sodium silicate/polymer composite gels for the prevention of spontaneous combustion of coal. J. Hazard. Mater. 2019, 371, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Research on Characteristics of the Newly Developed Thickening Gels for Prevention and Control of Coal Spontaneous Combustion. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2017. (In Chinese). [Google Scholar]
- Miller, M.J.; Khilar, K.; Fogler, H.S. Aging of foamed gel used for subsurface permeability reduction. J. Colloid Interface Sci. 1995, 175, 88–96. [Google Scholar] [CrossRef]
- Qin, B.; Feng, L.; Jiang, W. Research progress on extinguishing foam of coal mine. Coal Sci. Technol. Mag. 2022, 43, 1–1226. (In Chinese) [Google Scholar]
- Xie, J.; Zhang, X.; Xu, S. Research Status and Development Trend of Foam Fire Prevention Materials. Coal Technol. 2023, 42, 129–132. (In Chinese) [Google Scholar]
- Zhao, L. Laboratory experimental researches on profile control by gelling foaming fluids. Oilfield Chem. 1990, 7, 347–350. (In Chinese) [Google Scholar]
- Zhao, R.; Yue, X.; Hou, J. Feasibility Study of Authigenic Gas Gelling/Foaming Fluid as Indepth Profiling Agent Injected in Single Slug. Oilfield Chem. 2005, 22, 362–365. (In Chinese) [Google Scholar]
- Tian, Z. Research on the Theory and Technology of Foamed Gel Using for Fire Prevention. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2009. (In Chinese). [Google Scholar]
- Qin, B.T.; Zhang, L.L.; Lu, Y. Experimental studies on preparation of multi-phase foamed gel for preventing spontaneous combustion of coal. J. Cent. South Univ. Sci. Technol. 2013, 44, 4652–4657. (In Chinese) [Google Scholar]
- Zheng, C. Study on Preparation and Performance of New Gel Foam for Preventing Coal Spontaneous Combustion. Ph.D. Thesis, Xi’an University of Science and Technology, Xi’an, China, 2020. (In Chinese). [Google Scholar]
- Shi, L. Study on the Theory and Properties of Colloidal Foam for Controlling and Preventing Coal Spontaneous Combustion. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 2019. (In Chinese). [Google Scholar]
- Zhong, D. Study on Formation Mechanism and Inhibitory Property of Gel Foam for Coal Mine Fire Prevention and Extinguishing. Ph.D. Thesis, Hunan University of Science and Technology, Xiangtan, China, 2019. (In Chinese). [Google Scholar]
- Ji, Y. Study on Mechanism of Antioxidant Foamed Gel for Preventing Spontaneous Combustion of Coal in Gob. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2022. (In Chinese). [Google Scholar]
- Cai, C. Study on Preparation and Characteristics of Highly Stable Foam Gel for Prevention and Control of Coal Spontaneous Combustion. Shandong Coal Sci. Technol. 2022, 40, 112–114120. (In Chinese) [Google Scholar]
- Xue, D.; Hu, X.; Cheng, W.; Wei, J.; Zhao, Y.; Shen, L. Fire prevention and control using gel-stabilization foam to inhibit spontaneous combustion of coal: Characteristics and engineering applications. Fuel 2020, 264, 116903. [Google Scholar] [CrossRef]
- Chen, J.; Jia, B.; Fu, S.; Wen, Y.; Liang, Y.; Tian, F. Novel PFA-Based Inorganic Three-Phase Foam for Inhibiting Coal Spontaneous Combustion. ACS Omega 2023, 8, 24615–24623. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.T.; Zhang, L.L.; Lu, Y. Preparation and inhibition characteristic of multi-phase foamed gel for preventing spontaneous combustion of coal. Adv. Mater. Res. 2013, 634, 3678–3682. [Google Scholar] [CrossRef]
- Han, C.; Nie, S.; Liu, Z.; Liu, S.; Zhang, H.; Li, J.; Zhang, H.; Wang, Z. A novel biomass sodium alginate gel foam to inhibit the spontaneous combustion of coal. Fuel 2022, 314, 122779. [Google Scholar] [CrossRef]
- Hou, C.; Zhou, Y.; Luo, H. The Analysis of the Solidification Mechanisms & Water Resistance Improvement Accesses of the Water-Glass. Ceramics 2011, 15, 18–21. (In Chinese) [Google Scholar]
- Xi, Z.; Wang, X.; Wang, X.; Wang, L.; Li, D.; Guo, X.; Jin, L. Self-hardening thermoplastic foam for the inhibition of coal oxidation at low temperatures. Combust. Sci. Technol. 2019, 191, 1942–1959. (In Chinese) [Google Scholar] [CrossRef]
- Wu, X. Preparation of water glass gel foam fire-fighting materials for mining. Inn. Mong. Coal Econ. 2020, 24, 128–129. (In Chinese) [Google Scholar]
- Wu, X.; Nie, S.; Ha, C. Study on preparation and performance of gel foam material for prevention and control of coal mine fire. J. Saf. Sci. Technol. 2022, 18, 78–84. (In Chinese) [Google Scholar]
- Lu, W.; Zhang, X.; Yuan, Y.; Qi, G.; Hu, X.; Li, J.; Liang, Y.; Guo, B. Study on the characteristics and mechanism of a new type of antioxidant gel foam for coal spontaneous combustion prevention. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127254. [Google Scholar] [CrossRef]
- Xue, D.; Hu, X.; Cheng, W.; Wu, M.; Shao, Z.; Li, Y.; Zhao, Y.; Zhang, K. Carbon dioxide sealing-based inhibition of coal spontaneous combustion: A temperature-sensitive micro-encapsulated fire-retardant foamed gel. Fuel 2020, 266, 117036. [Google Scholar] [CrossRef]
- Lu, X.X.; Xue, X.; Wang, C.Y.; Shi, G.Y.; Xing, Y.; Han, Y. Investigation on the suppression characteristic of deoxidization gel foam on coal spontaneous combustion. Fire Mater. 2022, 46, 651–665. [Google Scholar] [CrossRef]
- Li, D. Progress in the Application of Polyacrylamide. Guangdong Chem. Ind. 2022, 49, 9186–9190. (In Chinese) [Google Scholar]
- Shen, Y.; Wang, D.; Wang, Q. Development of a Kind of Foamed Gel and Its Sealing and Inhibition Characteristics. Saf. Coal Mines 2017, 48, 28–31. (In Chinese) [Google Scholar]
- Fan, X.L.; Ma, L.; Sheng, Y.J.; Liu, X.X.; Wei, G.M.; Liu, S.M. Experimental investigation on the characteristics of XG/GG/HPAM gel foam and prevention of coal spontaneous combustion. Energy 2023, 284, 128710. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, G.; Li, F.; Guo, Y.; Chen, C.; Chen, C.; Li, R.; Yang, Z. A novel H-TiO2/gel co-stabilized three-dimensional network synergistic fire-retardant foam gel for coal-pile. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129642. [Google Scholar] [CrossRef]
- Ma, L.; Fan, X.; Wei, G.; Sheng, Y.; Liu, S.; Liu, X. Preparation and characterization of antioxidant gel foam for preventing coal spontaneous combustion. Fuel 2023, 338, 127270. [Google Scholar] [CrossRef]
- Wang, G.; Yan, G.; Zhang, X.; Du, W.; Huang, Q.; Sun, L.; Zhang, X. Research and development of foamed gel for controlling the spontaneous combustion of coal in coal mine. J. Loss Prev. Process Ind. 2016, 44, 474–486. [Google Scholar] [CrossRef]
- Chen, W.; Gao, J.; Ma, J. Research Progress of Sodium Alginate Composite Hydrogel. J. Beijing Inst. Fash. Technol. Nat. Sci. Ed. 2022, 42, 101–108. (In Chinese) [Google Scholar]
- Han, C.; Nie, S.; Liu, Z.; Yang, J.; Zhang, H.; Zhang, H.; Li, J.; Wang, Z. A Novel Highly Stable Biomass Gel Foam Based on Double Cross-Linked Structure for Inhibiting Coal Spontaneous Combustion. Energies 2022, 15, 5207. [Google Scholar] [CrossRef]
- Vahabi, H.; Shabanian, M.; Aryanasab, F.; Mangin, R.; Laoutid, F.; Saeb, M.R. Inclusion of modified lignocellulose and nano-hydroxyapatite in development of new bio-based adjuvant flame retardant for poly (lactic acid). Thermochem. Acta 2018, 666, 51–59. [Google Scholar] [CrossRef]
- Han, C.; Nie, S.; Liu, Z.; Yang, J.; Zhang, H.; Li, J.; Zhang, H. Study on the Performance of a Novel Highly Stable Nano-Hydroxyapatite Gel Foam to Inhibit Coal Spontaneous Combustion. Combust. Sci. Technol. 2022, 1–15. [Google Scholar] [CrossRef]
- Wu, M.; Liang, Y.; Zhao, Y.; Wang, W.; Hu, X.; Tian, F.; He, Z.; Li, Y.; Liu, T. Preparation of new gel foam and evaluation of its fire extinguishing performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127443. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, H.; Han, C.; Li, J.; Yang, J.; Zhang, H.; Dai, G.; Su, H. Preparation of New Eco-Friendly Gel Foam Based on Biomass Pectin Material for Fire Prevention of Coal. Combust. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B. Experimental research on gel-stabilized foam designed to prevent and control spontaneous combustion of coal. Fuel 2019, 254, 115558. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, K.; Zhang, Y.; Dong, Z.; Wang, J. Study on preparation and characteristics of CMC/ZrCit/GDL firefighting gel foam. Coal Sci. Technol. 2023, 51, 122–129. (In Chinese) [Google Scholar]
- Dai, Q.; Zhao, W.; Fan, C. Preparation of water glass gel foam fire-fighting materials for mining. Oil-Garfield Surf. Eng. 2010, 29, 15–16. (In Chinese) [Google Scholar]
- Cao, Q.; Shen, J.; Guo, W. Effects of foaming agent on properties of foamed gel. Adv. Compos. Lett. 2020, 29, 0963693519881649. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, B. Rheological characteristics of foamed gel for mine fire control. Fire Mater. 2016, 40, 246–260. [Google Scholar] [CrossRef]
- Wang, Z. Study on Flow Characteristics and Field Application of Foamed Gel Using for Fire Prevention. Datong Coal Sci. Technol. 2019, 1, 16–18. (In Chinese) [Google Scholar]
- Cao, Q.; Cao, Q. Effects of temperature on the properties of the new gel foam. In Advances in Machinery, Materials Science and Engineering Application; IOS Press: Amsterdam, The Netherlands, 2022; pp. 95–103. [Google Scholar]
- Sheng, Y.; Zhang, H.; Ma, L.; Wang, Z.; Hu, D.; Zhang, S. Rheological Properties of Gel Foam Co-Stabilized with Nanoparticles, Xanthan Gum, and Multiple Surfactants. Gels 2023, 9, 534. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B.; Hao, Y.; Li, H. Experimental investigation of the flow and extinguishment characteristics of gel-stabilized foam used to control coal fire. Energy 2022, 247, 123484. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, B.; Shi, B.; Wu, Q.; Wang, J. The fire extinguishing performances of foamed gel in coal mine. Nat. Hazards 2016, 81, 1957–1969. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Wang, Z.; Wang, D.; Zhan, H. Experimental investigation of the mechanism of foaming agent concentration affecting foam stability. J. Surfactants Deterg. 2017, 20, 1443–1451. [Google Scholar] [CrossRef]
- Xu, C.; Wang, D.; Wang, H.; Hu, J.; Zhu, X.; Zhang, Y. Effect of partially hydrolyzed polyacrylamide on the solution and foam properties of sodium alcohol ether sulfate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 51–60. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, D.; Craig, V.S.J. The interaction of particles with surfactant thin films: Implications for dust suppression. Langmuir 2019, 35, 7641–7649. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, D.; Wang, H.; Ma, L.; Zhu, X.; Zhu, Y.; Zhang, Y.; Liu, F. Experimental investigation of coal dust wetting ability of anionic surfactants with different structures. Process Saf. Environ. Prot. 2019, 121, 69–76. [Google Scholar] [CrossRef]
- Zhu, S. Study on characteristics of foam gel for prevention and control of coal spontaneous combustion. Coal Sci. Technol. 2019, 47, 223–228. (In Chinese) [Google Scholar]
- Save, V.; Pangarkar, V.G. Characterization of colloidal gas aprons. Chem. Eng. Commun. 1994, 127, 35–54. [Google Scholar] [CrossRef]
- Wang, J.; Yan, T.; Zheng, C. Development of a kind of gel foam and its thermal stability. Saf. Coal Mines 2021, 52, 42–46. (In Chinese) [Google Scholar]
- Yan, Y.L.; Qu, C.T.; Zhang, N.S.; Yang, Z.G.; Liu, L. A study on the kinetics of liquid drainage from colloidal gas aphrons (CGAs). Colloids Surf. A Physicochem. Eng. Asp. 2005, 259, 167–172. (In Chinese) [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B.; Xu, Y.; Hao, M.; Shao, X.; Zhuo, H. Experimental investigation of the drainage characteristic and stability mechanism of gel-stabilized foam used to extinguish coal fire. Fuel 2022, 313, 122685. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, Y.; Huang, X. Stabilization of foams solely with polyoxyethylene-type nonionic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2008, 331, 239–244. (In Chinese) [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Wang, Z.; Zhang, X. Research on the fire extinguishing performance of new gel foam for preventing and controlling the spontaneous combustion of coal gangue. Environ. Sci. Pollut. Res. 2023, 30, 88548–88562. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, H.; Meng, G.; Chen, X. Research on gelling and fire extinguishing characteristic of a new-style foamed gel. Fire Saf. Sci. 2013, 22, 219–225. [Google Scholar]
- Zhang, L.; Qin, B. Study on the gelation of foamed gel for preventing the spontaneous combustion of coal. J. Spectrosc. 2014, 2014, 163742. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, G.; Li, F.; Chen, C.; Chen, C.; Li, R.; Zou, R.; Zhang, M. Response Surface Analysis (RSA) optimization of temperature-resistant gel foam fabrication and performance evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130260. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, G.; Li, F.; Chen, C.; Chen, C.; Li, R.; Zou, R.; Zhang, M. Research of Inhibition Characteristic of Foam Gel. Saf. Coal Mines 2009, 40, 8–10. (In Chinese) [Google Scholar]
- Li, F.; Zhang, L.; Wei, J. Study on preparation and characteristics of gel foam for preventing fire. Min. Saf. Environ. Prot. 2022, 49, 34–38. (In Chinese) [Google Scholar]
- Wang, J.; Liu, X.; Zheng, C. Experimental research on the resistance performance of a new type of gel foam material. Min. Saf. Environ. Prot. 2021, 48, 30–3339. (In Chinese) [Google Scholar]
- Lu, X.; Zhao, H.; Xue, X.; Han, Y. Experimental Research on Inhibition Characteristic of Foamed Gel on Raw Coal Spontaneous Combustion. Saf. Coal Mines 2019, 50, 33–37. (In Chinese) [Google Scholar]
- Huang, Z.; Quan, S.; Hu, X.; Zhang, Y.; Gao, Y.; Ji, Y.; Qi, X.; Yin, Y. An environmentally friendly antioxidant foamed gel for inhibiting spontaneous combustion of coal. Combust. Sci. Technol. 2023, 195, 4144–4165. [Google Scholar] [CrossRef]
- Lu, W.; Cao, H.; Sun, X.; Hu, X.; Li, J.; Li, J.; Kong, B. A Review on the Types, Performance, and Environmental Protection of Filling and Plugging Materials for Prevention and Control of Coal Spontaneous Combustion in China. Combust. Sci. Technol. 2022. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tian, Z.; Ye, Q.; Lu, Y. Research Progress of Gel Foam Extinguishing Agent in Coal Mines. Fire 2023, 6, 470. https://doi.org/10.3390/fire6120470
Zhang Y, Tian Z, Ye Q, Lu Y. Research Progress of Gel Foam Extinguishing Agent in Coal Mines. Fire. 2023; 6(12):470. https://doi.org/10.3390/fire6120470
Chicago/Turabian StyleZhang, Yan, Zhaojun Tian, Qing Ye, and Yi Lu. 2023. "Research Progress of Gel Foam Extinguishing Agent in Coal Mines" Fire 6, no. 12: 470. https://doi.org/10.3390/fire6120470
APA StyleZhang, Y., Tian, Z., Ye, Q., & Lu, Y. (2023). Research Progress of Gel Foam Extinguishing Agent in Coal Mines. Fire, 6(12), 470. https://doi.org/10.3390/fire6120470