Engineering Attributes of Ternary Geopolymer Mortars Containing High Volumes of Palm Oil Fuel Ash: Impact of Elevated Temperature Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix Design
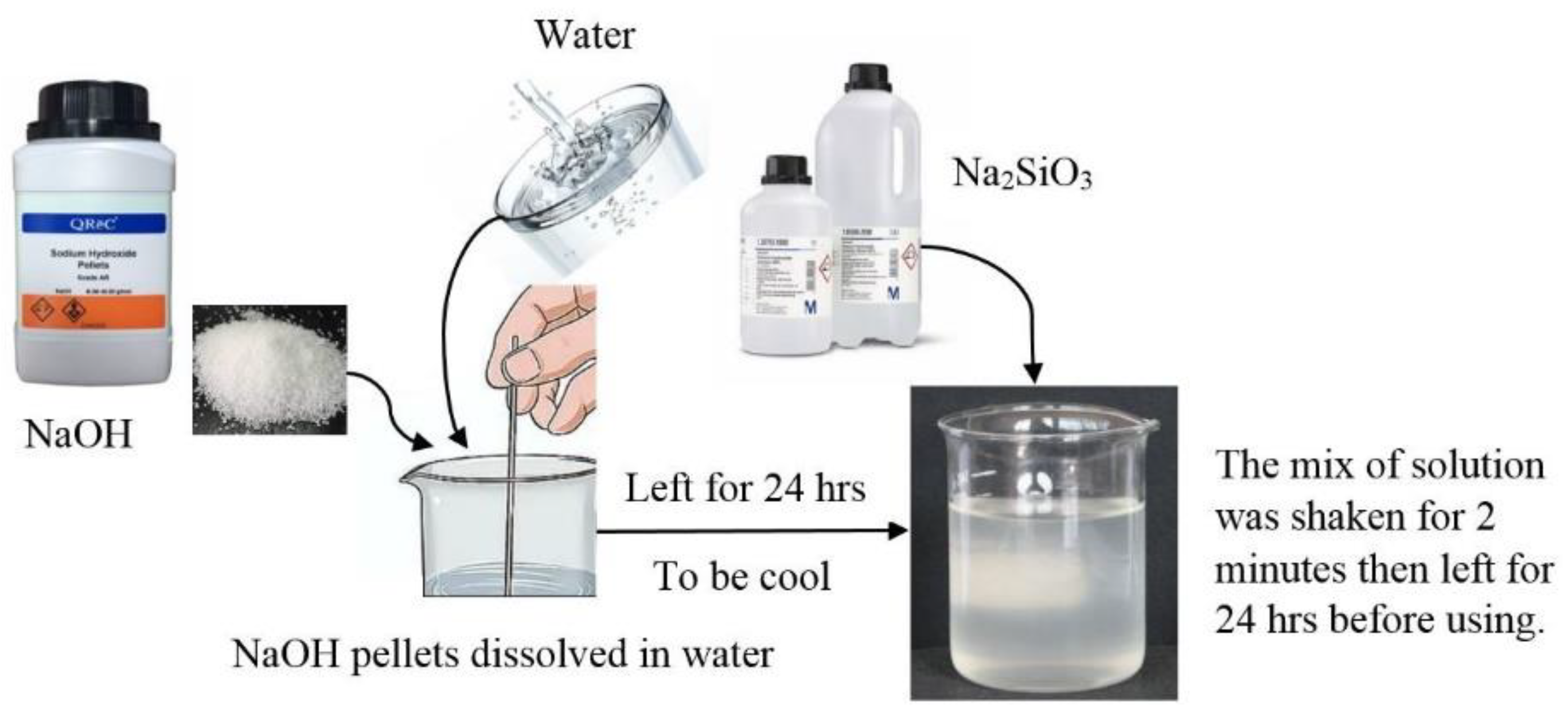
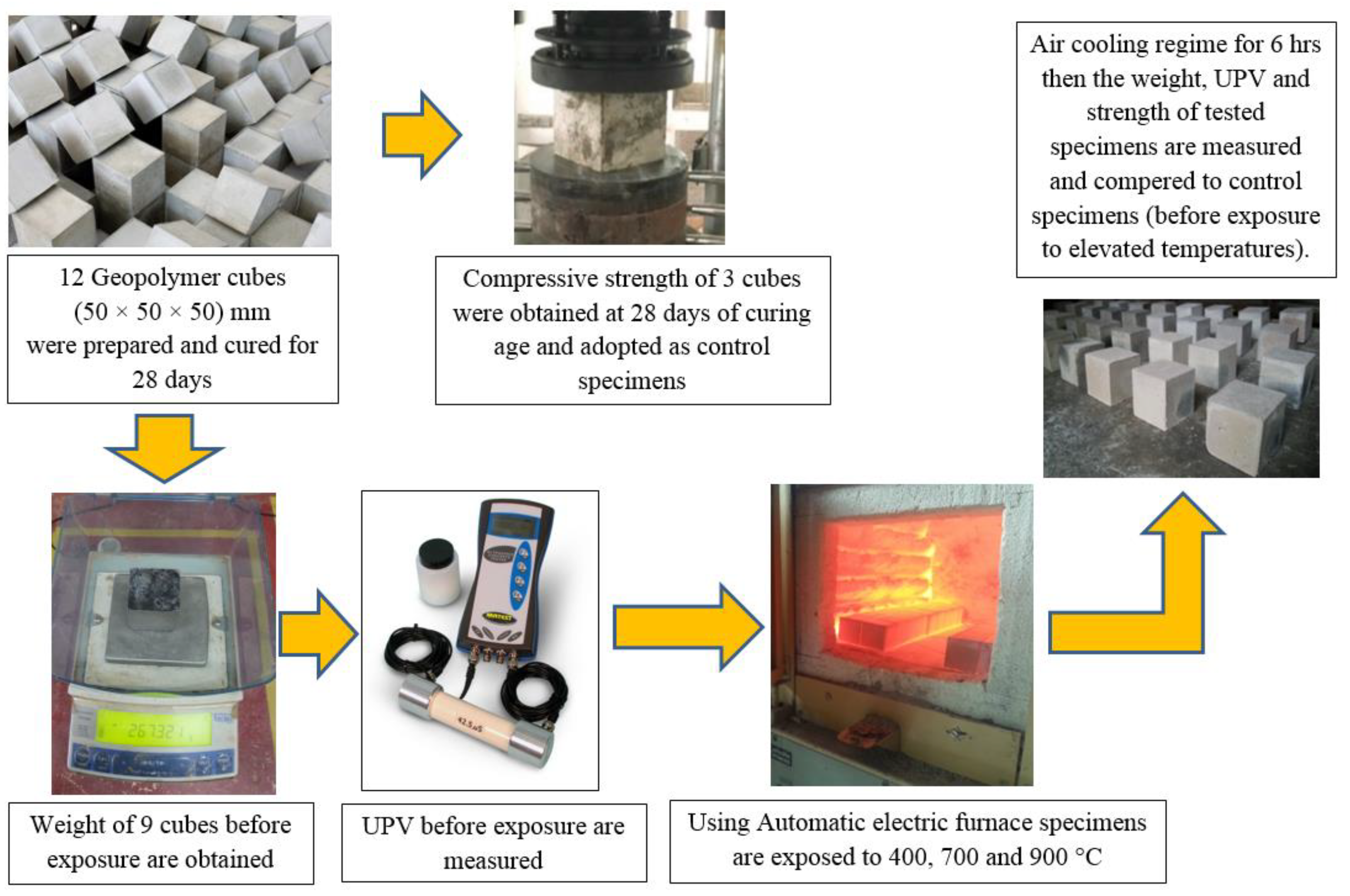
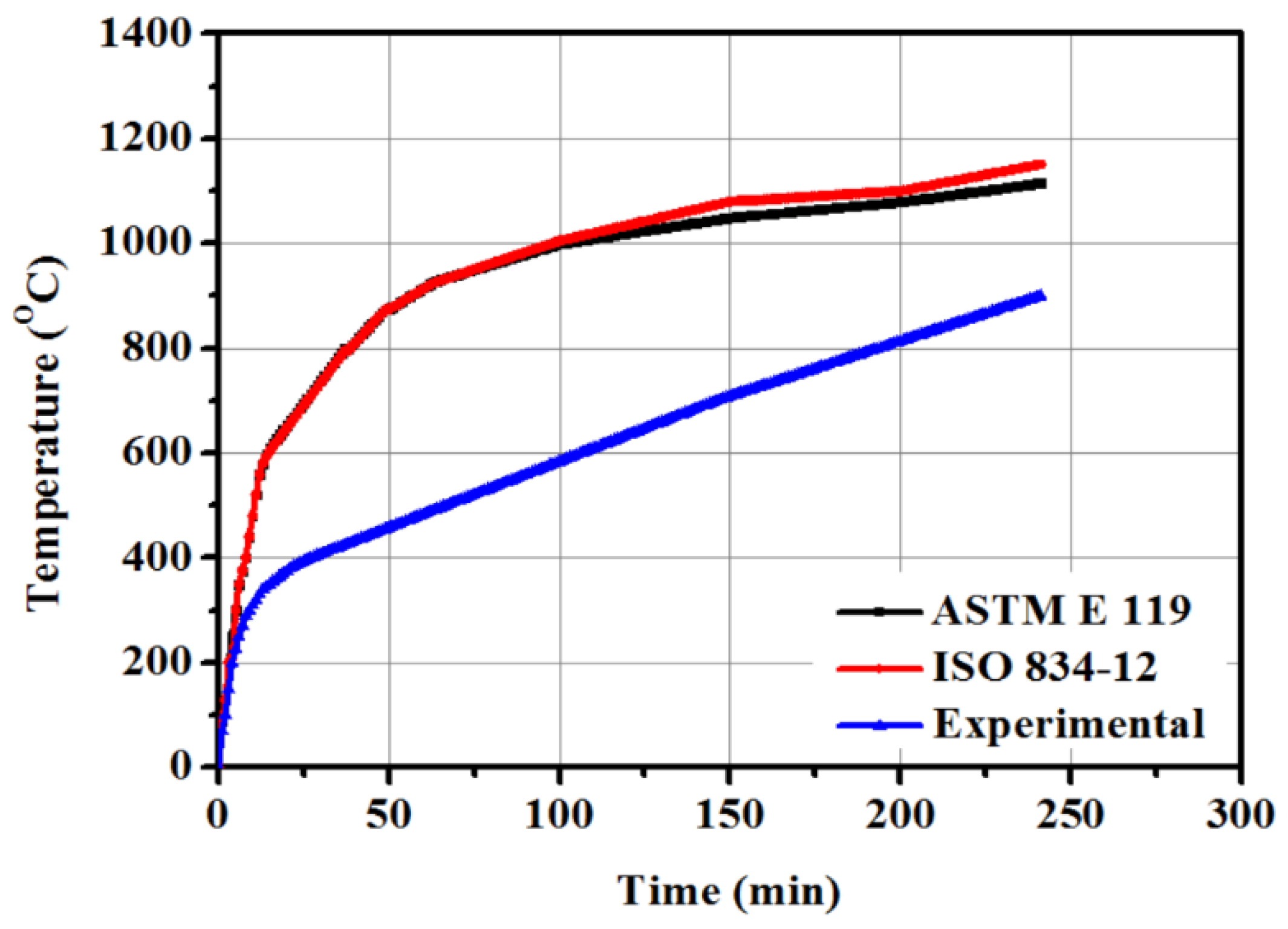
2.3. Tests Procedure
3. Results and Discussion
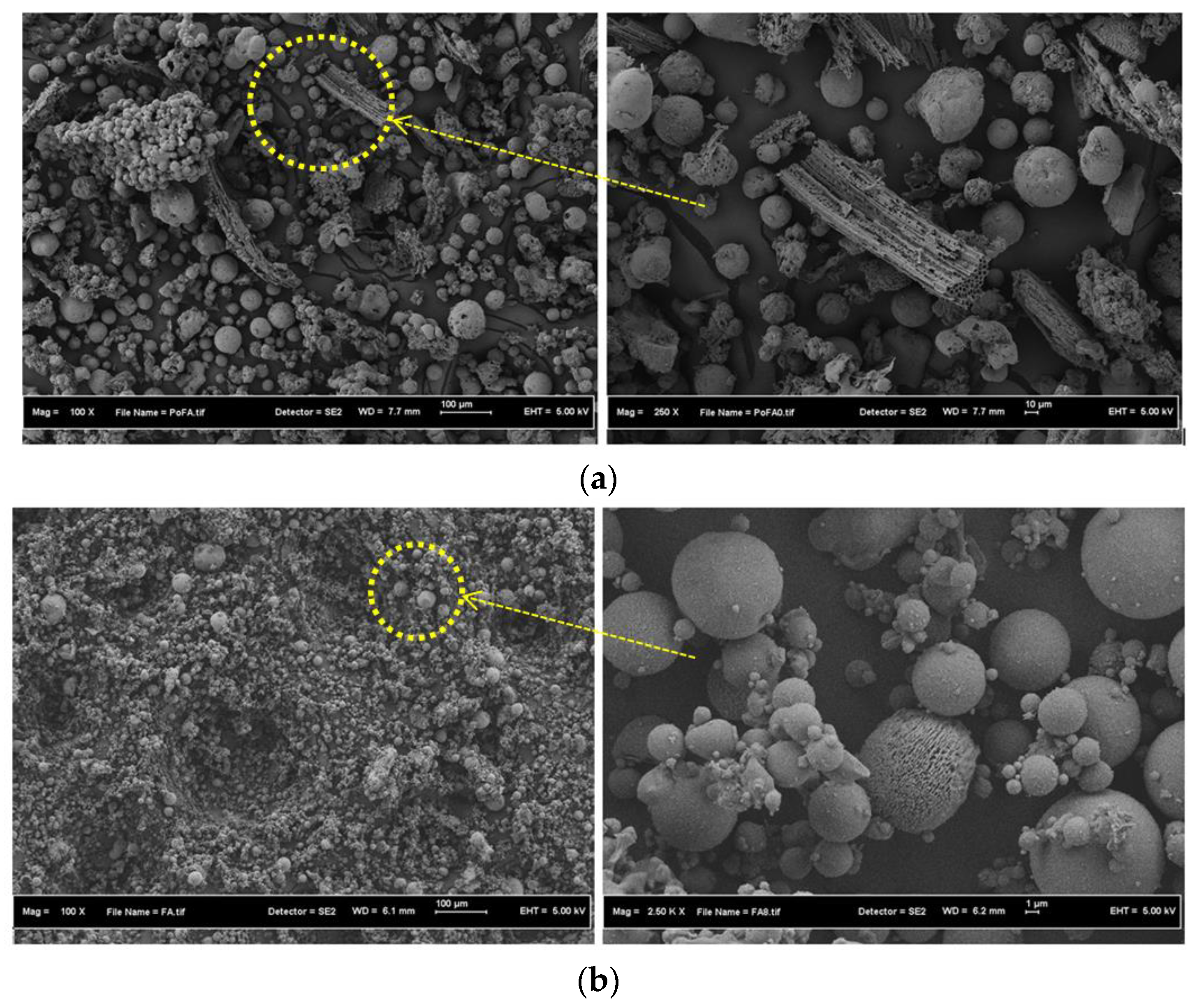
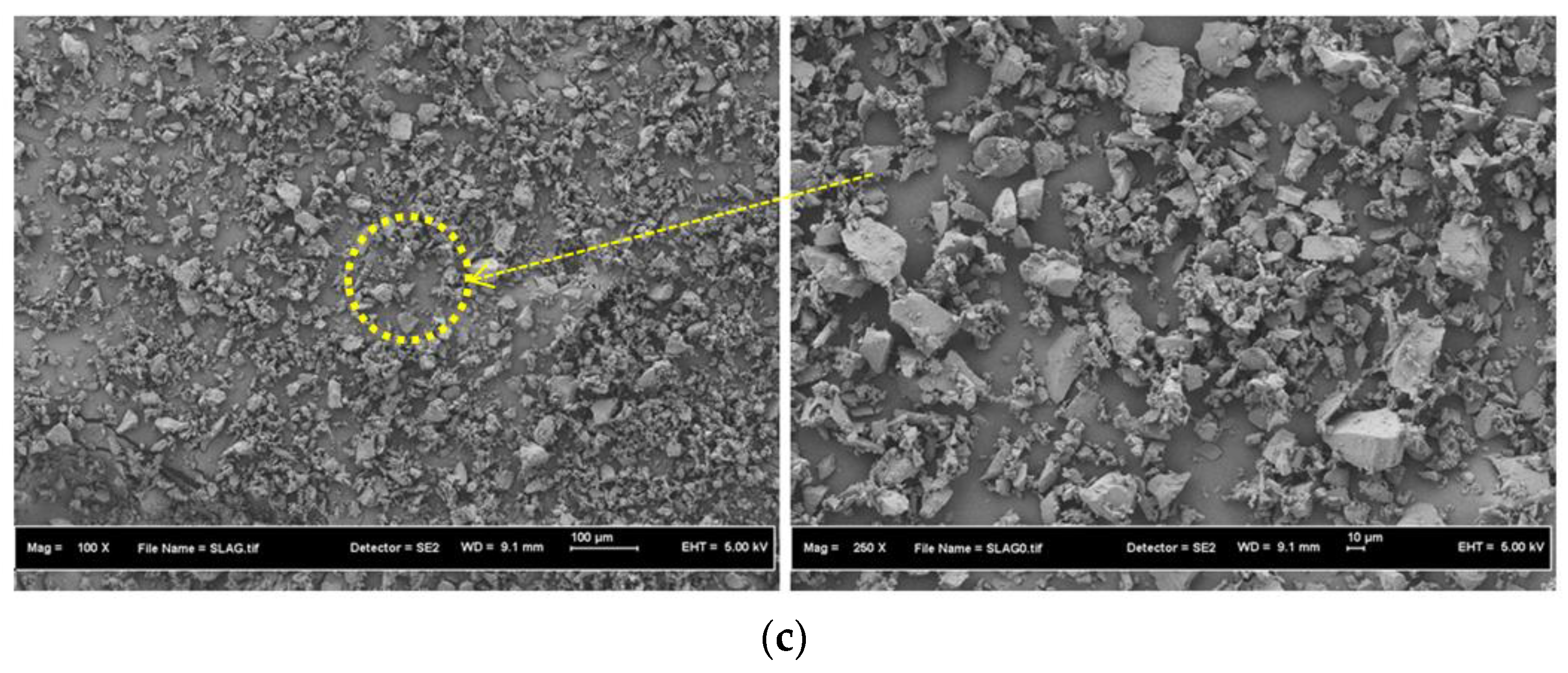
3.1. Materials Properties
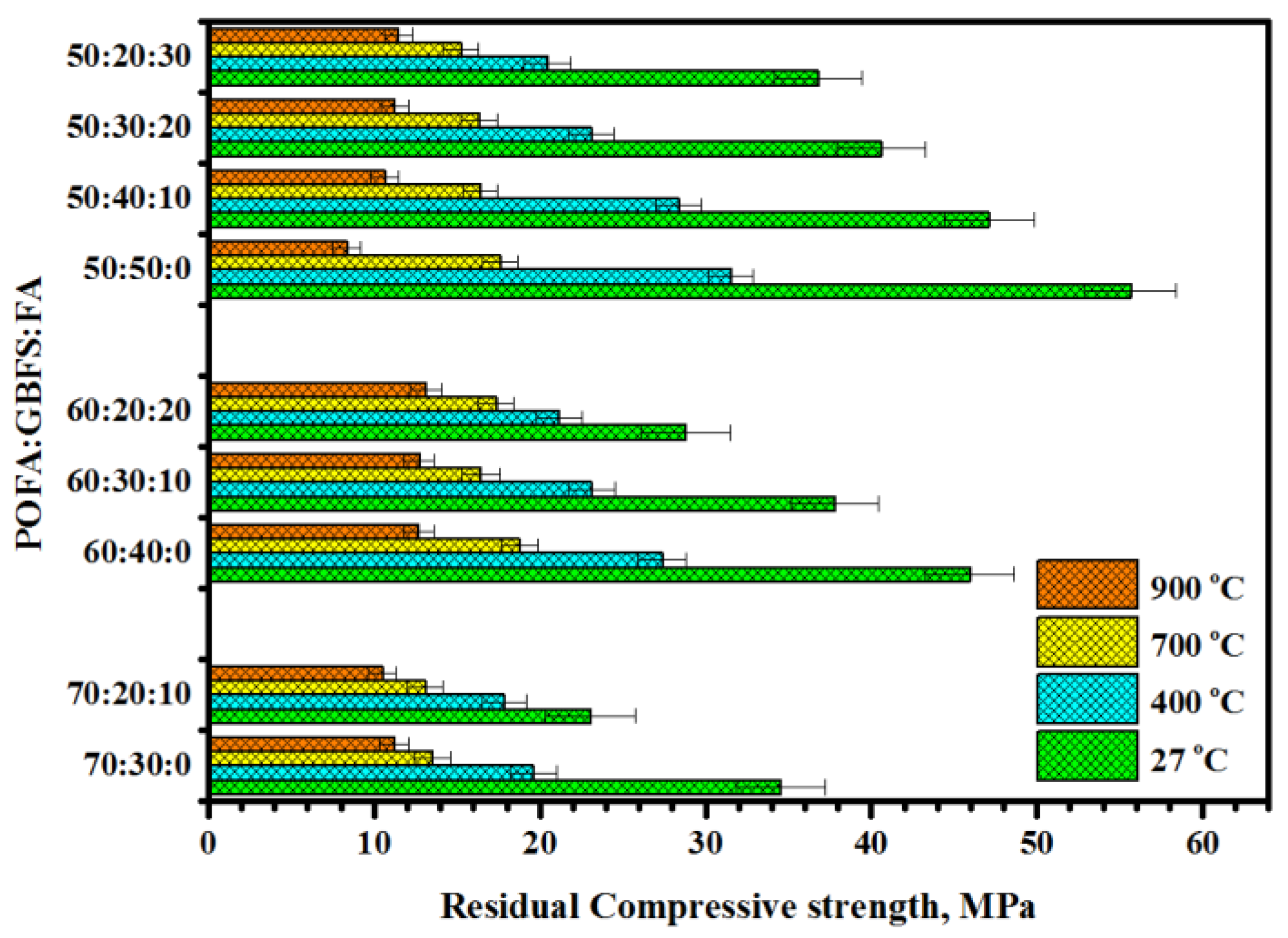
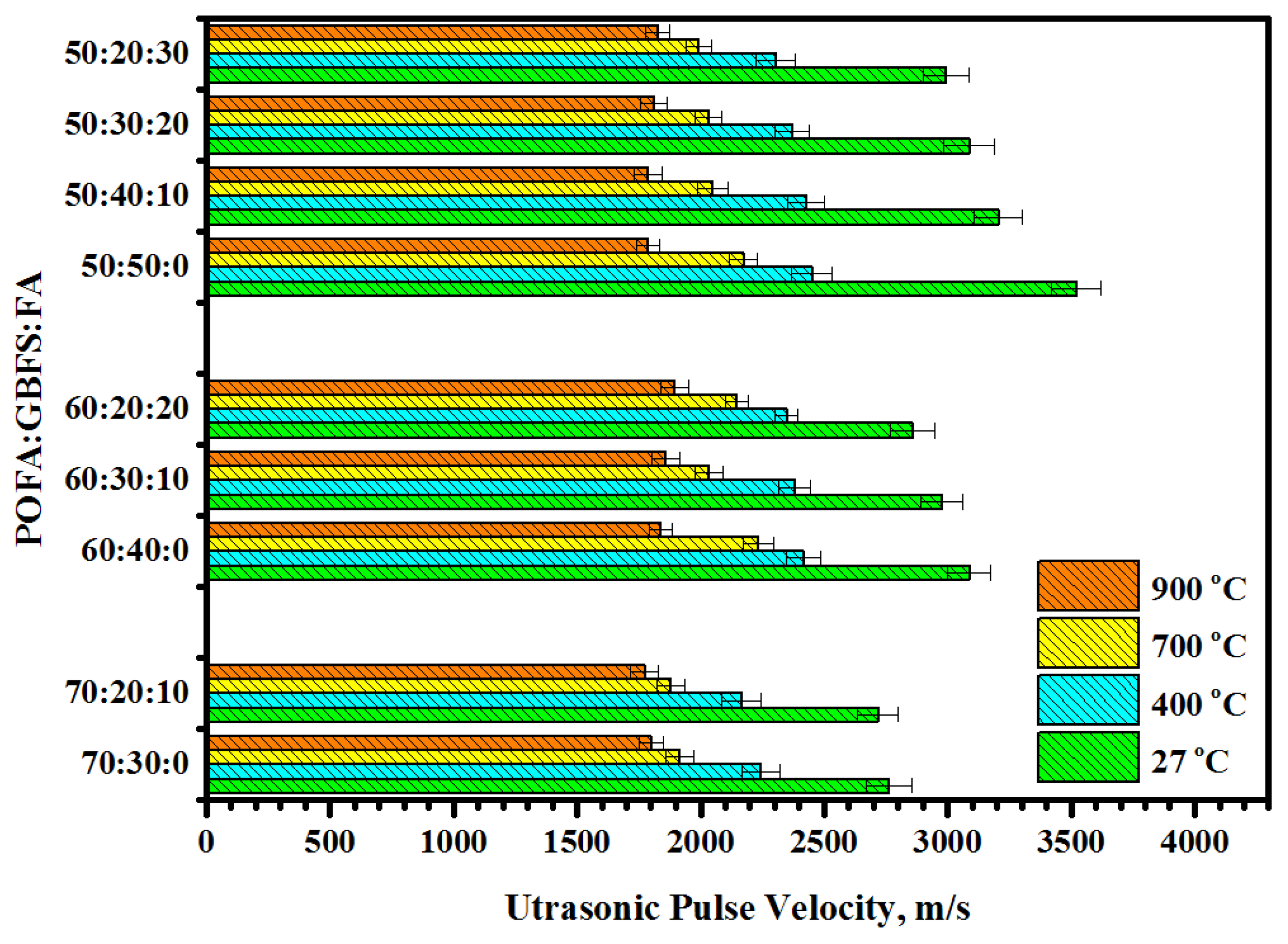
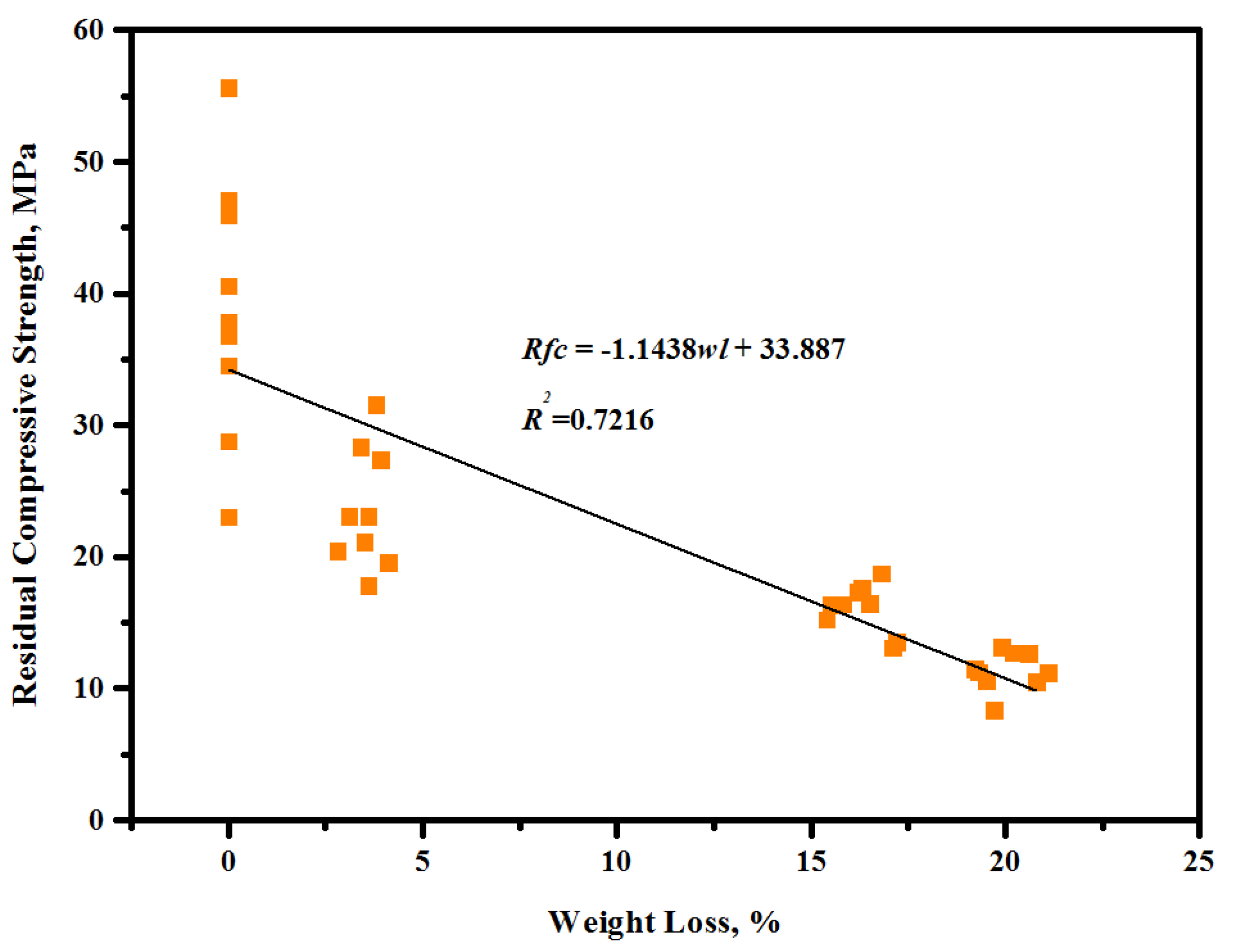
3.2. Residual Compressive Strength
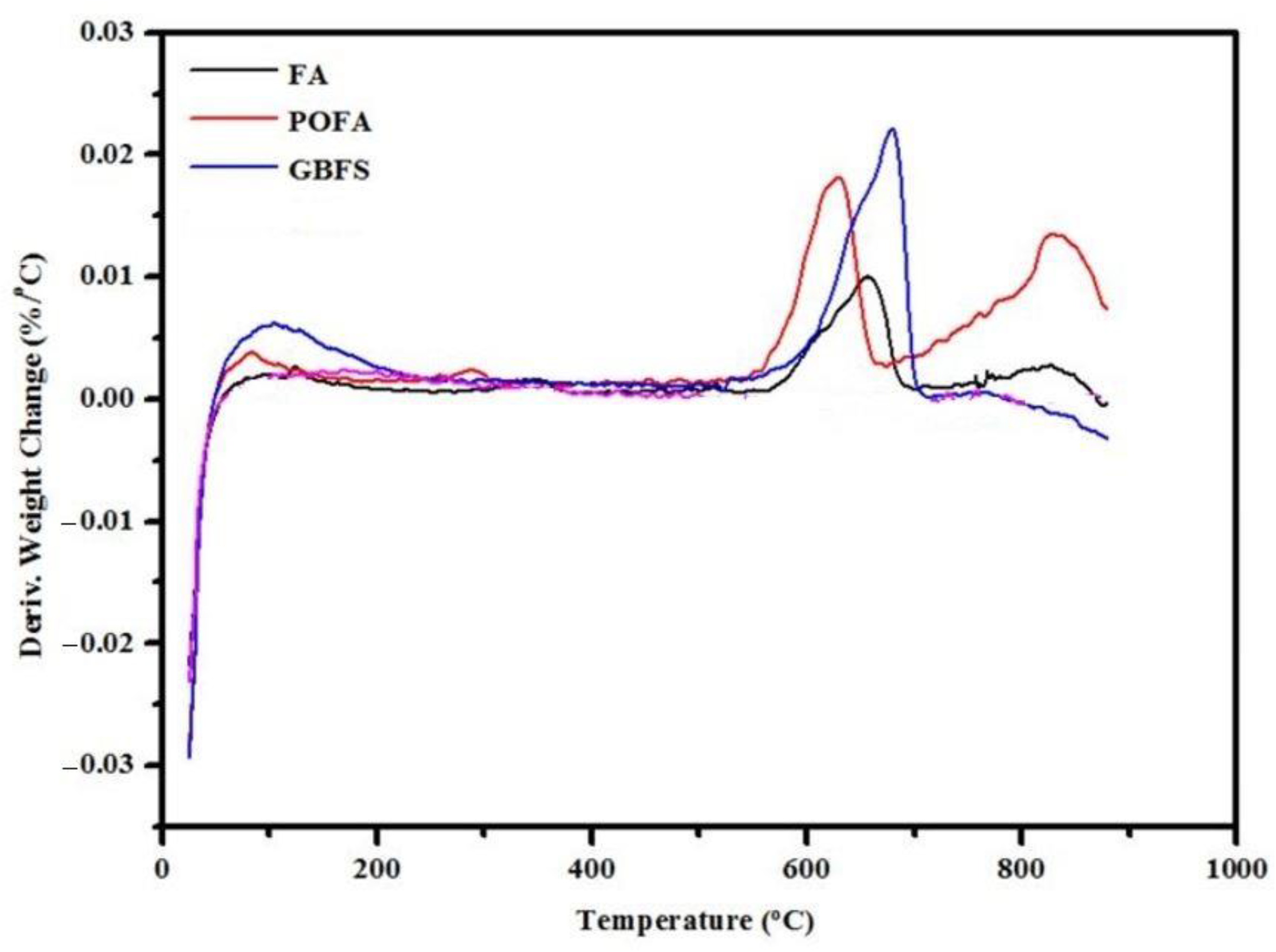
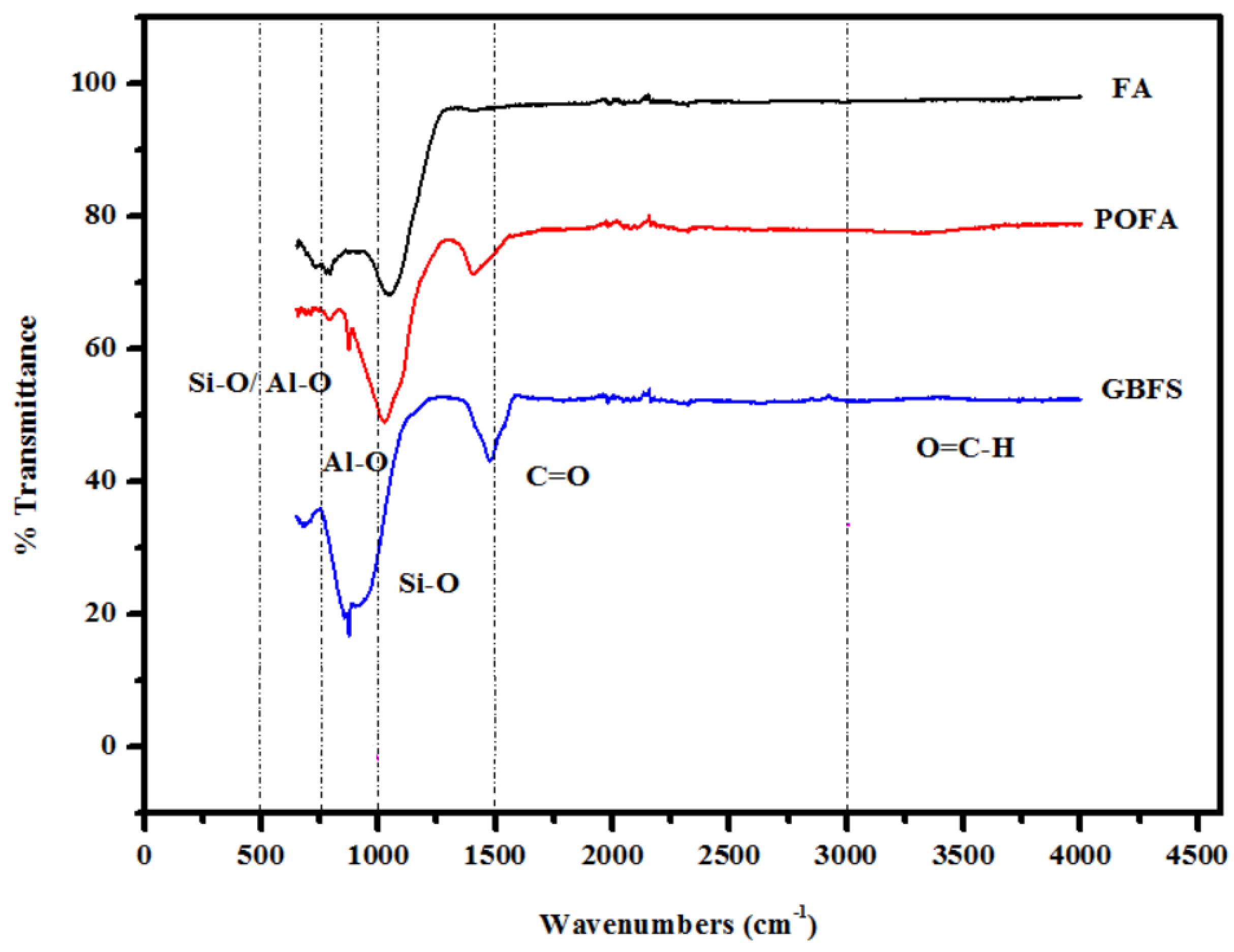
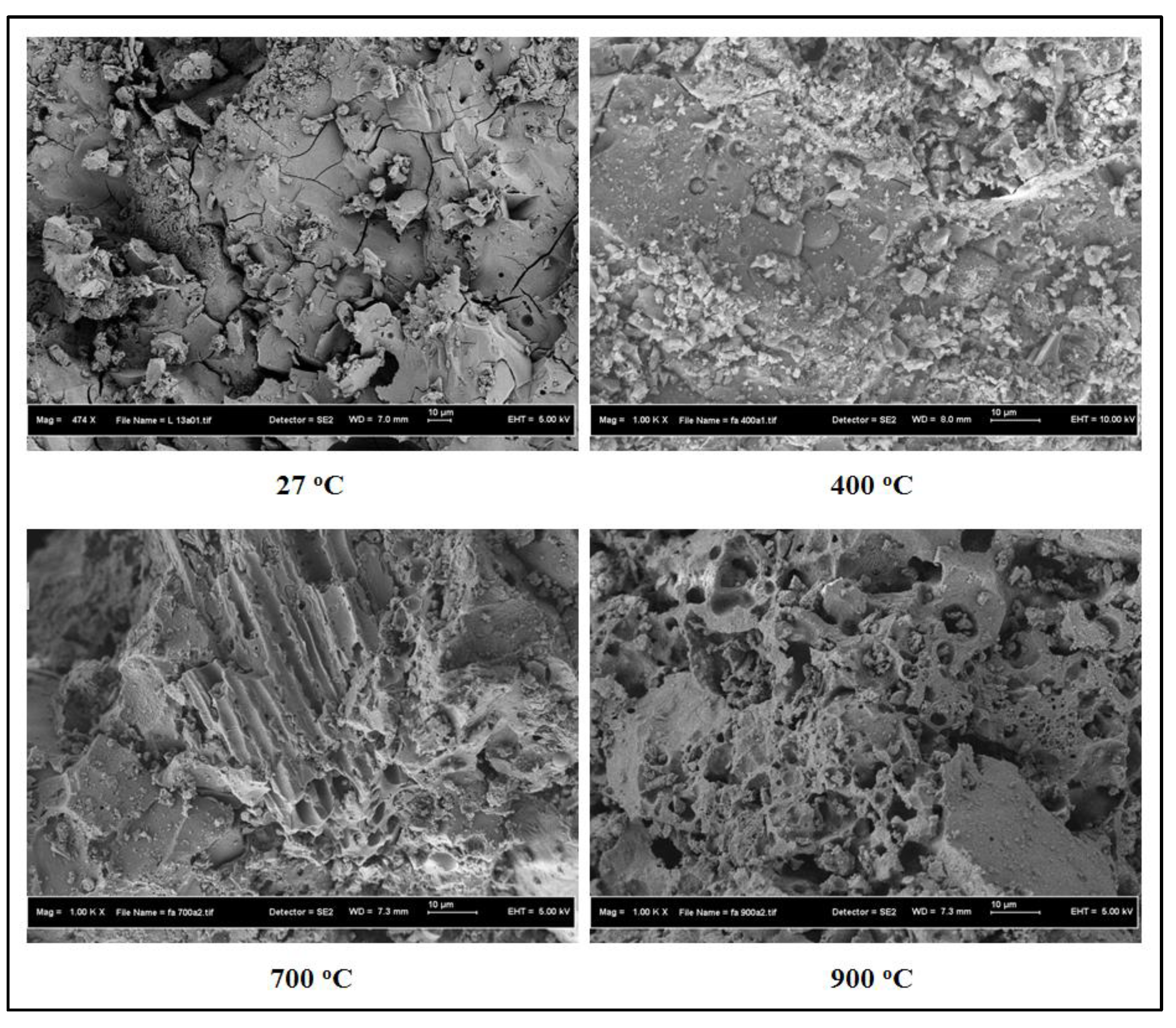
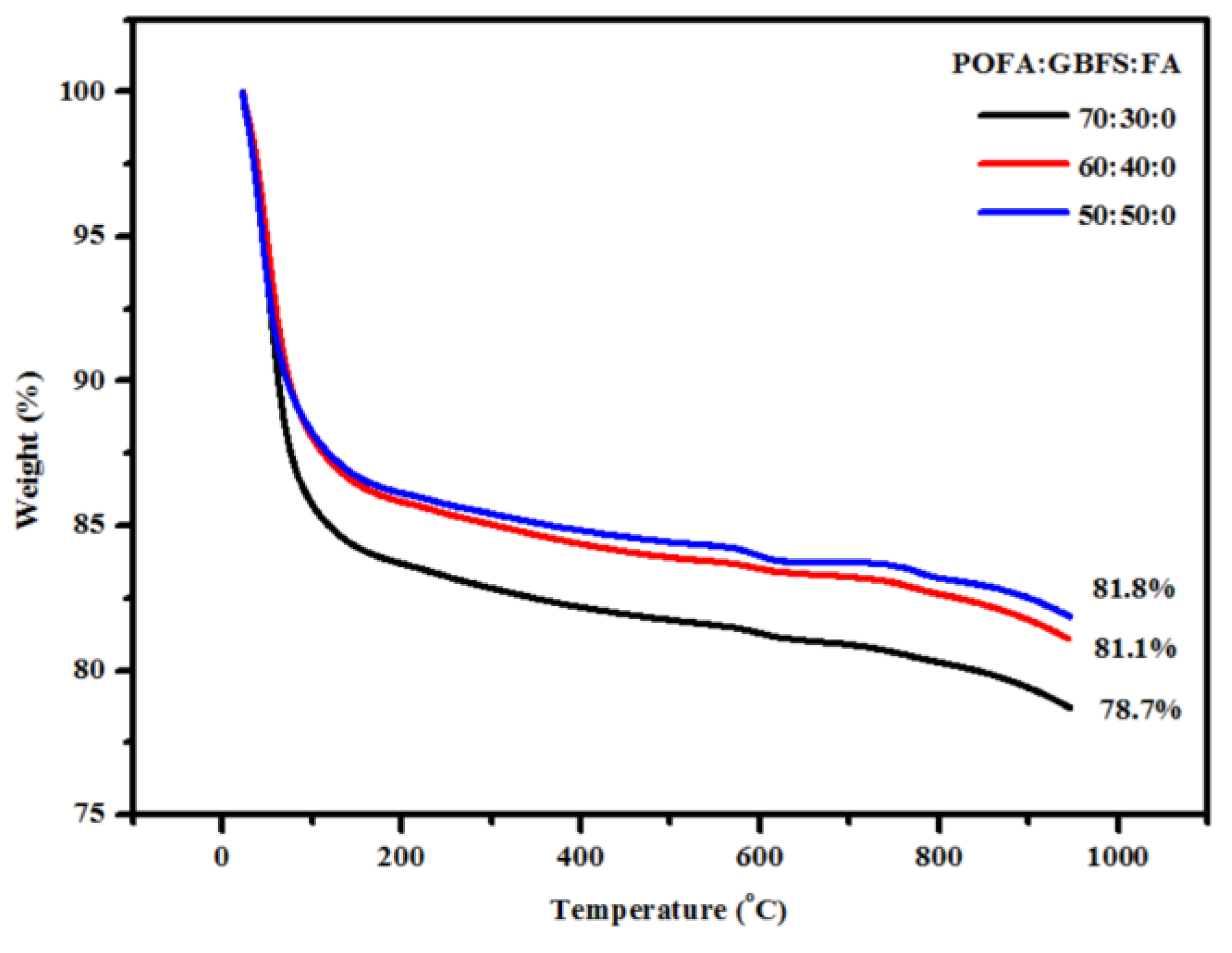
3.3. SEM Images and TGA Curves
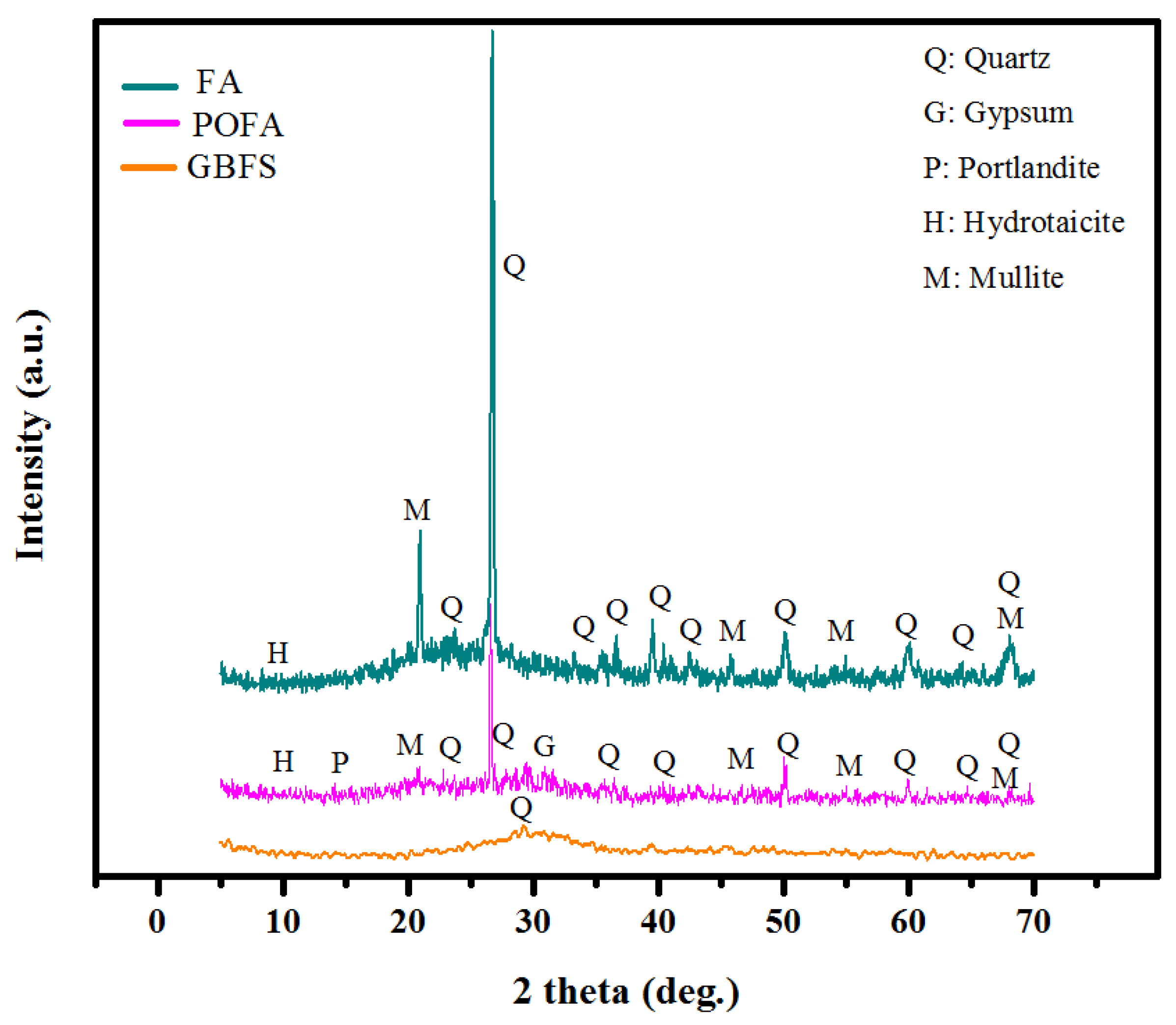
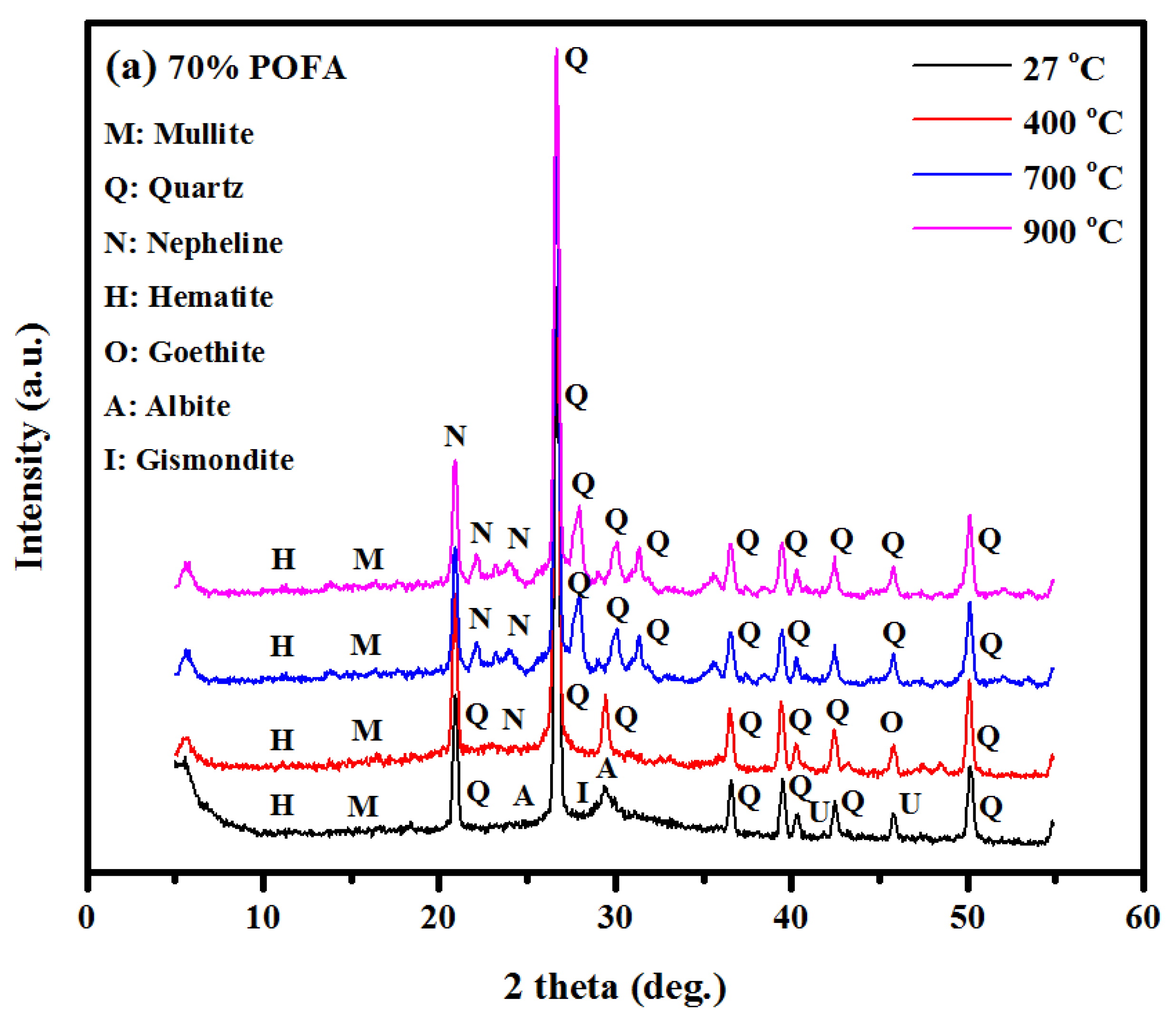
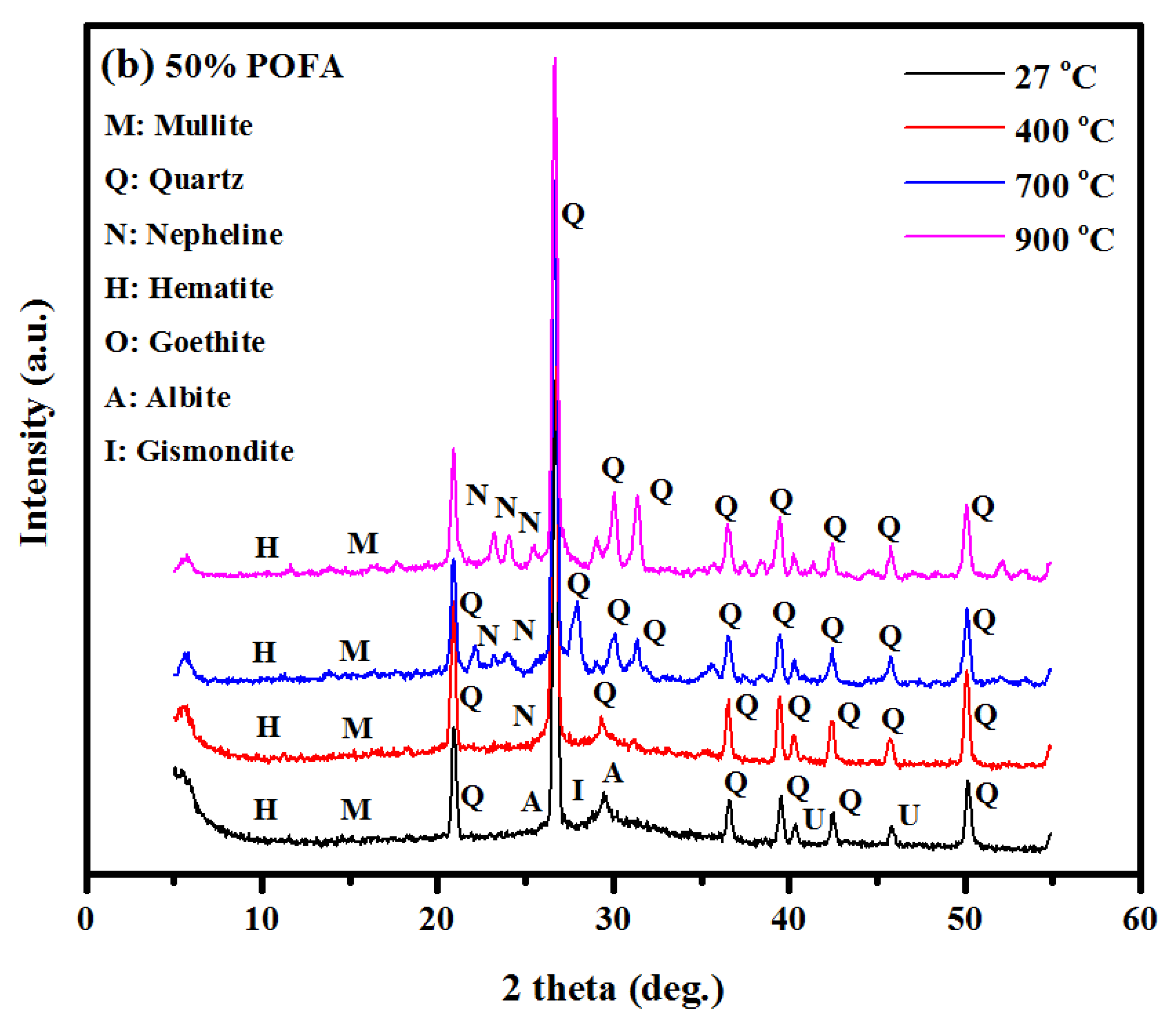
3.4. XRD Analysis
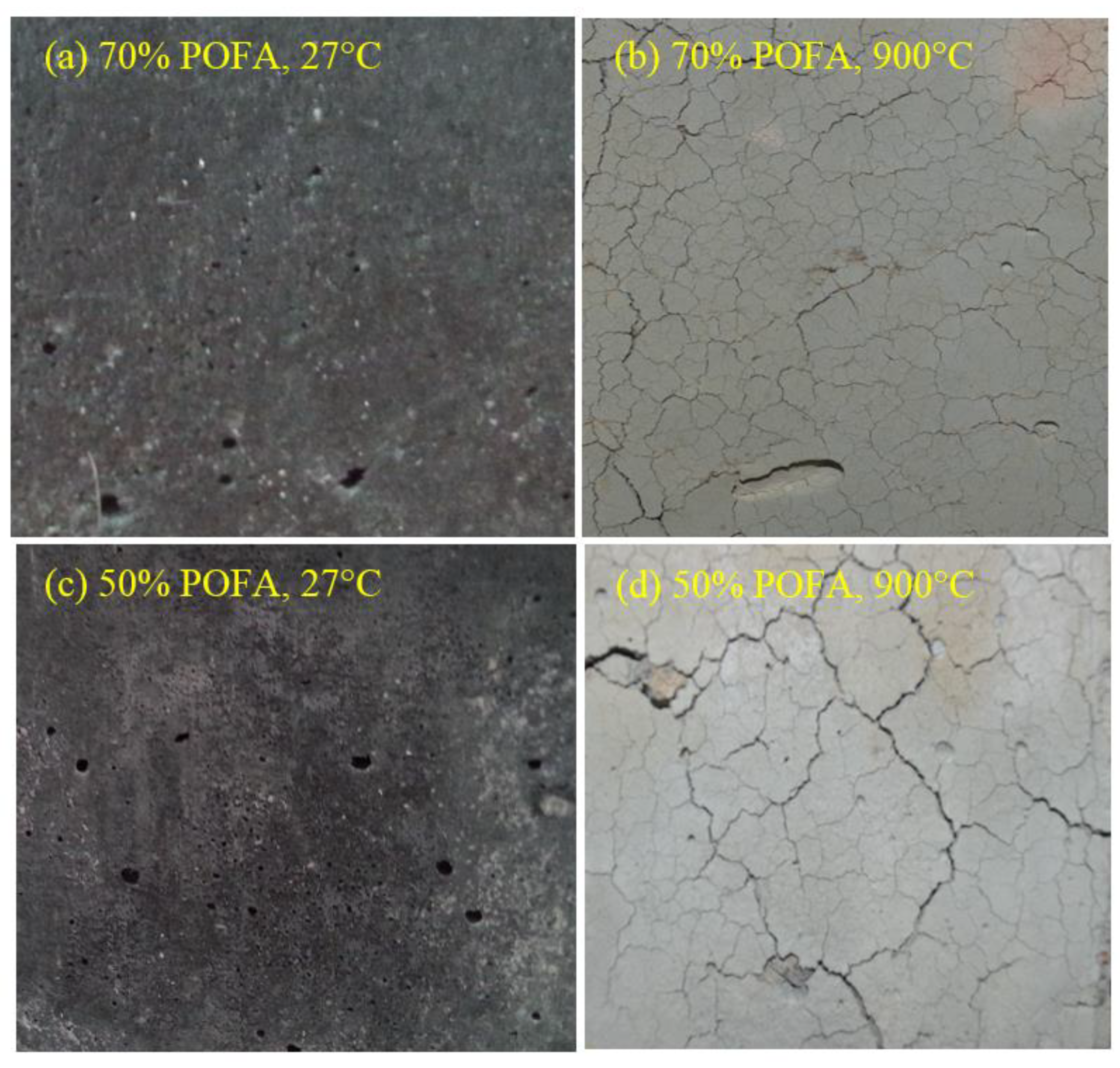
3.5. Visual Appearance
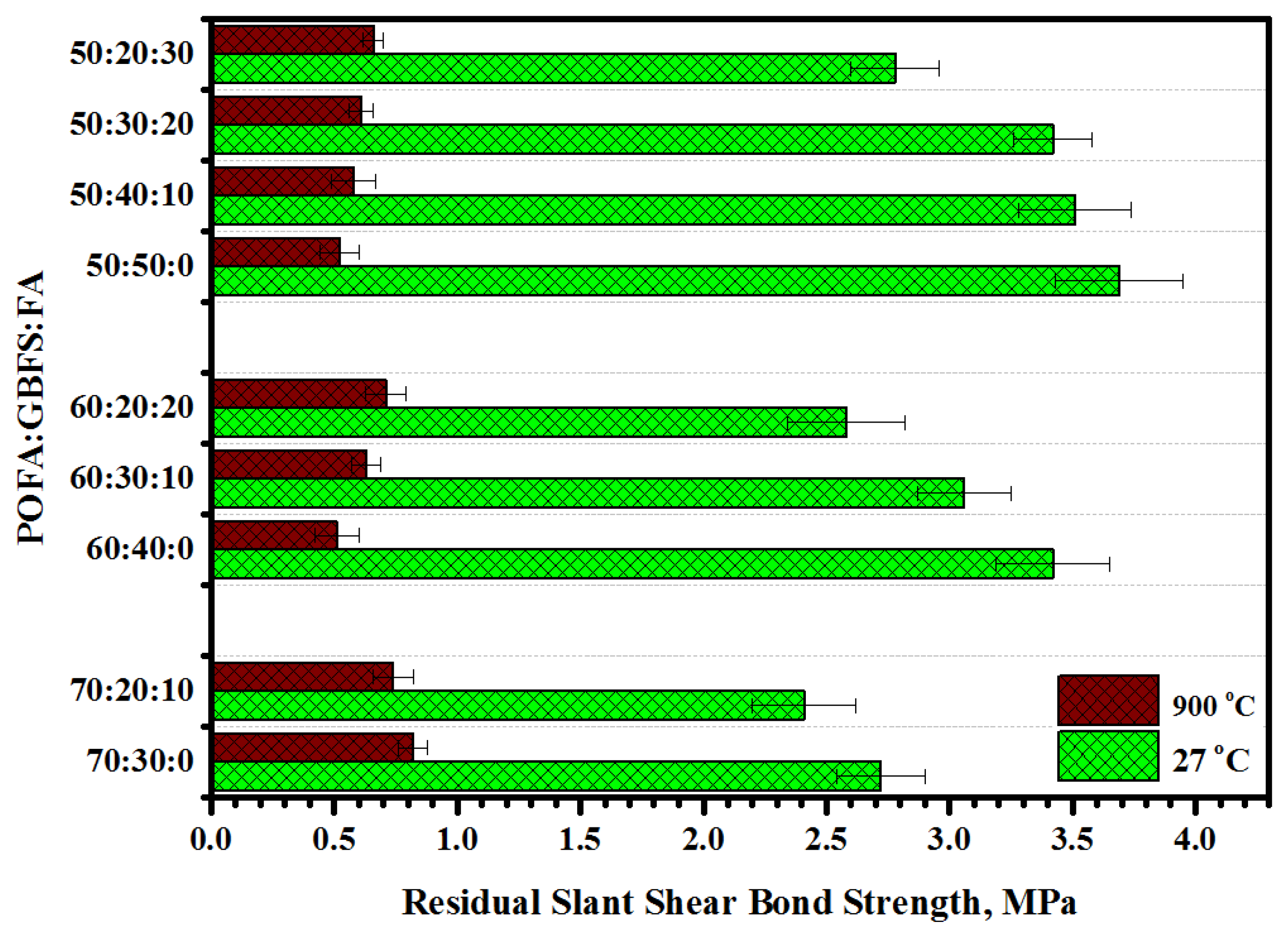
3.6. Slant Shear Bond Strength
4. Conclusions
- The proposed geopolymers made with a high volume of POFA (enriched in silica and alumina) showed high durability performance against elevated temperature exposure;
- The thermal resistance of TGPMs made with various POFA contents (50 to 70%) was significantly improved when exposed to high temperatures up to 900 °C;
- The existence of the crystalline phases, porosity, wide surface area of the tiny spherical particles, formation of various gels due to enhanced geopolymerization, and less water demand were the main factors for such improvement in the engineering characteristics of the designed TGPMs;
- TGPMs made using a high volume of POFA and FA as slag replacement showed considerable improvement in the residual strength and UPV values at elevated temperatures;
- The microstructure results of TGPM containing 70% POFA at high temperature exposure displayed an increase in thermal stability;
- The loss of bond strength of the TGPM mixes enclosing high levels of POFA, FA, and GBFS was remarkably reduced when heated up to 900 °C;
- The effects of heat intensity on the color appearance of the studied TGPMs at elevated temperatures were quantified in terms of surface discoloration, indicating their practical benefits under fire hazards;
- It is asserted that POFA (an eco-friendly and sustainable agricultural waste material) can suitably be recycled to make high-performance sustainable TGPMs demanded by the construction industries, providing immense benefits to the economy due to their durability in civil structures, high strength performance, and resistance to elevated heat exposure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GPMs | geopolymer mortars |
| TGPMs | ternary geopolymer mortars |
| GPBs | geopolymer binders |
| OPC | ordinary Portland cement |
| FA | fly ash |
| POFA | palm oil fuel ash |
| GBFS | ground blast furnace slag |
| AS | aluminum silicate |
| LOI | loss in ignition |
| FS | flexural strength |
| STS | splitting tensile strength |
| CS | compressive strength |
| RCS | residual compressive strength |
| SSBS | slant shear bond strength |
| TGA | Thermogravimetric analysis |
| XRF | X-ray fluorescence |
| XRD | X-ray diffraction |
| SEM | scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
References
- Tangchirapat, W.; Saeting, T.; Jaturapitakkul, C.; Kiattikomol, K.; Siripanichgorn, A. Use of waste ash from palm oil industry in concrete. Waste Manag. 2007, 27, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Huseien, G.F.; Ismail, M.; Tahir, M.M.; Mirza, J.; Khalid, N.H.A.; Asaad, M.A.; Husein, A.A.; Sarbini, N.N. Synergism between palm oil fuel ash and slag: Production of environmental-friendly alkali activated mortars with enhanced properties. Constr. Build. Mater. 2018, 170, 235–244. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.; Hussein, A.A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Behnia, A.; Alengaram, U.J.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar. Mater. Des. 2014, 59, 532–539. [Google Scholar] [CrossRef]
- Ranjbar, N.; Mehrali, M.; Alengaram, U.J.; Metselaar, H.S.C.; Jumaat, M.Z. Compressive strength and microstructural analysis of fly ash/palm oil fuel ash based geopolymer mortar under elevated temperatures. Constr. Build. Mater. 2014, 65, 114–121. [Google Scholar] [CrossRef]
- Awal, A.A.; Hussin, M.W. The effectiveness of palm oil fuel ash in preventing expansion due to alkali-silica reaction. Cem. Concr. Compos. 1997, 19, 367–372. [Google Scholar] [CrossRef]
- Alengaram, U.J.; Mahmud, H.; Jumaat, M.Z. Enhancement and prediction of modulus of elasticity of palm kernel shell concrete. Mater. Des. 2011, 32, 2143–2148. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z. A review on the use of agriculture waste material as lightweight aggregate for reinforced concrete structural members. Adv. Mater. Sci. Eng. 2014, 2014, 365197. [Google Scholar] [CrossRef]
- Opiso, E.M.; Tabelin, C.B.; Maestre, C.V.; Aseniero, J.P.J.; Park, I.; Villacorte-Tabelin, M. Synthesis and characterization of coal fly ash and palm oil fuel ash modified artisanal and small-scale gold mine (ASGM) tailings based geopolymer using sugar mill lime sludge as Ca-based activator. Heliyon 2021, 7, e06654. [Google Scholar] [CrossRef]
- Liu, M.Y.J.; Alengaram, U.J.; Santhanam, M.; Jumaat, M.Z.; Mo, K.H. Microstructural investigations of palm oil fuel ash and fly ash based binders in lightweight aggregate foamed geopolymer concrete. Constr. Build. Mater. 2016, 120, 112–122. [Google Scholar] [CrossRef]
- Awal, A.A.; Hussin, M.W. Effect of palm oil fuel ash in controlling heat of hydration of concrete. Procedia Eng. 2011, 14, 2650–2657. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rukzon, S.; Sirivivatnanon, V. Resistance to chloride penetration of blended Portland cement mortar containing palm oil fuel ash, rice husk ash and fly ash. Constr. Build. Mater. 2008, 22, 932–938. [Google Scholar] [CrossRef]
- Salami, B.A.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Durability performance of Palm Oil Fuel Ash-based Engineered Alkaline-activated Cementitious Composite (POFA-EACC) mortar in sulfate environment. Constr. Build. Mater. 2017, 131, 229–244. [Google Scholar] [CrossRef]
- Salih, M.A.; Ali, A.A.A.; Farzadnia, N. Characterization of mechanical and microstructural properties of palm oil fuel ash geopolymer cement paste. Constr. Build. Mater. 2014, 65, 592–603. [Google Scholar] [CrossRef]
- Islam, A.; Alengaram, U.J.; Jumaat, M.Z.; Bashar, I.I. The development of compressive strength of ground granulated blast furnace slag-palm oil fuel ash-fly ash based geopolymer mortar. Mater. Des. 2014, 56, 833–841. [Google Scholar] [CrossRef]
- Ariffin, M.; Bhutta, M.; Hussin, M.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Karim, M.; Zain, M.F.M.; Jamil, M.; Lai, F. Fabrication of a non-cement binder using slag, palm oil fuel ash and rice husk ash with sodium hydroxide. Constr. Build. Mater. 2013, 49, 894–902. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Evolution of alkaline activated ground blast furnace slag–ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 55, 387–393. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Effects of H2O/Na2O molar ratio on the strength of alkaline activated ground blast furnace slag-ultrafine palm oil fuel ash based concrete. Mater. Des. 2014, 56, 158–164. [Google Scholar] [CrossRef]
- Salih, M.A.; Farzadnia, N.; Ali, A.A.A.; Demirboga, R. Development of high strength alkali activated binder using palm oil fuel ash and GGBS at ambient temperature. Constr. Build. Mater. 2015, 93, 289–300. [Google Scholar] [CrossRef]
- Salih, M.A.; Farzadnia, N.; Ali, A.A.A.; Demirboga, R. Effect of different curing temperatures on alkali activated palm oil fuel ash paste. Constr. Build. Mater. 2015, 94, 116–125. [Google Scholar] [CrossRef]
- Yusuf, T.O.; Ismail, M.; Usman, J.; Noruzman, A.H. Impact of blending on strength distribution of ambient cured metakaolin and palm oil fuel ash based geopolymer mortar. Adv. Civ. Eng. 2014, 2014, 658067. [Google Scholar] [CrossRef]
- Ismail, M.; Yusuf, T.O.; Noruzman, A.H.; Hassan, I. Early strength characteristics of palm oil fuel ash and metakaolin blended geopolymer mortar. Adv. Mater. Res. 2013, 690, 1045–1048. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; MacKenzie, K. Preparation and characterisation of fly ash based geopolymer mortars. Constr. Build. Mater. 2010, 24, 1906–1910. [Google Scholar] [CrossRef]
- Hussin, M.; Bhutta, M.; Azreen, M.; Ramadhansyah, P.; Mirza, J. Performance of blended ash geopolymer concrete at elevated temperatures. Mater. Struct. 2015, 48, 709–720. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Sata, V.; Hanjitsuwan, S.; Ridtirud, C.; Hatanaka, S.; Chindaprasirt, P. High calcium fly ash geopolymer mortar containing Portland cement for use as repair material. Constr. Build. Mater. 2015, 98, 482–488. [Google Scholar] [CrossRef]
- Dombrowski, K.; Buchwald, A.; Weil, M. The influence of calcium content on the structure and thermal performance of fly ash based geopolymers. J. Mater. Sci. 2007, 42, 3033–3043. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Adhesion characterization of tungsten mine waste geopolymeric binder. Influence of OPC concrete substrate surface treatment. Constr. Build. Mater. 2008, 22, 154–161. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-activated ground fly ash geopolymer cured at ambient temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Zuhua, Z.; Xiao, Y.; Huajun, Z.; Yue, C. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
- Lee, W.; Van Deventer, J. The effects of inorganic salt contamination on the strength and durability of geopolymers. Colloids Surf. Physicochem. Eng. Asp. 2002, 211, 115–126. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kodur, V.; Qi, S.L.; Wu, B. Characterizing the bond strength of geopolymers at ambient and elevated temperatures. Cem. Concr. Compos. 2015, 58, 40–49. [Google Scholar] [CrossRef]
- Amran, M.; Huang, S.-S.; Debbarma, S.; Rashid, R.S. Fire resistance of geopolymer concrete: A critical review. Constr. Build. Mater. 2022, 324, 126722. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Mirza, J.; Tahir, M.M.; Asaad, M.A.; Ismail, M.; Shah, K.W. Waste ceramic powder incorporated alkali activated mortars exposed to elevated Temperatures: Performance evaluation. Constr. Build. Mater. 2018, 187, 307–317. [Google Scholar] [CrossRef]
- Klima, K.; Schollbach, K.; Brouwers, H.; Yu, Q. Thermal and fire resistance of Class F fly ash based geopolymers–A review. Constr. Build. Mater. 2022, 323, 126529. [Google Scholar] [CrossRef]
- Lahoti, M.; Tan, K.H.; Yang, E.-H. A critical review of geopolymer properties for structural fire-resistance applications. Constr. Build. Mater. 2019, 221, 514–526. [Google Scholar] [CrossRef]
- Rickard, W.D.; Temuujin, J.; van Riessen, A. Thermal analysis of geopolymer pastes synthesised from five fly ashes of variable composition. J. Non-Cryst. Solids 2012, 358, 1830–1839. [Google Scholar] [CrossRef]
- Shah, K.W.; Huseien, G.F. Bond strength performance of ceramic, fly ash and GBFS ternary wastes combined alkali-activated mortars exposed to aggressive environments. Constr. Build. Mater. 2020, 251, 119088. [Google Scholar] [CrossRef]
- Mhaya, A.M.; Shahidan, S.; Zuki, S.S.M.; Huseien, G.F.; Azmi, M.A.M.; Ismail, M.; Mirza, J. Durability and Acoustic Performance of Rubberized Concrete Containing POFA as Cement Replacement. Sustainability 2022, 14, 15510. [Google Scholar] [CrossRef]
- Huseien, G.F.; Asaad, M.A.; Abadel, A.A.; Ghoshal, S.K.; Hamzah, H.K.; Benjeddou, O.; Mirza, J. Drying shrinkage, sulphuric acid and sulphate resistance of high-volume palm oil fuel ash-included alkali-activated mortars. Sustainability 2022, 14, 498. [Google Scholar] [CrossRef]
- Hamzah, H.K.; Huseien, G.F.; Asaad, M.A.; Georgescu, D.P.; Ghoshal, S.; Alrshoudi, F. Effect of waste glass bottles-derived nanopowder as slag replacement on mortars with alkali activation: Durability characteristics. Case Stud. Constr. Mater. 2021, 15, e00775. [Google Scholar] [CrossRef]
- Memon, S.A.; Lo, T.Y.; Barbhuiya, S.; Xu, W. Development of form-stable composite phase change material by incorporation of dodecyl alcohol into ground granulated blast furnace slag. Energy Build. 2013, 62, 360–367. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, C.; Duan, P.; Liu, Y.; Zhang, Z.; Qiu, X.; Li, D. A comparative study of high-and low-Al2O3 fly ash based-geopolymers: The role of mix proportion factors and curing temperature. Mater. Des. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Sitarz, M.; Mozgawa, W.; Handke, M. Vibrational spectra of complex ring silicate anions—Method of recognition. J. Mol. Struct. 1997, 404, 193–197. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.; Smith, J. Mechanism of polysialation in the incorporation of zirconia into fly ash-based geopolymers. Ind. Eng. Chem. Res. 2000, 39, 2925–2934. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Dick, W.A. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]






| Material | FA | POFA | GBFS |
|---|---|---|---|
| SiO2 | 57.20 | 64.20 | 30.8 |
| Al2O3 | 28.81 | 4.25 | 10.9 |
| Fe2O3 | 3.67 | 3.13 | 0.64 |
| CaO | 5.16 | 10.20 | 51.8 |
| MgO | 1.48 | 5.90 | 4.57 |
| K2O | 0.94 | 8.64 | 0.36 |
| Na2O | 0.08 | 0.10 | 0.45 |
| SO3 | 0.10 | 0.09 | 0.06 |
| LOI | 0.12 | 1.73 | 0.22 |
| SiO2:Al2O3 | 1.98 | 15.11 | 2.82 |
| No. | Binder (Mass %) | B:A * | S:B ** | SiO2:Al2O3 | CaO:SiO2 | CaO:Al2O3 | ||
|---|---|---|---|---|---|---|---|---|
| POFA | GBFS | FA | ||||||
| 1 | 70 | 30 | 0 | 1.0 | 0.40 | 8.63 | 0.42 | 3.61 |
| 2 | 20 | 10 | 1.0 | 0.40 | 7.04 | 0.32 | 2.23 | |
| 3 | 60 | 40 | 0 | 1.0 | 0.40 | 7.32 | 0.53 | 3.86 |
| 4 | 30 | 10 | 1.0 | 0.40 | 6.13 | 0.41 | 2.54 | |
| 5 | 20 | 20 | 1.0 | 0.40 | 5.33 | 0.31 | 1.66 | |
| 6 | 50 | 50 | 0 | 1.0 | 0.40 | 6.25 | 0.65 | 4.08 |
| 7 | 40 | 10 | 1.0 | 0.40 | 5.34 | 0.52 | 2.80 | |
| 8 | 30 | 20 | 1.0 | 0.40 | 4.72 | 0.41 | 1.94 | |
| 9 | 20 | 30 | 1.0 | 0.40 | 4.27 | 0.31 | 1.31 | |
| Mix POFA:GBFS:FA | After 400 °C | After 700 °C | After 900 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ave. | SD | CV | Ave. | SD | CV | Ave. | SD | CV | |
| 70:30:00 | 19.6 | 1.42 | 0.0724 | 13.51 | 1.12 | 0.0829 | 11.2 | 0.88 | 0.0785 |
| 70:20:10 | 17.82 | 1.34 | 0.0752 | 13.08 | 1.08 | 0.0825 | 10.5 | 0.84 | 0.0801 |
| 60:40:00 | 27.37 | 1.46 | 0.0533 | 18.76 | 1.13 | 0.0602 | 12.68 | 0.91 | 0.0717 |
| 60:30:10 | 23.12 | 1.43 | 0.0618 | 16.42 | 1.16 | 0.0706 | 12.72 | 0.92 | 0.0723 |
| 60:20:20 | 21.16 | 1.38 | 0.0653 | 17.36 | 1.07 | 0.0616 | 13.12 | 0.94 | 0.0716 |
| 50:50:00 | 31.54 | 1.34 | 0.0425 | 17.6 | 1.05 | 0.0596 | 8.34 | 0.86 | 0.1031 |
| 50:40:10 | 28.37 | 1.37 | 0.0483 | 16.41 | 1.06 | 0.0645 | 10.64 | 0.84 | 0.0789 |
| 50:30:20 | 23.11 | 1.39 | 0.0601 | 16.34 | 1.09 | 0.0667 | 11.24 | 0.82 | 0.0729 |
| 50:20:30 | 20.46 | 1.41 | 0.0689 | 15.22 | 1.08 | 0.0709 | 11.46 | 0.83 | 0.0724 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huseien, G.F.; Kubba, Z.; Ghoshal, S.K. Engineering Attributes of Ternary Geopolymer Mortars Containing High Volumes of Palm Oil Fuel Ash: Impact of Elevated Temperature Exposure. Fire 2023, 6, 340. https://doi.org/10.3390/fire6090340
Huseien GF, Kubba Z, Ghoshal SK. Engineering Attributes of Ternary Geopolymer Mortars Containing High Volumes of Palm Oil Fuel Ash: Impact of Elevated Temperature Exposure. Fire. 2023; 6(9):340. https://doi.org/10.3390/fire6090340
Chicago/Turabian StyleHuseien, Ghasan Fahim, Ziyad Kubba, and Sib Krishna Ghoshal. 2023. "Engineering Attributes of Ternary Geopolymer Mortars Containing High Volumes of Palm Oil Fuel Ash: Impact of Elevated Temperature Exposure" Fire 6, no. 9: 340. https://doi.org/10.3390/fire6090340
APA StyleHuseien, G. F., Kubba, Z., & Ghoshal, S. K. (2023). Engineering Attributes of Ternary Geopolymer Mortars Containing High Volumes of Palm Oil Fuel Ash: Impact of Elevated Temperature Exposure. Fire, 6(9), 340. https://doi.org/10.3390/fire6090340







