Experimental and Numerical Studies on the Explosion Characteristics of Ethanol–Air Mixtures under Aviation Conditions
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Setup
2.2. Experimental Conditions
2.3. Experimental Data Processing
3. Numerical Simulations
4. Results and Discussion
4.1. Maximum Explosion Pressures
4.2. Maximum Rates of Pressure Rise
4.3. Explosion Times and Fast Burning Times
4.4. Heat Loss
4.5. Sensitivity Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| p0 | initial pressure |
| pmax | maximum explosion pressure, including pmax,ad and pmax,exp |
| pmax,ad | maximum explosion pressure in adiabatic conditions |
| pmax,exp | maximum explosion pressure in experimental conditions |
| T0 | initial temperature |
| average flame temperature | |
| V0 | explosion vessel volume |
| x | ethanol volume concentration in ethanol–air mixture |
| n0 | total mole number of the mixture before combustion |
| ne | total mole number of the mixture after combustion |
| nl | mole number of the limiting component in the mixture |
| ne/n0 | |
| rl | nl/n0 |
| the stoichiometric coefficient of the limiting component in the mixture | |
| the modified combustion heat (at constant volume and T0) after considering the endothermic process | |
| the average molar heat capacity at constant volume of the gaseous mixture at the end of combustion, averaged for the temperature range to | |
| qtr | the heat amount transferred to the vessel wall and lost outside the system at the end of combustion |
| the adiabatic coefficient of the gaseous mixture at the end of combustion | |
| (dp/dt)max | maximum rate of pressure rise |
| θmax | explosion time |
| θf | fast burning time |
| proportion of heat loss | |
| a | slope of the fitted line pmax − 1/T0 |
| b | intercept of the fitted line pmax − 1/T0 |
| R2 | coefficient of determination of the fitted line pmax − 1/T0 |
| φ | the equivalence ratio of the ethanol–air mixture, φ = ([fuel]/[O2])real/([fuel]/[O2])stoich |
References
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Geng, L.; Bi, L.; Li, Q.; Chen, H.; Xie, Y. Experimental study on spray characteristics, combustion stability, and emission performance of a CRDI diesel engine operated with biodiesel–ethanol blends. Energy Rep. 2021, 7, 904–915. [Google Scholar] [CrossRef]
- Mohammed, M.K.; Balla, H.H.; Al-Dulaimi, Z.M.H.; Kareem, Z.S.; Al-Zuhairy, M.S. Effect of ethanol-gasoline blends on SI engine performance and emissions. Case Stud. Therm. Eng. 2021, 25, 100891. [Google Scholar] [CrossRef]
- Prasath, K.; Manivannan, S.; Marimuthu, S.; Kannan, C.R.; Das, A.D. Experimental investigation of ethanol blended diesel fuel in single cylinder CI engine. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Prathipati, R.; Aditya Harshavardhan, K.H.; KarthikVarma, D.; Siddabathula, V.; Ramachandran, B. An empirical research on the investigation of DI diesel engine fuelled by ethanol. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Coronado, C.J.; Carvalho, J.O., Jr.; Andrade, J.C.; Mendiburu, A.Z.; Cortez, E.V.; Carvalho, F.S.; Goncalves, B.; Quintero, J.C.; Velasquez, E.I.; Silva, M.H.; et al. Flammability limits of hydrated and anhydrous ethanol at reduced pressures in aeronautical applications. J. Hazard. Mater. 2014, 280, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, W.; Wang, J.; Rao, D.; Chen, X.; Ma, H.; Zeng, W. Study on the laminar burning velocity of ethanol/RP-3 aviation kerosene premixed flame. Combust. Flame 2022, 238, 111921. [Google Scholar] [CrossRef]
- Xu, J. An explosion accident caused by the ethanol vapor vent pipe set in the room. Labour Prot. 2019, 3, 62–63. (In Chinese) [Google Scholar]
- Brooks, M.R.; Crowl, D.A. Flammability envelopes for methanol, ethanol, acetonitrile and toluene. J. Loss Prev. Process. 2007, 20, 144–150. [Google Scholar] [CrossRef]
- Coronado, C.J.; Carvalho, J.A., Jr.; Andrade, J.C.; Cortez, E.V.; Carvalho, F.S.; Santos, J.C.; Mendiburu, A.Z. Flammability limits: A review with emphasis on ethanol for aeronautical applications and description of the experimental procedure. J. Hazard. Mater. 2012, 241–242, 32–54. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Q. Numerical study of influence of initial pressures and temperatures on the lower flammability limits of oxygenated fuels in air. J. Loss Prev. Process. 2016, 41, 40–47. [Google Scholar] [CrossRef]
- Velasquez, E.I.G.; Coronado, C.J.R.; Cartagena, J.C.Q.; Carvalho, J.A.; Mendiburu, A.Z.; Andrade, J.C.; Cortez, E.V.; Santos, J.C. Prediction of flammability limits for ethanol-air blends by the Kriging regression model and response surfaces. Fuel 2017, 210, 410–424. [Google Scholar] [CrossRef]
- Bradley, D.; Lawes, M.; Mansour, M.S. Explosion bomb measurements of ethanol-air laminar gaseous flame characteristics at pressures up to 1.4 MPa. Combust. Flame 2009, 156, 1462–1470. [Google Scholar] [CrossRef]
- Cammarota, F.; Di Benedetto, A.; Di Sarli, V.; Salzano, E. The effect of hydrogen addition on the explosion of ethanol/air mixtures. Chem. Eng. Trans. 2012, 26, 405–410. [Google Scholar]
- Li, Q.; Cheng, Y.; Huang, Z. Comparative assessment of the explosion characteristics of alcohol-air mixtures. J. Loss Prev. Process. 2015, 37, 91–100. [Google Scholar] [CrossRef]
- Mitu, M.; Brandes, E. Influence of pressure, temperature and vessel volume on explosion characteristics of ethanol/air mixtures in closed spherical vessels. Fuel 2017, 203, 460–468. [Google Scholar] [CrossRef]
- Mitu, M.; Brandes, E.; Hirsch, W. Mitigation effects on the explosion safety characteristic data of ethanol/air mixtures in closed vessel. Process. Saf. Environ. 2018, 117, 190–199. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, C.; Shi, C.; Zhao, D.; Chen, J.; Lei, P.; Zhang, Y. Experimental study on deflagration of ethanol vapor from pool with different location and area in tunnel. J. Loss Prev. Process. 2019, 60, 213–220. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Zhao, X.; Lei, P.; Nie, Y. Experimental study on the influence of obstacle aspect ratio on ethanol liquid vapor deflagration in a narrow channel. Int. J. Therm. Sci. 2020, 153, 106354. [Google Scholar] [CrossRef]
- Xu, C.; Wu, S.; Li, Y.; Chu, S.; Wang, C. Explosion characteristics of hydrous bio-ethanol in oxygen-enriched air. Fuel 2020, 271, 117604. [Google Scholar] [CrossRef]
- ASTM E681-04; Standard Test Method for Concentration Limits of Flammability of Chemicals (Vapors and Gases). American Society of Testing Materials: West Conshohocken, PA, USA, 2004.
- Razus, D.; Brinzea, V.; Mitu, M.; Movileanu, C.; Oancea, D. Temperature and pressure influence on maximum rates of pressure rise during explosions of propane-air mixtures in a spherical vessel. J. Hazard. Mater. 2011, 190, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G.; Burke, S.M.; Davies, V.A.; Parajuli, B.; Metcalfe, W.K.; Curran, H.J. Autoignition of ethanol in a rapid compression machine. Combust. Flame 2014, 161, 1164–1171. [Google Scholar] [CrossRef]
- Egolfopoulos, F.N.; Du, D.X.; Law, C.K. A study on ethanol oxidation kinetics in laminar premixed flames, flow reactors, and shock tubes. Symp. (Int.) Combust. 1992, 24, 833–841. [Google Scholar] [CrossRef]
- Pekalski, A.A.; Schildberg, H.P.; Smallegange, P.S.D.; Lemkowitz, S.M.; Zevenbergen, J.F.; Braithwaite, M.; Pasman, H.J. Determination of the Explosion Behaviour of Methane and Propene in Air or Oxygen at Standard and Elevated Conditions. Process. Saf. Environ. 2005, 83, 421–429. [Google Scholar] [CrossRef]
- Dupont, L.; Accorsi, A. Explosion characteristics of synthesised biogas at various temperatures. J. Hazard. Mater. 2006, 136, 520–525. [Google Scholar] [CrossRef]
- Huzayyin, A.S.; Moneib, H.A.; Shehatta, M.S.; Attia, A.M.A. Laminar burning velocity and explosion index of LPG–air and propane–air mixtures. Fuel 2008, 87, 39–57. [Google Scholar] [CrossRef]
- Razus, D.; Brinzea, V.; Mitu, M.; Oancea, D. Temperature and pressure influence on explosion pressures of closed vessel propane-air deflagrations. J. Hazard. Mater. 2010, 174, 548–555. [Google Scholar] [CrossRef]
- Oancea, D.; Gosa, V.; Ionescu, N.I.; Popescu, D. An experimental method for the measurement of adiabatic maximum pressure during an explosive gaseous combustion. Rev. Roum. Chim. 1985, 30, 767–776. [Google Scholar]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Temperature and Pressure Influence on Ethane–Air Deflagration Parameters in a Spherical Closed Vessel. Energy Fuels 2012, 26, 4840–4848. [Google Scholar] [CrossRef]
- Dahoe, A.E.; Zevenbergen, J.F.; Lemkowitz, S.M.; Scarlett, B. Dust explosions in spherical vessels: The role of flame thickness in the validity of the ‘cube-root law’. J. Loss Prev. Process. 1996, 9, 33–44. [Google Scholar] [CrossRef]
- Van den Bulck, E. Closed algebraic expressions for the adiabatic limit value of the explosion constant in closed volume combustion. J. Loss Prev. Process. 2005, 18, 35–42. [Google Scholar] [CrossRef]
- Cammarota, F.; Di Benedetto, A.; Russo, P.; Salzano, E. Experimental analysis of gas explosions at non-atmospheric initial conditions in cylindrical vessel. Process. Saf. Environ. 2010, 88, 341–349. [Google Scholar] [CrossRef]
- Gieras, M.; Klemens, R. Experimental Studies of Explosions of Methane-Air Mixtures in a Constant Volume Chamber. Combust. Sci. Technol. 2009, 181, 641–653. [Google Scholar] [CrossRef]
- Cammarota, F.; Di Benedetto, A.; Di Sarli, V.; Salzano, E. Influence of initial temperature and pressure on the explosion behavior of n-dodecane/air mixtures. J. Loss Prev. Process. 2019, 62, 103920. [Google Scholar] [CrossRef]
- Wang, T.; Luo, Z.; Wen, H.; Cheng, F.; Liu, L.; Su, Y.; Liu, C.; Zhao, J.; Deng, J.; Yu, M. The explosion enhancement of methane-air mixtures by ethylene in a confined chamber. Energy 2021, 214, 119042. [Google Scholar] [CrossRef]
- Mohammad, A.; Juhany, K.A. Laminar burning velocity and flame structure of DME/methane + air mixtures at elevated temperatures. Fuel 2019, 245, 105–114. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, C.; Xu, C.; Yu, Z. Experimental and Numerical Study on the Effect of Hydrogen Addition on Laminar Burning Velocity of Ethanol–Air Mixtures. Energies 2022, 15, 3114. [Google Scholar] [CrossRef]


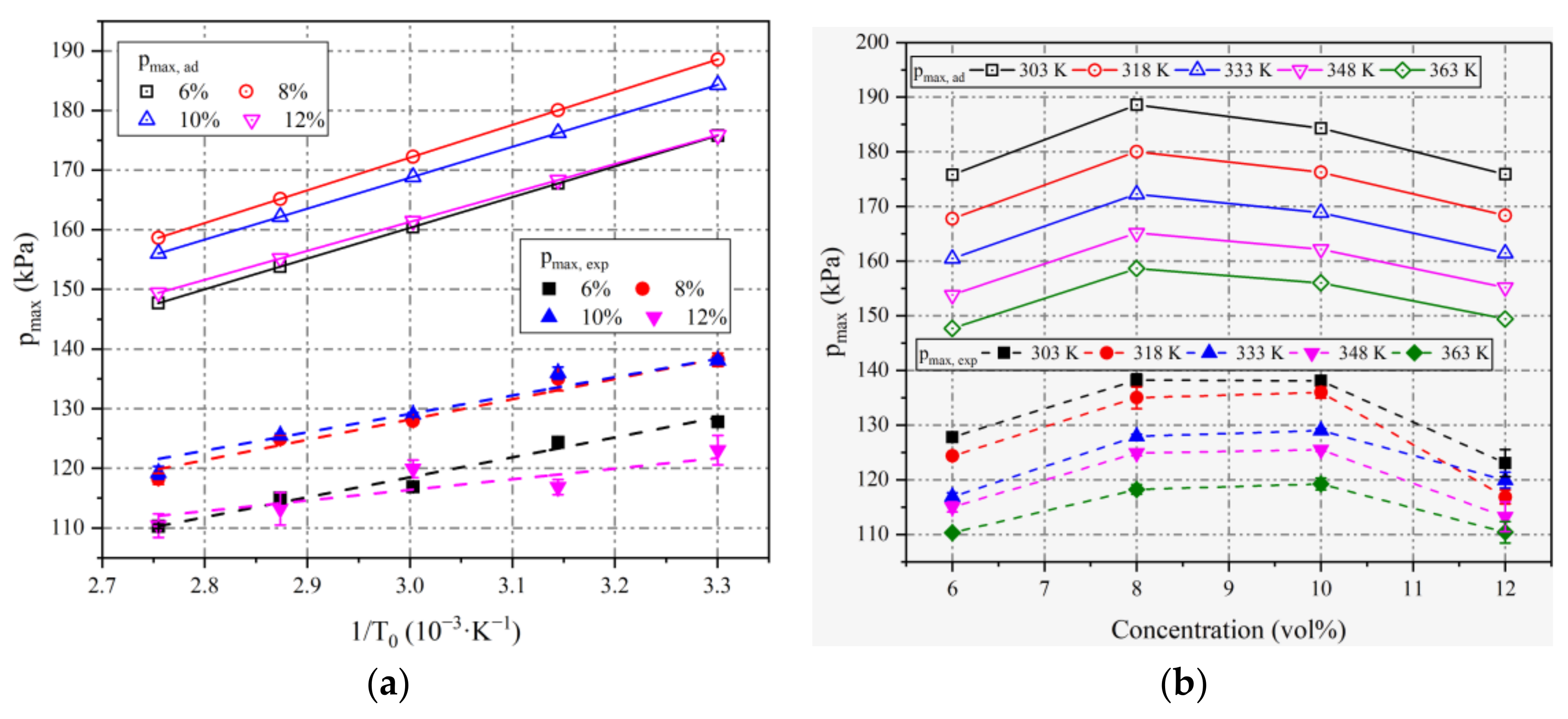
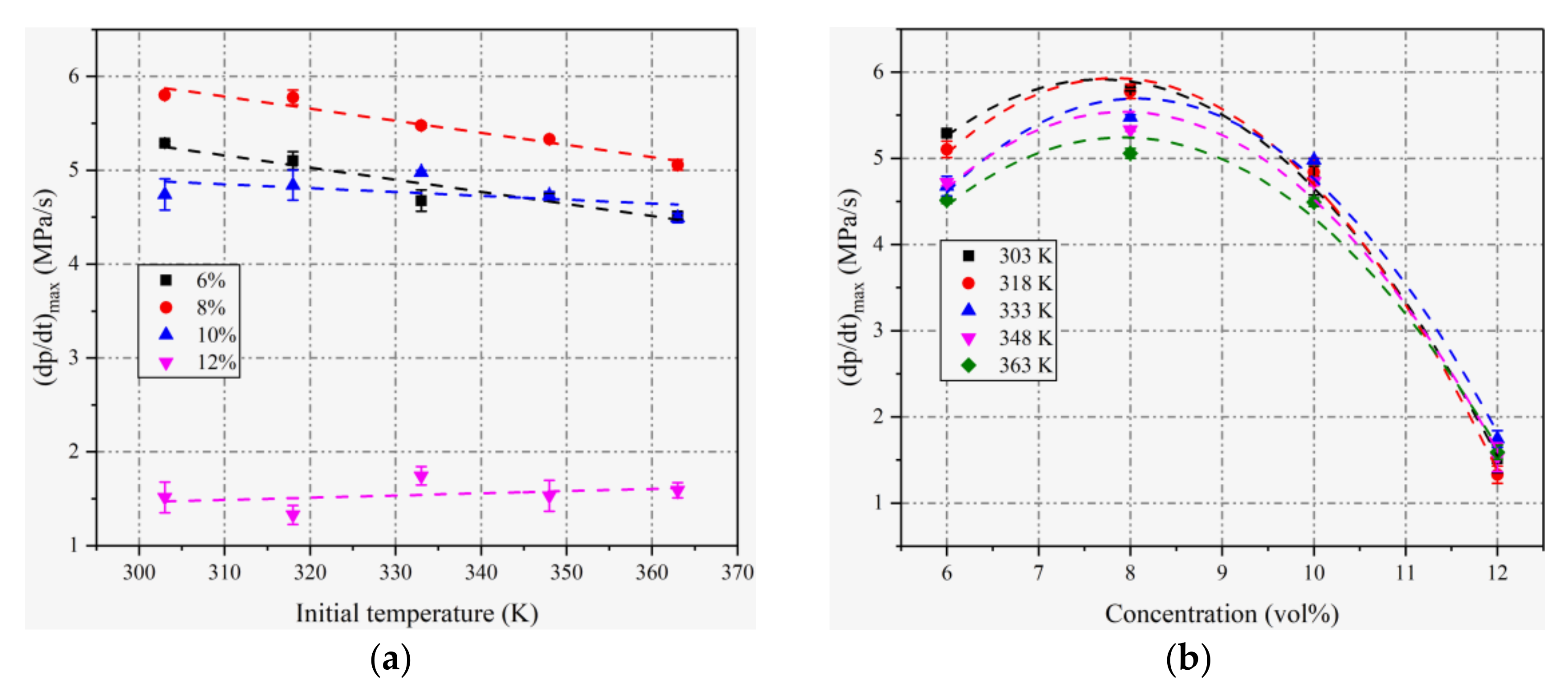
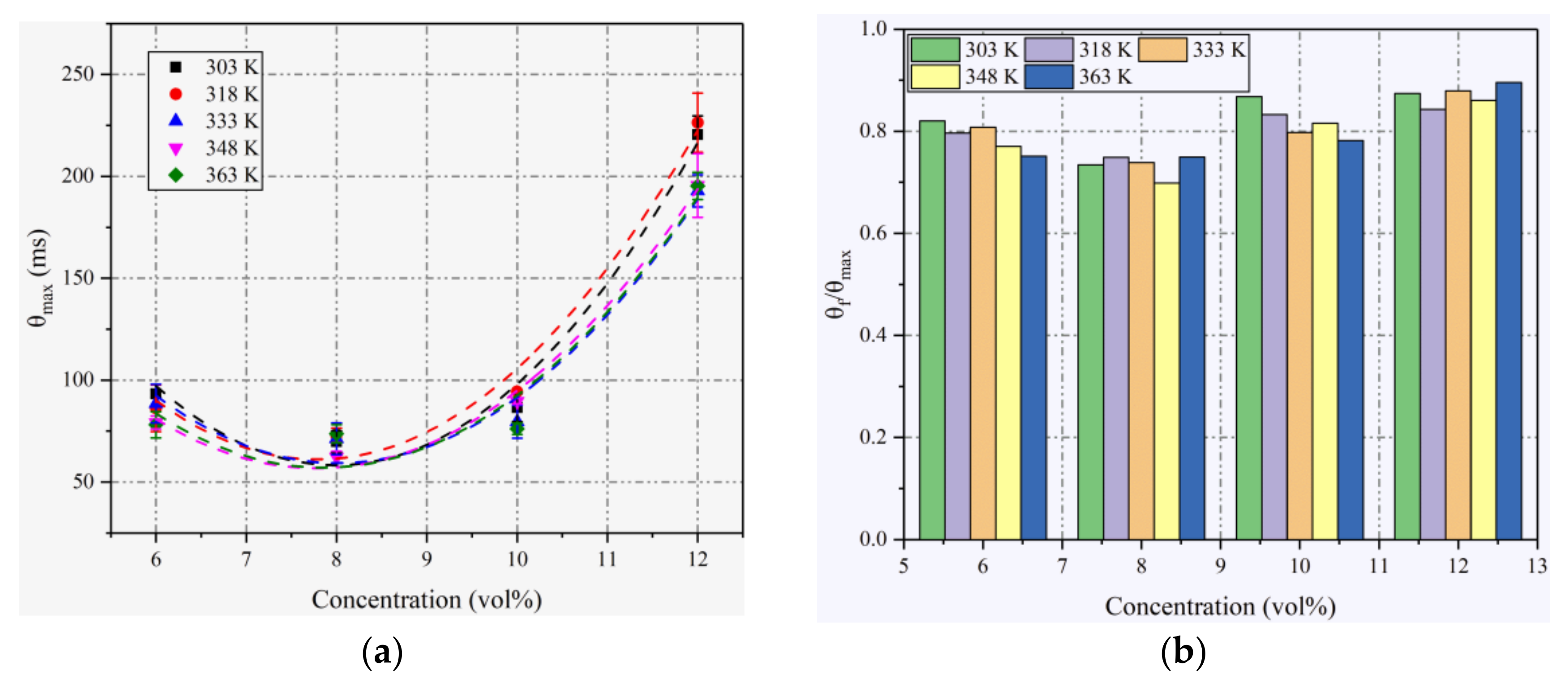


| Initial Pressure | Initial Temperatures | Concentration (vol%) |
|---|---|---|
| 20 kPa | 303 K, 318 K, 333 K, 348 K, 363 K | 6%, 8%, 10%, 12% |
| Equation | + b | |||
|---|---|---|---|---|
| Data Source | Concentration | Slope (a) | Intercept (b) | Coefficient of Determination (R2) |
| Simulation (pmax,ad) | 6% | 51.50 ± 0.01 | 5.80 ± 0.02 | 1 |
| 8% | 54.83 ± 0.00 | 7.62 ± 0.00 | 1 | |
| 10% | 51.84 ± 0.01 | 13.20 ± 0.02 | 1 | |
| 12% | 48.53 ± 0.02 | 15.73 ± 0.05 | 1 | |
| Experiment (pmax,exp) | 6% | 33.44 ± 1.25 | 18.17 ± 3.67 | 0.9853 |
| 8% | 34.00 ± 2.26 | 26.19 ± 6.70 | 0.9610 | |
| 10% | 30.80 ± 1.18 | 36.72 ± 3.54 | 0.9834 | |
| 12% | 17.79 ± 4.83 | 63.01 ± 14.69 | 0.5913 | |
| R1 | H + O2 <=> O + OH | R135 | CH3 + OH <=> CH2OH + H |
| R9 | H + O2(+M) <=> HO2(+M) | R145 | CH3 + HO2 <=> CH3O + OH |
| R12 | HO2 + H <=> OH + OH | R146 | CH3 + HO2 <=> CH4 + O2 |
| R13 | H2 + O2 <=> H + HO2 | R189 | CH3 + CH3(+M) <=> C2H6(+M) |
| R15 | HO2 + OH <=> H2O + O2 | R327 | C2H2 + H(+M) <=> C2H3(+M) |
| R27 | CO + OH <=> CO2 + H | R332 | C2H3 + H <=> C2H2 + H2 |
| R30 | HCO + M <=> H + CO + M | R363 | C2H5OH + H <=> SC2H4OH + H2 |
| R32 | HCO + H <=> CO + H2 | R383 | SC2H4OH <=> CH3CHO + H |
| R127 | CH3 + H(+M) <=> CH4(+M) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, X.; Zhang, Z.; Zheng, K.; Wang, X.; Wang, J. Experimental and Numerical Studies on the Explosion Characteristics of Ethanol–Air Mixtures under Aviation Conditions. Fire 2023, 6, 349. https://doi.org/10.3390/fire6090349
Ning X, Zhang Z, Zheng K, Wang X, Wang J. Experimental and Numerical Studies on the Explosion Characteristics of Ethanol–Air Mixtures under Aviation Conditions. Fire. 2023; 6(9):349. https://doi.org/10.3390/fire6090349
Chicago/Turabian StyleNing, Xiaoyao, Zhihui Zhang, Kangxue Zheng, Xuehui Wang, and Jian Wang. 2023. "Experimental and Numerical Studies on the Explosion Characteristics of Ethanol–Air Mixtures under Aviation Conditions" Fire 6, no. 9: 349. https://doi.org/10.3390/fire6090349
APA StyleNing, X., Zhang, Z., Zheng, K., Wang, X., & Wang, J. (2023). Experimental and Numerical Studies on the Explosion Characteristics of Ethanol–Air Mixtures under Aviation Conditions. Fire, 6(9), 349. https://doi.org/10.3390/fire6090349






