Thermal Decomposition and Kinetic Analysis of Amazonian Woods: A Comparative Study of Goupia glabra and Manilkara huberi
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Samples Preparation and Anatomical Identification
2.3. Thermal Analysis
2.4. Kinetic Study
2.4.1. Arrhenius Model
2.4.2. Kissinger Model
2.5. Fourier-Transform Infrared Spectroscopy with Attenuated Reflectance (FTIR-ATR) Analysis
2.6. Direct Infusion Mass Spectrometry (DIMS) Analysis
3. Results and Discussion
3.1. Species Identification
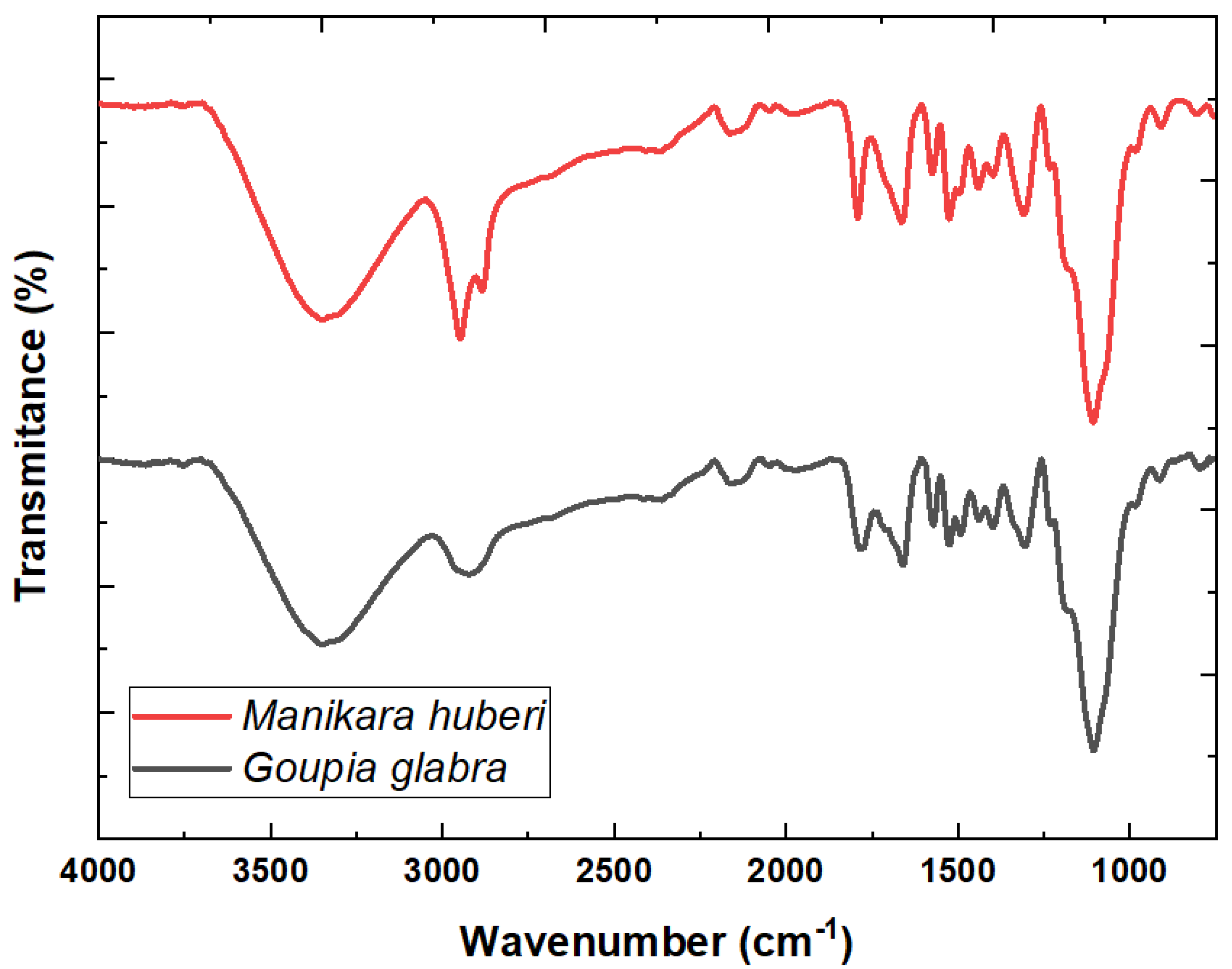
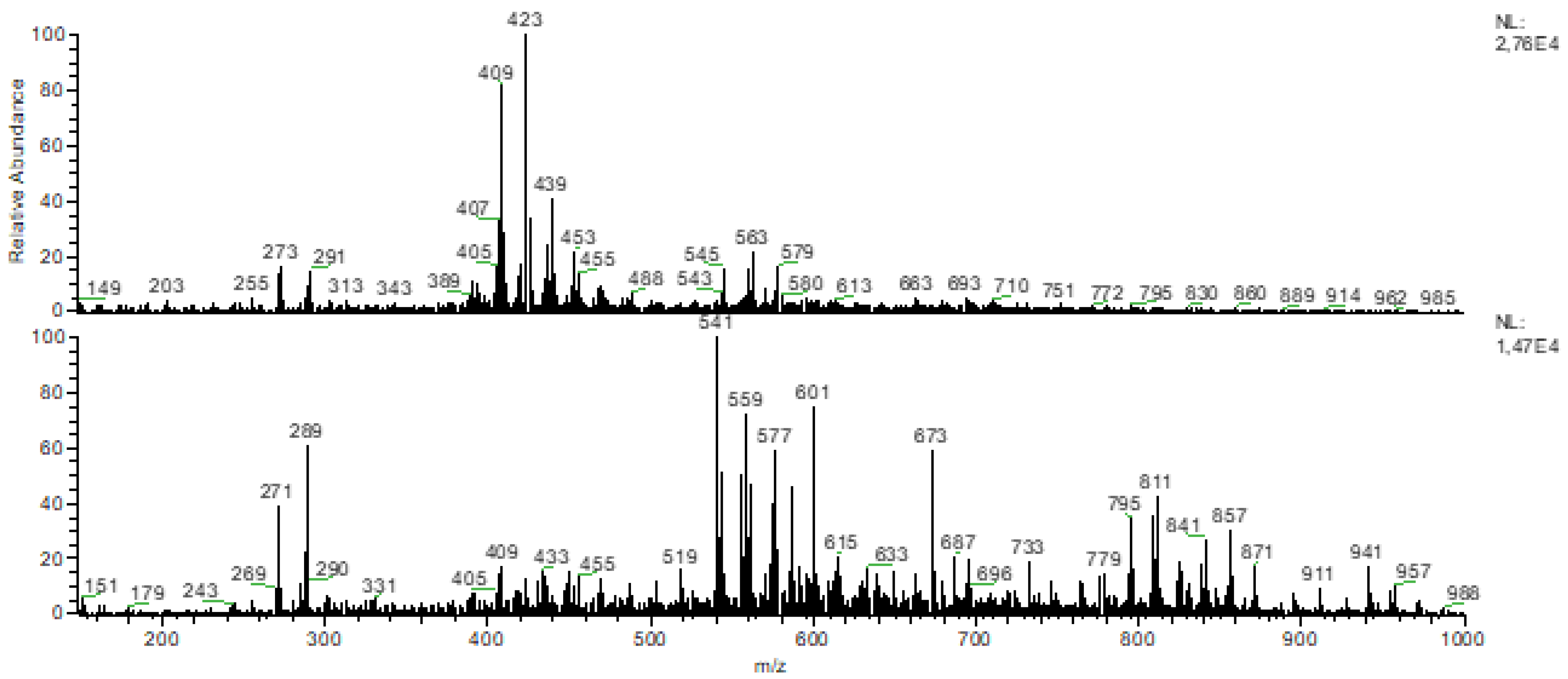
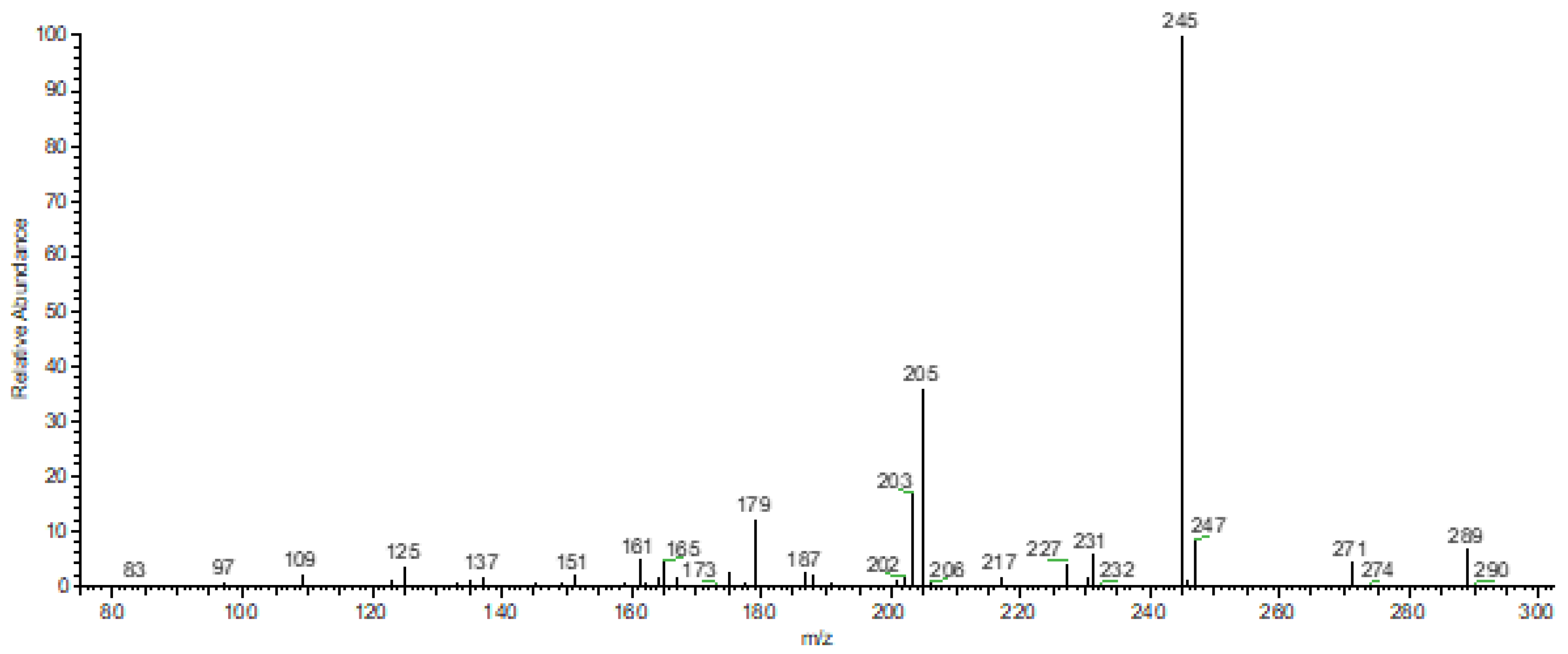
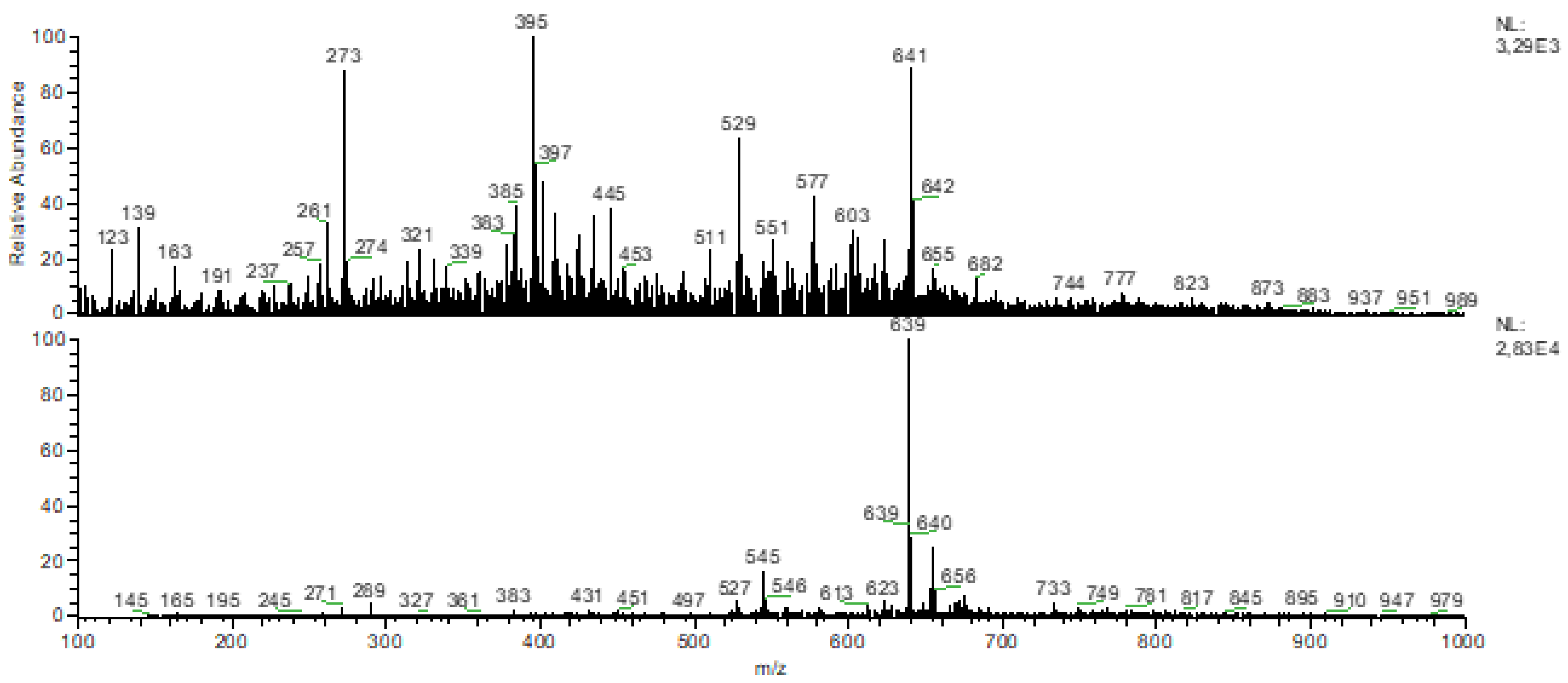
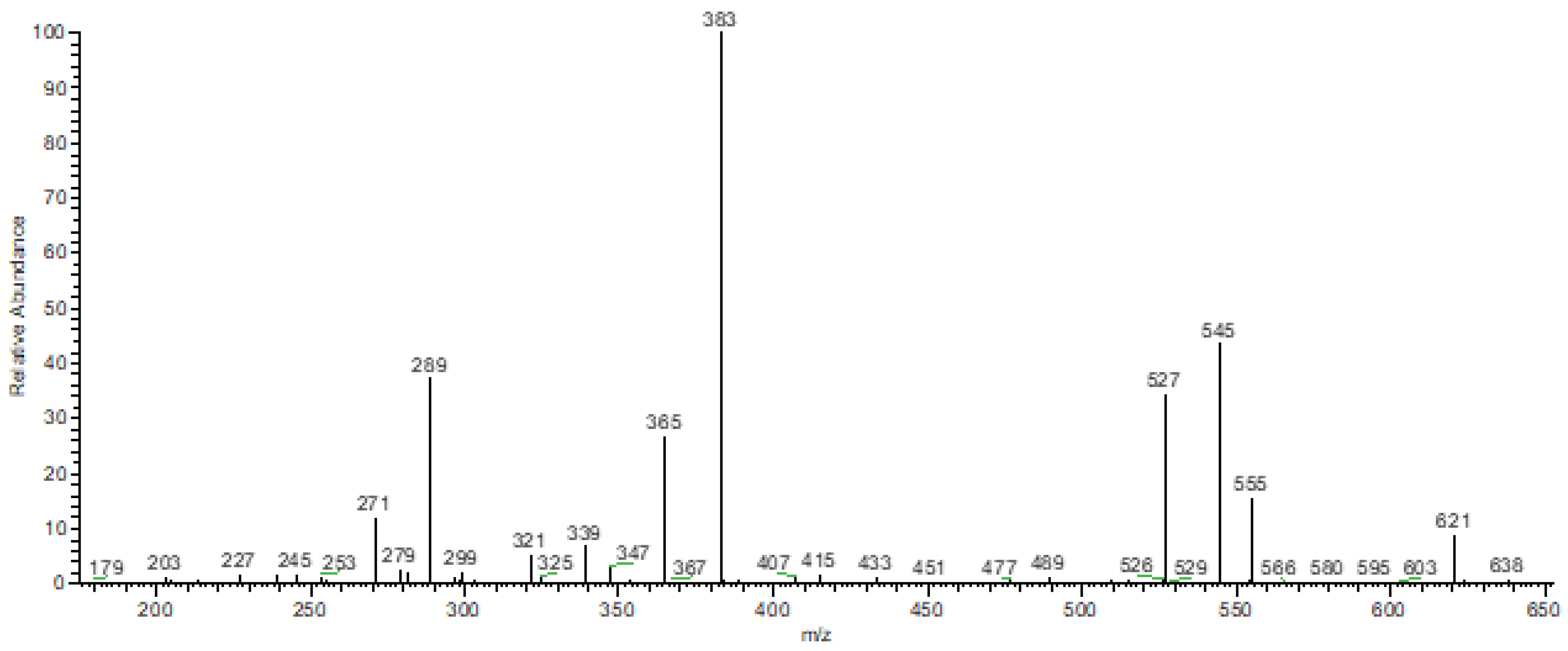
3.2. FTIR-ATR and DIMS Analysis
3.3. Thermal Evaluation
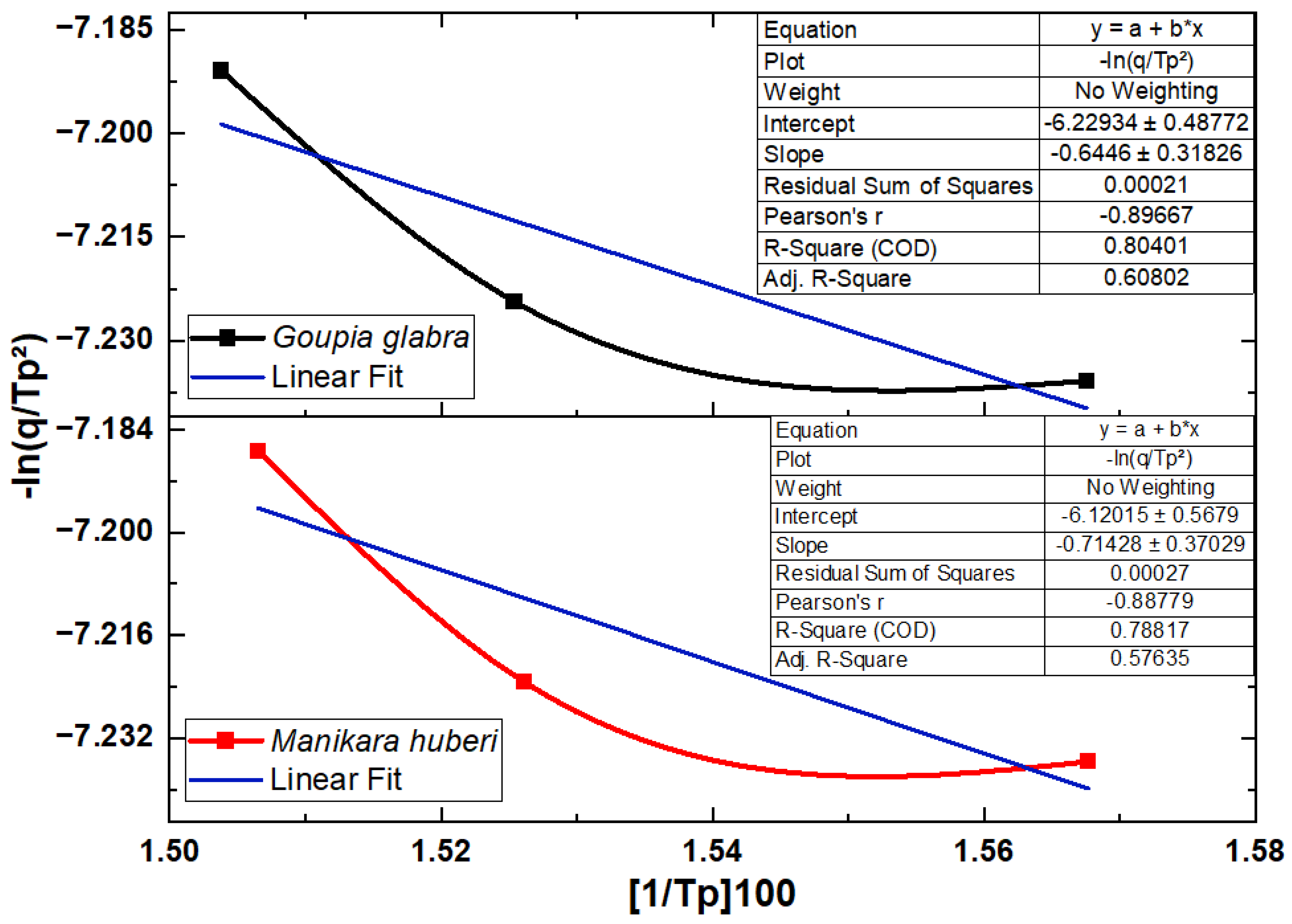
3.4. Kinetic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demirbaş, A. Estimating of structural composition of wood and non-wood biomass samples. Energy Sources 2005, 27, 761–767. [Google Scholar] [CrossRef]
- Hon, D.N.S.; Chang, S.T. Surface Degradation of Wood by Ultraviolet Light. J. Polym. Sci. 1984, 22, 2227–2241. [Google Scholar] [CrossRef]
- Tao, W.; Shi, S.; Kroll, C.N. Influences of wood preservation, lumber size, and weather on field leaching of red pine lumber. J. Hazard. Mater. 2013, 260, 296–304. [Google Scholar] [CrossRef]
- Li, X. Physical, Chemical, and Mechanical Properties of Bamboo and Its Utilization Potential for Fiberboard Manufacturing; Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 2004. [Google Scholar]
- Ferreira, G.C.; Gomes, J.I.; Hopkins, M.J.G. Estudo anatômico das espécies de Leguminosae comercializadas no estado do Pará como “angelim”. Acta Amaz. 2004, 34, 387–398. [Google Scholar] [CrossRef]
- Hirai, E.H.; de Carvalho, J.O.P.; Pinheiro, K.A.O. Pinheiro, Comportamento Populacional De Cupiúba (Goupia glabra Aubl.) Em Floresta De Terra Firme Na Fazenda Rio Capim, Paragominas (PA). Rev. Ciências Agrárias Amaz. J. Agric. Environ. Sci. 2007, 47, 89–102. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas do Brasil. 2016. Available online: https://www.nhbs.com/arvores-brasileiras-volume-1-manual-de-identificacao-e-cultivo-de-plantas-arboreas-nativas-do-brasil-brazilian-trees-volume-1-identification-and-cultivation-manual-of-native-brazilian-arboreal-plants-book (accessed on 20 September 2024).
- Florsheim, S.M.B.; IF—Instituto Florestal. Identificação Macrsocópica de Madeiras Comerciais do Estado de São Paulo. 2020. Available online: https://repositorio.cetesb.sp.gov.br/handle/123456789/2519 (accessed on 20 September 2024).
- Flora e Funga do Brasil. Available online: https://floradobrasil.jbrj.gov.br/reflora/listaBrasil/PrincipalUC/PrincipalUC.do;jsessionid=2C57D2C037147236F7D692FD8FFAA6A2#CondicaoTaxonCP (accessed on 20 September 2024).
- Pinto, L.A.D.A. Forest Management Criteria for Manilkara huberi (Ducke) A. Chev. (Sapotaceae) in Upland Forests of Central Amazon Based on Dendrochronological Study. Environ. Sci. Proc. 2020, 3, 105. [Google Scholar] [CrossRef]
- Silveira, D.; Gavrilov, A.; Kanzaki, L. Botanical Characterisation, Geographical Distribution and Phytochemistry Analysis of Manilkara huberi (Ducke) Stanhl Autochtonous in Amapa State, Brazil. Proc. Natl. Acad. Sci. USA 2011, 2, 34–39. Available online: http://libcat.bas-net.by/xfile/v_bio/2011/2/40tsj0.pdf#page=34 (accessed on 20 September 2024).
- Mvondo, R.R.N.; Meukam, P.; Jeong, J.; Meneses, D.D.S.; Nkeng, E.G. Influence of water content on the mechanical and chemical properties of tropical wood species. Results Phys. 2017, 7, 2096–2103. [Google Scholar] [CrossRef]
- Peemoeller, H.; Kamke, F.A. Absolute Moisture Content Determination of Aspen Wood Below the Fiber Saturation Point using Pulsed NMR. Holzforschung 1994, 48, 474–479. [Google Scholar] [CrossRef]
- Gallio, E.; Zanatta, P.; Duarte Ribes, D.; Beltrame, R.; Alberto Gatto, D. Higroscopicidade da madeira de três espécies florestais deterioradas por térmitas Nasutitermes. Pesqui. Florest. Bras. 2018, 38, 1–7. [Google Scholar] [CrossRef]
- Candelier, K.; Thevenon, M.F.; Petrissans, A.; Dumarcay, S.; Gerardin, P.; Petrissans, M. Control of wood thermal treatment and its effects on decay resistance: A review. Ann. For. Sci. 2016, 73, 571–583. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Zheng, C.; Lee, D.H.; Liang, D.T. In-depth investigation of biomass pyrolysis based on three major components: Hemicellulose, cellulose and lignin. Energy Fuels 2006, 20, 388–393. [Google Scholar] [CrossRef]
- Pereira, B.L.C.; Carneiro, A.C.O.; Carvalho, A.M.M.L.; Trugilho, P.F.; Melo, I.C.N.A.; Oliveira, A.C. Study of thermal degradation of Eucalyptus wood by thermogravimetry and calorimetry. Rev. Arvore 2013, 37, 567–576. [Google Scholar] [CrossRef]
- Klock, U.; Muñiz, G.I.B.; Anzaldo, J.H.; Andrade, A. Química da madeira. Available online: https://idoc.pub/documents/apostila-quimica-da-madeira-134wqog68w47 (accessed on 20 September 2024).
- Oliveira, J.T.D.S.; Silva, J.D.C. Variação radial da retratibilidade e densidade básica da madeira de Eucalyptus saligna Sm. Rev. Árvore 2003, 27, 381–385. [Google Scholar] [CrossRef]
- de Oliveira, R.M. Utilização de Técnicas de Caracterização de Superfícies em Madeiras Tratadas Termicamente. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2009. [Google Scholar] [CrossRef]
- Chen, R.; Kirsh, Y. The Analysis of Thermally Stimulated Processes; Pergamon Press: Oxford, UK, 1981; Available online: https://www.sciencedirect.com/book/9780080229300/the-analysis-of-thermally-stimulated-processes (accessed on 20 September 2024).
- Wilson, L.; Yang, W.; Blasiak, W.; John, G.R.; Mhilu, C.F. Thermal characterization of tropical biomass feedstocks. Energy Convers. Manag. 2011, 52, 191–198. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Brown, M.E. Introduction to Thermal Analysis; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Sá, I.S.C.; da Silva, F.M.A.; Nunomura, R.D.C.S. Simplex centroid mixture design, DI-ESI-MS, and chemometric analysis-guided isolation of parinarioidin C from bark of Brosimum parinarioides (Moraceae). Phytochem. Lett. 2022, 50, 25–30. [Google Scholar] [CrossRef]
- Amaral Reis, C.; Minini, D.; Da Silva, E.L.; De Cademartori, P.H.G.; Vidaurre, G.B.; Morrone, S.R.; De Muñiz, G.I.B.; Nisgoski, S. Comparative wood and charcoal anatomy of Manilkara sp.: Contribution for market inspections. Holzforschung 2024, 78, 503–518. [Google Scholar] [CrossRef]
- do Nascimento, L.B.; das Neves Brandes, A.F.; Valente, F.D.W.; Tamaio, N. Anatomical identification of commercialized wood in the state of Rio de Janeiro, Brazil. Rev. Bras. Bot. 2017, 40, 291–329. [Google Scholar] [CrossRef]
- Reis, C.A.; da Silva, E.L.; Minini, D.; de Muñiz, G.I.B.; Morrone, S.R.; Nisgoski, S. Preliminary study of colorimetry as an auxiliary tool for Manilkara spp. wood discrimination. Eur. J. Wood Wood Prod. 2023, 81, 1119–1133. [Google Scholar] [CrossRef]
- John, M.J.; Thomas, S. Biofibres and biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Jesus, E.; Franca, T.; Calvani, C.; Lacerda, M.; Gonçalves, D.; Oliveira, S.L.; Marangoni, B.; Cena, C. Making wood inspection easier: FTIR spectroscopy and machine learning for Brazilian native commercial wood species identification. RSC Adv. 2024, 14, 7283–7289. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Rhourri-Frih, B.; Renimel, I.; Chaimbault, P.; André, P.; Herbette, G.; Lafosse, M. Pentacyclic triterpenes from Manilkara bidentata resin. Isolation, identification and biological properties. Fitoterapia 2013, 88, 101–108. [Google Scholar] [CrossRef]
- Tibe, O.; Meagher, L.P.; Fraser, K.; Harding, D.R.K. Condensed tannins and flavonoids from the forage legume sulla (Hedysarum coronarium). J. Agric. Food Chem. 2011, 59, 9402–9409. [Google Scholar] [CrossRef]
- Savic, I.; Nikolic, V.; Savic, I.; Nikolic, L.; Jovic, M.; Jovic, M. The qualitative analysis of the green tea extract using ESI-MS method. Savrem. Tehnol. 2014, 3, 30–37. [Google Scholar] [CrossRef]
- Mo, S.; Dong, L.; Hurst, W.J.; Van Breemen, R.B. Quantitative analysis of phytosterols in edible oils using APCI liquid chromatography-tandem mass spectrometry. Lipids 2013, 48, 949–956. [Google Scholar] [CrossRef]
- Font, R.; Fullana, A.; Conesa, J. Kinetic models for the pyrolysis and combustion of two types of sewage sludge. J. Anal. Appl. Pyrolysis 2005, 74, 429–438. [Google Scholar] [CrossRef]
- Jelemenský, Ľ. Calculation of Kinetic Parameters of the Thermal Decomposition of Wood by Distributed Activation Energy Model (DAEM). Available online: https://www.academia.edu/122057478/Calculation_of_Kinetic_Parameters_of_the_Thermal_Decomposition_of_Wood_by_Distributed_Activation_Energy_Model_DAEM_ (accessed on 20 September 2024).
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Misse, S.E.; Brillard, A.; Brilhac, J.F.; Obonou, M.; Ayina, L.M.; Schönnenbeck, C.; Caillat, S. Thermogravimetric analyses and kinetic modeling of three Cameroonian biomass. J. Therm. Anal. Calorim. 2018, 132, 1979–1994. [Google Scholar] [CrossRef]
- Fan, Y.; Lu, D.; Wang, J.; Kawamoto, H. Thermochemical behaviors, kinetics and bio-oils investigation during co-pyrolysis of biomass components and polyethylene based on simplex-lattice mixture design. Energy 2022, 239, 122234. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Kait, C.F.; Ullah, Z.; Nasrullah, A.; Khan, M.I.; Gonfa, G.; Ahmad, P.; Muhammad, N. Kinetics and thermodynamic parameters of ionic liquid pretreated rubber wood biomass. J. Mol. Liq. 2016, 223, 754–762. [Google Scholar] [CrossRef]
- Youssef, M.A.; Sefain, M.Z.; El-Kalyoubi, S.F. Thermal behaviour of cellulose acetate. Thermochim. Acta 1989, 150, 33–38. [Google Scholar] [CrossRef]
- Ramiah, M.V. Thermogravimetric and differential thermal analysis of cellulose, hemicellulose, and lignin. J. Appl. Polym. Sci. 1970, 14, 1323–1337. [Google Scholar] [CrossRef]
- Tsujiyama, S.I.; Miyamori, A. Assignment of DSC thermograms of wood and its components. Thermochim. Acta 2000, 351, 177–181. [Google Scholar] [CrossRef]
- Dill, I.; Kraepelin, G. Palo podrido: Model for extensive delignification of wood by Ganoderma applanatum. Appl. Environ. Microbiol. 1986, 52, 1305–1312. [Google Scholar] [CrossRef]
- Tsujiyama, S.I. Differential scanning calorimetric analysis of the lignin-carbohydrate complex degraded by wood-rotting fungi. J. Wood Sci. 2001, 47, 497–501. [Google Scholar] [CrossRef]
- Kaloustian, J.; Pauli, A.M.; Pastor, J. Etude comparative de cinq vegetaux mediterraneens par analyses thermique et chimique. J. Therm. Anal. Calorim. 1998, 53, 57–69. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Bai, J.; Chen, X.; Shao, J.; Jia, C.; Wang, Q. Study of breakage of main covalent bonds during co-pyrolysis of oil shale and alkaline lignin by TG-FTIR integrated analysis. J. Energy Inst. 2019, 92, 512–522. [Google Scholar] [CrossRef]
- Lyons Ceron, A.; Ochieng, R.; Sarker, S.; Järvik, O.; Konist, A. Co-Pyrolysis of Woody Biomass and Oil Shale—A Kinetics and Modelling Study. Energies 2024, 17, 1055. [Google Scholar] [CrossRef]
- Eloy, E.; Bandera, E.; Mangini, T.D.S.; Trevisan, R.; Candaten, L.; Zanchetta, L.D.S. Thermal modification influences the mechanical resistance of wood from forestry species. Ciência Rural 2023, 53, e20210467. [Google Scholar] [CrossRef]
- Výbohová, E.; Kučerová, V.; Andor, T.; Balážová, Ž.; Veľková, V. The Effect of Heat Treatment on the Chemical Composition of Ash Wood. BioResources 2018, 13, 8394–8408. [Google Scholar] [CrossRef]
- Liu, L.C. Chemistry in Action: Making Molecular Movies with Ultrafast Electron Diffraction and Data Science; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]



| Process | Tpeak (°C) | Tonset (°C) | TEndset (°C) | ΔH (mJ) |
|---|---|---|---|---|
| Goupia glabra | ||||
| Peak 1 | 71.47 | 30.97 | 104.42 | −1103.06 |
| Peak 2 | 331.45 | 271.27 | 384.21 | 12633.37 |
| Peak 3 | 466.08 | 422.37 | 480.77 | 18652.66 |
| Manilkara huberi | ||||
| Peak 1 | 76.08 | 45.08 | 106.72 | −1441.68 |
| Peak 2 | 366.41 | 317.08 | 414.10 | 9648.04 |
| Heat Flux (°C/min) | Sample | Temperature (°C) | Mass (mg-%) | α | Slope |
|---|---|---|---|---|---|
| 20 | Goupia glabra | T0 = 22.62 | W0 = (10.53–100) | 0.15 | 3.00 ± 0.01 |
| Tt = 117.53 | Wt = (9.63–91.51) | ||||
| Tf = 377.39 | Wf = (4.42–42.04) | ||||
| 40 | T0 = 24.14 | W0 = (9.98–100) | 0.11 | 3.10 ± 0.02 | |
| Tt = 104.97 | Wt = (9.17–91.97) | ||||
| Tf = 396.98 | Wf = (2.61–26.19) | ||||
| 60 | T0 = 25.95 | W0 = (10.35–100) | 0.10 | 3.20 ± 0.03 | |
| Tt = 112.54 | Wt = (9.53–92.10) | ||||
| Tf = 404.02 | Wf = (2.42–23.43) | ||||
| 20 | Manilkara huberi | T0 = 19.75 | W0 = (10.38–100) | 0.14 | 3.04 ± 0.01 |
| Tt = 127.17 | Wt = (9.46–91.18) | ||||
| Tf = 380.56 | Wf = (3.95–38.09) | ||||
| 40 | T0 = 23.76 | W0 = (9.97–100) | 0.10 | 3.11 ± 0.02 | |
| Tt = 108.73 | Wt = (9.18–92.11) | ||||
| Tf = 404.59 | Wf = (2.61–23.06) | ||||
| 60 | T0 = 22.01 | W0 = (9.72–100) | 0.11 | 3.28 ± 0.03 | |
| Tt = 138.36 | Wt = (8.82–90.76) | ||||
| Tf = 414.06 | Wf = (1.75–18.09) |
| Sample | Model | Heat Flux (°C/min) | Activation Energy (KJ/mol.K) |
|---|---|---|---|
| Goupia glabra (cupiúba) | Arrhenius | 20 | 53.46 |
| 40 | 59.42 | ||
| 60 | 61.45 | ||
| Kissinger | 20, 40, 60 | 53.59 | |
| Manilkara huberi (maçaranduba) | Arrhenius | 20 | 58.18 |
| 40 | 59.52 | ||
| 60 | 62.77 | ||
| Kissinger | 20, 40, 60 | 59.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junior, M.D.V.; Guerrero, F.; da Silva, F.M.A.; Ramos, G.Q.; Matos, R.S.; Țălu, Ș.; Trong, D.N.; da Fonseca Filho, H.D. Thermal Decomposition and Kinetic Analysis of Amazonian Woods: A Comparative Study of Goupia glabra and Manilkara huberi. Fire 2024, 7, 390. https://doi.org/10.3390/fire7110390
Junior MDV, Guerrero F, da Silva FMA, Ramos GQ, Matos RS, Țălu Ș, Trong DN, da Fonseca Filho HD. Thermal Decomposition and Kinetic Analysis of Amazonian Woods: A Comparative Study of Goupia glabra and Manilkara huberi. Fire. 2024; 7(11):390. https://doi.org/10.3390/fire7110390
Chicago/Turabian StyleJunior, Mark Dany Veloso, Fidel Guerrero, Felipe Moura Araújo da Silva, Glenda Quaresma Ramos, Robert Saraiva Matos, Ștefan Țălu, Dung Nguyen Trong, and Henrique Duarte da Fonseca Filho. 2024. "Thermal Decomposition and Kinetic Analysis of Amazonian Woods: A Comparative Study of Goupia glabra and Manilkara huberi" Fire 7, no. 11: 390. https://doi.org/10.3390/fire7110390
APA StyleJunior, M. D. V., Guerrero, F., da Silva, F. M. A., Ramos, G. Q., Matos, R. S., Țălu, Ș., Trong, D. N., & da Fonseca Filho, H. D. (2024). Thermal Decomposition and Kinetic Analysis of Amazonian Woods: A Comparative Study of Goupia glabra and Manilkara huberi. Fire, 7(11), 390. https://doi.org/10.3390/fire7110390









