A Thermal Characteristics Study of Typical Industrial Oil Based on Thermogravimetric-Differential Scanning Calorimetry (TG-DSC)
Abstract
:1. Introduction
2. Experiment Section
2.1. Material Material
2.2. Measurement
2.2.1. Measurement Methods
2.2.2. Measurement Equipment
2.2.3. Measurement Procedure
3. Results
3.1. TG-DSC Analysis of Oil
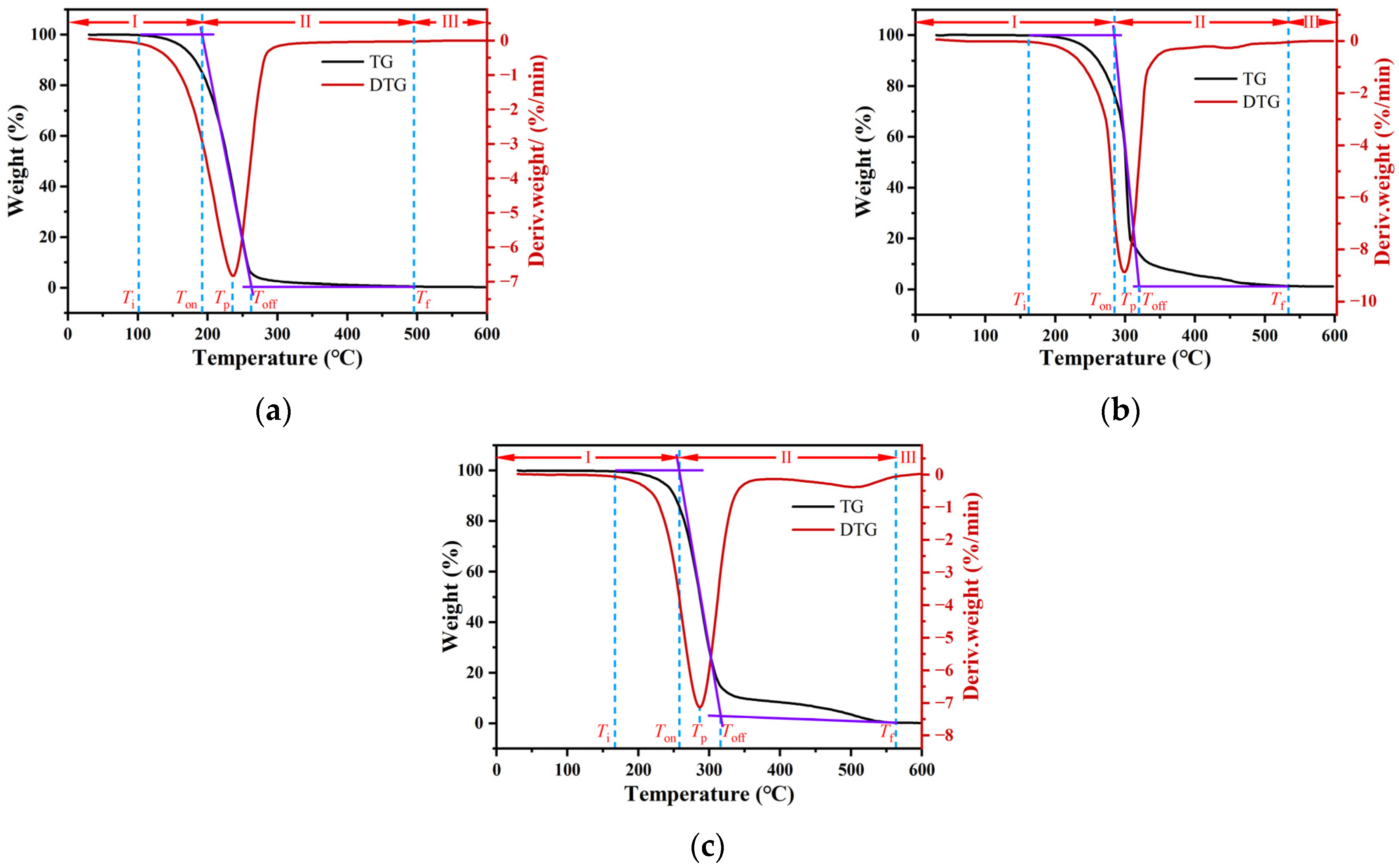
3.1.1. Typical TG-DTG Curves and Thermal Weight Loss Characteristic Parameters
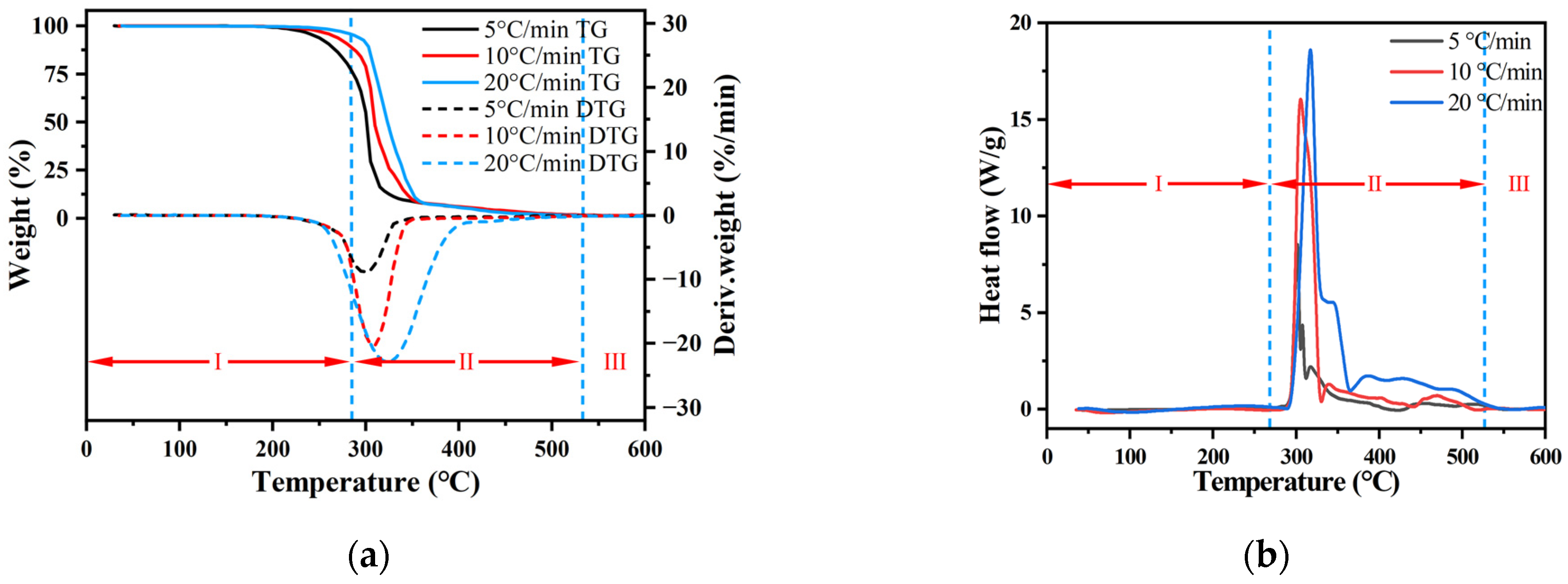
3.1.2. TG-DSC Analysis of Transformer Oil
3.1.3. The TG-DSC Analysis of Engine Oil
3.1.4. The TG-DSC Analysis of Hydraulic Oil
3.2. Dynamic Analysis and Mechanism Model Establishment
3.2.1. Basis of Dynamics Analysis
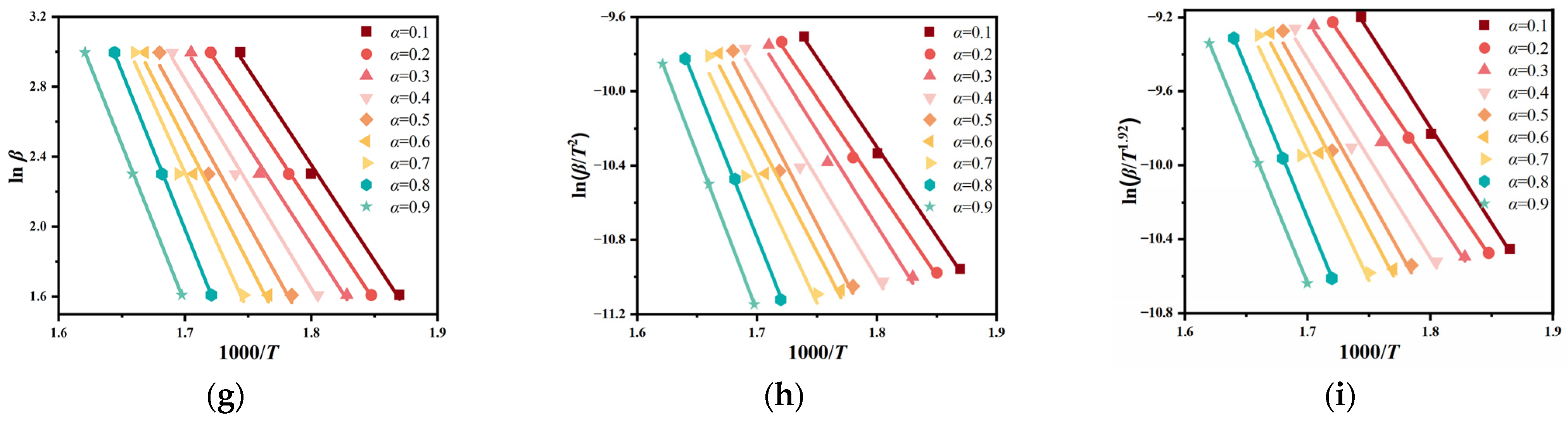
3.2.2. Kinetic Parameters
3.2.3. The Model of the Kinetic Mechanism
3.3. Determination of Kinetic Parameters and Mechanism Model
3.3.1. Preliminary Determination of Kinetic Parameters
3.3.2. Determination of Kinetic Mechanism Model
4. Summary
- (1)
- Thermogravimetric analysis of industrial oils, represented by TG-DTG curves, revealed a singular peak during the thermal decomposition process. The peak signifies an initial increase in the evaporation and oxidation reaction rate, peaking at the maximum and then gradually tapering off. By introducing characteristic temperatures from thermogravimetric analysis, the mass loss process of industrial oils was divided into three main stages. The primary evaporation and oxidation reaction of industrial oils mainly occurred in stage II, where the mass loss for transformer oil, engine oil, and hydraulic oil ranged between approximately 80–84%, 73–79%, and 86–89%, respectively.
- (2)
- Thermal analysis, evidenced by the TG, DTG, and DSC curves, exhibits a notable shift towards higher temperatures with an increase in the heating rate. The peak temperature of the DSC curve was higher than that of the DTG curve, indicating that the heat changes lagged behind the mass loss during the thermal decomposition of the oils. Moreover, as the heating rate escalated, the peak values of both DTG and DSC curves augmented, highlighting that a higher heating rate not only increased the thermal decomposition but also aggravated the evaporation and oxidation reactions of oils.
- (3)
- Three model-free iso-conversational methods, FWO, KAS, and Starink methods, were used to calculate the apparent activation energy of the oils. The trend calculated using different methods exhibited a slight difference, with an increase in the conversion rate. The apparent activation energy of engine oil, hydraulic oil, and transformer oil was determined to be 110.50, 105.13, and 60.95 kJ/mol, respectively. Additionally, the Master-plot method aided in identifying the kinetic mechanism model, with the reaction order model (Fn) emerging as the most suitable for describing the evaporative oxidation reaction of these industrial oils in an air atmosphere, corresponding to the kinetic mechanism function f(α) = (1 − α)n.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, R.; Weng, G.M. Sustainable energy resources for driving methane conversion. Adv. Energy Mater. 2023, 13, 2301734. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2023; International Energy Agency (IEA): Paris, France, 2023; Available online: https://www.iea.org/reports/world-energy-outlook-2023 (accessed on 24 October 2023).
- Narayanan, D.K.; Ravoof, A.A.; Jayapriya, J.; Revathi, G.; Murugan, M. Hazards in oil, gas, and petrochemical industries. In Crises in Oil, Gas and Petrochemical Industries; Elsevier: Amsterdam, The Netherlands, 2023; pp. 71–99. [Google Scholar]
- Li, M.; Xu, Z.; Luo, Q.; Wang, C. Investigation of bicubic flame radiation model of continuously opposed spilling fire over n-butanol fuel. Energy 2023, 272, 127144. [Google Scholar] [CrossRef]
- Addo, E.K.; Kabo-bah, A.T.; Diawuo, F.A.; Debrah, S.K. The role of nuclear energy in reducing greenhouse gas (GHG) emissions and energy security: A systematic review. Int. J. Energy Res. 2023, 2023, 8823507. [Google Scholar] [CrossRef]
- Cherwoo, L.; Gupta, I.; Flora, G.; Verma, R.; Kapil, M.; Arya, S.K.; Ravindran, B.; Khoo, K.S.; Bhatia, S.K.; Chang, S.W.; et al. Biofuels an alternative to traditional fossil fuels: A comprehensive review. Sustain. Energy Technol. Assess. 2023, 60, 103503. [Google Scholar] [CrossRef]
- Seifi, H.; Gholami, T.; Seifi, S.; Ghoreishi, S.M.; Salavati-Niasari, M. A review on current trends in thermal analysis and hyphenated techniques in the investigation of physical, mechanical and chemical properties of nanomaterials. J. Anal. Appl. Pyrolysis 2020, 149, 104840. [Google Scholar] [CrossRef]
- Peñalver, R.; Arroyo-Manzanares, N.; López-García, I.; Hernández-Córdoba, M. An overview of microplastics characterization by thermal analysis. Chemosphere 2020, 242, 125170. [Google Scholar] [CrossRef]
- Francis Billaud, J.G. Lucie Coniglio Pyrolysis of secondary raw material from used frying oils. arXiv 2007, arXiv:0709.4340. [Google Scholar]
- Januszewicz, K.; Hunicz, J.; Kazimierski, P.; Rybak, A.; Suchocki, T.; Duda, K.; Mikulski, M. An experimental assessment on a diesel engine powered by blends of waste-plastic-derived pyrolysis oil with diesel. Energy 2023, 281, 128330. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Mohamed, A.; Ali Abdelnaby, M. Pyrolysis kinetic behavior and TG-FTIR-GC–MS analysis of end-life ultrafiltration polymer nanocomposite membranes. Chem. Eng. J. 2022, 428, 131181. [Google Scholar] [CrossRef]
- Kong, W.; Shen, B.; Ma, J.; Kong, J.; Feng, S.; Wang, Z.; Xiong, L. Pyrolysis of spirulina platensis, tetradesmus obliquus and chlorella vulgaris by TG-FTIR and Py-GC/MS: Kinetic analysis and pyrolysis behaviour. Energy 2022, 244, 123165. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, S.; Zhu, J.; Lyu, Q.; Wei, K.; Huang, Q.; Li, G.; Xia, H. TG-MS study on co-combustion characteristics and coupling mechanism of coal gasification fly ash and coal gangue by ECSA®. Fuel 2022, 314, 123086. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, S.; Hu, X.; Song, W.; Cai, J.; Xu, Q. Restraining effect of nitrogen on coal oxidation in different stages: Non-isothermal TG-DSC and EPR research. Int. J. Min. Sci. Technol. 2020, 30, 387–395. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, T.F.; Zhang, Y.; Peng, X.Q.; Song, W.; Yang, J.S.; Yuan, C.D. Oxidation behaviors of Hongqian heavy crude oil characterized by TG-DSC-FTIR-MS within full temperature regions. Fuel 2023, 353, 129242. [Google Scholar] [CrossRef]
- Pires, J. Simultaneous Thermogravimetry-Differential Scanning Calorimetry (TG-DSC) in Nanoporous Materials: Examples of Data for Zeolites, Metal-Organic Frameworks (MOFs), Clay Based and Mesostructured Solids. J. Inorg. Organomet. Polym. Mater. 2024, 34, 3346–3359. [Google Scholar] [CrossRef]
- Ostasz, A.; Lyszczek, R.; Sztanke, K.; Sztanke, M. TG-DSC and TG-FTIR Studies of Annelated Triazinylacetic Acid Ethyl Esters-Potential Anticancer Agents. Molecules 2023, 28, 1735. [Google Scholar] [CrossRef]
- Osypiuk, D.; Bartyzel, A.; Cristóvao, B. Synthesis, thermal behaviour and evaluation of the antioxidant capacity of ZnII complexes with polydentate Schiff bases. J. Mol. Struct. 2023, 1294, 136337. [Google Scholar] [CrossRef]
- Ren, N.; Wang, F.; Zhang, J.J.; Zheng, X.F. Progress in thermal analysis kinetics. Acta Phys.-Chim. Sin. 2020, 36, 1905062. [Google Scholar] [CrossRef]
- Rattana-Amron, T.; Klamchuen, A. Kinetic analysis of oxidation characteristics in synthetic motor oil. Pet. Sci. Technol. 2022, 40, 604–625. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Pau, D.; Li, K.; Xie, K.; Zou, Y. Pyrolysis kinetics and determination of organic components and N-alkanes yields of Karamay transformer oil using TG, FTIR and Py-GC/MS analyses. Fuel 2021, 306, 121691. [Google Scholar] [CrossRef]
- Zhao, S.; Pu, W.-F.; Su, L.; Shang, C.; Song, Y.; Li, W.; He, H.-Z.; Liu, Y.-G.; Liu, Z.-Z. Properties, combustion behavior, and kinetic triplets of coke produced by low-temperature oxidation and pyrolysis: Implications for heavy oil in-situ combustion. Pet. Sci. 2021, 18, 1483–1491. [Google Scholar] [CrossRef]
- Sun, W.; Lin, W.-C.; You, F.; Shu, C.-M.; Qin, S.-H. Prevention of green energy loss: Estimation of fire hazard potential in wind turbines. Renew. Energy 2019, 140, 62–69. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, S.; Zhang, L.; Deng, J.; Peng, X.; Cheng, H. New insights into the oxidation behaviors of crude oils and their exothermic characteristics: Experimental study via simultaneous TGA/DSC. Fuel 2018, 219, 141–150. [Google Scholar] [CrossRef]
- Gundogar, A.S.; Kok, M.V. Thermal characterization, combustion and kinetics of different origin crude oils. Fuel 2014, 123, 59–65. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Zhang, Y.; Li, Y.; Tam, W.C.; Kong, D.; Deng, J. New insights into the ignition characteristics of liquid fuels on hot surfaces based on TG-FTIR. Appl. Energy 2024, 360, 122827. [Google Scholar] [CrossRef]
- Zhao, S.; Tang, X.; Pu, W.; He, M.; Yang, Y. Kinetic modeling for upgrading of heavy crude oil via thermal cracking in porous media: SARA fractions and gas compositions. Fuel 2024, 371, 132087. [Google Scholar] [CrossRef]
- Zhao, A.X.; Tang, X.J.; Zang, Z.H.; Liu, J.H. The pyrolytic experiment and thermal dynamics analysis of insulating oil. In Proceedings of the 2013 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Chenzhen, China, 20–23 October 2013; pp. 89–92. [Google Scholar]
- Mishra, A.; Kumari, U.; Turlapati, V.Y.; Siddiqi, H.; Meikap, B.C. Extensive thermogravimetric and thermo-kinetic study of waste motor oil based on iso-conversional methods. Energy Convers. Manag. 2020, 221, 113194. [Google Scholar] [CrossRef]
- Irmak Aslan, D.; Parthasarathy, P.; Goldfarb, J.L.; Ceylan, S. Pyrolysis reaction models of waste tires: Application of master-plots method for energy conversion via devolatilization. Waste Manag. 2017, 68, 405–411. [Google Scholar] [CrossRef]
- Chen, J.; Mu, L.; Jiang, B.; Yin, H.; Song, X.; Li, A. TG/DSC-FTIR and Py-GC investigation on pyrolysis characteristics of petrochemical wastewater sludge. Bioresour. Technol. 2015, 192, 1–10. [Google Scholar] [CrossRef]





| Experimental Oils | Density (kg/m3) | Flash Point (°C) | Tilting Point (°C) | Kinematic Viscosity (mm2/s) |
|---|---|---|---|---|
| Transformer oil | 885 | 143 | <−24 | 9.7 |
| Engine oil | 841 | 232 | −36 | 89.2 |
| Hydraulic fluid | 860 | 240 | −15 | 45.8 |
| Experimental Oils | Temperature Range/°C | Heating Rate/(°C/min) | Atmosphere | Gas Flow /(mL/min) |
|---|---|---|---|---|
| Transformer oil | 30~600 | 5 | air | 100 |
| 10 | ||||
| 20 | ||||
| Engine oil | 30~600 | 5 | air | 100 |
| 10 | ||||
| 20 | ||||
| Hydraulic fluid | 30~600 | 5 | air | 100 |
| 10 | ||||
| 20 |
| Heating Rates (°C/min) | Ti (°C) | Ton (°C) | Tp (°C) | Toff (°C) | Tf (°C) | Stage I (%) | Stage II (%) | Residual Mass (%) | Tp,DSC (°C) | Peak of Heat Flow (W/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 100 | 192 | 237 | 265 | 495 | 15.13 | 83.45 | 1.42 | 260 | 0.76 |
| 10 | 108 | 218 | 260 | 285 | 516 | 16.65 | 81.52 | 1.83 | 288 | 1.12 |
| 20 | 117 | 237 | 285 | 314 | 538 | 17.29 | 80.59 | 2.12 | 336 | 3.99 |
| Heating Rates (°C/min) | Ti (°C) | Ton (°C) | Tp (°C) | Toff (°C) | Tf (°C) | Stage I (%) | Stage II (%) | Residual Mass (%) | Tp,DSC (°C) | Peak of Heat Flow (W/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 164 | 287 | 300 | 316 | 537 | 25.64 | 73.12 | 1.24 | 294 | 8.52 |
| 10 | 182 | 296 | 307 | 319 | 554 | 21.04 | 77.57 | 1.39 | 312 | 15.90 |
| 20 | 214 | 302 | 323 | 344 | 569 | 19.90 | 79.03 | 1.07 | 318 | 18.37 |
| Heating Rates (°C/min) | Ti (°C) | Ton (°C) | Tp (°C) | Toff (°C) | Tf (°C) | Stage I (%) | Stage II (%) | Residual Mass (%) | Tp,DSC (°C) | Peak of Heat Flow (W/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 147 | 252 | 287 | 322 | 564 | 10.88 | 88.94 | 0.18 | 302 | 3.66 |
| 10 | 177 | 282 | 310 | 335 | 577 | 11.98 | 87.48 | 0.54 | 305 | 11.50 |
| 20 | 206 | 304 | 320 | 342 | 593 | 12.91 | 86.54 | 0.55 | 310 | 21.29 |
| Reaction Mechanism Model | Sign | f(α) | g(α) |
|---|---|---|---|
| Reaction order model | F0 | 1 | α |
| F1 | 1 − α | −ln (1 − α) | |
| F2 | (1 − α)2 | (1 − α)−1 − 1 | |
| F3 | (1 − α)3 | [(1 − α)−1 − 1]/2 | |
| Diffusion model | D1 | α/2 | α2 |
| D2 | [−ln (1 − α)]−1 | [(1 − α) ln (1 − α)] + α | |
| D3 | 3 (1 − α)2/3/[2 (1 − (1 − α)1/3)] | [1 − (1 − α)1/3]2 | |
| Power function | P2 | 2α1/2 | α1/2 |
| P3 | 3α2/3 | α1/3 | |
| P4 | 4α3/4 | α1/4 | |
| Nucleation model | A1.5 | 3/2 (1 − α) [−ln (1 − α)]1/3 | [−ln (1 − α)]2/3 |
| A2 | 2 (1 − α) [−ln (1 − α)]1/2 | [−ln (1 − α)]1/2 | |
| A3 | 3 (1 − α) [−ln (1 − α)]2/3 | [−ln (1 − α)]1/3 | |
| A4 | 4 (1 − α) [−ln (1 − α)]3/4 | [−ln (1 − α)]1/4 |
| Industrial Oils | α | FWO | KAS | Starink | Average Value | |||
|---|---|---|---|---|---|---|---|---|
| Ea | R2 | Ea | R2 | Ea | R2 | Ea | ||
| transformer oil | 0.1 | 62.97 | 0.98 | 59.65 | 0.97 | 58.76 | 0.98 | 60.46 |
| 0.2 | 63.02 | 0.99 | 58.26 | 0.99 | 59.44 | 0.99 | 60.24 | |
| 0.3 | 62.80 | 0.99 | 57.65 | 0.99 | 58.83 | 0.99 | 59.76 | |
| 0.4 | 62.65 | 0.99 | 57.32 | 0.99 | 58.32 | 0.99 | 59.43 | |
| 0.5 | 62.74 | 0.99 | 58.82 | 0.99 | 58.63 | 0.99 | 60.07 | |
| 0.6 | 64.22 | 0.99 | 58.71 | 0.99 | 59.02 | 0.99 | 60.65 | |
| 0.7 | 65.03 | 0.98 | 59.44 | 0.99 | 59.75 | 0.99 | 61.40 | |
| 0.8 | 66.16 | 0.98 | 61.86 | 0.98 | 60.83 | 0.97 | 62.95 | |
| 0.9 | 67.28 | 0.97 | 61.56 | 0.97 | 61.87 | 0.97 | 63.57 | |
| Average value | 64.10 | 0.98 | 59.25 | 0.98 | 59.49 | 0.98 | 60.95 | |
| engine oil | 0.1 | 102.77 | 0.99 | 100.90 | 0.98 | 84.57 | 0.99 | 96.08 |
| 0.2 | 105.06 | 0.99 | 104.96 | 0.99 | 93.27 | 0.99 | 101.10 | |
| 0.3 | 113.01 | 0.98 | 111.30 | 0.98 | 110.08 | 0.99 | 111.46 | |
| 0.4 | 121.07 | 0.99 | 117.78 | 0.99 | 116.61 | 0.99 | 118.49 | |
| 0.5 | 124.23 | 0.98 | 123.65 | 0.99 | 127.68 | 0.98 | 125.19 | |
| 0.6 | 123.05 | 0.97 | 121.13 | 0.92 | 125.59 | 0.94 | 123.26 | |
| 0.7 | 117.38 | 0.93 | 113.90 | 0.95 | 113.10 | 0.94 | 114.79 | |
| 0.8 | 115.38 | 0.91 | 112.11 | 0.92 | 108.27 | 0.92 | 111.92 | |
| 0.9 | 106.96 | 0.94 | 105.88 | 0.92 | 95.13 | 0.95 | 102.66 | |
| Average value | 114.32 | 0.96 | 112.40 | 0.96 | 108.26 | 0.97 | 110.50 | |
| hydraulic oil | 0.1 | 86.65 | 0.99 | 79.95 | 0.99 | 86.06 | 0.99 | 84.22 |
| 0.2 | 86.21 | 0.99 | 79.71 | 0.99 | 81.68 | 0.99 | 82.53 | |
| 0.3 | 88.45 | 0.98 | 85.33 | 0.97 | 84.08 | 0.99 | 85.95 | |
| 0.4 | 94.54 | 0.98 | 89.14 | 0.96 | 89.35 | 0.97 | 91.01 | |
| 0.5 | 102.34 | 0.96 | 103.04 | 0.96 | 98.81 | 0.96 | 101.40 | |
| 0.6 | 110.64 | 0.97 | 102.07 | 0.96 | 104.59 | 0.97 | 105.77 | |
| 0.7 | 125.18 | 0.97 | 114.00 | 0.92 | 116.57 | 0.96 | 118.58 | |
| 0.8 | 142.53 | 0.99 | 134.48 | 0.99 | 134.85 | 0.99 | 137.29 | |
| 0.9 | 143.15 | 0.99 | 140.46 | 0.99 | 134.75 | 0.99 | 139.42 | |
| Average value | 108.85 | 0.98 | 103.12 | 0.97 | 103.42 | 0.98 | 105.13 | |
| Industrial Oils | Heating Rate (°C/min) | Ea (kJ/mol) | A (min−1) | f(α) | R2 |
|---|---|---|---|---|---|
| transformer oil | 5 | 60.95 | 3.12 × 105 | (1 − α)0.11 | 0.9952 |
| 10 | 3.50 × 105 | (1 − α)0.12 | 0.9996 | ||
| 20 | 3.52 × 105 | (1 − α)0.14 | 0.9993 | ||
| engine oil | 5 | 110.50 | 2.14 × 109 | (1 − α)0.48 | 0.9882 |
| 10 | 2.66 × 109 | (1 − α)0.50 | 0.9926 | ||
| 20 | 2.24 × 109 | (1 − α)0.51 | 0.9791 | ||
| hydraulic oil | 5 | 105.13 | 6.41 × 108 | (1 − α)0.35 | 0.9971 |
| 10 | 8.12 × 108 | (1 − α)0.36 | 0.9911 | ||
| 20 | 7.16 × 108 | (1 − α)0.37 | 0.9759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Qian, Y.; Zhong, G.; Wu, S.; Pan, S. A Thermal Characteristics Study of Typical Industrial Oil Based on Thermogravimetric-Differential Scanning Calorimetry (TG-DSC). Fire 2024, 7, 401. https://doi.org/10.3390/fire7110401
Zhao Y, Qian Y, Zhong G, Wu S, Pan S. A Thermal Characteristics Study of Typical Industrial Oil Based on Thermogravimetric-Differential Scanning Calorimetry (TG-DSC). Fire. 2024; 7(11):401. https://doi.org/10.3390/fire7110401
Chicago/Turabian StyleZhao, Yaohong, Yihua Qian, Guobin Zhong, Siyuan Wu, and Siwei Pan. 2024. "A Thermal Characteristics Study of Typical Industrial Oil Based on Thermogravimetric-Differential Scanning Calorimetry (TG-DSC)" Fire 7, no. 11: 401. https://doi.org/10.3390/fire7110401
APA StyleZhao, Y., Qian, Y., Zhong, G., Wu, S., & Pan, S. (2024). A Thermal Characteristics Study of Typical Industrial Oil Based on Thermogravimetric-Differential Scanning Calorimetry (TG-DSC). Fire, 7(11), 401. https://doi.org/10.3390/fire7110401





