Experimental Estimation of Turbulent Flame Velocity in Gasoline Vapor Explosion in Multi-Branch Pipes
Abstract
1. Introduction
2. Theoretical Estimation Method for Turbulent Flame Velocity
3. Experimental Methods and Data
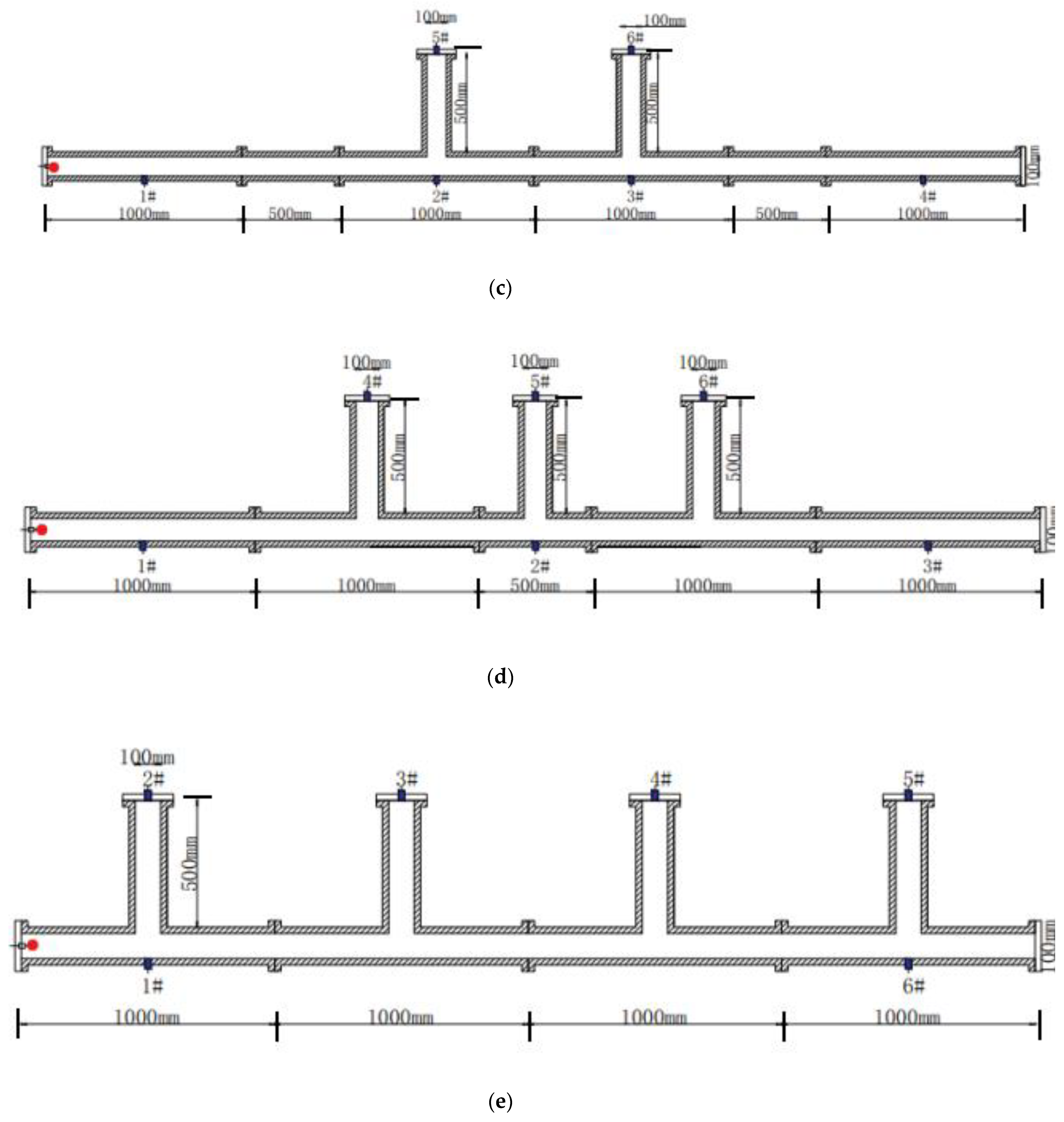
3.1. Experimental Devices and Methods
3.2. Acquisition of Experimental Data
4. Theoretical Calculation of Other Data
4.1. Maximum Flame Front Area
4.2. Laminar Flame Velocity and Gas Adiabatic Index
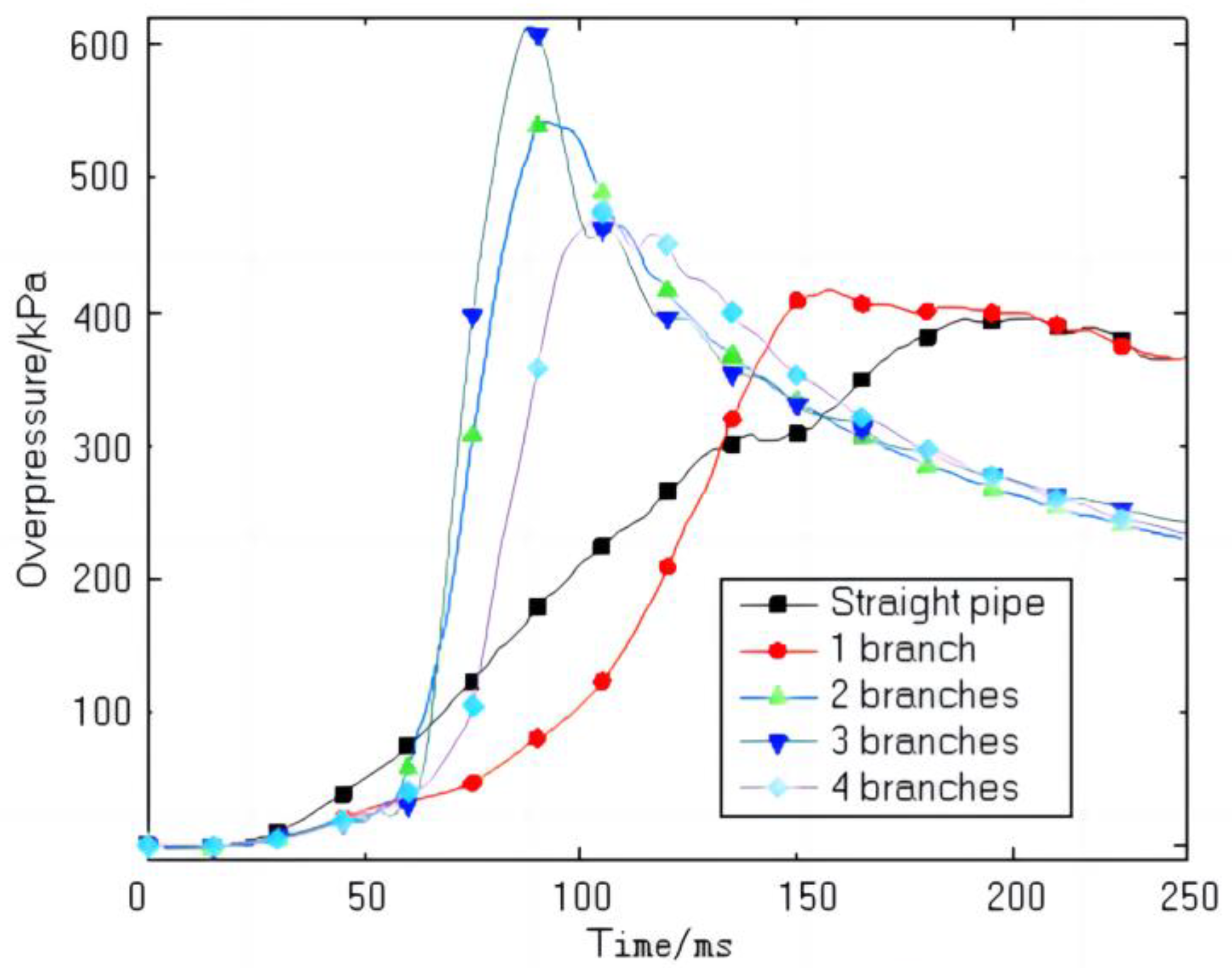
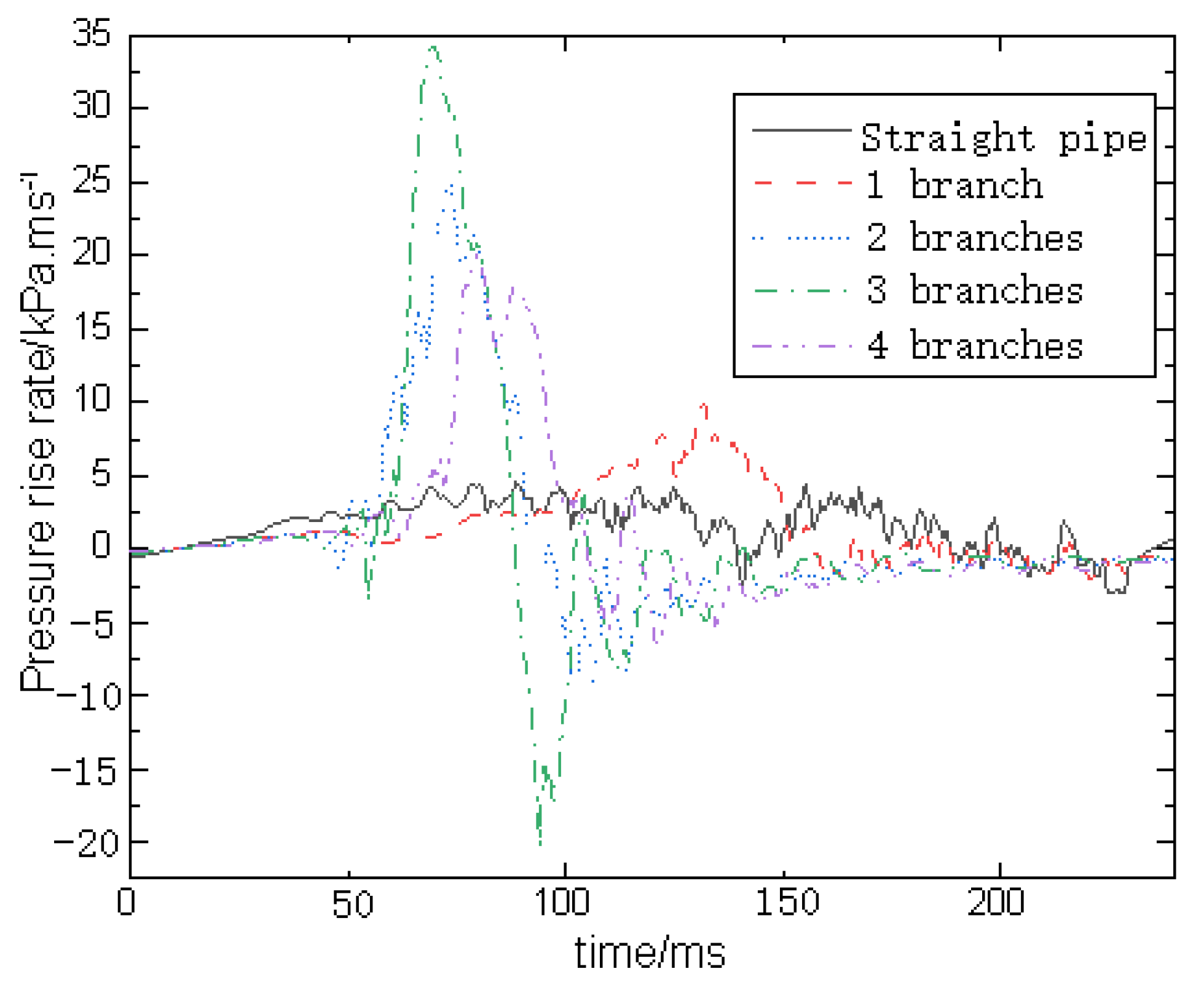
5. Turbulent Flame Velocity Estimation Results and Discussion
6. Conclusions
- The current formula for the maximum overpressure rise rate does not take into account the influence of turbulence, which inevitably leads to deviation when this formula is applied to multi-branch-structured pipe. Considering the influence of turbulence, it is feasible to estimate the turbulent flame velocity of explosions in multi-branch pipes by modifying the formula.
- Compared with straight pipelines, the turbulent flame velocities of explosions in pipes with one, two, three, and four branches are 1.01, 1.64, 1.82, and 1.62 times higher than those in a straight pipe.
- The turbulent flame velocity increases from 8.29 to 15.06 m/s as the number of branch pipes increases from 0 to 3. However, an increase in the number of branch pipes does not always lead to an increase in turbulent flame velocity. When the number of branches increased from three to four, the turbulent flame velocity decreased from 15.06 to 13.39 m/s.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.S.; Zhou, L.; Cai, Y.X.; Zhou, D.L.; Chen, R.; Qin, X.Z. Explosion suppression experiment of oil storage vessel based on porous explosion suppression materials. Oil Gas Storage Transp. 2023, 42, 1158–1165. [Google Scholar]
- Zhang, B.Y.; Cui, J.R. Experimental study on explosion propagation barrier properties of metal foamed with different ex-plosion surface structures for methane gas. Explos. Shock. Waves 2023, 43, 168–178. [Google Scholar]
- Liang, H.; Wang, T.; Luo, Z.; Wang, X.; Kang, X.; Deng, J. Risk Assessment of Liquefied Petroleum Gas Explosion in a Limited Space. ACS Omega 2021, 38, 24683–24692. [Google Scholar] [CrossRef]
- Gao, N. Effect of Initial Temperature on Free Radicals of Gas Explosion in Restricted Space. Adv. Mater. Res. 2013, 2657, 798–799. [Google Scholar] [CrossRef]
- Cui, Y.H.; Jiang, J.C.; Yu, Y.; Zhang, Q.W. Initial Pressure Influence on Explosion Pressures of Methane-Air Deflagrations in Linked Vessels. Adv. Mater.Res. 2014, 3187, 936. [Google Scholar] [CrossRef]
- Li, Y.; Xu, H.L.; Wang, X.S. Experimental Study on the Influence of Initial Pressure on Explosion of Methane-coal Dust Mixtures. Procedia Eng. 2013, 62, 980–984. [Google Scholar] [CrossRef]
- Heidari, A.; Wen, J.X. Numerical simulation of flame acceleration and deflagration to detonation transition in hydrogen-air mixture. Int. J. Hydrog. Energy 2014, 39, 21317–21327. [Google Scholar] [CrossRef]
- Pan, Y.; Luke, A.L. Experimental and numerical studies on the response of stiffened plates subjected to gas explosions. J. Constr. Steel Res. 1999, 52, 171–193. [Google Scholar] [CrossRef]
- Choi, K.; Sakasai, H.; Nishimura, K. Minimum ignition energies of pure magnesium powders due to electrostatic discharges and nitrogen’s effect. J. Loss Prev. Process Ind. 2016, 41, 141–146. [Google Scholar] [CrossRef]
- Ferrara, G.; Willacy, S.; Phylaktou, H.; Andrews, G.; Di Benedetto, A.; Salzano, E.; Russo, G. Venting of gas explosion through relief ducts: Interaction between internal and external explosions. J. Hazard. Mater. 2008, 155, 358–368. [Google Scholar] [CrossRef]
- Tan, Y.X.; Xie, Y.Y.; Huo, Y.J.; Han, Y. Influence of different turbulence states on flame propagation process of ethanol vapor near explosive lower limit. J. North Univ. China (Nat. Sci. Ed.) 2018, 39, 89–98. [Google Scholar]
- Bauwens, C.R.; Chaffee, J.; Dorofeev, S. Effect of Ignition Location, Vent Size, and Obstacles on Vented Explosion Overpressures in Propane-Air Mixtures. Combust. Sci. Technol. 2010, 182, 1915–1932. [Google Scholar] [CrossRef]
- Arisova, V.N.; Trykov Yu, P.; Ponomareva, I.A. Structural changes in magnesium-aluminum composite obtained by explosion welding after bending and thermal treatment. Russ. J. Non-Ferr. Met. 2014, 55, 46–50. [Google Scholar] [CrossRef]
- Ponizy, B.; Henneton, N.; Claverie, A.; Veyssiere, B. Detailed investigation of flame transmission from a vessel to a discharge duct. Combust. Flame 2014, 161, 1348–1364. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Garcia-Agreda, A.; Russo, P.; Sanchirico, R. Combined Effect of Ignition Energy and Initial Turbulence on the Explosion Behavior of Lean Gas/Dust-Air Mixtures. Ind. Eng. Chem. Res. 2011, 51, 7663–7670. [Google Scholar] [CrossRef]
- Numerical Simulation of Flame Propagation Process of Premixed Gas in Bifurcation Pipeline; Harbin Engineering University: Harbin, China, 2015.
- Wang, K.; Su, M.; Wei, L.; Chen, S.; Kong, X.; Fang, Y. Effect of initial turbulence on explosion behavior of stoichiometric methane-ethylene-air mixtures in confined space. Process Saf. Environ.Prot. 2022, 161, 583–593. [Google Scholar] [CrossRef]
- Yang, S.G.; Cai, J.W.; Yang, Y.; Sun, W.S.; Men, J.M. Disaster effect of combustible Gas explosion in urban underground shallow buried pipe ditch (II): Influencing factor analysis and consequence assessment. Explos. Shock. Waves 2023, 43, 155–166. [Google Scholar]
- Li, G.Q.; Du, Y.; Bai, J. Numerical simulation of flame propagation characteristics of gas vapor explosion in T-shaped branch pipeline. China Saf. Sci. Technol. 2016, 12, 120–127. [Google Scholar]
- Wang, H.; Chen, T. Experimental and Numerical Study of the Impact of Initial Turbulence on the Explosion Behavior of Methane-Air Mixtures. Chem. Eng. Technol. 2021, 44, 1195–1205. [Google Scholar] [CrossRef]
- Xie, Y.Y. Effect of Turbulence on Explosion Limit of Combustible Gas (Vapor) and Flame Propagation Process; North University of China: Taiyuan, China, 2017. [Google Scholar]
- Jian, C.G.; Lin, B.Q.; Song, Z.C.; Zhou, S.N. Induction of turbulence and its effect on flame and explosion wave in gas explosion process. J. Exp. Mech. 2004, 19, 39–44. [Google Scholar]
- Pu, Y.K.; Jarosinski, J.; Johnson, V.G.; Kauffman, C.W. Turbulence effects on dust explosions in the 20-liter spherical vessel. Symp. Combust. 1991, 23, 843–849. [Google Scholar] [CrossRef]
- Dahoe, A.E.; Zevenbergen, J.F.; Lemkowitz, S.M.; Scarlett, B. Dust explosions in spherical vessels: The role of flame thickness in the validity of the ‘cube-root law’. J. Loss Prev. Process Ind. 1996, 9, 33–44. [Google Scholar] [CrossRef]
- Cammarota, F.; Benedetto, A.D.; Russo, P.; Salzano, E. Experimental analysis of gas explosions at non-atmospheric initial conditions in cylindrical vessel. Process Saf. Environ. Prot. 2010, 88, 341–349. [Google Scholar] [CrossRef]
- Christophe, C.; Geoffrey, S. On the “tulip flame” phenomenon. Combust. Flame 1996, 105, 225–238. [Google Scholar]
- Metghalchi, M.; Keck, J.C. Burning velocities of mixtures of air with methanol, isooctane, and indolene at high pressure and temperature. Combust. Flame 1982, 48, 191–210. [Google Scholar] [CrossRef]
- Zhang, P.L.; Qi, S.; Wang, S.M.; Cheng, S.G. Calculation and analysis of adiabatic combustion temperature of No. 93 gasoline vapor. J. Logist. Eng. Coll. 2017, 33, 33–39. [Google Scholar]
- Zhang, P.L.; Xiao, J.; Wang, J.; Li, Y.X. Branch pipe length and the number of the influence of the gas vapor explosion overpressure features. J. Saf. Environ. 2022, 48, 2412–2419. [Google Scholar]





| Initial Condition | Value of Initial Parameter |
|---|---|
| Lower flammable limit (v %) | 1.0 |
| Octane rating | 92 |
| Specific gravity | 0.725 |
| Upper flammable limit (v %) | 2.7 |
| Average molecular formula | C8.1H14.05 |
| Average heating value (kJ/kg) | 44,000 |
| Branch Number | Overpressure Peak Value (kPa) | Peak Boost Rate (kPa·ms−1) |
|---|---|---|
| 0 | 396.00 | 4.54 |
| 1 | 416.53 | 9.93 |
| 2 | 542.19 | 25.41 |
| 3 | 613.74 | 34.39 |
| 4 | 474.89 | 20.04 |
| Combustible Gas | (cm·s−1) | (cm·s−1) | |
|---|---|---|---|
| Methanol | 1.11 | 43.70 | −157.22 |
| Propane | 1.08 | 40.11 | −186.48 |
| Isooctane | 1.13 | 33.72 | −110.82 |
| RMFD-303 | 1.13 | 35.58 | −140.45 |
| Branch Number | Turbulent Flame Velocity (m/s) | Laminar Flame Velocity (m/s) | ||
|---|---|---|---|---|
| 0 | 8.29 | 0.15 | 54.94 | 1.00 |
| 1 | 8.32 | 0.15 | 55.04 | 1.01 |
| 2 | 13.62 | 0.15 | 90.23 | 1.64 |
| 3 | 15.06 | 0.15 | 99.80 | 1.82 |
| 4 | 13.39 | 0.15 | 88.74 | 1.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.; Zhang, P.; Duan, J.; Xiang, S.; Shen, T.; Yang, C. Experimental Estimation of Turbulent Flame Velocity in Gasoline Vapor Explosion in Multi-Branch Pipes. Fire 2024, 7, 37. https://doi.org/10.3390/fire7020037
Lin K, Zhang P, Duan J, Xiang S, Shen T, Yang C. Experimental Estimation of Turbulent Flame Velocity in Gasoline Vapor Explosion in Multi-Branch Pipes. Fire. 2024; 7(2):37. https://doi.org/10.3390/fire7020037
Chicago/Turabian StyleLin, Keyu, Peili Zhang, Jimao Duan, Shuo Xiang, Ting’ao Shen, and Chaoshan Yang. 2024. "Experimental Estimation of Turbulent Flame Velocity in Gasoline Vapor Explosion in Multi-Branch Pipes" Fire 7, no. 2: 37. https://doi.org/10.3390/fire7020037
APA StyleLin, K., Zhang, P., Duan, J., Xiang, S., Shen, T., & Yang, C. (2024). Experimental Estimation of Turbulent Flame Velocity in Gasoline Vapor Explosion in Multi-Branch Pipes. Fire, 7(2), 37. https://doi.org/10.3390/fire7020037






