Study on Explosion Mechanism of Dimethyl Ether/H2-Blended Gas Based on Chemical Kinetics Method
Abstract
:1. Introduction
2. Research Methods and Pyrolysis Oxidation Mechanism Model
2.1. Research Methods
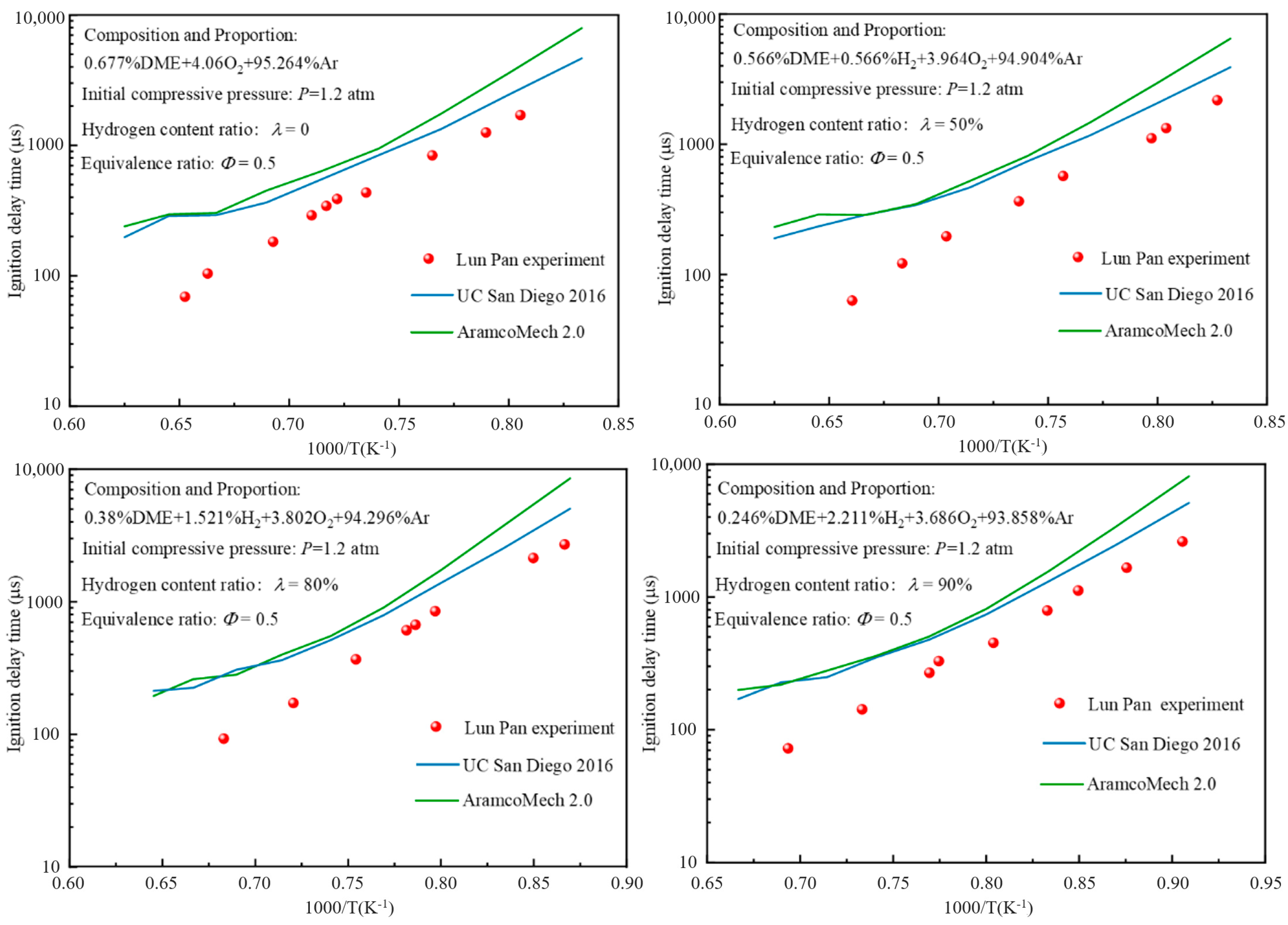
2.2. Modeling and Validation of Pyrolytic Oxidation Mechanism
3. Results and Discussion
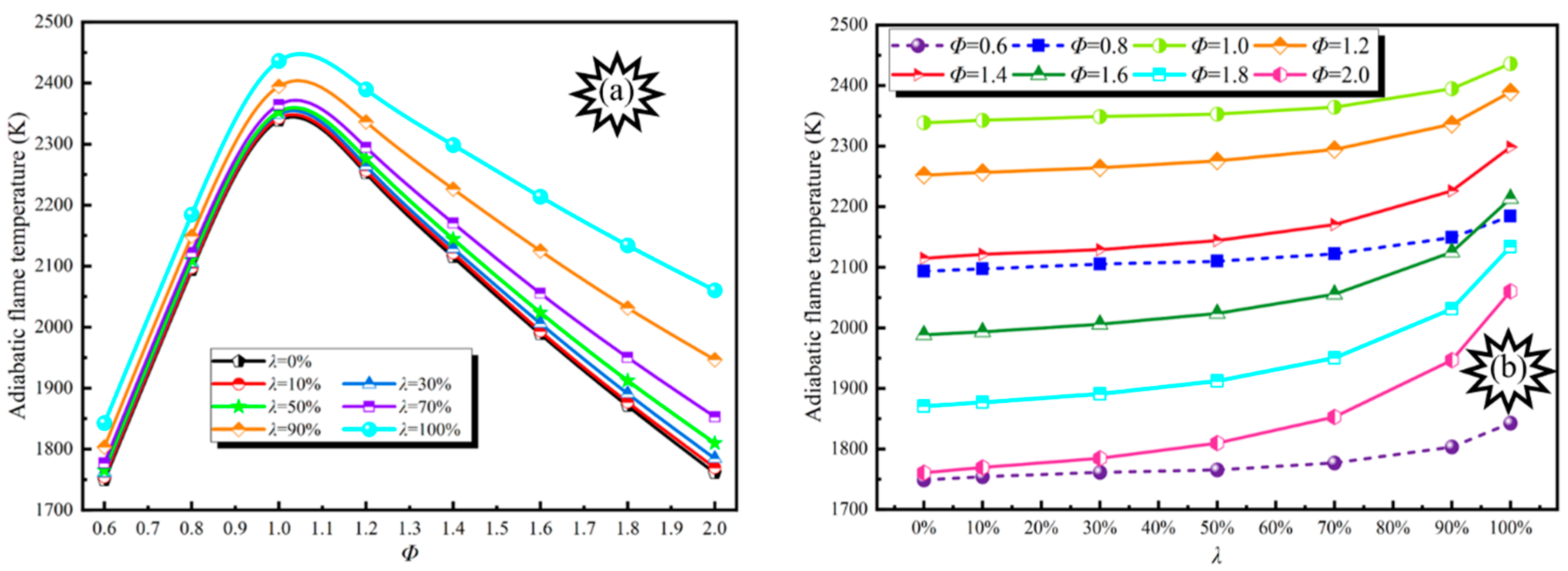
3.1. Adiabatic Flame Temperature
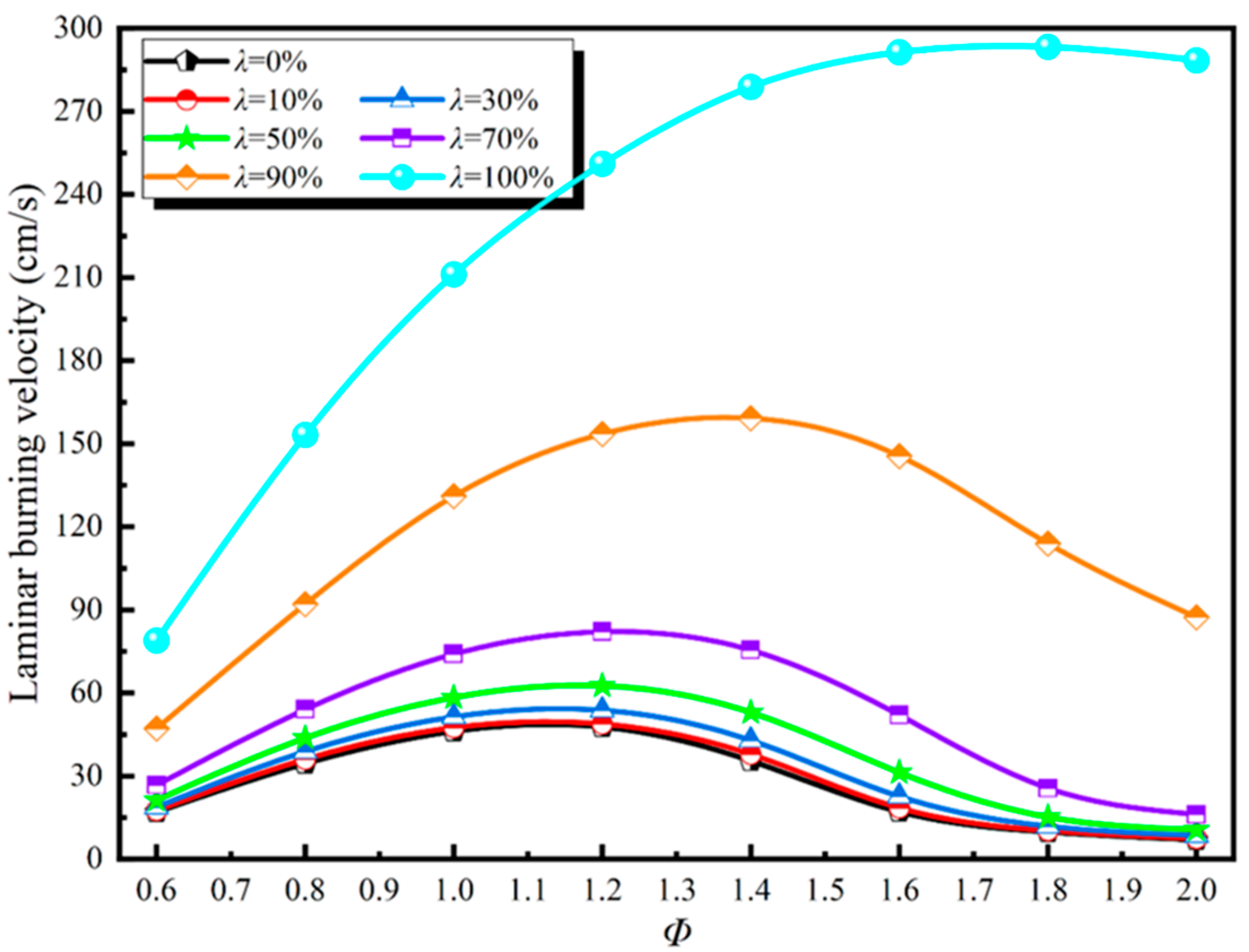
3.2. Laminar Flame Speed
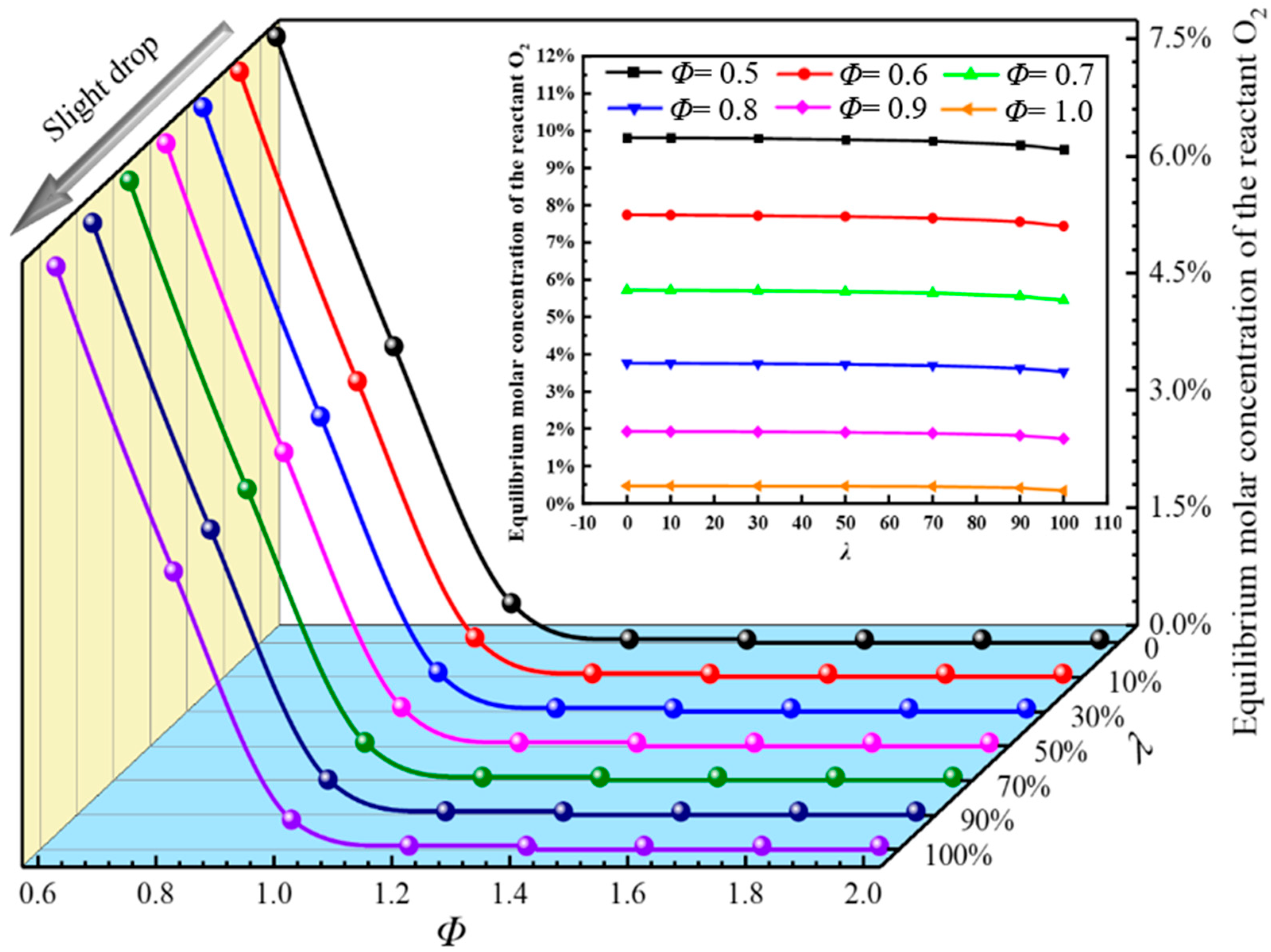
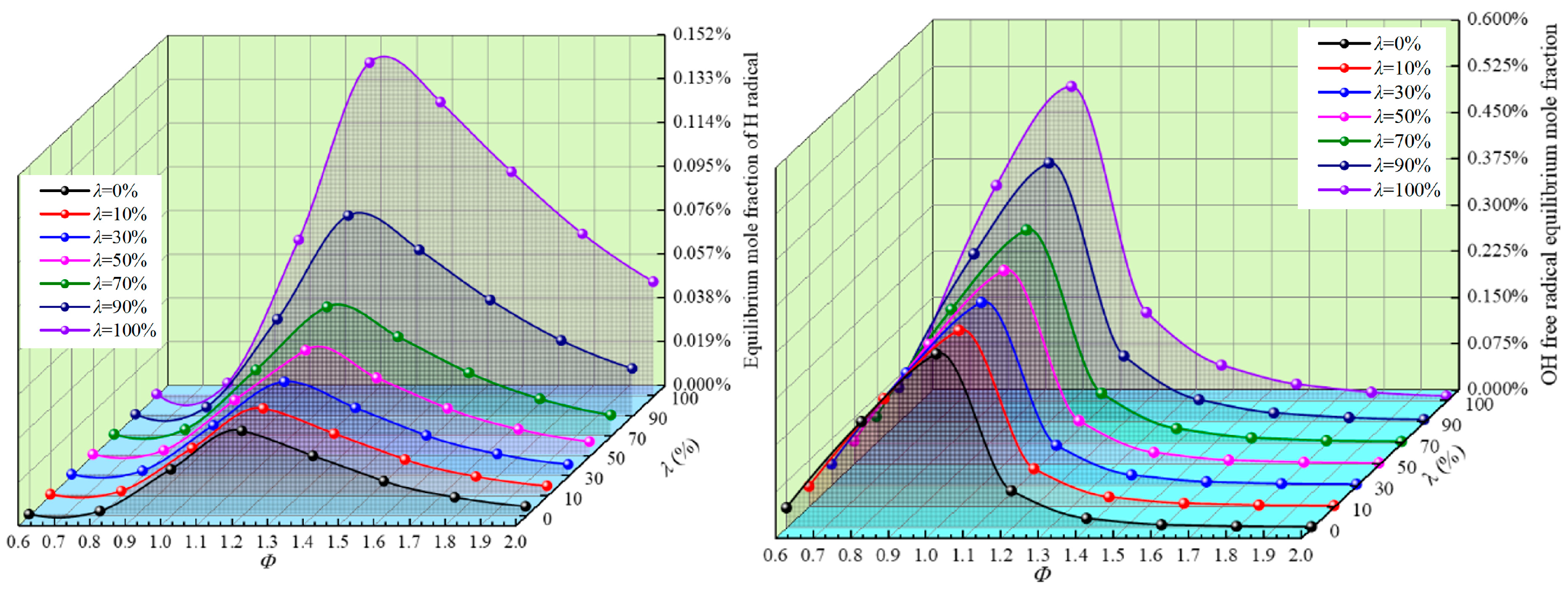
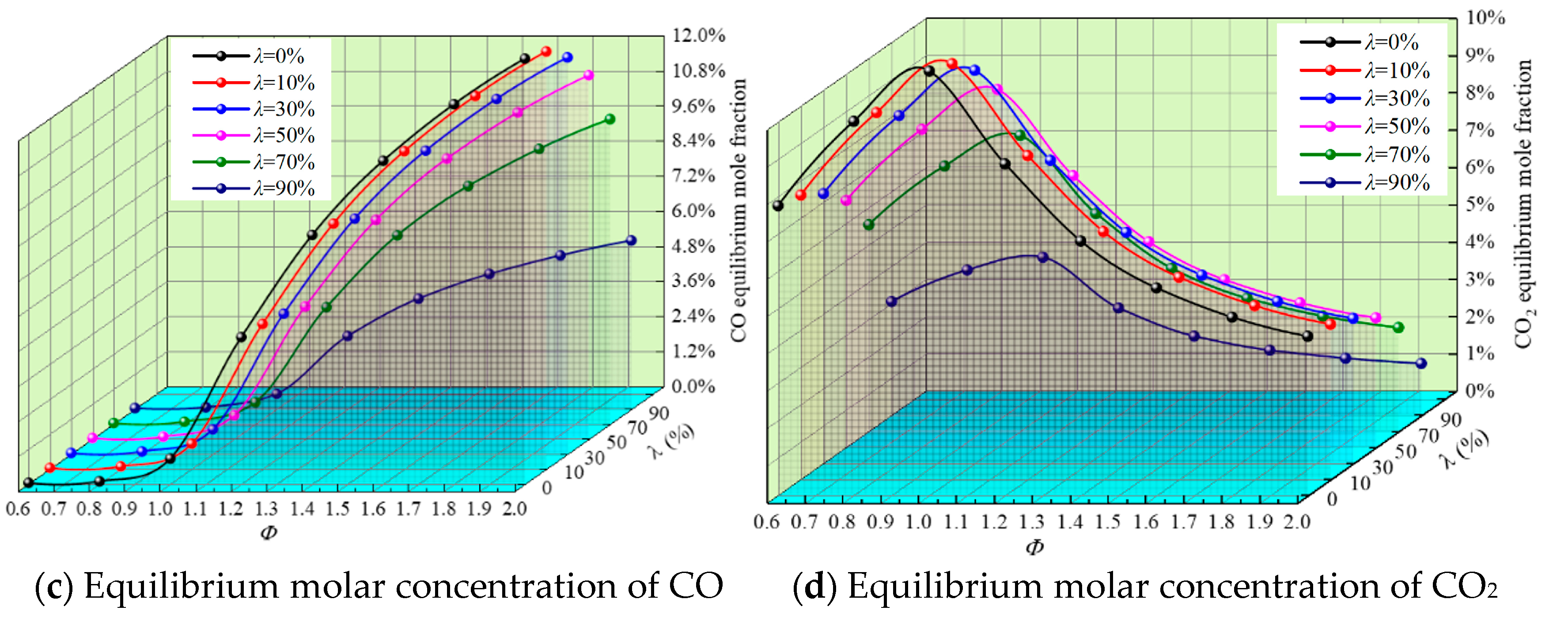
3.3. Equilibrium Molar Concentration
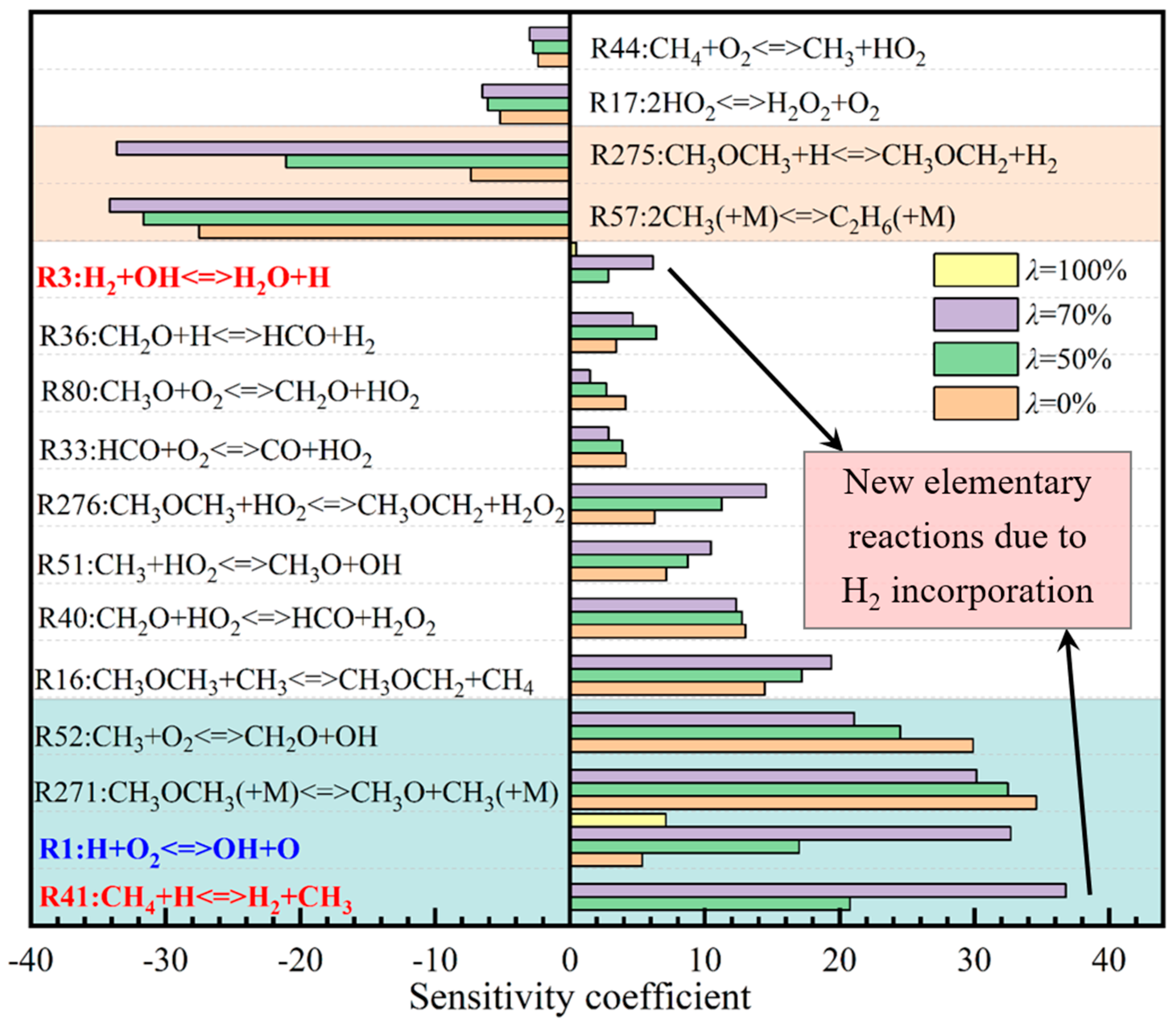
3.4. Sensitivity Analysis of Elementary Reactions
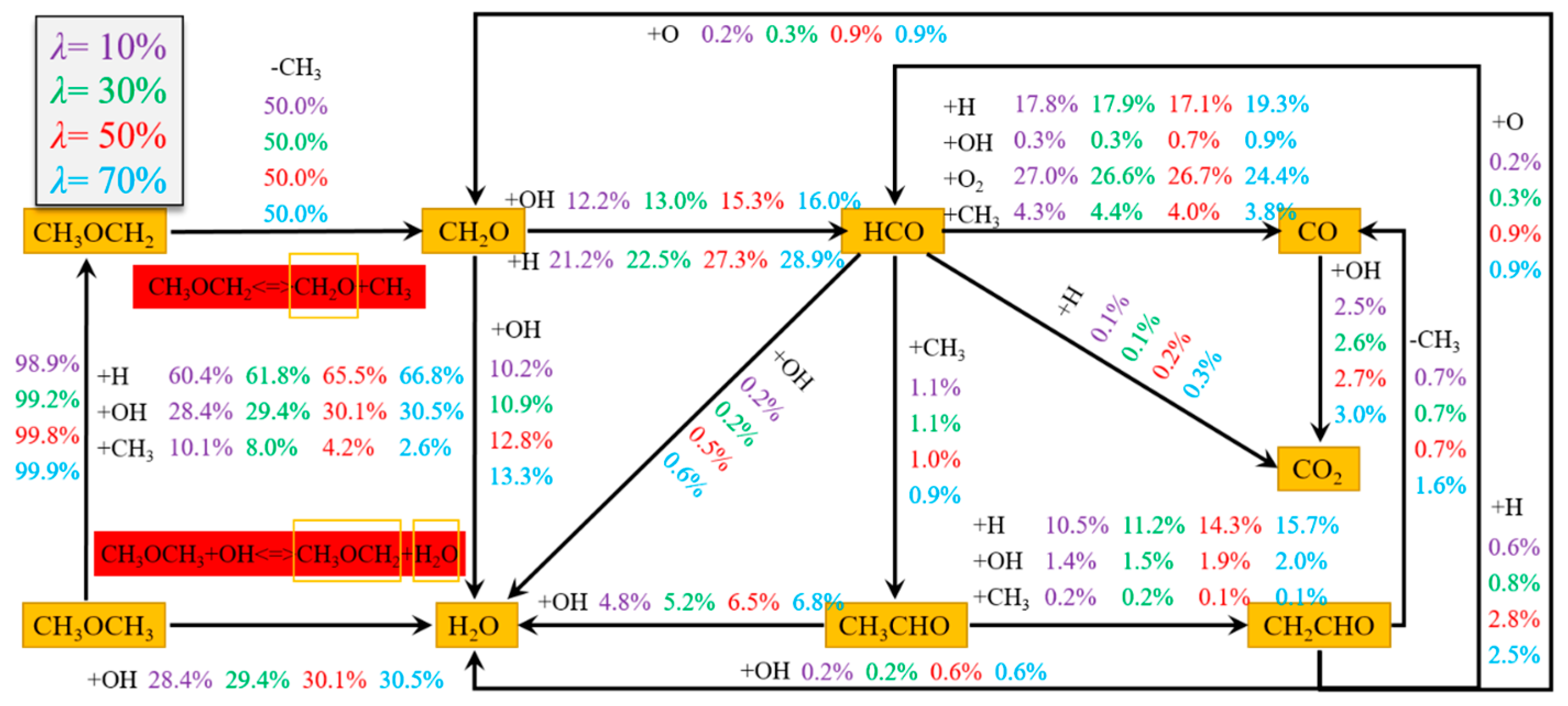
3.5. Chemical Reaction Path
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pingkuo, L.; Xue, H. ScienceDirect Comparative analysis on similarities and differences of hydrogen energy development in the World ’ s top 4 largest economies: A novel framework. Int. J. Hydrogen Energy 2022, 47, 9485–9503. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.; Ling, W. Policy optimization of hydrogen energy industry considering government policy preference in China. Sustain. Prod. Consum. 2022, 33, 890–902. [Google Scholar] [CrossRef]
- Zhou, G.; Kong, Y.; Zhang, Q.; Li, R.; Qian, X.; Zhao, H.; Ding, J.; Li, Y.; Yang, S.; Liu, Y. Effect of dimensionless vent ratio on the flame-shock wave evolution dynamics of blended LPG/DME gas explosion venting. Fuel 2024, 358, 130205. [Google Scholar] [CrossRef]
- Mogi, T.; Horiguchi, S. Explosion and detonation characteristics of dimethyl ether. J. Hazard. Mater. 2009, 164, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, S.; Gray, J.A.T.; Reiss, J.; Moeck, J.P.; Paschereit, O.C.; Sesterhenn, J. Detonation initiation in pipes with a single obstacle for mixtures of hydrogen and oxygen-enriched air. Combust. Flame 2018, 198, 290–304. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, Y.; Kong, Y.; Zhang, Q.; Sun, Y.; Wang, Y.; Ding, J. Study on explosion dynamics and kinetic mechanism of DME/H2 blended gas at typical fuel-lean/rich concentrations. Case Stud. Therm. Eng. 2022, 40, 102444. [Google Scholar] [CrossRef]
- Winter, C.J. Hydrogen energy—Abundant, efficient, clean: A debate over the energy-system-of-change. Int. J. Hydrogen Energy 2009, 34, 1–52. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, Y.; Kong, Y.; Zhang, Q.; Qian, X.; Liu, Z.; Wang, K.; Liu, Y.; Yang, S.; Li, Y. Influence of equivalence ratio and H2 blended ratio on explosion propagation characteristics of DME/H2 blended gas in closed narrow space. Int. J. Hydrogen Energy 2023, 48, 30132–30143. [Google Scholar] [CrossRef]
- Mogi, T.; Shiina, H.; Wada, Y.; Dobashi, R. Investigation of the properties of the accidental release and explosion of liquefied dimethyl ether at a filling station. J. Loss Prev. Process Ind. 2013, 26, 32–37. [Google Scholar] [CrossRef]
- Wei, S.; Yu, M.; Pei, B.; Ma, Z.; Li, S.; Kang, Y. Effect of hydrogen enrichment on the laminar burning characteristics of dimethyl-ether / methane fuel: Experimental and modeling study. Fuel 2021, 305, 121475. [Google Scholar] [CrossRef]
- Rezgui, Y.; Guemini, M. ScienceDirect Effect of hydrogen addition on equimolar dimethyl ether / iso -octane / oxygen / argon premixed flames. Int. J. Hydrogen Energy 2017, 42, 29557–29573. [Google Scholar] [CrossRef]
- Zhang, K.; Du, S.; Chen, H.; Wang, J.; Zhang, J.; Guo, Y.; Guo, J. Effect of hydrogen concentration on the vented explosion of hydrogen–air mixtures in a 5-m-long duct. Process Saf. Environ. Prot. 2022, 162, 978–986. [Google Scholar] [CrossRef]
- Youn, I.M.; Park, S.H.; Roh, H.G.; Lee, C.S. Investigation on the fuel spray and emission reduction characteristics for dimethyl ether (DME) fueled multi-cylinder diesel engine with common-rail injection system. Fuel Process. Technol. 2011, 92, 1280–1287. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Xiao, F.; Li, D. Combustion and emission characteristics of a diesel engine with DME as port premixing fuel under different injection timing. Energy Convers. Manag. 2014, 77, 52–60. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Q.; Yu, J.; Zhang, Y.; Zeng, K.; Miao, H.; Jiang, D. Measurement of laminar burning velocity of dimethyl ether–air premixed mixtures. Fuel 2007, 86, 2360–2366. [Google Scholar] [CrossRef]
- Wang, Y.L.; Holley, A.T.; Ji, C.; Egolfopoulos, F.N.; Tsotsis, T.T.; Curran, H.J. Propagation and extinction of premixed dimethyl-ether/air flames. Proc. Combust. Inst. 2009, 32, 1035–1042. [Google Scholar] [CrossRef]
- Moradi, J.; Gharehghani, A.; Mirsalim, M. Numerical comparison of combustion characteristics and cost between hydrogen, oxygen and their combinations addition on natural gas fueled HCCI engine. Energy Convers. Manag. 2020, 222, 113254. [Google Scholar] [CrossRef]
- Li, H.; Xiao, H. Effect of H2 addition on laminar burning velocity of NH3/DME blends by experimental and numerical method using a reduced mechanism. Combust. Flame 2023, 257, 113000. [Google Scholar] [CrossRef]
- Cong, X.; Ji, C.; Wang, S. Investigation into engine performance of a hydrogen-dimethyl ether spark-ignition engine under various dimethyl ether fractions. Fuel 2021, 306, 121429. [Google Scholar] [CrossRef]
- Pan, L.; Hu, E.; Zhang, J.; Zhang, Z.; Huang, Z. Experimental and kinetic study on ignition delay times of DME/H2/O2/Ar mixtures. Combust. Flame 2014, 161, 735–747. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, H.; Wu, H.; Xu, Y. Ignition properties of lean DME/H2 mixtures at low temperatures and elevated pressures. Fuel 2018, 226, 545–554. [Google Scholar] [CrossRef]
- Prince, J.C.; Williams, F.A. A short reaction mechanism for the combustion of dimethyl-ether. Combust. Flame 2015, 162, 3589–3595. [Google Scholar] [CrossRef]
- Curran, H.J. Developing detailed chemical kinetic mechanisms for fuel combustion. Proc. Combust. Inst. 2019, 37, 57–81. [Google Scholar] [CrossRef]
- Kéromnès, A.; Metcalfe, W.K.; Heufer, K.A.; Donohoe, N.; Das, A.K.; Sung, C.J.; Herzler, J.; Naumann, C.; Griebel, P.; Mathieu, O.; et al. An experimental and detailed chemical kinetic modeling study of hydrogen and syngas mixture oxidation at elevated pressures. Combust. Flame 2013, 160, 995–1011. [Google Scholar] [CrossRef]
- Burke, U.; Metcalfe, W.K.; Burke, S.M.; Heufer, K.A.; Dagaut, P.; Curran, H.J. A detailed chemical kinetic modeling, ignition delay time and jet-stirred reactor study of methanol oxidation. Combust. Flame 2016, 165, 125–136. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, X.; Li, R.; Zhou, G.; Sun, Y.; Ma, Y.; Kong, Y. Explosion characteristics and chemical kinetics of blended LPG/DME clean fuel based on pyrolysis and oxidation mechanism model. Fuel 2022, 320, 123896. [Google Scholar] [CrossRef]
- Peng, C.; Zou, C.; Ren, J.; Lin, Q.; Xia, W.; Luo, J.; Xiao, Y. Ignition delay times of C2H4, C2H4/n-C4H10, and n-C4H10/i-C4H10 under O2/CO2 atmospheres: Shock tube experiments and kinetic model. Combust. Flame 2023, 254, 112825. [Google Scholar] [CrossRef]
- Burnett, M.A.; Daniels, C.; Wei, L.; Wooldridge, M.S.; Wang, Z. A computational and experimental study of the effects of thermal boundary layers and negative coefficient chemistry on propane ignition delay times. Combust. Flame 2023, 257, 112415. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, C.; Wan, K.; Gao, Z.; Vervisch, L.; Domingo, P.; He, Y.; Wang, Z.; Lee, C.H.; Cai, Q.; et al. Prediction of ignition delay times of Jet A-1/hydrogen fuel mixture using machine learning. Aerosp. Sci. Technol. 2022, 127, 107675. [Google Scholar] [CrossRef]
- Aliyu, M.; Abdelhafez, A.; Nemitallah, M.A.; Said, S.A.M.; Habib, M.A. Effects of adiabatic flame temperature on flames’ characteristics in a gas-turbine combustor. Energy 2022, 243, 123077. [Google Scholar] [CrossRef]
- He, X.; Liu, Z.; Jiang, H.; Yang, Q.; Jiang, Z.; Feng, G.; Zhao, C. Super adiabatic flame temperature phenomenon for NH3/O2/N2 mixtures. Fuel 2023, 346, 128264. [Google Scholar] [CrossRef]
- Clará, R.A.; Marigliano, A.C.G.; Campos, V.d.V.; Sólimo, H.N. Density, viscosity, vapour-liquid equilibrium, excess molar enthalpy, and their correlations of the binary system [1-pentanol + R-(+)-limonene] over the complete concentration range, at different temperatures. Fluid Phase Equilib. 2010, 293, 151–156. [Google Scholar] [CrossRef]
- Yang, Q.C.; Qiu, D.F.; Dang, Y.L.; Qiao, Z.P.; Xu, Z. wei Phase equilibrium systems of CsX + MnX2 + H2O (X = Cl, Br) at 298.15 K and standard molar enthalpy of formation of CsMnX3·2H2O and Cs2MnX4·2H2O. J. Chem. Thermodyn. 2021, 161, 106541. [Google Scholar] [CrossRef]
- Mu, X.; Cong, H.; Shao, Z.; Yuan, Z.; Zhu, B.; Zhang, K.; Bi, M.; Wang, X. Experimental and theoretical research on the inhibition performance of ethanol gasoline/air explosion by C6F12O. J. Loss Prev. Process Ind. 2023, 83, 105088. [Google Scholar] [CrossRef]
- Yu, M.; Li, S.; Li, H.; Han, S.; Wang, F.; Lou, R.; Zheng, K.; Yu, Y. Numerical evaluation of the influence of initial pressure/temperature on the explosion properties and soot formation of methane/coal volatiles mixtures. Fuel 2023, 331, 125698. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, Y.; Guo, B.; Song, S.; Bai, J. Explosion dynamics of premixed LPG/H2 fuel in a confined space. Int. J. Hydrogen Energy 2023, 48, 36211–36221. [Google Scholar] [CrossRef]
- Shang, S.; Bi, M.; Gao, W. Investigation on synergistic suppression of hydrogen explosion behaviors by ethylene and carbon dioxide. J. Loss Prev. Process Ind. 2024, 87, 105214. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Kong, Y.; Zhang, Q.; Huang, Q.; Wei, Z.; Lu, H. Study on Explosion Mechanism of Dimethyl Ether/H2-Blended Gas Based on Chemical Kinetics Method. Fire 2024, 7, 328. https://doi.org/10.3390/fire7090328
Zhou Y, Kong Y, Zhang Q, Huang Q, Wei Z, Lu H. Study on Explosion Mechanism of Dimethyl Ether/H2-Blended Gas Based on Chemical Kinetics Method. Fire. 2024; 7(9):328. https://doi.org/10.3390/fire7090328
Chicago/Turabian StyleZhou, Yong, Yang Kong, Qi Zhang, Qi Huang, Zhikai Wei, and Huaheng Lu. 2024. "Study on Explosion Mechanism of Dimethyl Ether/H2-Blended Gas Based on Chemical Kinetics Method" Fire 7, no. 9: 328. https://doi.org/10.3390/fire7090328
APA StyleZhou, Y., Kong, Y., Zhang, Q., Huang, Q., Wei, Z., & Lu, H. (2024). Study on Explosion Mechanism of Dimethyl Ether/H2-Blended Gas Based on Chemical Kinetics Method. Fire, 7(9), 328. https://doi.org/10.3390/fire7090328





