Abstract
Extinguishing methanol fires poses significant challenges due to methanol’s high toxicity, polarity, and fluidity. While conventional fire suppressants, such as alcohol-resistant firefighting foam, water mist and dry powders, can extinguish methanol fires, they fail to prevent the spread of liquid methanol, creating a risk of environmental contamination as the mixture of suppressants and methanol flows into surrounding soil and water resources. To address this issue, a novel kind of composite dry powder has been developed to effectively combat methanol pool fires. The powder can not only rapidly extinguish flames but also transform liquid methanol into gel-like substances, significantly reducing the hazards caused by the flow of harmful liquids. Laboratory experiments identify an optimal mass ratio of 0.16 between the composite powder and methanol to achieve complete flame extinction and liquid solidification. The superior performance of as-prepared composite powder could be mainly ascribed to the cooperation of metallic salts, polymers, and silica additives. Additionally, the powder is effective for extinguishing ethanol fires, making it a valuable tool for the emergency management of alcohol fires in leakage incidents.
1. Introduction
The increasing focus on environmental protection and clean energy has spurred the utilization of methanol as a fuel due to its low carbon and clean-burning properties. Methanol is now widely used in vehicles, boilers, and stoves, contributing to the reduction of harmful emissions [1]. However, methanol presents serious risks when accidents, such as spills, occur during processing, transportation, and storage. Due to its toxicity and flammability, spilled methanol can pollute the environment and, if ignited, lead to fires and even explosions [2,3].
With a flash point of about 11 °C, methanol ignites more easily than many other fuels, and its wide flammability range heightens the risk of fire under various conditions. Unlike gasoline or other hydrocarbons, methanol burns with minimal smoke or soot, resulting in a harder-to-spot flame. While methanol fires are not inherently more frequent, they are a known hazard in industries where methanol is used, such as chemical manufacturing, laboratories, and fuel production. The primary risks of methanol fires stem from their invisibility, flammability, and toxicity, requiring strict handling and storage precautions.
Although methanol fires may not occur more often than other types, their unique characteristics make them particularly hazardous if not promptly identified and addressed. The rapid spread of methanol spill fires can expand the burning area in a short time, posing a serious threat to energy utilization and social security. Moreover, spilled methanol would flow around and further contaminate the soil or water body wherever it reaches, even after the fire was extinguished. Therefore, to completely extinguish the methanol fires, the extinguishing agents not only need to knock down the fires but also solidify the liquid methanol to prevent its continuous flow.
Efforts to mitigate the dangers of methanol fires have been focusing on developing and implementing effective fire suppression techniques. Foam is a primary agent for extinguishing fires in areas where flammable liquids are handled. However, methanol is more difficult to control than typical hydrocarbon fuels because it easily mixes with water, causing the foam blanket to break down and the fuel surface to be exposed again. Alcohol-resistant foams (ARF) have been developed to address this issue. These foams use polymers that form a protective film to prevent the foam from collapsing when in contact with polar flammable liquids like methanol. Large-scale experiments have shown the effectiveness of alcohol-resistant aqueous film-forming foam (AR-AFFF) and alcohol-resistant protein foam (AR-FP) in extinguishing methanol pool fires [4]. Li tested six types of foam on methanol pool fires in a 1.5 m diameter circular steel pan and found that all the ARF outperformed their counterparts without anti-resistant constituents in controlling methanol fires [5]. Zhi studied the interaction between ARF and 14 kinds of polar flammable liquids and found that methanol fires need a longer time to extinguish the edge fires of its naturally high saturated vapor pressure [6]. Other methods, such as water mist and dry powders, were also used to suppress methanol fires. Water mist works by cooling the fire and diluting the methanol, with smaller droplets being more effective in flame suppression for a fixed amount of water mist [7]. In the dry powder usage guide, it was also mentioned that dry powder can be used to extinguish liquid fuel fires. The National Fire Protection Association (NFPA) included information about the use of dry chemical extinguishers for flammable liquid fires, including methanol [8]. Recently, Zhu and coworkers have assessed the effectiveness of dry water to extinguish methanol pool fires [9]. However, among the above-mentioned fire suppressants, whether AFFF foam, mist water, or dry powder, none could effectively stop the flow of methanol. Even if the fire was extinguished, the danger of methanol could not be completely eliminated. Mixtures of methanol liquid and fire suppressants may spread, potentially contaminating surrounding areas such as soil or water.
On the other hand, while recent innovations in fire suppression technology, such as the introduction of AI or robotic systems [10,11], have gained attention, the extinguishing agents used in these technologies are still largely based on traditional substances like water mist, dry powders, and foam. Although new extinguishing agents, like fluorine-free foam, new dry powders, and gel-based suppressants, have emerged [12,13,14], they may not be suitable for extinguishing methanol fires due to inherent limitations, such as their performance or cost concerns. Therefore, using materials specifically designed for methanol fire suppression is essential for effective firefighting.
To address the challenges of methanol fire suppression and spill containment, a novel composite dry powder has been developed here. This dry powder is designed to not only extinguish methanol fires but also solidify the spilled liquid, preventing its spreading. By carefully selecting metallic salts, polymers, and additives, the composite powder can minimize risks by both extinguishing the fire and solidifying the fuel, thereby preventing further contamination. This approach offers an efficient and cost-effective method for managing methanol spills, especially in areas where foam or other liquid-based suppression methods are not ideal. The use of such composite powder reduces the need for additional liquid absorption materials, providing a safer, faster, and more efficient solution for handling methanol spills and preventing environmental hazards.
2. Materials and Methods
2.1. Materials Preparation and Characterization
All chemical agents were used without further purification. Potassium bicarbonate (KHCO3, denoted as KC) and acrylic-based polymers (denoted as AC) were obtained from Shanghai Pharmaceuticals Group, while hydrophobic silicon dioxide (SiO2, denoted as HS) was sourced from Degussa. Several types of AC could be used, such as cross-linked polyacrylic acid (CL-PAA), polylactic acid-acrylic copolymers (PLA-AC), and polyacrylic acid-polyglutamic acid copolymers (PAA-PGA). The composite powders were prepared using a straightforward two-step stirring method. In the first step, specific amounts of KC and AC were mixed and stirred thoroughly. In the second step, HS, in varying mass ratios, was added to the former mixture and stirred for several additional minutes to ensure uniform distribution. Commercially available sodium bicarbonate powders (denoted as BC, with 82.0% sodium bicarbonate content and 18.0% additives) were purchased from Zhejiang Yuntian FireFighting Equipment Co., Ltd., Hangzhou, China.
The powder morphology was analyzed using a Schottky Field Emission Scanning Electron Microscope (Gemini SEM 500, Zeiss, Oberkochen, Germany) equipped with Aztec electronic dispersive spectroscopy (EDS) for elemental composition analysis. The hydrophobicity of the powder was characterized by measuring the apparent contact angles of water droplets on compacted powder tablets using a contact angle meter (SL-250, KINO, Shanghai, China). Tablets were prepared by compacting the powder at 3.0 MPa. Each sample’s contact angle was determined from three measurements, with the average value reported as the final result. The flowability of the composite powder was assessed using the static angle of repose. The powder was allowed to flow through a funnel onto a base to form a stationary heap (base diameter: 50 mm, the height of the funnel bottom above the base: 40 mm). This procedure was repeated three times, and images of the heaps were analyzed to determine the slope angle relative to the horizontal. The averaged angles of repose were compared across samples under identical conditions.
2.2. Fire Tests
Fire-extinguishing tests were conducted in a confined space measuring 3 m × 3 m × 3 m. The experimental setup was similar to the previous one [15]. In a typical test, 200 mL of methanol was placed in a rectangular pan with a side length of 20 cm. The methanol pool fire was allowed to burn freely for about 70 s before powder discharge.
The powder container had an internal diameter of 60 mm and a height of 100 mm. A cone-shaped nozzle with an internal diameter of 6 mm and a cone angle of 60° was used. For each test, 50 g of powder samples were loaded into the container and pressurized with nitrogen to a gauge pressure of 0.06 MPa. The mass-based flow rate of the composite powders at a discharging pressure of 0.06 MPa was measured as 12 g/s, keeping approximately equal for all the samples. The powder was discharged onto the fire at a 60° angle, with the nozzle positioned 0.40 m perpendicular to the fuel pan. The valve of the powder container was turned off immediately after the fire was extinguished. Fire extinction time and powder consumption were recorded. After the fire was extinguished, the smoke vent was activated, and the room was cleaned.
Flame temperatures were measured using K-type thermocouples, which were positioned along a vertical trunk perpendicular to the pan, spaced 15 cm from the fuel surface. The mass variation of the agents consumed for fire suppression was recorded with a scale having an uncertainty of 0.1 g. The entire suppression process was documented using a video camera. Each test was repeated three times to obtain an average result.
3. Results and Discussions
3.1. Properties Characterization of the Composite Powder
3.1.1. Morphology of the Composite Particles
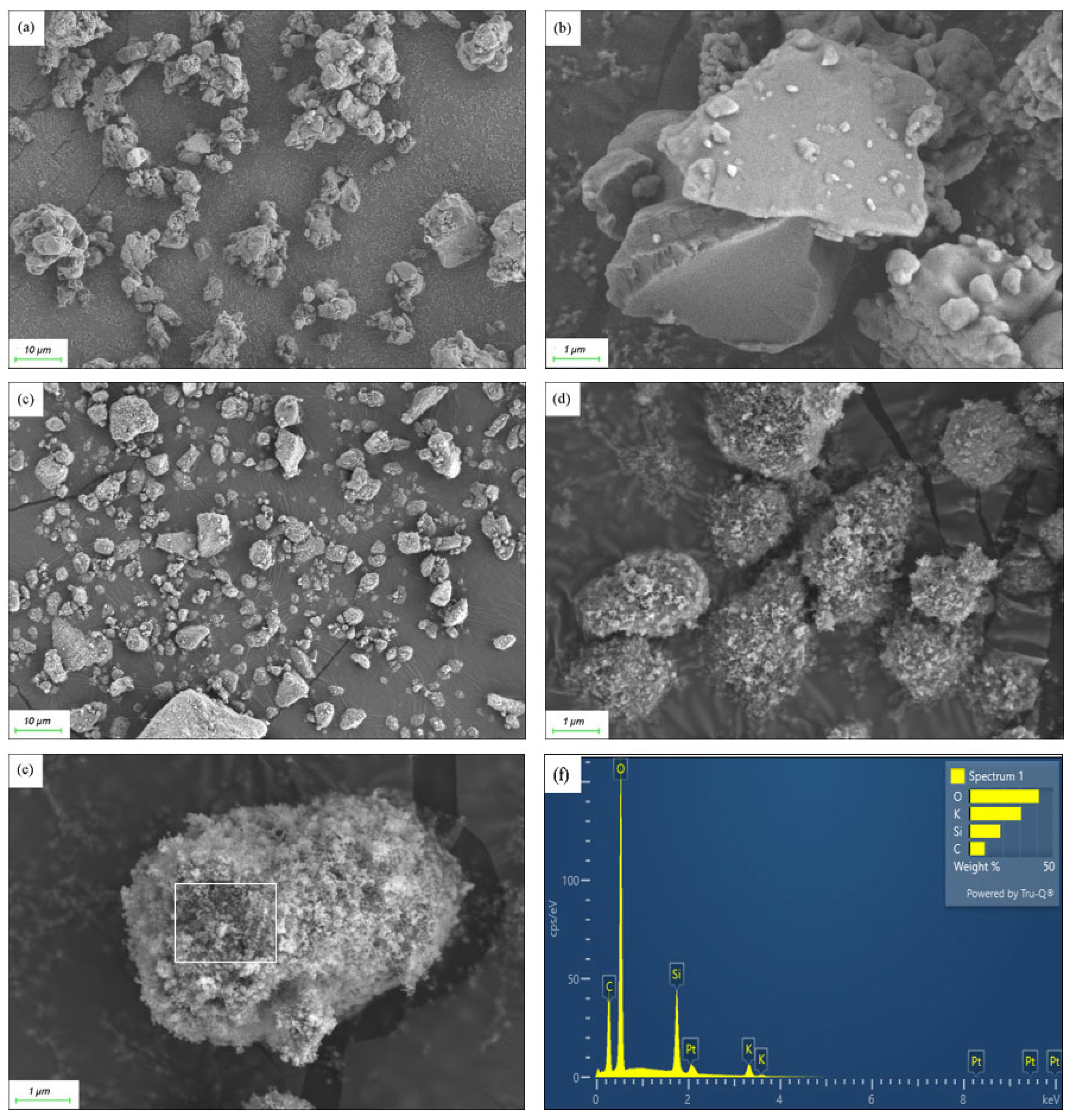
The SEM image in Figure 1a showed that before the addition of hydrophobic SiO2, the mixture of KC and AC formed irregular aggregate particles, with sizes ranging from approximately 2 to 8 μm. These particles appeared to be clustered together in an irregular manner. A magnified SEM image in Figure 1b revealed that the metallic salt particles had relatively smooth crystal surfaces.
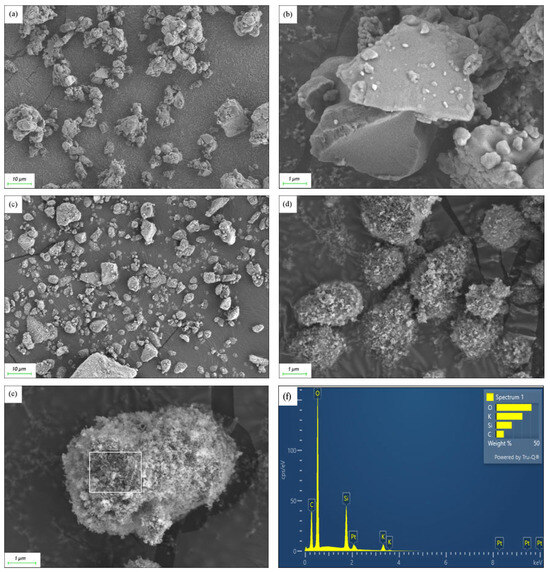
Figure 1.
(a,b) SEM images of the KC-AC composite without silica addition; (c–e) SEM images of the composite with the addition of silica; (f) EDS pattern of the particle surface as marked in (e).
The addition of hydrophobic SiO2 nanoparticles improved the dispersion of the composite particles. The aggregates seen in Figure 1a now were dispersed more evenly, as seen in Figure 1c. Further observation revealed that the surfaces of the composite particles became rougher with the introduction of silica. SEM images of Figure 1d,e showed that the surfaces of these micron-sized particles were thoroughly coated by silica nanoparticles, approximately 10 nm in size, enhancing the particle surface texture. As revealed by Figure 1f, EDS analysis of the area marked in Figure 1e confirmed the presence of key elements such as silicon (Si), carbon (C), oxygen (O), and potassium (K), in agreement with the original constitution of SiO2, KHCO3, and AC polymers. Furthermore, this analysis supported the core-shell structure of the composite, where metallic salt and polymer acted as the core, and silica nanoparticles formed the shell.
3.1.2. Hydrophobicity and Flowability of the Composite Particles
As shown in the above SEM images, nanosized hydrophobic silica formed a coating around the particles, creating core-shell structures. These coatings served a dual purpose: providing moisture protection and enhancing flowability, which were two critical properties for their reliable performance under varying environmental conditions [16].
Since the primary functional materials—metallic salts and polymers—are soluble in water, the hydrophobic coating is essential as a barrier against moisture absorption. To determine the optimal silica mass ratio in the composite fire suppressants, samples with hydrophobic silica contents of 0, 2.0%, 4.0%, 6.0%, 8.0%, and 10.0% (denoted as MPS-0, 1, 2, 3, 4, 5, respectively) were prepared and evaluated.
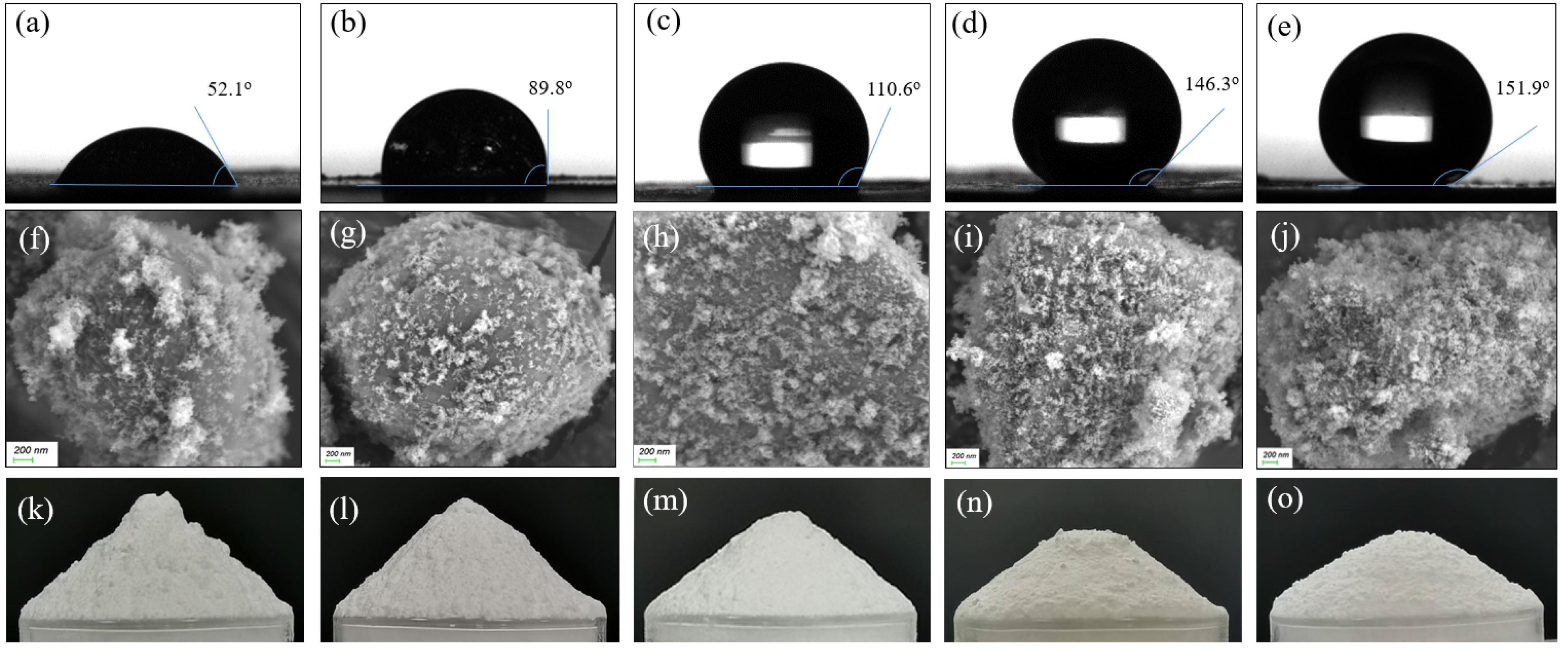
Figure 2 compares the contact angles of the samples. When a water droplet contacted the tablet MPS-0 without silica, it was rapidly absorbed, leaving no visible trace, and thus no image was shown. With increasing SiO2 content, the moisture resistance of the composite powders improved progressively, as indicated by the rising contact angles. As shown in Figure 2a,b, composite particles with silica content no more than 4.0 wt% exhibited limited hydrophobicity, with contact angles remaining below 90°. When the silica content increased to 6.0 wt%, the contact angle rose progressively, exceeding 110° (Figure 2c). Further increasing the silica content to 8.0 wt% resulted in a contact angle of 146.3° (Figure 2d), indicating its good hydrophobic effect. Although raising the silica content to 10.0 wt% increased the contact angle to 151.9° (Figure 2e), the improvement in hydrophobicity became much smaller.
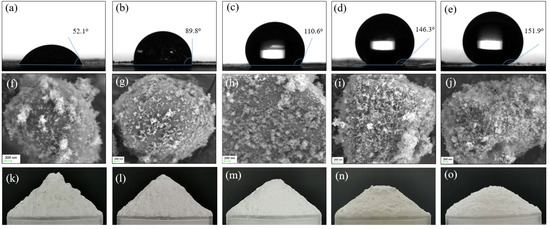
Figure 2.
Images of the contact angle (top row), SEM (middle row), and the repose angle (bottom row) of the samples with different contents of silica: MPS-1 (a,f,k); MPS-2 (b,g,l); MPS-3 (c,h,m); MPS-4 (d,i,n); MPS-5 (e,j,o).
Figure 2 also presents SEM images of the five samples with varying silica content, offering insights into the surface characteristics of these composite samples. As shown in Figure 2f,g, the sample surfaces could not be fully covered by hydrophobic silica particles even with the HS content reaching 4.0%, resulting in small contact angles. At a silica content of 6.0%, most of the surface area was coated by HS nanoparticles, leaving very small surface exposure (Figure 2h). This corresponded to an increased contact angle of 110.6°, indicating a more hydrophobic surface. At 8.0% silica content, the surface was nearly fully covered by silica nanoparticles (Figure 2i). Further increases in silica content to 10.0% appeared to result in a thicker coating but did not significantly alter the overall surface coating (Figure 2j).
The flowability of the composites was evaluated using the angle of repose method, a commonly used technique for granular materials [17]. Figure 2k–o illustrates the heaps formed by the samples with varying silica content, showing a gradual decrease in the angle of repose as silica content increased. This reduction indicated improved flowability of the powders. Specifically, as shown in Figure 2k, sample MPS-1 (2.0% silica) exhibited a repose angle of 43.2°. With higher silica content, the angles further decreased: MPS-2 (4.0% silica) had an angle of 40.8°, MPS-3 (6.0% silica) dropped to 35.2°, and the repose angle of MPS-4 (8.0% silica) reached 29.6°, reflecting greatly enhanced flowability. Beyond 8.0% silica content, the angle of repose showed no notable decrease, indicating diminishing flowability improvement.
The key to these enhancements lies in the superfine hydrophobic silica particles, approximately 10 nm in size, which feature a large surface area. This characteristic allowed the silica nanoparticles to separate composite particles effectively, reducing particle cohesion and preventing agglomeration [16,18]. Furthermore, the silica particles lowered friction between powder particles, facilitating smoother flow [18,19]. These benefits were particularly valuable for mixtures like metallic salts and cross-linked polymer powders, which tended to clump due to high cohesion [20]. By addressing these challenges, silica nanoparticles not only enhanced hydrophobicity but also significantly improved the flowability of the composite powders, ensuring their effectiveness across diverse applications.
Given that the primary functions of the composite agent were fire extinguishment and methanol solidification, excessive silica content would reduce the proportion of the main active ingredient. Also, the composition flowability would get worse due to an increase in the number of contacts and additional friction forces acting between the particles of the additive [21,22]. Therefore, the study suggested that a silica content of around 8.0% achieved the optimal balance between full surface coverage and maintaining the effectiveness of the functional agents. Consequently, the optimal silica content for the composite was determined to be around 8.0%.
3.2. Fire-Extinguishing Tests of the Composite Powder
A series of laboratory-scale extinguishing tests were conducted to assess the performance of the composite sample in both fire suppression and solidification of methanol. For comparison, three samples with varying mass ratios of metallic salts to cross-linked polymers (9:1, 8:2, and 7:3, denoted as MPS-6,7,8, respectively). In all three samples, the silica content was fixed at 8.0%. Additionally, conventional BC powders were tested under similar conditions.
3.2.1. Fire-Extinguishing Performance
Table 1 lists the composition, fire extinction time, and the agent mass consumed for flame extinguishment of the four samples. It was shown that sample MPS-6 obviously exhibited superior fire-extinguishing performances to the other samples in terms of fire extinction time and agent mass consumed. The average fire extinction time of MPS-6 was 1.8 s, while a longer time was needed for the other two samples of MPS-7 and MPS-8. Correspondingly, it was found that the required agent mass of MPS-6 was less than that of the other two samples. The firefighting effectiveness of BC dry powder is roughly equivalent to that of MPS-6, though slightly weaker. This slight difference may be due to the fact that sodium bicarbonate in the composition has a lower extinguishing capacity compared to potassium bicarbonate.

Table 1.
Composition and Fire Extinguishing performance of the four samples.
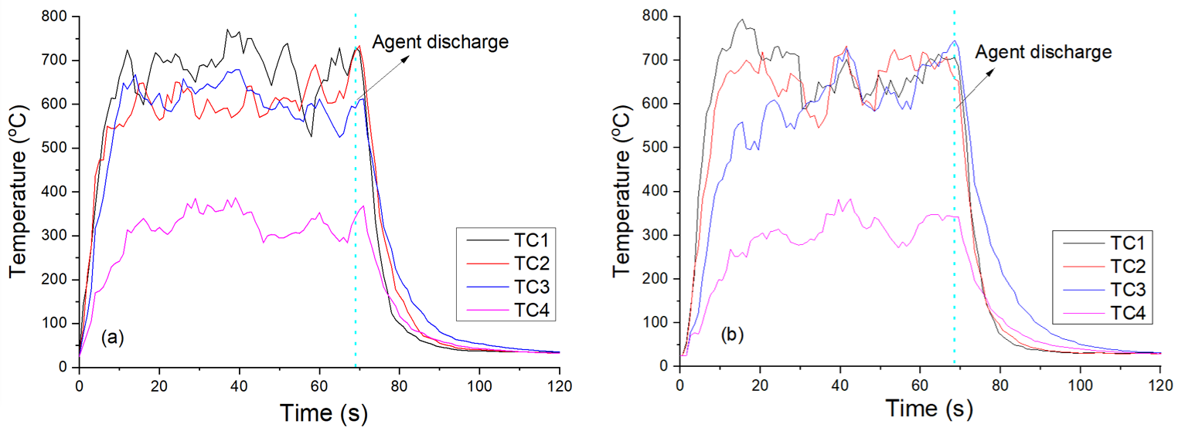
In this composite fire-extinguishing medium, the metal salts played the primary role in extinguishing the fire, while the polymers did not contribute to fire-extinguishing properties. MPS-8 contained the lowest metal salt content, resulting in a decreased extinguishing efficiency, the longest extinguishing time, and the highest amount of fire-extinguishing agent used. MPS-7 had a metal salt content between the other two, and its extinguishing efficiency was also intermediate. Figure 3 shows typical images of the fire-extinguishing process for MPS-7 and MPS-8, from which the changes in the flame shape during the fire-extinguishing process could be observed. In comparison to those of MPS-7, it was noteworthy that some small sparks can be seen in the flame suppressed by MPS-8, which could be caused by the ignition of the polymer. Since polymers can be ignited under certain conditions [23], excessive addition of polymers in the composite would weaken the overall fire-extinguishing efficiency, resulting in a longer fire-extinguishing time and more agent mass. Figure 4 displays the corresponding temperature changes measured by thermocouples in the tests of MPS-7 and 8, demonstrating the differences in the fire-extinguishing process for the two agents. Similarly, the temperature decreasing rate in Figure 4a was faster than that of Figure 4b, implying its relatively better fire-extinguishing capacity than MPS-8.

Figure 3.
Typical images of fire suppression process with different composite powders: (a) MPS-7, (b) MPS-8.
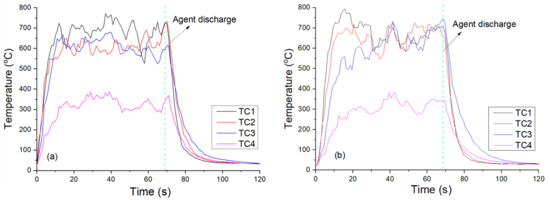
Figure 4.
Temperature variations recorded by thermocouples in the tests with the two samples as fire-extinguishing agents: (a) MPS-7, (b) MPS-8.
3.2.2. Liquid Solidification Performance of the Composite Particles
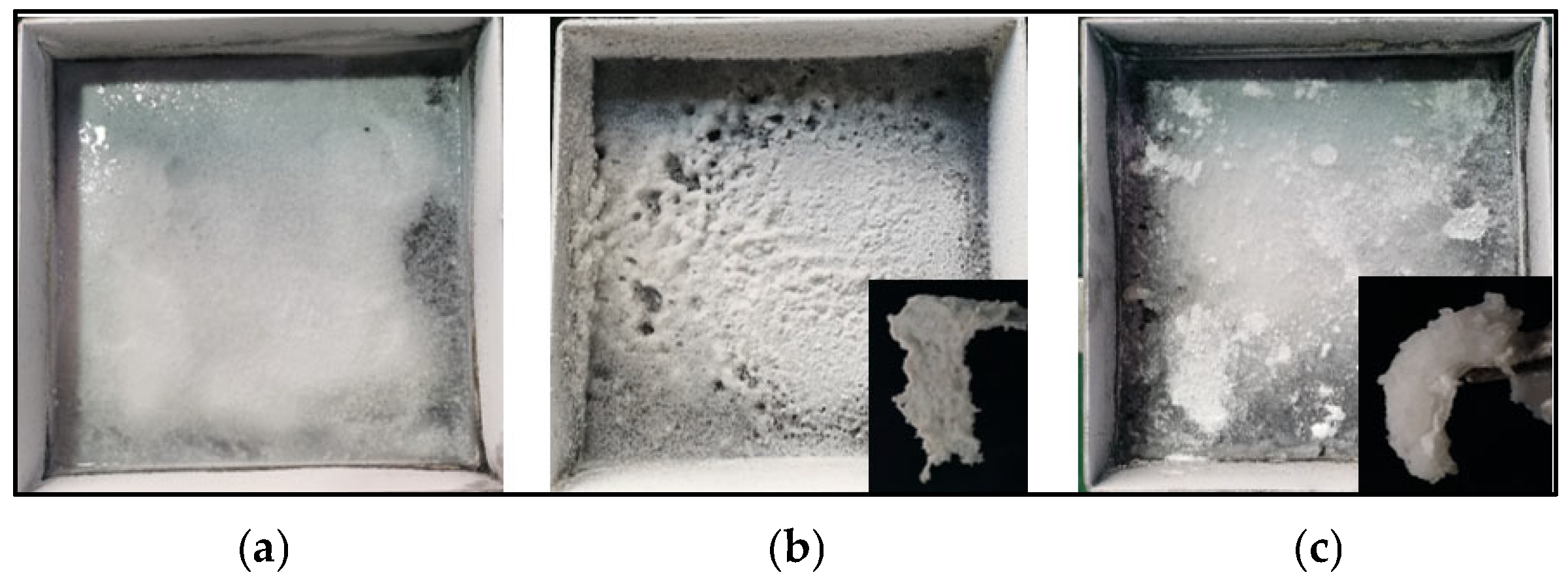
Although the excessive addition of polymers weakened the overall fire-extinguishing efficiency of the composite, the presence of polymers was crucial for the rapid solidification of liquid methanol. Figure 5 shows the situation of the mixture of the remaining fuel and powders in the oil pan after fire extinguishment with the three media. In Figure 5a, the powder settled at the bottom of the pan, and the upper layer of methanol remained in a flowing state. In Figure 5b, there is almost no flowing methanol, and the liquid methanol has been solidified into white gel. In Figure 5c, the completed solidified gel state is also shown. Inset images in Figure 5b,c show the morphology of the gel formed by the powders and methanol. The gel could be lifted and maintained a stable gel-like solid form for a long time. For MPS-6, an additional 13.3 g was needed for a complete solidification. Therefore, for the three MPS samples, the mass ratio of composite powders employed over methanol to achieve a complete flame extinction and liquid solidification was 0.23, 0.16, and 0.20, respectively. For sample MPS-6, although the fire was extinguished quickly and the extinguishing time was short, due to the low polymer content in the sample, a larger amount of powder was needed to achieve full solidification of the liquid methanol. For sample MPS-8, the extinguishing time was longer, and more powder was used for firefighting. However, due to the higher polymer content in the sample, less powder was required for the solidification of methanol after extinguishing the fire. Overall, sample MPS-7 required the least amount of powder to achieve both firefighting and full solidification of the liquid methanol. Under the current conditions, an optimal mass ratio of 0.16 was achieved for sample MPS-7 to ensure complete flame extinction and liquid solidification based on laboratory experiments. As for BC powders, they could extinguish the fire but could not solidify ethanol. Even excessive amounts of BC were ineffective in preventing the flow of methanol.
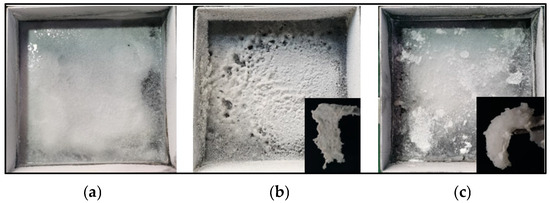
Figure 5.
Images of the mixture of methanol and different composite powders in the fuel pan of 3 fire tests: (a) MPS-6, (b) MPS-7, and (c) MPS-8.
As-prepared composite powders showed a similar effect on ethanol fires, as they can quickly extinguish the fire and simultaneously solidify the ethanol, achieving the goal of thoroughly eliminating the hazard. The optimized mass ratio of the composite powder to ethanol was measured as 0.18 under the same experimental conditions.
Figure 6 presented SEM images of the gel-like substances formed after fire extinguishment. From these images, the adhesive framework can be observed, which encapsulates all the particles together (Figure 6a,b). Due to the polymer film coating, the surface of the solid particles becomes smooth, as seen in Figure 6b,c. The magnified image of Figure 6d clearly reveals pores on the surface, which could be due to the release of methanol. When prepared for SEM observation, methanol would escape from the composite, leaving behind voids or pores in the material.

Figure 6.
Typical SEM images of the gel-like product after fire tests: (a) a panoramic image, (b) image of the linked particles, (c) magnified image of the particle surface, (d) details of the particle surface.
3.3. Mechanism of the Composite Powders in Fighting Methanol Pool Fires
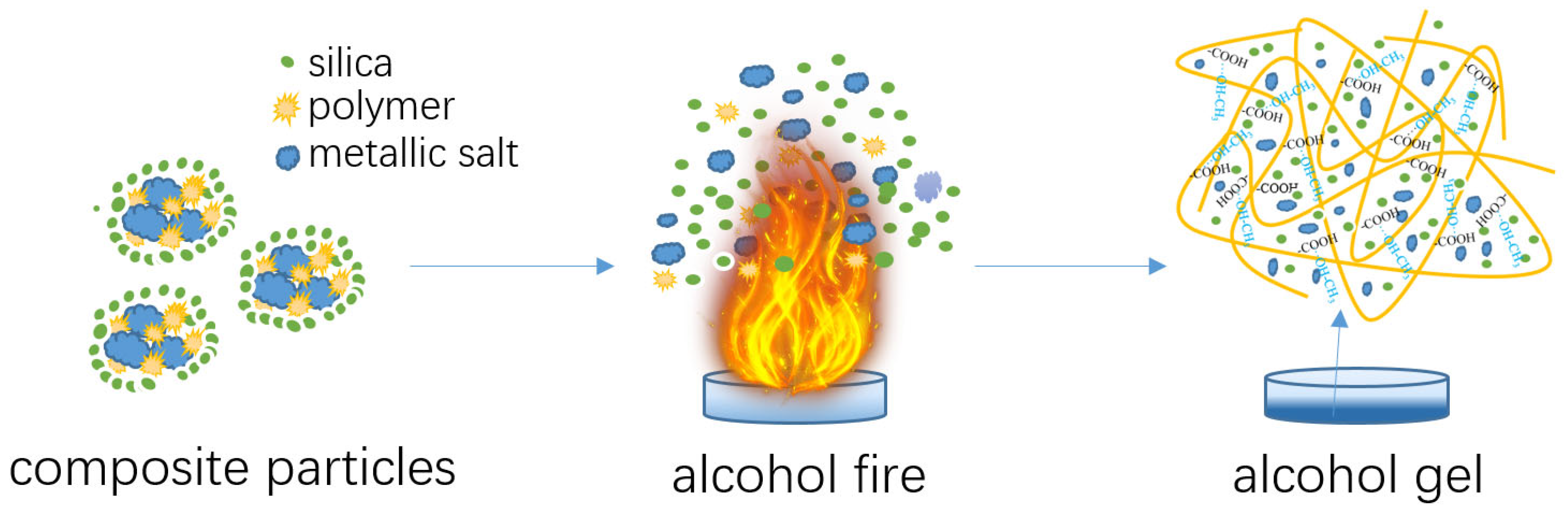
The core-shell structured composite powders demonstrated a dual function of extinguishing methanol/ethanol fire and solidifying liquid alcohol. Figure 7 schematically illustrated the interaction process between the composite powders and alcohol flame, as well as between the composite powders and liquid alcohol.
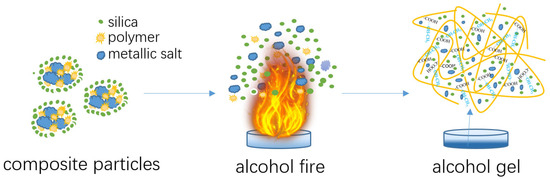
Figure 7.
Schematic illustration of the behaviors of composite particles in flame extinguishment and the solidification of alcohol liquid.
As shown in Figure 7, the composite powder exhibited a core-shell structure, with the inner cores consisting of a micron-scale mixture of metal salts and polymers and the outer layer composed of hydrophobic silica nanoparticles. The silica coating imparted excellent water repellency and flowability to the composite particles. This made the composite powder easy to handle and ensured efficient dispersion in the fire environment. When the powder was released into the methanol flames, the core-shell structure disintegrated under intense heat. This breakdown allowed the metal salts in the inner core to begin their thermochemical reactions. Specifically, the metallic bicarbonates present in the composite underwent endothermic decomposition reactions, which absorbed a significant amount of heat from the surrounding environment, lowering the temperature of the flame and contributing to the suppression of the fire. Meanwhile, when the metal salts decomposed under heat, the resulting potassium ions played a critical role in capturing the free radicals in the flame. By interacting with the radicals of hydroxyl (OH·), hydrogen (H·), and alkyl radicals, which sustain the chain reaction of combustion, the metal ions effectively interrupted the combustion process, preventing the chain reactions that would otherwise continue fueling the fire [24].
In addition to the effects of metal salts, the silica nanoparticles in the composite powder also played an important role in the fire-extinguishing mechanism [25,26]. Silica nanoparticles, which made up the outer shell of the composite, helped in the process of radical scavenging. The surface defects on the silica particles provided active sites for capturing free radicals, further suppressing the combustion reaction. This combination of chemical and physical mechanisms enhanced the composite’s fire-extinguishing capability, allowing the composite to efficiently control and fast extinguish the fire.
The polymer itself did not contribute to extinguishing the fire and may even ignite. However, when its content was relatively low, and the powder matrix primarily consisted of strongly fire-extinguishing metal salts, the polymer did not promote combustion. When the powder particles penetrated the flames and fell into the methanol pool, the dry polymer powder’s coiled carbon chains unfolded. The carboxyl groups on the polymer chains interacted with the hydroxyl groups in methanol through hydrogen bonding, forming cross-linked networks that rapidly solidified methanol or ethanol [27,28]. During this solidification process, any undecomposed or partially decomposed metal salt particles and silica nanoparticles were encapsulated within the solidified framework, forming a solid composite that no longer flowed.
In summary, the composite powders function through the synergistic contributions of their key components. Metallic salts serve as the primary fire-extinguishing agents by facilitating heat absorption and capturing combustion free radicals, which help disrupt the combustion process. Acrylic-based polymers play a crucial role in the solidification process, forming a gel-like structure upon contact with liquid alcohol. The formation of gel compounds immobilizes the fuel source, preventing further spread and reducing the risk of flowing. Silica nanoparticles enhance moisture resistance and improve flowability, both of which are critical for maintaining the composite’s reliability under varying environmental conditions. Additionally, silica nanoparticles also aid in fire suppression by scavenging combustion radicals. The combined action of these components results in an integrated mechanism where the fire is rapidly extinguished while the alcohol fuel is simultaneously solidified, minimizing secondary hazards such as fuel migration or re-ignition. This synergistic effect ensures effective suppression and containment, making the composite material highly practical for real-world fire emergencies.
3.4. Potential Environmental Impacts and Applications
The environmental impact, cost, ease of application, and long-term storage stability are crucial factors in assessing the practical application of the composite. Although comprehensive experiments on these factors have not yet been performed, a preliminary discussion was carried out, focusing on the chemical composition of raw materials, the fabrication process, and the products formed after fire extinguishment, with an emphasis placed on toxicity and sustainability. The possible advantages of using the composite over the separate application of two dry powders were also stated, highlighting its efficiency and integrated functionality.
The composite powders consist of potassium salts, silicon dioxide, and acrylic-based polymers, which are all environmentally friendly materials with minimal toxicity or risk to soil and water. Furthermore, the resulting gel-like solid can be easily collected and transported to designated disposal sites. When dissolved in water, methanol or ethanol can be recovered through filtration and distillation, as no chemical reactions occur between the alcohol molecules and the polymers. Other components, such as metallic salts, silica, and polymers, can be extracted, recycled, and reused using techniques like filtration and crystallization. Overall, this composition is environmentally sustainable, establishing the composite as a green fire-extinguishing material.
The raw materials for the composite powders are readily available, and the preparation process is simple and cost-effective. The production cost is comparable to that of conventional powders. Additionally, the developed composite powders exhibit properties similar to those of traditional dry powders, including good hydrophobicity and flowability, which makes them easy to store and directly fill into fire extinguishers without requiring hardware modifications. Due to their high mass efficiency and compact volume, solid composite powders are particularly well-suited for responding to fires caused by methanol or ethanol leakage during preparation, storage, and transportation. These characteristics highlight the composite’s practical advantages in fire-extinguishing applications in various scenarios.
Compared to the use of separate dry powders for fire suppression and a subsequent methanol solidification process, the composite dry powder offers a more efficient and integrated solution. This composite powder is specifically designed to simultaneously extinguish fires and solidify methanol, eliminating the need for two distinct processes. In contrast, using separate powders for fire suppression and methanol solidification can be less efficient, leading to additional complexity in both handling and application. The composite powder streamlines the entire process by combining both fire suppression and fuel immobilization in one solution, making it more effective and practical for real-world fire emergencies. This dual-action approach not only simplifies the fire response process but also enhances the overall performance and ease of application, reducing the risk of errors and increasing reliability in critical situations.
However, the current study was conducted under controlled laboratory conditions, which may limit the evaluation of the extinguishing agent’s performance in real fire scenarios. These limitations stem mainly from resource constraints and the need to ensure safety during testing. Larger-scale fire-extinguishing experiments are planned to verify the composites’ effectiveness. Additionally, long-term stability tests of the composite powder will be conducted to assess its storage conditions, shelf life, and sustained efficacy over time, all of which are essential for practical applications.
4. Conclusions
To tackle the challenges posed by methanol fires, which can easily flow and spread, a novel composite dry powder fire-extinguishing agent was designed and prepared. This agent could not only rapidly extinguish flames but also quickly solidify liquid methanol, thereby effectively eliminating the hazards caused by methanol fires. This overcomes the limitations of traditional extinguishing agents such as foam, dry powder, and fine water mist, which could extinguish fires but failed to eliminate their residual hazards.
- The composite powder consisted of metal salts, polymers, and hydrophobic silica nanoparticles, which could be prepared through a simple mixing process. The silica particles spontaneously coated the surfaces of the metal salt and polymer composites, forming core-shell structured particles.
- When the silica nanoparticle content was 8.0–10.0%, the composite dry powder exhibited excellent hydrophobicity and flowability, with a hydrophobic angle exceeding 150° and a repose angle below 30°. The silica particles played a dual role by coating the particles to impart hydrophobicity and promote flowability.
- When the content ratio of metal salt to polymer in the composite particles approached 8:2, the particles demonstrated optimal fire-extinguishing performance and solidification effectiveness, with an application mass ratio of powders to methanol of 0.16. Excessive polymer content weakened the fire-extinguishing performance, while insufficient polymer content reduced the solidification effect.
- When applied, the metal salts and hydrophobic silica in the composite particles primarily functioned to extinguish the fire, while the polymer solidified the methanol. The -COOH groups in the polymer formed a hydrogen-bonded gel network with the -OH groups in methanol, encapsulating the dry powder particles to form a gel-like structure. This prevented methanol from continuing to flow, thereby reducing the risk.
This composite powder is also suitable for extinguishing ethanol fires, providing both extinguishing and solidifying functions. This approach not only mitigated fire hazards but also reduced the environmental risks associated with alcohol contamination, making it a valuable tool for managing alcohol-based fire incidents.
Author Contributions
Conceptualization, X.N. and Z.Z.; methodology, X.N. and K.Z.; formal analysis, W.W. and X.N.; investigation, S.H. and W.W.; data curation, S.H.; writing—original draft preparation, X.N.; review and editing, X.N. and Z.Z.; visualization, Z.Z.; supervision, Z.Z.; funding acquisition, X.N. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. U1933126) and Fundamental Research Funds for the Central Universities of China (No. WK2320000039).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MPS | Composite of metallic salt, polymer and silica |
| KC | Potassium bicarbonate |
| AC | Acrylic-based polymer |
| HS | Hydrophobic silica |
| SEM | Scanning electronic microscopy |
| EDS | Energy Dispersive X-ray Spectroscopy |
References
- Methanol: A Future-Proof Fuel for Clean Energy. Available online: https://www.methanol.org (accessed on 11 December 2024).
- Etemad, H.; Choi, J.H. Fire, explosion and safety Hazard identification (HAZID) of the entire methanol dual fueled system and ship. J. Korean Soc. Mar. Eng. 2017, 41, 992–1005. [Google Scholar] [CrossRef]
- Log, T.; Moi, A.L. Ethanol and Methanol Burn Risks in the Home Environment. Int. J. Environ. Res. Public Health 2018, 15, 2379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Bi, Y.; Xu, D.; Zhi, H.; Qiu, P. Experimental investigation of foam spread and extinguishment of the large-scale methanol pool fire. J. Hazard. Mater. 2015, 287, 87–92. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, H.; Zhao, J.; Zhang, Y.; Hu, L. Experimental Research on the Effectiveness of Different Types of Foam of Extinguishing Methanol/Diesel Pool Fires. Combust. Sci. Technol. 2024, 196, 1791–1809. [Google Scholar] [CrossRef]
- Zhi, H.; Bao, Y.; Wang, L.; Mi, Y. Extinguishing performance of alcohol-resistant firefighting foams on polar flammable liquid fires. J. Fire Sci. 2020, 38, 53–74. [Google Scholar] [CrossRef]
- Prasa, K.; Li, C.; Kailasanath, K. Simulation of water mist suppression of small scale methanol liquid pool fires. Fire Saf. J. 1999, 33, 185–212. [Google Scholar] [CrossRef]
- National Fire Protection Association. NFPA 17: Dry Chemical Extinguishing Systems; National Fire Protection Association: Quincy, MA, USA, 2022. [Google Scholar]
- Wu, Z.; Chai, G.; Zhu, G. Experimental study on the effectiveness of dry water material to suppress oil pool fire and analysis of fire extinguishing mechanism. J. Therm. Anal. Calorim. 2024, 149, 10193–10212. [Google Scholar] [CrossRef]
- Wilk-Jakubowski, J.; Stawczyk, P.; Ivanov, S.; Stankov, S. High-power acoustic fire extinguisher with artificial intelligence platform. Int. J. Comput. Vis. Robot. 2022, 2, 236–249. [Google Scholar] [CrossRef]
- Diwanji, M.; Hisvankar, S.; Khandelwal, C. Autonomous Fire Detecting and Extinguishing Robot. In Proceedings of the 2nd International Conference on Intelligent Communication and Computational Techniques (ICCT), Jaipur, India, 28–29 September 2019; pp. 327–329. [Google Scholar] [CrossRef]
- Sheng, Y.; Hu, D.; Li, Y.; Zhang, S.; Wang, T.; Zhao, Q. Tuning thermal stability of fluorine-free foam by nano-MDH inorganic flame retardant. J. Mol. Liq. 2024, 415, 126328. [Google Scholar] [CrossRef]
- Huang, A.C.; Cao, F.C.; Ma, X.Y. A Comparative Approach Study on the Thermal and Calorimetric Analysis of Fire-Extinguishing Powders. Safety 2024, 10, 31. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Z.; Zhao, J.; Li, R. Preparation of novel gel foam and its fire suppression performance against gasoline pool fires. Energy 2024, 304, 132148. [Google Scholar] [CrossRef]
- Ni, X.; Zhang, S.; Zheng, Z.; Wang, X.S. Application of water@silica core-shell particles for suppressing gasoline pool fires. J. Hazard. Mater. 2018, 341, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Shamsutdinov, A.S.; Kondrashova, N.B.; Valtsifer, I.V.; Bormashenko, E.; Huo, Y.; Saenko, E.V.; Pyankova, A.V.; Valtsifer, V.A. Manufacturing, Properties, and Application of Nanosized Superhydrophobic Spherical Silicon Dioxide Particles as a Functional Additive to Fire Extinguishing Powders. Ind. Eng. Chem. Res. 2021, 60, 11905–11914. [Google Scholar] [CrossRef]
- Willibald, C.; Löwe, H.; Theile, T.; Dual, J.; Schneebeli, M. Angle of repose experiments with snow: Role of grain shape and cohesion. J. Glaciol. 2019, 66, 658–666. [Google Scholar] [CrossRef]
- Müller, A.K.; Ruppel, J.; Drexel, C.P.; Zimmermann, I. Precipitated silica as flow regulator. Eur. J. Pharm. Sci. 2008, 34, 303–308. [Google Scholar] [CrossRef]
- Zimmerman, I.; Eber, M.; Meyer, K. Nanomaterials as flow regulators in dry powders. Z. Phys. Chem. 2004, 218, 51–102. [Google Scholar] [CrossRef]
- Karde, V.; Panda, S.; Ghoroi, C. Surface modification to improve powder bulk behavior under humid conditions. Powder Technol. 2015, 278, 181–188. [Google Scholar] [CrossRef]
- Qu, L.; Morton, D.A.V.; Zhou, Q. Particle engineering via mechanical dry coating in the design of pharmaceutical solid dosage forms. Curr. Pharm. Des. 2015, 21, 5802–5814. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Zimmerman, I. Effect of glidants in binary powder mixtures. Powder Technol. 2004, 139, 40–54. [Google Scholar] [CrossRef]
- Kashiwagi, T. Polymer combustion and flammability—Role of the condensed phase. Symp. Int. Combust 1994, 25, 1423–1437. [Google Scholar] [CrossRef]
- Ewing, C.T.; Beyler, C.; Carhar, H.W. Extinguishment of class B flames by thermal and chemical actions; Principles underlying a complete theory; Prediction of flame extinguishing effectiveness. J. Fire Prot. Eng. 1994, 6, 23–54. [Google Scholar] [CrossRef]
- Krasnyansky, M. Studies of fundamental physical-chemical mechanisms and processes of flame extinguishing by powder aerosols. Fire Mater. 2008, 32, 27–47. [Google Scholar] [CrossRef]
- He, S.; Ruan, C.; Shi, Y.; Chen, G.; Ma, Y.; Dai, H.; Chen, X.; Yang, X. Insight to hydrophobic SiO2 encapsulated SiO2 gel: Preparation and application in fire extinguishing. J. Hazard. Mater. 2021, 405, 124216. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Chakraborty, S.; Berab, R.; Mandal, A.; Ayan, D.; Chakrabarty, D.; Rene, E.R.; Lens, P.N.L. Adsorptive removal of alcohols from aqueous solutions by N-tertiary butylacryl-amide (NtBA) and acrylic acid co-polymer gel. Mater. Today Commun. 2019, 21, 100653. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).