Abstract
Megafires are transforming western boreal forests, and many burned forests are salvage logged, removing more structure from landscapes and delaying forest regeneration. We studied forest-dwelling owls in a post-fire and salvage-logged landscape in central British Columbia, Canada, in 2018–2019 after the 2010 Meldrum Creek Fire and the 2017 Hanceville Fire. We examined owl habitat selection via call surveys compared to the habitats available in this landscape. Owl pellets were dissected to determine owl diets. We detected six owl species, of which Northern Saw-whet Owls (Aegolius acadicus) were the most common. Owls had weak and variable habitat selection within an 800 m radius of detections; all species used some burned area. Great Gray Owls (Strix nebulosa) and Great Horned Owls (Bubo virginanus) obtained more prey from mature forests (e.g., red-backed voles, Myodes gapperi, snowshoe hares, Lepus americanus) than other owls did, whereas other owls primarily consumed small mammals that were common in burned or salvaged areas. These results indicate a diverse community of owls can use landscapes within a decade after wildfire, potentially with some prey switching to take advantage of prey that use disturbed habitats. Despite that, owl numbers were low and some owls consumed prey that were not available in salvage-logged areas, suggesting that impacts on owls were more severe from the combination of fire and salvage logging than from fire alone.
Keywords:
boreal forest; British Columbia; diet; disturbance; habitat use; landscape; owls; salvage logging; wildfire 1. Background
Wildfire is the most significant natural disturbance in western boreal forests [1,2]. The severity and activity of wildfires are predicted to increase in the coming decades with the impacts of climate change and anthropogenic fire suppression [3,4,5,6,7]. Fire regimes have also become more severe with the loss of traditional Indigenous burning practices under colonial governments [8,9,10,11]. Wildfires may pose significant threats to species conservation [12,13], as fires contribute to habitat loss and transformation, leading to fundamental shifts in animal communities [14,15].
Salvage logging following wildfire is a common practice to help forestry companies recoup economic losses [16,17]. However, post-fire salvage logging is a cumulative disturbance that removes biological legacies, such as standing and fallen trees, that would normally remain on the post-fire landscape [18,19,20], thus slowing tree regeneration [21] and shaping post-fire habitats for decades following fire [22,23,24]. Although salvage logging has become common, more research is needed to understand the full suite of effects on tree regrowth, fuels management, and wildlife habitat [20,22,25].
As forested landscapes undergo large conversions from mature forest to post-disturbance habitats, the impacts on avian predators are likely to be substantial [15,19,26,27], due to both the loss of nesting habitat and changes in the distribution and abundance of prey [14]. Biological legacies such as coarse woody debris and standing dead trees create important habitat structure for wildlife [28], which are reduced via salvage logging. The impacts of stand-replacing disturbance on owls are thought to be strongly negative. Habitat suitability models for owls in British Columbia (BC) and Alberta indicate that old forest is necessary for Barred Owls (Strix varia Barton), Great Horned Owls (Bubo virginianus Gmelin), and Great Gray Owls (Strix nebulosa Forster) [29,30,31]. Models for Northern Saw-whet Owls (Aegolius acadicus Gmelin) indicate these birds use multi-layered forest (~40–80 years post-disturbance) as well as mature forest [32]. Thus, loss of the older forests creates worse conditions for many owls. These results hold even though many of these owls also forage in meadows or open terrain adjacent to forest.
There is limited research on owl responses to wildfire or post-fire salvage logging [33,34,35,36], although the loss of mature forest is likely negative for owls. Most of the previous owl–fire research focuses on the California spotted owl (Strix occidentalis occidentalis Xántus de Vésey [37,38,39]), showing complex patterns of how owls persist in burned landscapes, with mature trees remaining important but owls foraging in some disturbed areas as well.
Shifts in small mammal populations following disturbances such as wildfire shape owl habitat selection by influencing habitat quality and prey availability, potentially with different prey species and abundances in disturbed areas versus the unburned forest [37,38,39,40,41]. Long-eared Owls (Asio otus L.) and Northern Saw-whet Owls depend on a mosaic of forest and open grassland habitats, both for safe nesting and hunting [42,43]. This pattern could make burned areas into attractive foraging habitat for some owls if sufficient nesting habitats were nearby. Small mammals constitute the bulk of many owl species’ diets as they are energetically valuable prey [42,44,45,46]. Microtus spp. and Myodes spp. are important seasonal prey for many forest owls [47,48]. For example, diets composed primarily of small mammals support earlier seasonal breeding times in Northern Pygmy Owls (Glaucidium gnoma Wagler) than diets of mainly birds [49]. In Oregon, Northern Saw-whet Owls seldom used 2-year-old burned forest, while Barred Owls and Great Horned Owls were more likely to use areas burned with lower severity than with higher severity [36].
To better understand how wildfire and post-fire salvage logging impact wildlife, we studied the impacts of these compounded disturbances on owls in interior Douglas fir (Pseudotsuga menziesii (Mirb.) Franco) forest in central British Columbia, Canada. In our study area, 65% of the landscape had burned 1–9 years prior to our surveys, some with salvage logging thereafter. Within this study area, snowshoe hares (Lepus americanus Erxleben), red squirrels (Tamiasciurus hudsonicus Erxleben), and voles were all negatively affected by fires and even more negatively affected by salvage logging post-fire [50,51], thus leading to dramatic changes in owls’ prey distribution and abundance. Our focus was thus on assessing to what extent this landscape was able to support owls, rather than on any individual owl species. Specifically, we sought to (1) determine owl presence and describe habitat use in this disturbed landscape, and (2) determine owl diets for the species that were present.
2. Methods and Materials
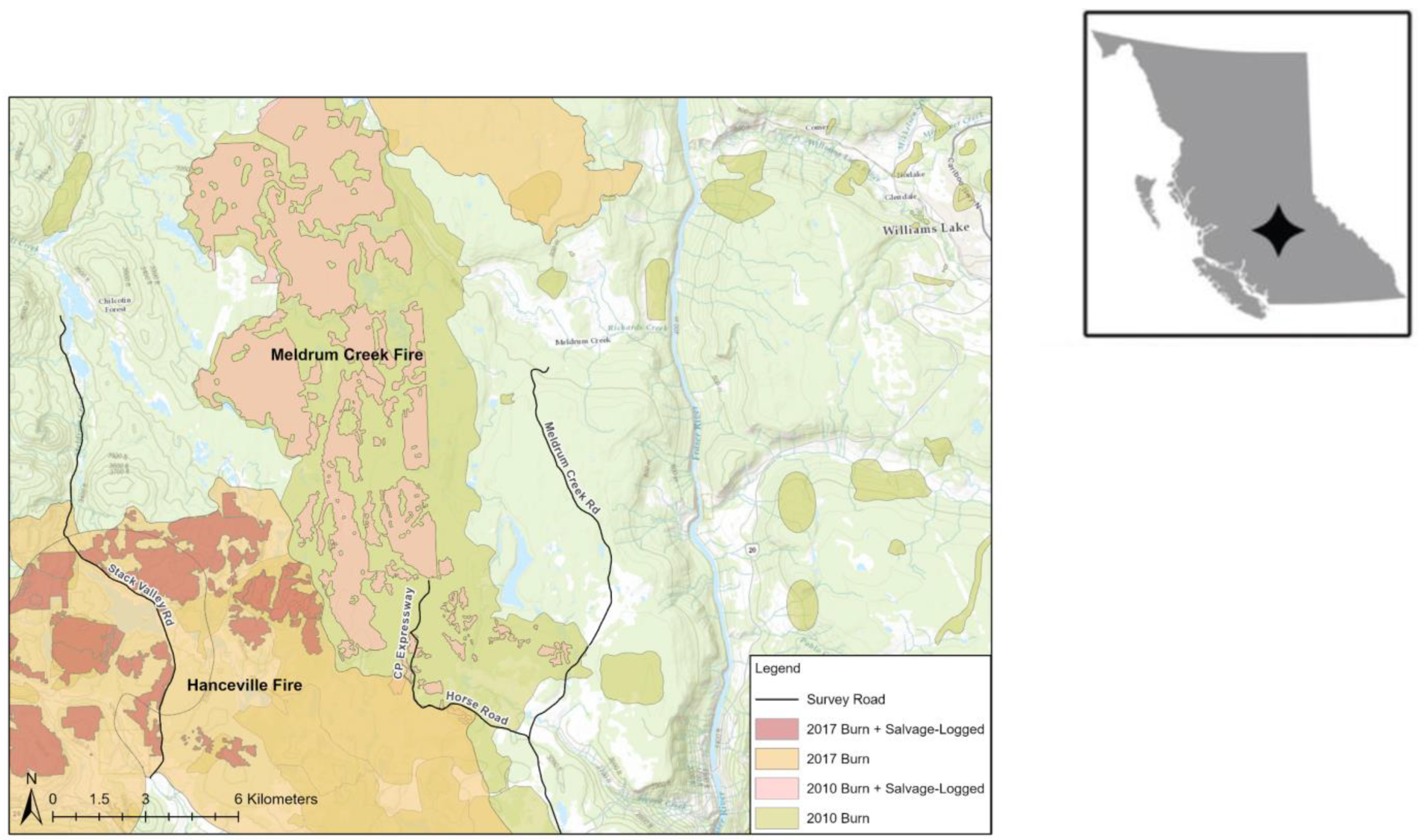
The study area was 39,866 ha in the Chilcotin Plateau, BC, on Department of National Defence Land and Crown land, at ~1000 m elevation. In 2010, the Meldrum Creek Fire Complex burned 47,293 ha of forest, and 6,876 ha of the burned area was salvage logged (Figure 1). In the study area, most of the areas with high- or moderate-severity fire damage were salvage logged, so we could not meaningfully analyze fire severity alone as a predictor of owl habitat use. In 2017, 241,160 ha southwest of the Meldrum Creek Fire burned in the Hanceville Fire, with about 15% of the Meldrum Creek Fire reburned a scant seven years after the prior fire. Salvage logging of the Hanceville Fire began in winter 2017–2018.
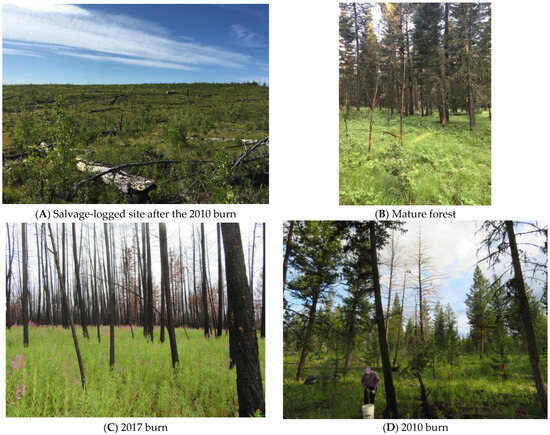
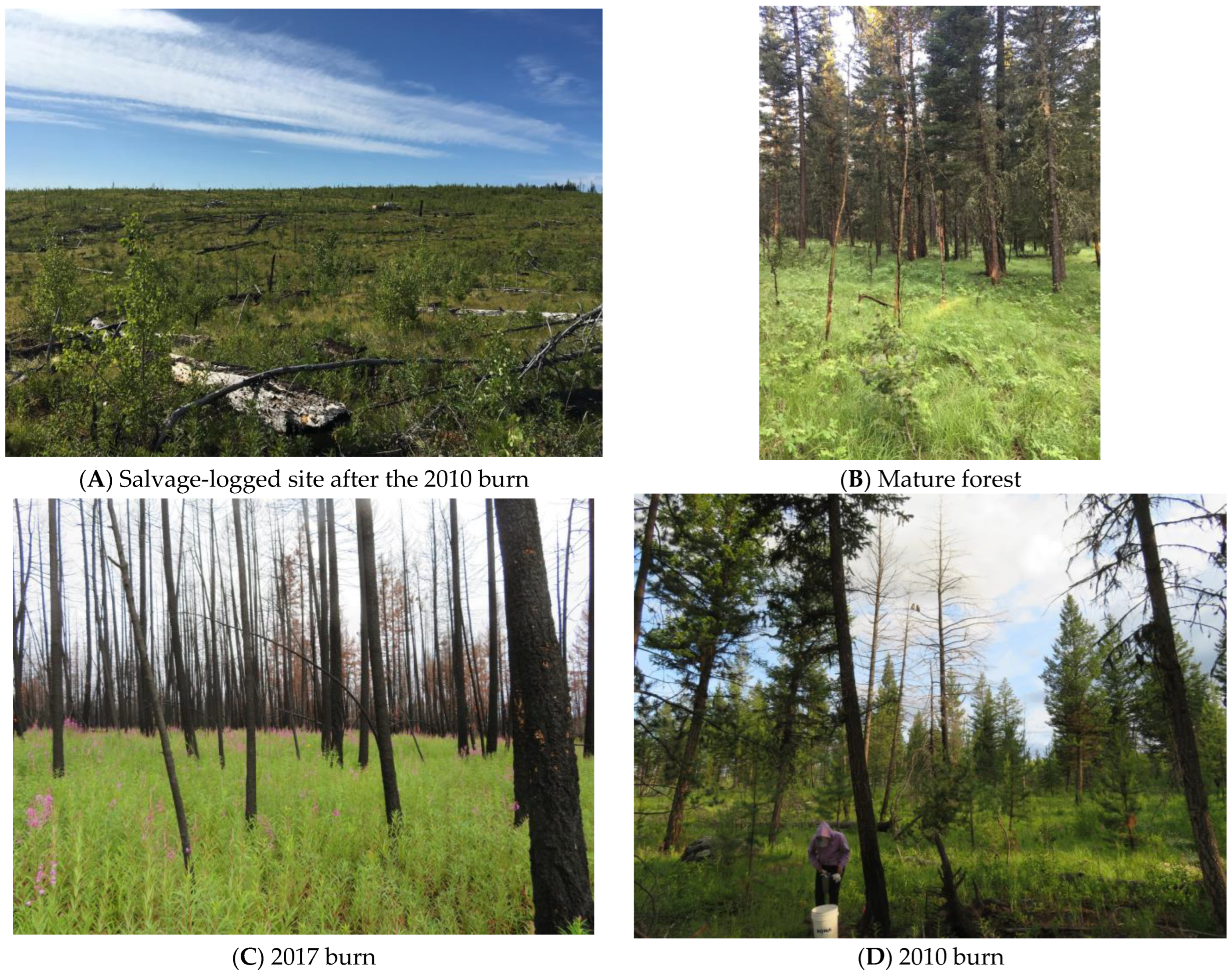
Figure 1.
Pictures of cover types within the study area. (A) Salvage logged, after the 2010 burn; (B) mature forest; (C) 2017 burn, (D) 2010 burn, with fledgling Great Gray Owls. All photographs were taken in 2018 or 2019 by A.J. Kelly.
The forest was mostly interior Douglas fir, with Engelmann spruce (Picea engelmannii Parry ex Engelm), lodgepole pine (Pinus contorta Douglas ex Loudon), and trembling aspen (Populus tremuloides Michx.) in a mosaic of seral stages. Grasslands and lakes and their adjacent wetlands comprised about 16% of the study area. Several local ranches grazed cattle over the entire study area. Standard logging, post-beetle kill salvage logging, and post-fire salvage logging were common and the forest is managed for harvest.
In this study area red squirrels, snowshoe hares, and red-backed voles (Myodes gapperi Vigors) essentially occurred only in mature forest [50,51]. In contrast, deermice (Peromyscus maniculatus Wagner) were abundant in the 2017 burned areas and 2010 burned and salvage-logged areas, and much scarcer in the 2010 burned areas and mature forest. Meadow voles (Microtus pennsylvanicus Ord) and yellow pine chipmunks (Tamias amoenus Allen) were most common in the 2010 burn and the 2010 salvaged burn [51]. Although we did not trap small mammals within grasslands, which likely also supported meadow voles, montane voles (M. montanus Peale), and long-tailed voles (M. longicaudus Merriam), these live-trapping results suggest that we can broadly infer owl hunting habitats in this landscape from their diets.
2.1. Owl Surveys
We conducted owl call surveys informed by BC provincial standards [52] between 12 April and 27 May 2018 and 2 April and 15 June 2019, i.e., throughout the main breeding season as weather allowed. Auditory methods are the primary survey method used for these cryptic nocturnal predators [53]. We selected three routes along roads that represented all habitat types and were spread throughout the study area (Figure 2). Each route consisted of 10 survey points spaced 1.6 km apart (spacing as used by [54] for a multi-owl study). Surveys started 30–60 min after sunset. We listened for owl calls for a minimum of four minutes at each survey point; we listened up to 17 min if no owls were initially detected (median five minutes), but the longer periods almost never led to more owl detections. Thus, points with no owls detected almost certainly reflected the genuine absence of owls rather than failure to detect owls that were present. If there was too much wind (above Beaufort 3), we did not survey, but returned on the next possible quiet night. In 2018, each route was surveyed two or three times as weather permitted, and in 2019, sites were surveyed three times.
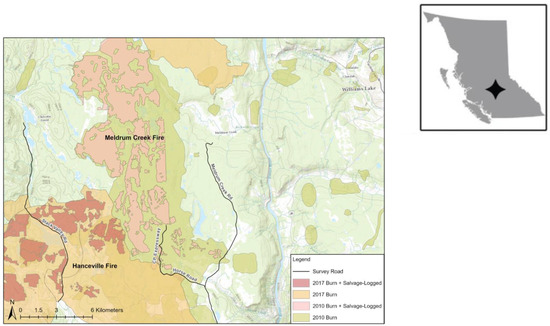
Figure 2.
The study area west of Williams Lake, British Columbia. Fires, salvage logging, and survey routes are shown in the large panel, and the small panel shows the study area location within BC. The northern part of the CP Expressway route is not shown because it crossed Department of National Defence lands and was not available on GIS layers.
2.2. Owl Habitat Use
We mapped the locations of owl detections in ArcGIS Pro version 3.3.2., both from call surveys and from when we saw owls when conducting fieldwork on small mammals [50,51]. We present owl detections from both years, but we used only 2019 survey detections for the habitat selection analysis because the study area was being salvage logged in 2018, thus changing available habitat throughout the survey period. We set 800 m radius buffers (201 ha) around each sighting or survey location because survey sites were located 1.6 km apart and these buffers would not overlap. We could not unambiguously determine the specific location from which owls were calling within each 201 ha area, but these circles reflect the home range composition of detected owls, as Northern Saw-whet Owls and Barred Owls have ~300 ha breeding season home ranges [55,56].
We determined the percentages of each of eight cover types constituting the study area as a whole: mature forest (23.6%), 2010 burn (14.2%), 2010 burn + salvage logged (10.0%), 2017 burn (17.3%), 2017 burn + salvage logged (7.0%), recent cut-block (≤40 yr since harvest, 11.9%), grassland (13.3%), and lakes (2.8%). If multiple disturbances occurred across different years in the same stand, the most recent disturbance type was taken as the cover type. The exception was grasslands, which were counted as grasslands even if they had burned, given the quick regeneration of grass and the fundamentally different habitat structure of grasslands to forests. We bounded the study area by creating a buffer of at least 1 km between the edge of the study area and observation buffers, thus ensuring we had adjacent available cover on all sides of each buffer.
2.3. Pellet Collection and Diet Analysis
We searched for owl pellets in areas where we detected owls during call surveys or located owls during the day, including at roost and nest sites. We dissected regurgitated pellets to determine owl diets [57,58,59]. Although pellet analyses may underrepresent insect and amphibian prey [60], bone, fur, and feathers are reliable markers for mammalian and avian prey in owl pellets [61,62], which were likely the main prey for these owl species. Further, analyzing diets from pellets enables use of pellets produced at any time in the year, rather than restricting sampling to the nesting season, as is required for analyses of prey remains and videography at nests [63].
We assigned pellets to owl species based on location of collection (e.g., near nests or perches) and pellet morphology [58.59]. We identified small mammal remains to species by dentition and skull attributes [64,65,66]. If teeth or jaws were absent, we used guard hairs to identify prey to genus if possible [67]. To determine the number of prey within each pellet, any pellet containing fur and up to one full skull or pelvis of the same species was considered to contain one individual. If there were multiple jaws or pelvises, we counted that total as representing the number of prey (e.g., two left mandibles indicated two prey).
2.4. Statistical Analysis
For the owl surveys, we determined the owl species present at each survey point, rather than analyzing every detection. Thus, if an owl was detected on one, two, or three survey occasions at the same point, that was all counted as owl presence at that point. This approach reflects owl territoriality, such that multiple detections of the same pair do not add information about where on the landscape owls are able to persist.
We calculated the average habitat composition for each owl species within the 201 ha circles around the points where we detected birds. We present owl habitats compared to the overall landscape composition because owls are highly mobile, and would easily be able to reach anywhere in the study area. We considered owls to exhibit biologically meaningful habitat choice if the cover types around their locations were strongly dissimilar to the distribution of cover types in the entire landscape, for example, if a cover type was largely absent near owl locations. We could not use inferential statistical tests due to low sample sizes (one landscape, owl detections low but variable by species). We analyzed diets first as the proportion of pellets containing each prey type (absolute frequency of occurrence: n of prey type/n pellets). Second, we calculated the proportion of each prey type (relative frequency of occurrence; n of prey type/n total prey). Third, we calculated the biomass of prey killed, using wet biomass estimates of each prey type and the number of each prey recorded. We used body masses primarily from [65] and secondarily from species accounts from the American Society of Mammalogists; we assigned the few songbirds the mass of 24.0 g [58].
3. Results
In 2018, the only owls we detected from 30 points surveyed 2–3 times each were Long-eared Owls, at four survey locations; we also found one nest. We saw one Short-eared Owl (Asio flammeus Pontoppidan) within the 2010 burn. Neither the Long-eared Owl nest area nor the Short-eared Owl observation area were impacted by the 2017 Hanceville Fire. In 2019, each site was surveyed three times and we detected Northern Saw-whet Owls at nine sites, Great Horned Owls at four sites, and Barred Owls at five sites (Figure 3). In 2019, we also located two roosting areas of Great Gray Owls that belonged to pairs with fledged chicks (Figure 4). We also located two roosting areas of Great Horned Owl pairs but could not confirm if they had reared chicks. We observed one adult Barred Owl at a roosting site, but did not find signs of a nest or fledglings.
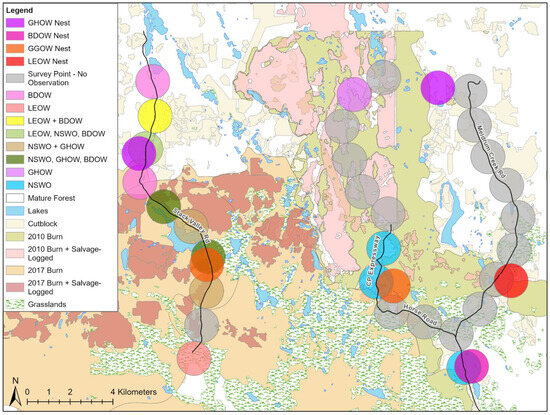
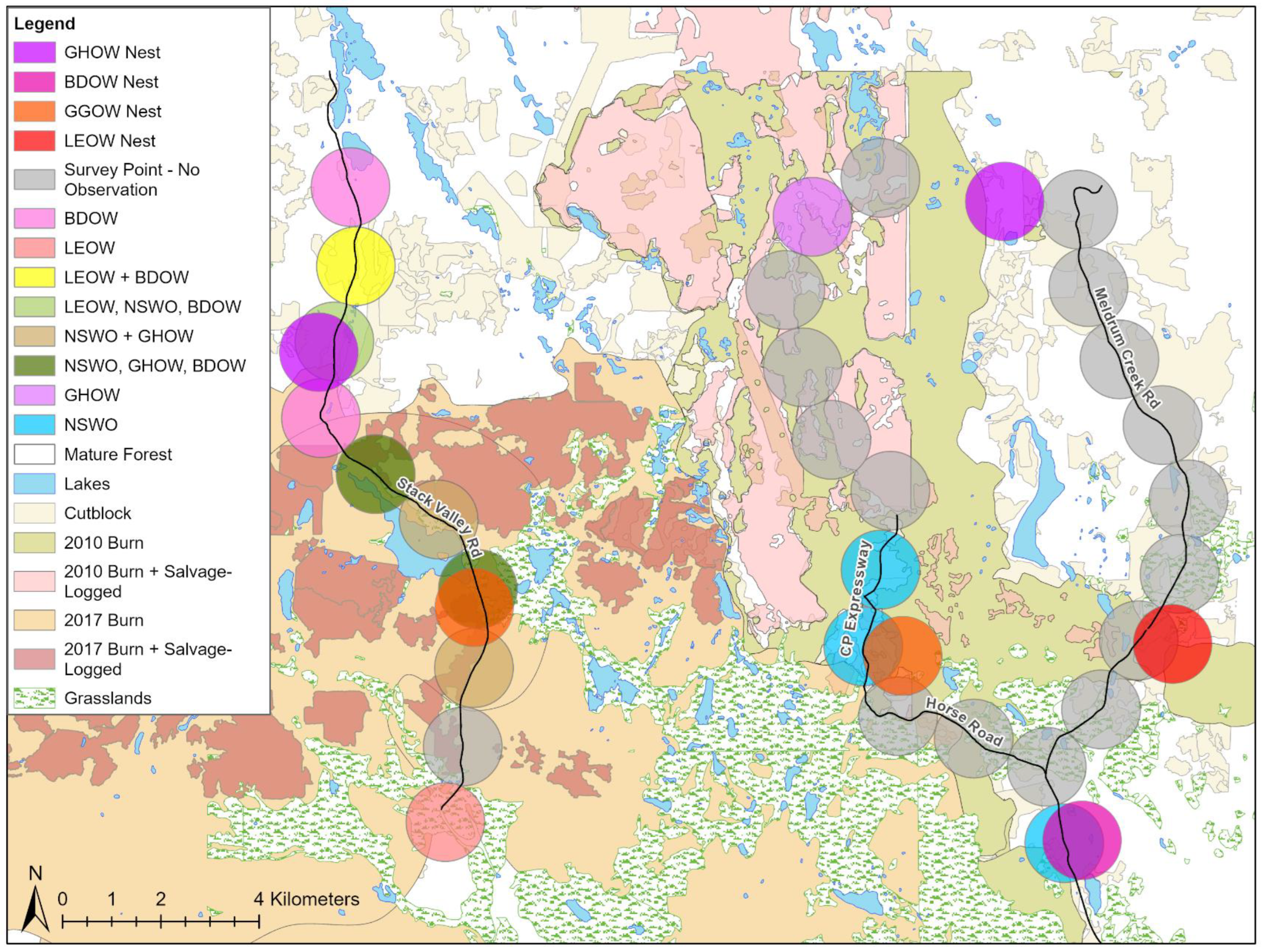
Figure 3.
A map of the study area west of Williams Lake, BC, showing where owls were detected in relation to different habitats. Each circle represents 201 ha around owl detections from auditory surveys, sightings, or nests. Gray circles show survey points with no detections. The map shows mature forest, and areas that were burned in 2010, burned in 2017, or burned and then were salvage logged after fire. BDOW = Barred Owl, GGOW = Great Gray Owl, GHOW = Great Horned Owl, LEOW = Long-eared Owl, NSWO = Northern Saw-whet Owl. Survey routes are shown, except for the northern part of the CP Expressway route as it was not available on GIS layers.
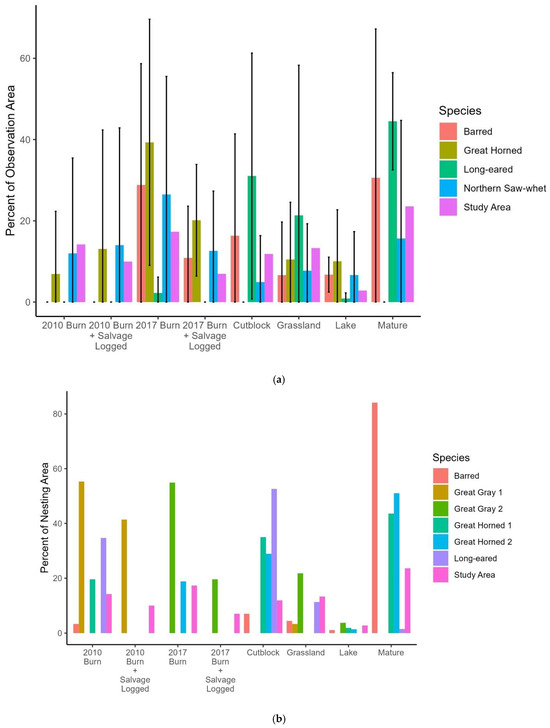
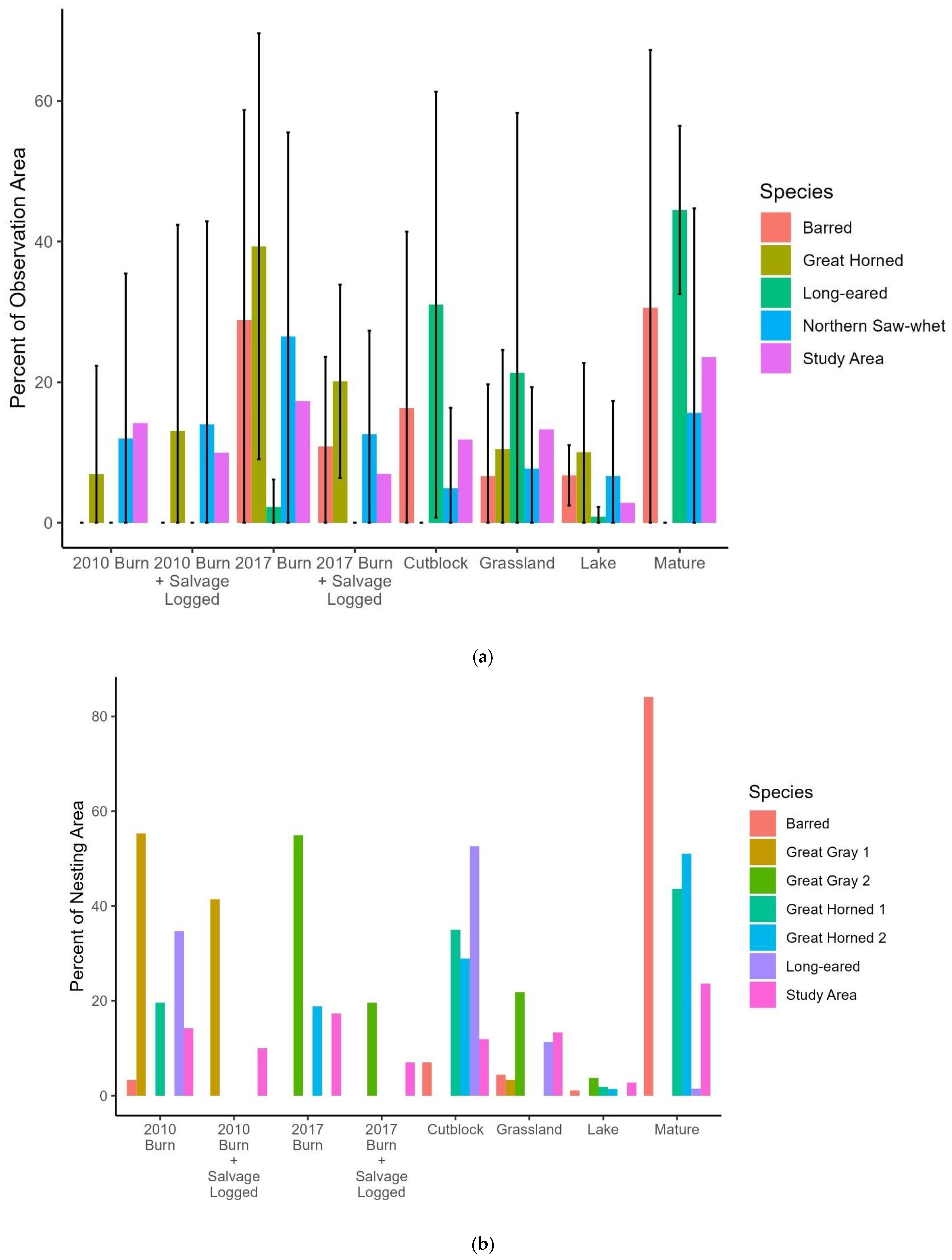
Figure 4.
Habitat use by owls in 2019 in the Chilcotin Plateau, BC. (a) Owl detections from hooting surveys, (b) owls detected on or near nests. The study area columns show the percentage of the landscape in each habitat type for the entire study area. For owl hooting survey locations, values are the average ±1 SD percentage of each habitat within an 800 m radius (201 ha) of the survey points where each species was detected. SL = salvage logged. Cut-blocks were clearcut ≤40 years prior, and mature refers to forest stands older than that.
The Northern Saw-whet Owl detections were in locations that were similar to the distribution of cover types in the study area as a whole (Figure 4); they and Great Horned Owls were the only species to use 2010 burn or 2010 burn + salvage. The four Great Horned Owl detections were at sites where 2017 burn and 2010 burn + salvage-logged areas were overrepresented. Long-eared Owls used disproportionately more cut-block (cut 0–40 years prior), grassland, and mature forest surrounding their locations than were available in the study area, but seldom used the other habitat types. The one Short-eared Owl we saw while undertaking other fieldwork was within the 2010 burn; the 201 ha area around it was 27.8% burn and 72.2% burn + salvaged. Barred Owls were detected only five times, but at survey stations where more of the area around each point was in the 2017 burn than was available in the landscape at large.
Owl Diets
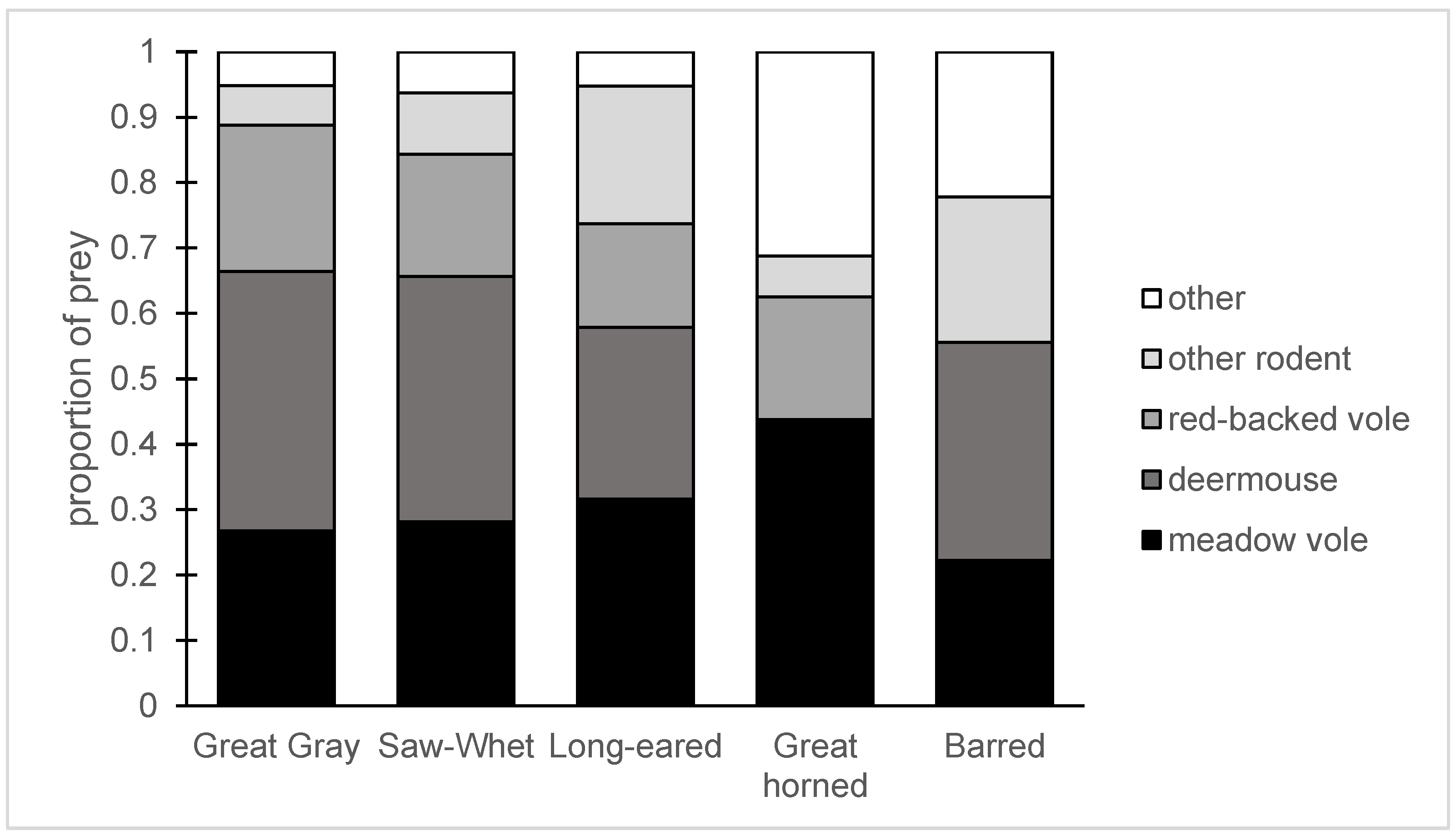
We collected 155 pellets from Great Horned (n = 9), Great Gray (n = 68), Northern Saw-whet (n = 43), Long-eared (n = 23), and Barred Owls (n = 12). The majority of these pellets were from 2019 (110 pellets), but we had pellets from both 2018 and 2019 for Northern Saw-whet Owls. Across all prey remains from all owl species, 23.2% of remains (58/250) could be assigned only to “unknown Microtus” or “small mammal”, but we have no reason to think there was a bias in which prey were identifiable. The owls essentially consumed small mammals (Figure 5); out of 250 identified prey, only 5 (2.0%) were small birds. A Northern Saw-whet Owl ate one grasshopper, and Great Horned Owls also consumed snowshoe hares. The rest of the prey were small mammals, primarily meadow voles, red-backed voles, deermice, and yellow pine chipmunks.
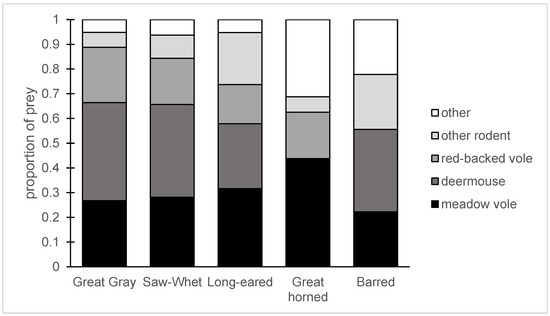
Figure 5.
The prey of five species of owl in a burned and salvage-logged landscape in central BC. This figure includes the 192 prey remains we could identify as snowshoe hare or rodent species, as well as shrews, Orthoptera, and passerines. “Other rodents” includes red squirrels, chipmunks, and other vole species. For Great Horned Owls, there were no deermice consumed and “other” means snowshoe hare. For the other owl species, “other” includes grasshoppers, shrews, and passerines.
Great Gray Owls had the most diverse diets, with at least 10 confirmed prey species (Table 1); the other owl species consumed 4–7 prey species. Great Gray Owls consumed five vole species, as well as one lemming, three shrews, two chipmunks, and three birds. Deermice were the most common prey, at 32.4% of individual animals consumed, followed by meadow voles (21.8%) and red-backed voles (18.3%). Long-eared Owls had a narrower diet (Table 2), as they primarily ate meadow voles (21.4%), deermice (17.9%), and chipmunks (14.3%). Chipmunks were 4 of 19 prey items, but because of their large body size, they represented 24.8% of prey biomass. Barred Owls had a high number of prey identifiable only to small mammal (18.8%) or Microtus (25.0%), but 18.8% of the prey were deermice and 12.5% meadow vole; Barred Owls also ate montane voles, red squirrels, and birds (Table 2).

Table 1.
Diets of Great Gray Owls in 2019. We had 68 pellets and 142 prey. AFO: absolute frequency of occurrence (% of pellets containing each prey type); RFO: relative frequency of occurrence (% of a given prey type out of all prey recorded).

Table 2.
Diets of Long-eared Owls and Barred Owls in 2019. For Long-eared Owls, we had 23 pellets and 28 prey; for Barred Owls, 12 pellets and 16 prey. AFO: absolute frequency of occurrence (% of pellets containing each prey type); RFO: relative frequency of occurrence (% of a given prey type out of all prey recorded). Cells with “—“ indicate no prey of that type were consumed.
Great Horned Owls primarily ate prey associated with mature forest (Table 3). Of 17 prey items we identified, five were snowshoe hares, three were red-backed voles, and one was a red squirrel. Although these owls also ate seven meadow voles, these comprised only 3.4% of the owls’ diet by biomass; hares were 92.1%. Notably, no deermice were detected in the diet of Great Horned Owls.

Table 3.
Diets of Great Horned Owls in 2019. We had nine pellets and 17 prey. AFO: absolute frequency of occurrence (% of pellets containing each prey type); RFO: relative frequency of occurrence (% of a given prey type out of all prey recorded). Three of the pellets containing snowshoe hare remains were collected at the same time and location, and could have reflected one snowshoe hare or separate hares.
Northern Saw-whet Owls are the only species for which we had pellets in both 2018 and 2019 (Table 4). In both years, the top three prey species were meadow voles, deermice, and red-backed voles, but the order shifted between years: deermice were the main prey item in 2019 (37.5%), but the third most common prey in 2018 (13.0%). In 2018, 9 of 23 prey (39.1%) could be identified only as “small mammal”; thus, we had few prey that were identifiable to species.

Table 4.
Diets of Northern Saw-whet Owls in 2018 and 2019. For 2018, we had 22 pellets and 23 prey; for 2019, 21 pellets and 24 prey. AFO: absolute frequency of occurrence (% of pellets containing each prey type); RFO: relative frequency of occurrence (% of a given prey type out of all prey recorded). Cells with “—“ indicate no prey of that type were consumed.
4. Discussion
Within a large landscape 1–9 years after severe wildfires and substantial salvage logging, we detected six species of owl, but all had few detections, and several were detected in only one year of the two years we surveyed. We heard Long-eared Owls and Great Horned Owls at 13% of survey points, Barred Owls at 17%, and Northern Saw-whet Owls at 30%. The owls we detected ranged from the small Northern Saw-whet Owl (~20 cm) to medium (Long-eared, Short-eared, Barred, ~35–50 cm) to large (Great Horned, Great Gray, ~50–80 cm). Unsurprisingly, larger owls consumed larger prey, e.g., snowshoe hares and red squirrels, whereas Northern Saw-whet Owls consumed small mammals. The analyses of owl diets suggested some habitat separation, with Barred Owls and Great Horned Owls eating prey associated with older and mature forests (snowshoe hares, red squirrels, red-backed voles), whereas the other owls consumed meadow voles, deermice, and chipmunks, all of which were reasonably common in the disturbed post-fire or post-fire and salvage-logged habitats [50,51].
In boreal forest in Alberta, Great Horned Owls were detected at 54% of acoustically surveyed sites in one study [43], and in 50% of sites in another [68]. In California, Great Horned Owls were negatively affected by severe fire 2–4 years prior [69], and in Oregon 2 years after severe fire, they were detected at only 12% of sites [36]. In line with these studies, we detected Great Horned Owls at 13% of survey locations, suggesting the combination of fire and salvage logging was strongly negative for Great Horned Owls. These owls use platform nests, which likely were less available after fire and salvage logging.
The Great Horned Owls primarily ate snowshoe hares, which are a major prey item for them in boreal forest [70]; because in our system snowshoe hares avoided salvage-logged sites [50], this result implies the Great Horned Owls were hunting in mature forest or in the 2010 burn, even though our few owl detections were in habitats dominated by the 2017 burn or where it had been salvage logged. Finding Great Horned Owls in habitat mosaics is not surprising; in Alberta, they were most abundant in landscapes where there was intermediate forest coverage (35–65% of the landscape [54]).
Short-eared Owls and Long-eared Owls are irruptive species that select areas with high vole abundances for their breeding sites each summer [71,72,73]. We saw one Short-eared Owl in 2019, detected Long-eared Owls at four survey points in 2018, and found one Long-eared Owl nest area. These are low values compared to contemporaneous research we conducted at a cattle ranch ~90 km to the southwest [58,59]. At the OK Ranch study area, we detected three Short-eared Owl nests in 2017 and one in 2019; owls or pellets or both were detected in all years 2016–2020, despite the much smaller study area (4,530 ha vs. 39,866 ha). This difference likely is also because Short-eared Owls are open-country rather than forest owls [74,75], and indeed our one sighting in this study was primarily in the 2010 burn + salvaged area, which had very little tree regeneration. At the OK Ranch we detected Long-eared Owls in 2017 and 2020, in contrast to the 2018 detections in this study; these birds often nest in trees adjacent to grasslands. In this burned landscape, the Long-eared Owls ate exclusively small mammals, with deermice, meadow voles, and chipmunks suggesting that they foraged in the burns or in the burned areas that were salvaged.
Great Gray Owls were detected via finding two roosting areas and documenting chicks. The habitats around these roost sites were almost entirely open, i.e., burned, burned and salvage logged, or grassland. This pattern echoes owl habitat use in California, where owls nested in large snags in dense forest that were adjacent to meadows [76], but owls also nested in burned forests shortly after fire. In our study area, Great Gray Owls ate a wide range of small mammals and a few birds. They ate some red-backed voles, indicative of mature forest, but deermice and meadow voles were each eaten more often than were the red-backed voles. Similarly, Great Gray Owls in Russia had diets dominated by open-country vole species [77], but the owls also consumed forest voles and shrews. This dietary breadth is common for Great Gray Owls, and may make it easier for them to use post-fire areas than for the owl species that are more specialized hunters. Indeed, Great Gray Owls in California actually appeared to be more likely to persist at previously occupied meadow forest sites within three years after fire than for sites that did not burn [76].
The Barred Owls ate a wide range of small mammalian prey, as well as small birds. They were detected in landscapes largely in the 2017 burn, salvage-logged 2017 burn, and mature forest. Regenerating post-fire habitats after the 2010 fire offered diverse small mammal communities and higher abundances of voles than mature, 2017 burn, and 2010 burn + salvage-logged sites [51], so we are not certain where the Barred Owls were hunting. In Oregon, Barred Owls used severely burned areas less often than other areas within a burn but were detected at 50% of sites overall [36], in contrast to our detection of these owls at sites with high amounts of the 2017 burn and salvage or 2017 burn. Barred Owls were present at 18% of survey sites in northern Alberta [68], similar to our 17%. Data for Barred Owls across western North America show wide habitat and diet generalization for this species in their expanded geographic range [55].
Northern Saw-whet Owls in this landscape ate a wide variety of small mammal prey. Out of our sample of 47 prey items, 6 small mammal species were identified, including both open-country prey (chipmunks) and mature forest prey (red-backed voles). This dietary breadth is typical, with owls in other regions also consuming small mammal prey, but eating a completely different suite of species [78].
The Northern Saw-whet Owls were located in areas that were an almost exact match to the distribution of habitats with the overall study area, with most owl detections occurring in locations that had a wide mix of forest and non-forest cover. This result echoes habitat use by these owls in Alberta, where they used landscapes containing a mix of agricultural or open areas and forest [43,54]. However, in sharp contrast, in Oregon, in severely burned parts of a large 2-year-old burn, Northern Saw-whet Owls were almost entirely absent [36], which might have been due to reduced prey, since snags were abundant and other owl species that use similar nests were present in the fire scar. Northern Saw-whet Owls were detected at 15% of survey sites in northern Alberta [43], at ~15–20% of survey sites in the Sierra Nevada Mountains in California [79], at 14% of sites 2 years after fire in Oregon [36], and at 30% of sites in Idaho [80]. In our surveys, we detected them 30% of the time, suggesting the mosaic of open areas and stands with some tree cover was favorable for their nesting and foraging. It would be interesting to have radio-tagged birds at burned sites to discern their habitat use at finer scales, and to determine where they are hunting.
4.1. Landscape Suitability for Owls
As a rough indicator of the effect of fires and salvage logging on owls, we can estimate a maximum number of owl territories by assuming non-overlapping nesting home ranges (as in [58]). If we assume the average owl pair has a home range of 300 ha that does not overlap other owls, if all habitats were suitable, then some ~133 pairs of birds could use the ~40,000 ha study area. We detected owls at only 15 of the 30 survey points. This result suggests that ~66 more pairs could have been there if fires and salvage logging had not reduced habitat quantity and quality. This very rough calculation might in fact underestimate the loss of owls: when habitats are poor, many animals use larger home ranges, and owls often have overlapping home ranges with other owl species: both patterns would make it more likely for owls to be detected. The fact we detected so few owls overall despite repeated efforts over two years suggests that the severe fire and salvage logging resulted in a substantial loss of owls within the first decade post-fire. We note that the increase in megafires over the last few decades is also converting much larger areas of forest than the historically smaller fires; landscape mosaics are substantially different now than they were in prior decades to centuries. Indeed, habitat loss is implicated as a major cause of substantial declines in the number of forest-dwelling birds in North America [81], and historic fire regimes with smaller, more frequent, and less severe fires were probably less damaging to owls than severe megafires [69]. This change in the scale and rapidity of disturbance calls for changes in conservation tactics, specifically to offer more protection to intact areas of mature forest as fewer and fewer such areas remain [82].
4.2. Conclusions
Critically, mature and old-growth forests are becoming ever more rare on landscapes as harvesting and fire remove older trees, which makes all remaining older forest more important to conserve. Foraging habitat is lost if owl species rely on prey that need mature forest. For owl species that require mature trees for reproduction or as perch sites, even small losses of remaining mature forest could have severe negative consequences via eliminating nesting habitat. Similarly, trees killed in fires can act as nests or perches for some owls, whereas logging that removes all standing trees (either living trees or as salvage harvest of dead trees) actively reduces site value for owls that require standing trees or snags. Our results suggest that post-fire salvage-logged areas are less suitable for owls than are burned areas, as witnessed by habitat distribution around owl detections and because several owl species relied on prey that were not present in salvage-logged areas. Thus, when conserving habitat for owls is a management goal, our results imply salvage logging after fire should be minimized.
As wildfires increase with the impacts of climate change and fire suppression, the demand for post-fire salvage-logging operations will likely increase. Fires and subsequent salvage logging occurring in quick succession in neighboring forest give small mammal communities little time to recover, and fewer remaining refugia to recover from. The ripple effects from these large-scale disturbances have the potential to impact owl species and their prey over large ranges of BC forests.
Author Contributions
A.J.K., F.I.D. and K.E.H. all contributed to research design and data analysis. A.J.K. conducted the fieldwork and wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was conducted while we were funded by the Habitat Conservation Trust Foundation for companion research on small mammals. We were also funded by a Natural Sciences and Engineering Research Council of Canada Discovery grant (312222) to K.E.H. and A.J.K. was funded by fellowships from the University of British Columbia Okanagan.
Data Availability Statement
Data are available from the authors upon reasonable request.
Acknowledgments
We thank our hardworking field assistants, E. Brown-Dussault, D. Hunter, E. Hanna, L. Oukil, J. Scherger, and J. Kobetitch. We thank the Suters and the Stowells for housing us through two field seasons.
Conflicts of Interest
The authors declare that they have no conflicts of interest with respect to this work.
References
- Agee, J.K. Disturbance ecology of North American boreal forests and associated northern mixed/subalpine forest. In Ecology and Conservation of Lynx in the United States; Ruggiero, L.F., Aubry, K.B., Buskirk, S.W., Koehler, G.M., Krebs, C.J., McKelvey, K.S., Squires, J.R., Eds.; University of Colorado Press: Boulder, CO, USA, 2000; pp. 39–82. [Google Scholar]
- Coogan, S.C.P.; Daniels, L.D.; Boychuk, D.; Burton, P.J.; Flannigan, M.D.; Gauthier, S.; Kafka, V.; Park, J.S.; Wotton, B.M. Fifty years of wildland fire science in Canada. Can. J. For. Res. 2021, 51, 283–302. [Google Scholar] [CrossRef]
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and earlier spring increase western U.S forest wildfires. Science 2006, 313, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Safford, H.D.; Crimmins, M.; Thode, A.E. Quantitative evidence for increasing fire forest fire severity in the Sierra Nevada and Southern Cascade Mountain, California and Nevada, USA. Ecosystems 2009, 12, 16–32. [Google Scholar] [CrossRef]
- Seidl, R.S.; Schelhaas, M.; Manfred, J.L. Unraveling the drivers of intensifying forest disturbance regimes in Europe. Glob. Change Biol. 2011, 17, 2842–2852. [Google Scholar] [CrossRef]
- Prichard, S.J.; Stevens-Rumann, C.S.; Hessburg, P.F. Shifting global fire regimes: Lessons from reburns and research needs. For. Ecol. Manag. 2017, 396, 217–233. [Google Scholar] [CrossRef]
- Hanes, C.C.; Wang, X.; Jain, P.; Parisien, M.-A.; Little, J.M.; Flannigan, M.D. Fire regime changes in Canada over the last half century. Can. J. For. Res. 2019, 49, 256–259. [Google Scholar] [CrossRef]
- Christianson, A. Social science research on Indigenous wildfire management in the 21st century and future research needs. Int. J. Wildland Fire 2015, 24, 190–200. [Google Scholar] [CrossRef]
- Liebmann, M.J.; Farella, J.; Roos, C.I.; Stack, A.; Martini, S.; Swetnam, T.W. Native American depopulation, reforestation, and fire regimes in the Southwest United States, 1492–1900 CE. Proc. Natl. Acad. Sci. USA 2016, 113, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, C.; Anderson, C.L.; Collingwood, A.; Sissions, R.; Dunn, C.J.; Meigs, G.W.; Hibbs, D.E.; Murphy, S.; Kuiper, S.D.; SpearChief-Morris, J.; et al. Out of the ashes: Ecological resilience to extreme wildfire, prescribed burns, and Indigenous burning in ecosystems. Front. Ecol. Evol. 2019, 7, 436. [Google Scholar] [CrossRef]
- Lake, F.K.; Christianson, A.C. Indigenous Fire Stewardship. In Encyclopedia of Wildfire and Wildland-Urban Interface (WUI) Fires; Mazello, S., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 649–722. [Google Scholar]
- Kelly, L.T.; Giljohann, K.M.; Duane, A.; Aquilu, N.; Archibald, S.; Batllori, E.; Bennett, A.F.; Buckland, S.T.; Canelles, Q.; Clarke, M.F.; et al. Fire and biodiversity in the Anthropocene. Science 2020, 370, 929. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; Lindenmayer, D.B. Temporal fragmentation of a critically endangered forest ecosystem. Austral Ecol. 2020, 45, 340–354. [Google Scholar] [CrossRef]
- Hutchen, J.; Volkmann, L.A.; Hodges, K.E. Experimental designs for studying small-mammal responses to fire in North American conifer forests. Int. J. Wildland Fire 2017, 26, 523–531. [Google Scholar] [CrossRef]
- Volkmann, L.; Hutchen, J.; Hodges, K.E. Trends in carnivore and ungulate fire ecology research in North American conifer forests. For. Ecol. Manag. 2020, 458, 117691. [Google Scholar] [CrossRef]
- Eng, M. Forest Stewardship in the Context of Large-Scale Salvage Operations: An Interpretation Paper; Technical Report 019; B.C. Ministry of Forests: Victoria, BC, Canada, 2004. [Google Scholar]
- Saint-Germain, M.; Greene, D.F. Salvage logging in the boreal and cordilleran forests of Canada: Integrating industrial and ecological concerns in management plans. For. Chron. 2009, 85, 120–134. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Lindenmayer, D.B.; Thorn, S.; Gustafsson, L. Salvage logging in the world’s forests: Interactions between natural disturbance and logging need recognition. Glob. Ecol. Bioegeogr. 2018, 27, 1140–1154. [Google Scholar] [CrossRef]
- Thorn, S.; Bässler, C.; Brandl, R.; Burton, P.J.; Cahall, R.; Campbell, J.L.; Castro, J.; Chio, C.-Y.; Cobb, T.; Donato, D.C.; et al. Impacts of salvage logging on biodiversity: A meta-analysis. J. Appl. Ecol. 2018, 55, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Bowd, E.J.; Banks, S.C.; Bissett, A.; May, T.W.; Lindenmayer, D.B. Direct and indirect disturbance impacts in forests. Ecol. Lett. 2021, 24, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Povak, N.A.; Churchill, D.J.; Cansler, C.A.; Hessburg, P.F.; Kane, V.R.; Kane, J.T.; Lutz, J.A.; Larson, A.J. Wildfire severity and postfire salvage harvest effects on long-term forest regeneration. Ecosphere 2020, 11, e03199. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Noss, R.F. Salvage logging, ecosystem processes, and biodiversity conservation. Conserv. Biol. 2006, 20, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Gayton, D.; Almuedo, L. Post-disturbance management of biodiversity in BC forests. BC J. Ecosyst. Manag. 2012, 13, 1–9. [Google Scholar] [CrossRef]
- Meigs, G.W.; Krawchuk, M.A. Composition and structure of forest fire refugia: What are the ecosystem legacies across burned landscapes? Forests 2018, 9, 243. [Google Scholar] [CrossRef]
- Nappi, A.; Drapeau, P.; Savard, J.P.L. Salvage logging after wildfire in the boreal forest: Is it becoming a hot issue for wildlife? For. Chron. 2004, 80, 67–74. [Google Scholar] [CrossRef]
- Geary, W.L.; Doherty, T.S.; Nimmo, D.G.; Tulloch, A.I.T.; Ritchir, E.G. Predator responses to fire: A global systematic review and meta-analysis. J. Anim. Ecol. 2019, 89, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Jager, H.I.; Long, J.W.; Malison, R.L.; Murphy, B.P.; Rust, A.; Silva, L.G.M.; Sollmann, R.; Steel, Z.L.; Bowen, M.D.; Dunham, J.B.; et al. Resilience of terrestrial and aquatic fauna to historical and future wildfire regimes in western North America. Ecol. Evol. 2021, 11, 12259–12284. [Google Scholar] [CrossRef] [PubMed]
- Farnell, I.; Elkin, C.; Lilles, E.; Roberts, A.M.; Venter, M. The effects of variable retention forestry on coarse woody debris dynamics and concomitant impacts on American marten habitat after 27 years. Can. J. For. Res. 2020, 50, 925–935. [Google Scholar] [CrossRef]
- Caswell, D. Species Habitat Model for Barred Owl. Available online: www.for.gov.bc.ca/hfd/library/fia/2008/LBIP_4765001a.pdf (accessed on 3 June 2025).
- Caswell, D. Species Habitat Model for Great Horned Owl. Available online: www.for.gov.bc.ca/hfd/library/fia/2008/LBIP_4765001b.pdf (accessed on 3 June 2025).
- Grassy Mountain Coal Project. Appendix C: Wildlife Habitat Suitability Models; Benga Mining Limited: Calgary, AB, Canada, 2016. Available online: www.ceaa-acee.gc.ca/050/documents/p80101/115629E.pdf (accessed on 3 June 2025).
- Caswell, D. Species Habitat Model for Northern Saw-Whet Owl. Available online: www.for.gov.bc.ca/hfd/library/fia/2008/LBIP_4765001c.pdf (accessed on 3 June 2025).
- Hannah, K.C.; Hoyt, J.S. Northern Hawk Owls and recent burns: Does burn age matter? Condor 2004, 106, 420–423. [Google Scholar] [CrossRef]
- Hinam, H.L.; Clair, C.C.S. High levels of habitat loss and fragmentation limit reproductive success by reducing home range size and provisioning rates of Northern saw-whet owls. Biol. Conserv. 2008, 141, 524–535. [Google Scholar] [CrossRef]
- Hannah, K.C. Call playbacks increase detection rates of northern hawk owls in recent burns. J. Raptor Res. 2009, 43, 241–244. [Google Scholar] [CrossRef]
- Duchac, L.S.; Lesmeister, D.B.; Dugger, K.M.; Davis, R.J. Differential landscape use by forest owls two years after a mixed-severity wildfire. Ecosphere 2021, 12, 203770. [Google Scholar] [CrossRef]
- Bond, M.L.; Lee, D.E.; Siegel, R.B.; Ward, J.P. Habitat use and selection by California Spotted Owls in a postfire landscape. J. Wildl. Manag. 2009, 73, 1116–1124. [Google Scholar] [CrossRef]
- Bond, M.L.; Bradley, C.; Lee, D.E. Foraging habitat selection by California Spotted Owls after fire. J. Wildl. Manag. 2016, 80, 1290–1300. [Google Scholar] [CrossRef]
- Ganey, J.L.; Kyle, S.C.; Rawlinson, T.A.; Apprill, D.L.; Ward, J.P. Relative abundance of small mammals in nest core areas and burned wintering areas of Mexican Spotted Owls in the Sacramento Mountains, New Mexico. Wilson J. Ornithol. 2014, 126, 47–52. [Google Scholar] [CrossRef]
- Sahores, M.; Trejo, A. Diet shift of Barn Owls (Tyto alba) after natural fires in Patagonia, Argentina. J. Raptor Res. 2004, 38, 174–177. [Google Scholar]
- Rockweit, J.T.; Franklin, A.B.; Carlson, P.C. Differential impacts of wildfire on the population dynamics of an old-forest species. Ecology 2017, 98, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Village, A. The diet and breeding of Long-eared Owls in relation to vole numbers. Bird Study 1981, 28, 214–224. [Google Scholar] [CrossRef]
- Domahidi, Z.; Shonfield, J.; Nielsen, S.E.; Spence, J.R.; Bayne, E.M. Spatial distribution of the Boreal Owl and Northern Saw-Whet Owl in the Boreal region of Alberta, Canada. Avian Conserv. Ecol. 2019, 14, 14. [Google Scholar] [CrossRef]
- Clark, R.J. A field study of the short-eared owl, Asio flammeus (Pontoppidan), in North America. Wildl. Monogr. 1975, 47, 3–67. [Google Scholar]
- Marti, C.D. A review of prey selection by the long-eared owl. Condor 1976, 78, 331–336. [Google Scholar] [CrossRef]
- Block, W.M.; Ganey, J.L.; Scott, P.E.; King, R. Prey ecology of Mexican spotted owls in pine-oak forests of northern Arizona. J. Wildl. Manag. 2005, 69, 618–629. [Google Scholar] [CrossRef]
- Cheveau, M.; Drapeau, P.; Imbeau, L. Owl winter irruptions as an indicator of small mammal population cycles in the boreal forest of eastern North America. Oikos 2004, 107, 190–198. [Google Scholar] [CrossRef]
- Selcuk, A.Y.; Bankoglu, K.; Kefelioglu, H. Comparison of winter diet of long-eared owls Asio otus (L., 1758) and short-eared owls Asio flammeus (Pontoppidan, 1763) (Ayes: Strigidae) in Northern Turkey. Acta Zool. Bulg. 2017, 69, 345–348. [Google Scholar]
- Deshler, J.F.; Murphy, M.T. The breeding biology of the northern pygmy-owl: Do the smallest of the small have an advantage? Condor 2012, 114, 314–322. [Google Scholar] [CrossRef]
- Kelly, A.J.; Hodges, K.E. Post-fire salvage logging reduces snowshoe hare and red squirrel density in early seral stages. For. Ecol. Manag. 2020, 473, 118272. [Google Scholar] [CrossRef]
- Kelly, A.J.; Hodges, K.E. Post-fire salvage logging alters impacts of recent wildfire on small mammal communities and abundances in summer. J. Mammal. 2022, 103, 1168–1181. [Google Scholar] [CrossRef]
- Hausleitner, D. Inventory Methods for Owl Surveys: Standards for Components of British Columbia’s Biodiversity No. 42. Prepared for: Ministry of Environment, Ecosystems Branch; Selkirk College: Castelgar, BC, Canada, 2006; iv + 52p. [Google Scholar]
- Andersen, D.E. Survey Techniques. In Raptor Research and Management Techniques; Bird, D.M., Bildstein, K.L., Eds.; Hancock House Publishers Ltd.: Surrey, BC, Canada, 2007; pp. 89–100. [Google Scholar]
- Grossman, S.R.; Hannon, S.J.; Sánchez-Azofeifa, A. Responses of Great Horned Owls (Bubo virginianus), Barred Owls (Strix varia), and Northern Saw-whet Owls (Aegolius acadicus) to forest cover and configuration in an agricultural landscape in Alberta, Canada. Can. J. Zool. 2008, 86, 1165–1172. [Google Scholar] [CrossRef]
- Livezey, K.B. Barred Owl habitat and prey: A review and synthesis of the literature. J. Raptor Res. 2007, 41, 177–201. [Google Scholar] [CrossRef]
- Waterhouse, F.L.; Doyle, F.I.; Turney, L.; Wijdeven, B.; Todd, M.; Bergman, C.; Vennesland, R.G. Spring and winter home ranges of the Haida Gwaii Northern Saw-Whet Owl (Aegolius acadicus brooksi). J. Raptor Res. 2017, 51, 153–164. [Google Scholar] [CrossRef]
- Marti, C.D.; Bechard, M.; Jaksic, F.M. Food habits. In Raptor Research and Management Techniques; Bird, D.M., Bildstein, K.L., Eds.; Hancock House Publishers Ltd.: Surrey, BC, Canada, 2007; pp. 129–152. [Google Scholar]
- Ormrod, A.; Doyle, F.I.; Lawson, K.J.; Hodges, K.E. Niche partitioning of avian predators in northern grasslands amended by biosolids. Ecol. Evol. 2021, 11, 6248–6259. [Google Scholar] [CrossRef] [PubMed]
- Meineke, J.; Doyle, F.I.; Hodges, K.E. Raptors benefit from biosolids applications on rangelands. Avian Conserv. Ecol. 2023, 18, 25. [Google Scholar] [CrossRef]
- Francksen, R.M.; Whittingham, M.J.; Baines, D. Assessing prey provisioned to Common Buzzard Buteo buteo chicks: A comparison of methods. Bird Study 2016, 63, 303–310. [Google Scholar] [CrossRef]
- Bocheński, Z.M. Owls, diurnal raptors, and humans: Signatures on avian bones. In Biosphere to Lithosphere; O’Connor, T., Ed.; Oxbow Books: Oxford, UK, 2005; pp. 31–45. [Google Scholar]
- Sharikov, A.; Kovinka, T.; Bragin, M. A comparative laboratory study of the preservation of different rodent bones in pellets of Strigiformes. Ornis Fenn. 2018, 95, 82–88. [Google Scholar] [CrossRef]
- Tornberg, R.; Reif, V. Assessing the diet of birds of prey: A comparison of prey items found in nests and images. Ornis Fenn. 2007, 84, 21–31. [Google Scholar]
- Korpimäki, E.; Norrdahl, K. Numerical and functional responses of Kestrels, Short-eared Owls, and Long-eared Owls to vole densities. Ecology 1991, 72, 814–826. [Google Scholar] [CrossRef]
- Nagorsen, D.W. Rodents and Lagomorphs of British Columbia; Royal British Columbia Museum: Victoria, BC, Canada, 2005. [Google Scholar]
- Heisler, L.M.; Somers, C.M.; Poulin, R.G. Owl pellets: A more effective alternative to conventional trapping for broad-scale studies of small mammal communities. Methods Ecol. Evol. 2016, 7, 96–103. [Google Scholar] [CrossRef]
- Foresman, K.R. Key to the Mammals of Montana; University of Montana: Missoula, MT, USA, 2001. [Google Scholar]
- Shonfield, J.; Bayne, E.M. Weak support for cumulative effects of industrial disturbance on three owl species in Alberta’s boreal forest. Avian Conserv. Ecol. 2023, 18, 9. [Google Scholar] [CrossRef]
- McGinn, K.; Zuckerberg, B.; Jones, G.M.; Wood, C.M.; Kahl, S.; Kelly, K.G.; Whitmore, S.A.; Kramer, H.A.; Barry, J.M.; Ng, E.; et al. Frequent, heterogenous fire supports a forest owl assemblage. Ecol. Appl. 2025, 35, e3080. [Google Scholar] [CrossRef] [PubMed]
- Rohner, C.; Doyle, F.I.; Smith, J.N.M. Great Horned Owls. In Ecosystem Dynamics of the Boreal Forest: The Kluane Project; Krebs, C.J., Boutin, S., Boonstra, R., Eds.; Oxford University Press: NewYork, NY, USA, 2001. [Google Scholar]
- Poulin, R.G.; Wellicome, T.I.; Todd, L.D. Synchronous and delayed numerical responses of a predatory bird community to a vole outbreak on the Canadian prairies. J. Raptor Res. 2001, 35, 288–295. [Google Scholar]
- Houston, C.S. Long-eared Owls, Asio otus: A review of North American banding. Can. Field Nat. 2005, 119, 395–402. [Google Scholar] [CrossRef]
- Wiggins, D.A.; Holt, D.W.; Leasure, S.M. Short-eared Owl (Asio flammeus), version 1.0. In Birds of the World; Billerman, S.M., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Booms, T.L.; Holroyd, G.L.; Gahbauer, M.A.; Trefry, H.E.; Wiggins, D.A.; Holt, D.W.; Johnson, J.A.; Lewis, S.B.; Larson, M.D.; Keyes, K.L.; et al. Assessing the status and conservation priorities of the Short-eared Owl in North America. J. Wildl. Manag. 2014, 78, 772–778. [Google Scholar] [CrossRef]
- Miller, R.A.; Buchanan, J.B.; Pope, T.L.; Carlisle, J.D.; Moulton, C.E.; Booms, T.L. Short-eared Owl land-use associations during the breeding season in the western United States. J. Raptor Res. 2022, 56, 273–286. [Google Scholar] [CrossRef]
- Siegel, R.B.; Eyes, S.A.; Tingley, M.W.; Wu, J.X.; Stock, S.L.; Medley, J.R.; Kalinowski, R.S.; Casas, A.; Lima-Baumbach, M.; Rich, A.C. Short-term resilience of Great Gray Owls to a megafire in California, USA. Condor 2019, 121, duy019. [Google Scholar] [CrossRef]
- Kropacheva, Y.E.; Smirnov, N.G.; Zykov, S.V.; Cheprakov, M.I.; Sadykova, N.O.; Bachurin, G.N. The diet of the Great Gray Owl, Strix nebulosa, at different levels of prey abundance during the nesting season. Russ. J. Ecol. 2019, 50, 43–49. [Google Scholar] [CrossRef]
- Woodruff, J.M.; Prince, B.A.; Bogiatto, R.J.; Hatfield, C.A. Notes on the fall-winter diet of Northern Saw-whet Owls in northern California. West. N. Am. Nat. 2020, 80, 74–75. [Google Scholar] [CrossRef]
- Groce, J.E.; Morrison, M.L. Habitat use by Saw-Whet Owls in the Sierra Nevada. J. Wildl. Manag. 2010, 74, 1523–1532. [Google Scholar] [CrossRef]
- Scholer, M.N.; Leu, M.; Belthoff, J.R. Factors associated with Flammulated Owl and Northern Saw-whet Owl occupancy in southern Idaho. J. Raptor Res. 2014, 48, 128–141. [Google Scholar] [CrossRef]
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Noss, R.F.; Thorn, S.; Bassler, C.; Leverkus, A.B.; Lindenmayer, D. Increasing disturbance demands new policies to conserve intact forest. Conserv. Lett. 2018, 12, e12449. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).