Imaging Recommendations for Diagnosis, Staging, and Management of Primary Central Nervous System Neoplasms in Adults
Abstract
:Simple Summary
Abstract
1. Introduction
2. Epidemiology
3. Clinical Presentation and Evaluation
4. Imaging Techniques for CNS Neoplasms
4.1. Computed Tomography (CT)
4.2. Magnetic Resonance Imaging (MRI)
4.3. Spinal Imaging
4.4. Ultrasonography (USG)
4.5. Nuclear Medicine
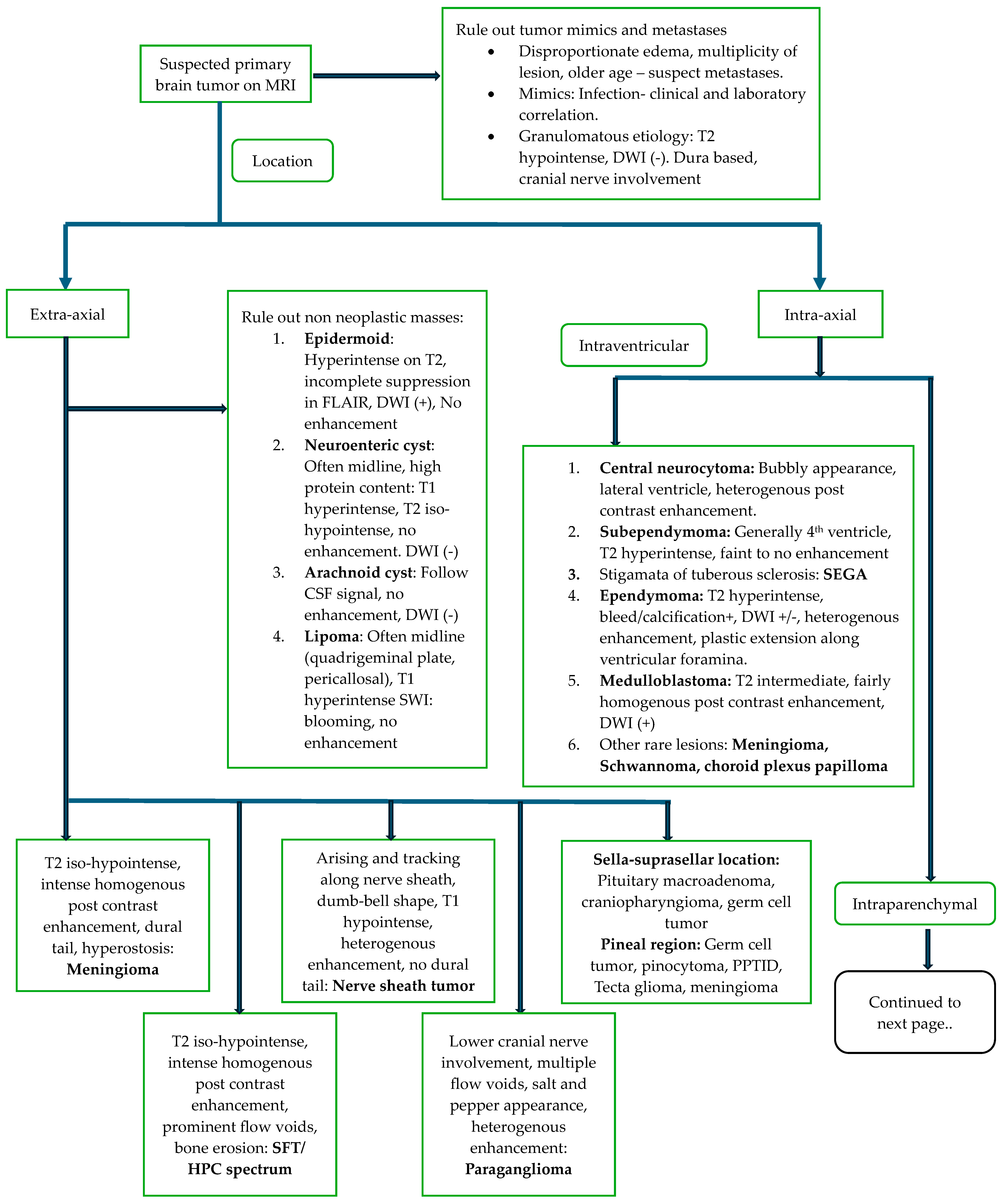
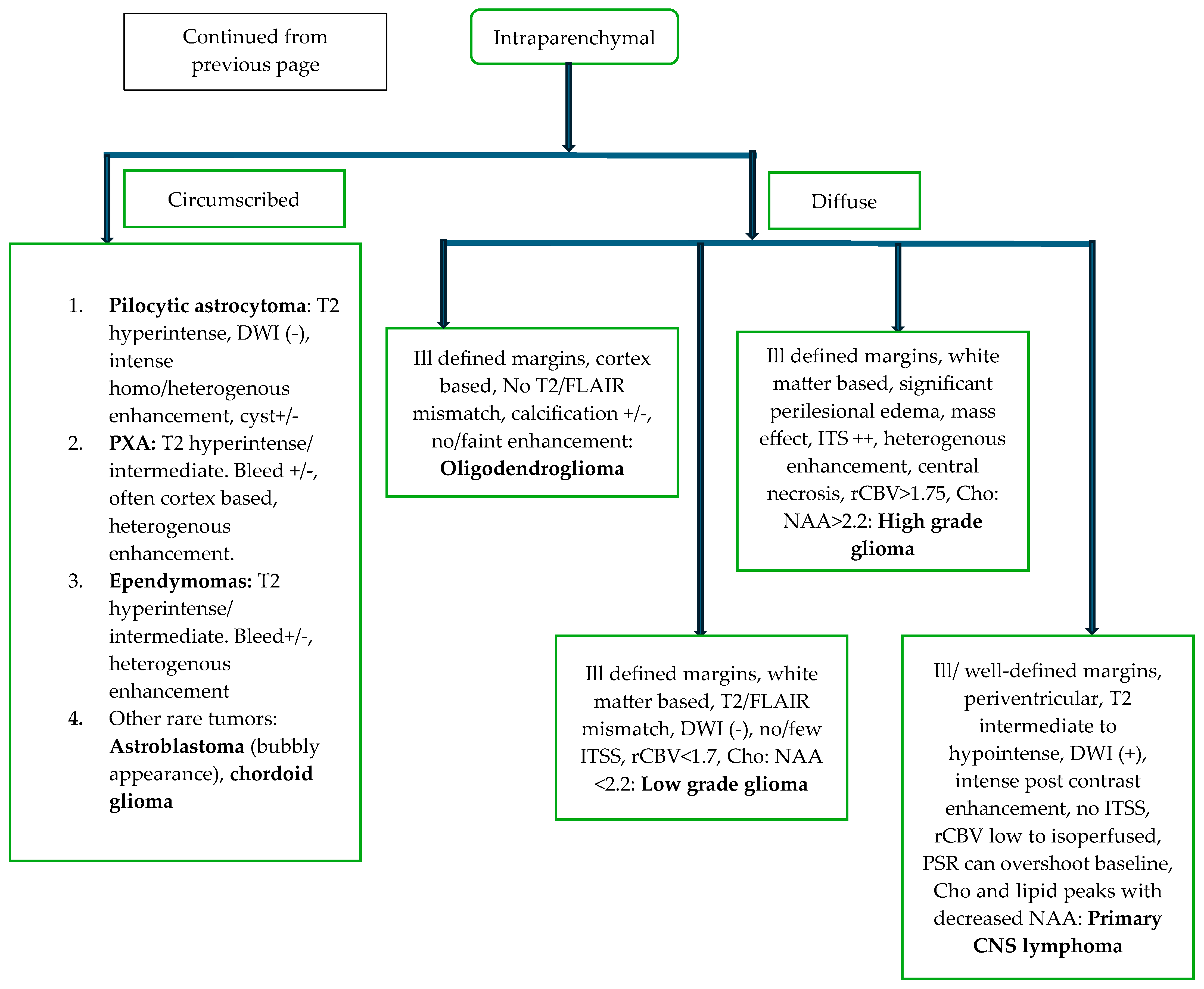
5. Role of Imaging in Primary CNS Neoplasms
5.1. Screening
5.2. Diagnosis
5.3. Intraoperative Imaging
5.4. Immediate Post-Operative Imaging
5.5. Follow Up Imaging
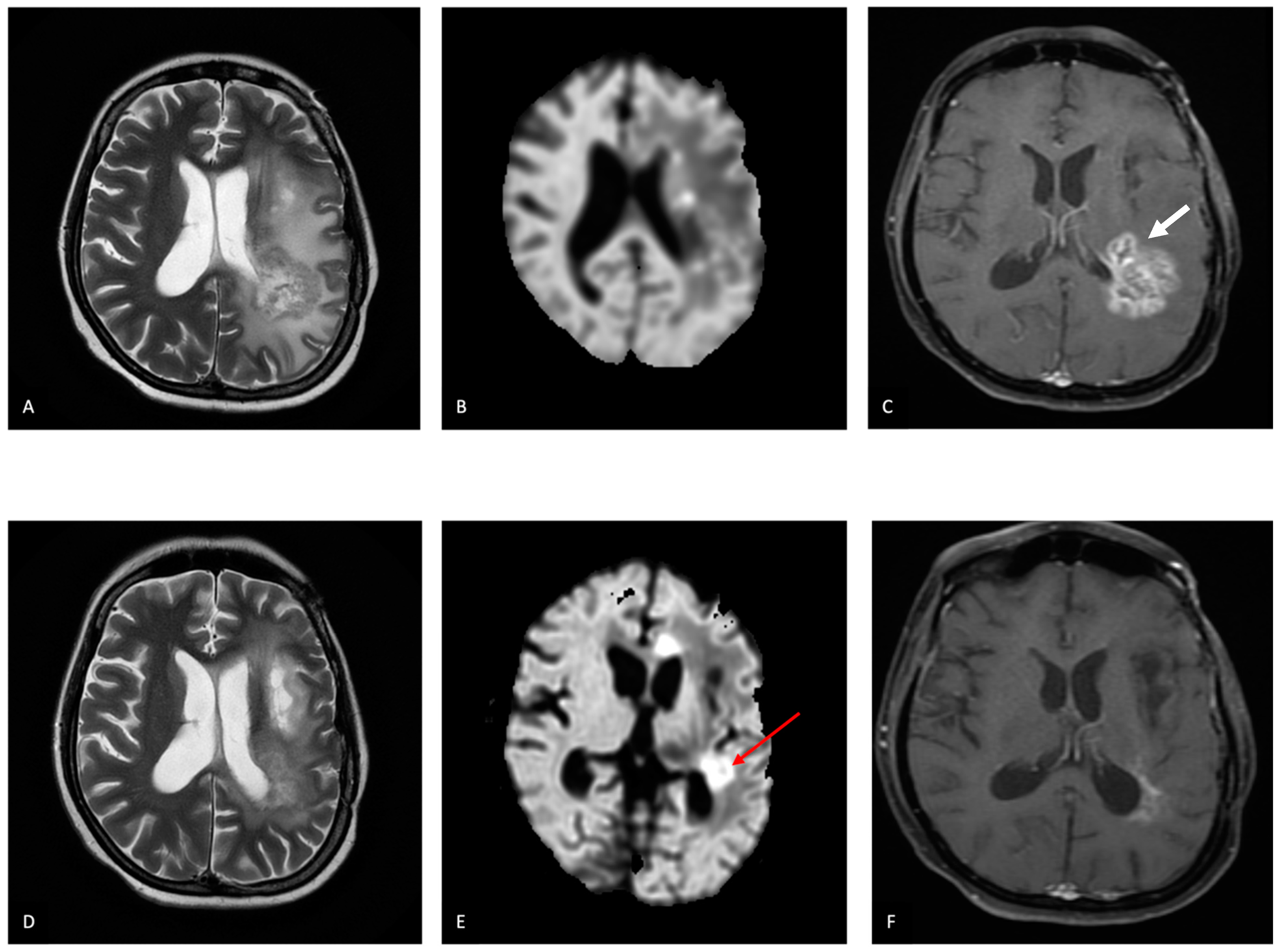
5.5.1. Glioma
5.5.2. Meningioma
5.5.3. Pituitary Tumors
5.6. Role of Nuclear Medicine
6. Role of Artificial Intelligence in Primary CNS Neoplasms
6.1. Pre-Treatment Prediction
6.2. Pseudoprogression
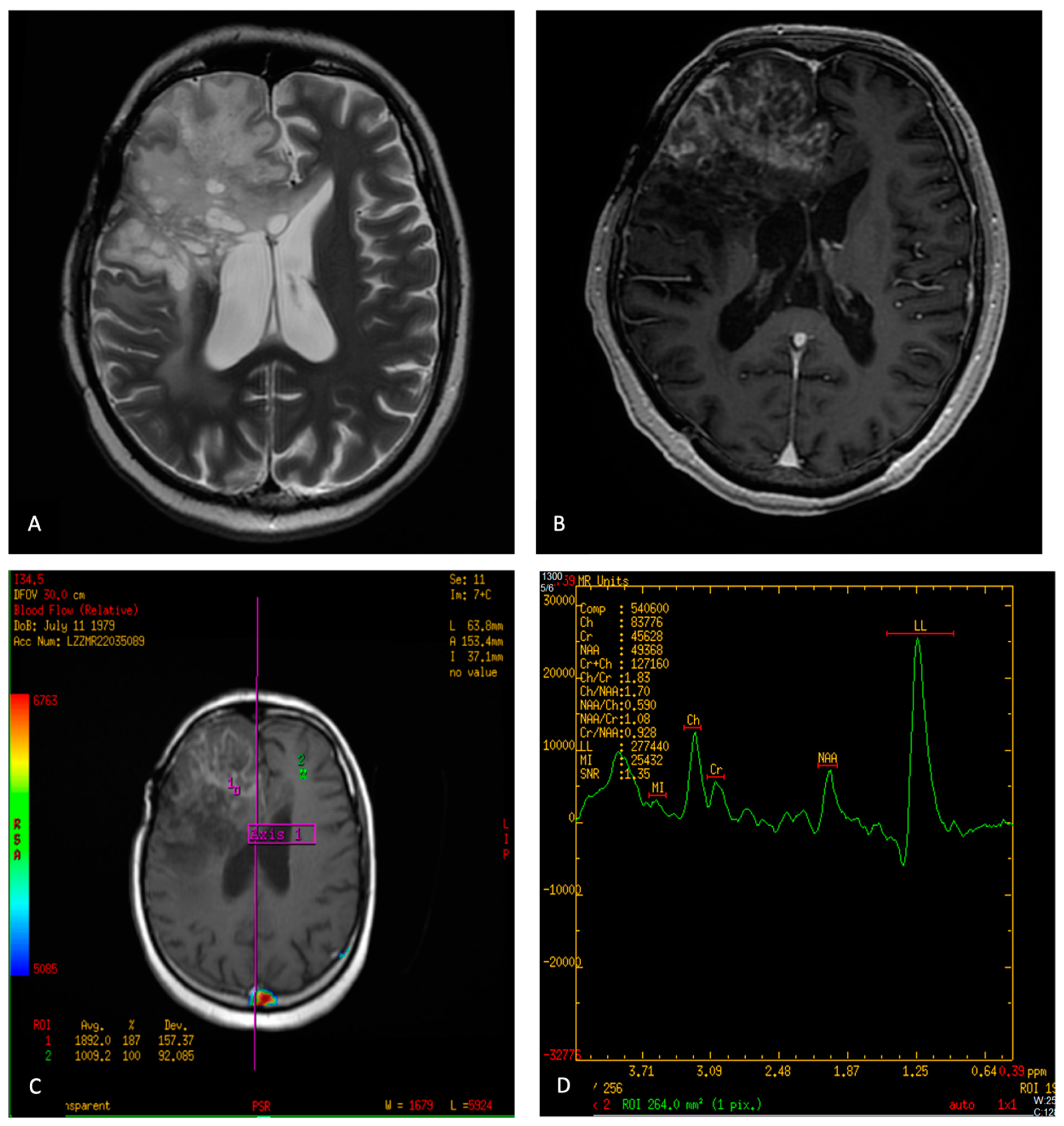
6.3. Radiation Necrosis (RN) and True Progression/Recurrence (Treatment Response Evaluation)
6.4. Role of AI in Imaging of Other Primary CNS Neoplasms
7. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022, 24 (Suppl. S5), v1–v95. [Google Scholar] [CrossRef]
- Dasgupta, A.; Gupta, T.; Jalali, R. Indian data on central nervous tumors: A summary of published work. South Asian J. Cancer 2016, 5, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Osborn, A.G. Osborn’s Brain: Imaging, Pathology and Anatomy, 2nd ed.Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Alomar, S.A. Clinical manifestation of central nervous system tumor. Semin. Diagn. Pathol. 2010, 27, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.P.; Ferreira, N.P.; Filho, A.A.P.; Filho, G.A.P.; Franciscatto, A.C. Stereotactic computed tomography–guided brain biopsy: Diagnostic yield based on a series of 170 patients. Surg. Neurol. 2006, 65, S27–S32. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Jyotsnarani, Y.; Uppin, S.G.; Susarla, R. Imaging features of primary tumors of the spine: A pictorial essay. Indian J. Radiol. Imaging 2016, 26, 279–289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhattacharya, K.; Rastogi, S.; Mahajan, A. Post-treatment imaging of gliomas: Challenging the existing dogmas. Clin. Radiol. 2024, 79, e376–e392. [Google Scholar] [CrossRef] [PubMed]
- Aydın, Z.B.; Aydın, H.; Birgi, E.; Hekimoğlu, B. Diagnostic Value of Diffusion-weighted Magnetic Resonance (MR) Imaging, MR Perfusion, and MR Spectroscopy in Addition to Conventional MR Imaging in Intracranial Space-occupying Lesions. Cureus 2019, 11, e6409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, J.; Xu, W.; Chen, X.; Zhang, J.; Xu, B. Role of magnetic resonance spectroscopy to differentiate high-grade gliomas from metastases. Tumor Biol. 2017, 39, 1010428317710030. [Google Scholar] [CrossRef]
- Neska-Matuszewska, M.; Bladowska, J.; Sąsiadek, M.; Zimny, A. Differentiation of glioblastoma multiforme, metastases and pri-mary central nervous system lymphomas using multiparametric perfusion and diffusion MR imaging of a tumor core and a peritumoral zone-Searching for a practical approach. PLoS ONE 2018, 13, e0191341. [Google Scholar] [CrossRef]
- Seo, M.; Choi, Y.; Soo Lee, Y.; Ahn, K.-J.; Kim, B.-S.; Park, J.-S.; Jeon, S.S. Glioma grading using multiparametric MRI: Head-to-head comparison among dynamic susceptibility contrast, dynamic contrast-enhancement, diffusion-weighted images, and MR spectroscopy. Eur. J. Radiol. 2023, 165, 110888. [Google Scholar] [CrossRef]
- Kong, L.-W.; Chen, J.; Zhao, H.; Yao, K.; Fang, S.-Y.; Wang, Z.; Wang, Y.-Y.; Li, S.-W. Intratumoral Susceptibility Signals Reflect Biomarker Status in Gliomas. Sci. Rep. 2019, 9, 17080. [Google Scholar] [CrossRef]
- Manan, A.A.; Yahya, N.; Idris, Z.; Manan, H.A. The Utilization of Diffusion Tensor Imaging as an Image-Guided Tool in Brain Tumor Resection Surgery: A Systematic Review. Cancers 2022, 14, 2466. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, T.N.; Andreoli, M.J.; Nair, V.A.; Yin, P.; Young, B.M.; Kundu, B.; Pankratz, J.; Radtke, A.; Holdsworth, R.; Kuo, J.S.; et al. Usage of fMRI for pre-surgical planning in brain tumor and vascular lesion patients: Task and statistical threshold effects on language lateralization. NeuroImage Clin. 2015, 7, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Asao, C.; Korogi, Y.; Kitajima, M.; Hirai, T.; Baba, Y.; Makino, K.; Kochi, M.; Morishita, S.; Yamashita, Y. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am. J. Neuroradiol. 2005, 26, 1455–1460. [Google Scholar] [PubMed]
- Hein, P.A.; Eskey, C.J.; Dunn, J.F.; Hug, E.B. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: Tumor recurrence versus radiation injury. AJNR Am. J. Neuroradiol. 2004, 25, 201–209. [Google Scholar] [PubMed]
- van Dijken, B.R.J.; van Laar, P.J.; Holtman, G.A.; van der Hoorn, A. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur. Radiol. 2017, 27, 4129–4144. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, L.; Wang, Q.; Zheng, X.; Wu, C.; Xu, B.-N. Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: A systematic review and meta-analysis. Eur. J. Radiol. 2014, 83, 2181–2189. [Google Scholar] [CrossRef]
- Barajas, R.F., Jr.; Chang, J.S.; Segal, M.R.; Parsa, A.T.; McDermott, M.W.; Berger, M.S.; Cha, S. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2009, 253, 486–496. [Google Scholar] [CrossRef]
- Clarke, J.L.; Chang, S. Pseudoprogression and pseudoresponse: Challenges in brain tumor imaging. Curr. Neurol. Neurosci. Rep. 2009, 9, 241–246. [Google Scholar] [CrossRef]
- Nguyen, H.S.; Milbach, N.; Hurrell, S.L.; Cochran, E.; Connelly, J.; Bovi, J.A.; Schultz, C.J.; Mueller, W.M.; Rand, S.D.; Schmainda, K.M.; et al. Progressing Bevacizumab-Induced Diffusion Restriction Is Associated with Coagulative Necrosis Surrounded by Viable Tumor and Decreased Overall Survival in Patients with Recurrent Glioblastoma. AJNR Am. J. Neuroradiol. 2016, 37, 2201–2208. [Google Scholar] [CrossRef]
- Chung, J.Y.; Lee, J.J.; Kim, H.J.; Seo, H.Y. Characterization of Magnetic Resonance Images for Spinal Cord Tumors. Asian Spine J. 2008, 2, 15–21. [Google Scholar] [CrossRef]
- Yuh, E.L.; Barkovich, A.J.; Gupta, N. Imaging of ependymomas: MRI and CT. Childs Nerv. Syst. 2009, 25, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.Y.; Chen, T.W.; Zhang, X.M.; Huang, X.H. GRE T2∗-weighted MRI: Principles and clinical applications. BioMed Res. Int. 2014, 2014, 312142. [Google Scholar] [CrossRef] [PubMed]
- Allam, K.E.; Elkhalek, Y.I.A.; Hassan, H.G.E.M.A.; Emara, M.A.E. Diffusion-weighted magnetic resonance imaging in differentiation between different vertebral lesions using ADC mapping as a quantitative assessment tool. Egypt. J. Radiol. Nucl. Med. 2022, 53, 155. [Google Scholar] [CrossRef]
- Ahmad, F.U.; Li, D.C.; Malcolm, J.G.; Rindler, R.S.; Baum, G.R.; Rao, A.; Khurpad, S.N. The role of diffusion tensor imaging in spinal pathology: A review. Neurol. India 2017, 65, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Y.A.; Chow, D.; Talbott, J.; Glastonbury, C.; Shah, V. Practical applications of CISS MRI in spine imaging. Eur. J. Radiol. Open 2019, 6, 231–242. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Yao, B.; Sun, P.; Hao, Y.; Piao, H.; Zhao, X. Application of Multiparametric Intraoperative Ultrasound in Glioma Surgery. BioMed Res. Int. 2021, 2021, 6651726. [Google Scholar] [CrossRef]
- Moiyadi, A.V.; Kannan, S.; Shetty, P. Navigated intraoperative ultrasound for resection of gliomas: Predictive value, influence on resection and survival. Neurol. India 2015, 63, 727–735. [Google Scholar] [CrossRef]
- Neugut, A.I.; Sackstein, P.; Hillyer, G.C.; Jacobson, J.S.; Bruce, J.; Lassman, A.B.; Stieg, P.A. Magnetic Resonance Imaging-Based Screening for Asymptomatic Brain Tumors: A Review. Oncologist 2018, 24, 375–384. [Google Scholar] [CrossRef]
- Prada, C.E.; Hufnagel, R.B.; Hummel, T.R.; Lovell, A.M.; Hopkin, R.J.; Saal, H.M.; Schorry, E.K. The Use of Magnetic Resonance Imaging Screening for Optic Pathway Gliomas in Children with Neurofibromatosis Type. J. Pediatr. 2015, 167, 851–856.e1. [Google Scholar] [CrossRef]
- Consul, N.; Amini, B.; Ibarra-Rovira, J.J.; Blair, K.J.; Moseley, T.W.; Taher, A.; Shah, K.B.; Elsayes, K.M. Li-Fraumeni Syndrome and Whole-Body MRI Screening: Screening Guidelines, Imaging Features, and Impact on Patient Management. Am. J. Roentgenol. 2021, 216, 252–263. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2021, 19, 77–102. [Google Scholar] [CrossRef] [PubMed]
- Engelhard, H.H.; Corsten, L.A. Leptomeningeal Metastasis of Primary Central Nervous System (CNS) Neoplasms. In Leptomeningeal Metastases; Abrey, L.E., Chamberlain, M.C., Engelhard, H.H., Eds.; Springer: Boston, MA, USA, 2005; pp. 71–85. [Google Scholar]
- Tiefenbach, J.; Lu, V.M.; Metzler, A.R.; Palejwala, A.; Haider, S.; Ivan, M.E.; Komotar, R.J.; Shah, A.H. The use of advanced neuroimaging modalities in the evaluation of low-grade glioma in adults: A literature review. Neurosurg. Focus 2024, 56, E3. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.-M.S.; Megally, H.I.; Khallaf, M.; Haseib, A. The combined role of MR spectroscopy and perfusion imaging in preoperative differentiation between high- and low-grade gliomas. Egypt. J. Radiol. Nucl. Med. 2019, 50, 72. [Google Scholar] [CrossRef]
- Villanueva-Meyer, J.E.; Mabray, M.C.; Cha, S. Current Clinical Brain Tumor Imaging. Neurosurgery 2017, 81, 397–415. [Google Scholar] [CrossRef]
- Al-Okaili, R.N.; Krejza, J.; Woo, J.H.; Wolf, R.L.; O’Rourke, D.M.; Judy, K.D.; Poptani, H.; Melhem, E.R. Intraaxial Brain Masses: MR Imaging–based Diagnostic Strategy—Initial Experience. Radiology 2007, 243, 539–550. [Google Scholar] [CrossRef]
- Singhal, V. Clinical Approach to Acute Decline in Sensorium. Indian J. Crit. Care Med. 2019, 23 (Suppl. S2), S120–S123. [Google Scholar]
- Maschio, M.; Aguglia, U.; Avanzini, G.; Banfi, P.; Buttinelli, C.; Capovilla, G.; Luisa Casazza, M.M.; Colicchio, G.; Coppola, A.; Costa, C.; et al. Management of epilepsy in brain tumors. Neurol. Sci. 2019, 40, 2217–2234. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Rao, K.; Pastorino, S.; Kesari, S. Corticosteroids in brain cancer patients: Benefits and pitfalls. Expert Rev. Clin. Pharmacol. 2011, 4, 233–242. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Akshulakov, S.K.; Kerimbayev, T.T.; Biryuchkov, M.Y.; Urunbayev, Y.A.; Farhadi, D.S.; Byvaltsev, V.A. Current Trends for Improving Safety of Stereotactic Brain Biopsies: Advanced Optical Methods for Vessel Avoidance and Tumor Detection. Front. Oncol. 2019, 9, 947. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, K.-N.; Wang, Q.; Li, G.; Zeng, F.; Zhang, Y.; Wu, F.; Chai, R.; Wang, Z.; Zhang, C.; et al. Chinese Glioma Genome Atlas (CGGA): A Comprehensive Resource with Functional Genomic Data from Chinese Glioma Patients. Genom. Proteom. Bioinform. 2021, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McFaline-Figueroa, J.R.; Lee, E.Q. Brain Tumors. Am. J. Med. 2018, 131, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-Oncol. 2021, 23, 1821–1834. [Google Scholar] [CrossRef] [PubMed]
- Molitch, M.E. Diagnosis and Treatment of Pituitary Adenomas: A Review. JAMA 2017, 317, 516–524. [Google Scholar] [CrossRef]
- von Baumgarten, L.; Illerhaus, G.; Korfel, A.; Schlegel, U.; Deckert, M.; Dreyling, M. The Diagnosis and Treatment of Primary CNS Lymphoma. Dtsch. Arztebl. Int. 2018, 115, 419–426. [Google Scholar] [CrossRef]
- Hoang-Xuan, K.; Deckert, M.; Ferreri, A.J.M.; Furtner, J.; Perez-Larraya, J.G.; Henriksson, R.; Hottinger, A.F.; Kasenda, B.; Lefranc, F.; Lossos, A.; et al. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro-Oncol. 2023, 25, 37–53. [Google Scholar] [CrossRef]
- Kamepalli, H.; Kalaparti, V.; Kesavadas, C. Imaging Recommendations for the Diagnosis, Staging, and Management of Adult Brain Tumors. Indian J. Med. Paediatr. Oncol. 2023, 44, 026–038. [Google Scholar] [CrossRef]
- Riche, M.; Amelot, A.; Peyre, M.; Capelle, L.; Carpentier, A.; Mathon, B. Complications after frame-based stereotactic brain biopsy: A systematic review. Neurosurg. Rev. 2021, 44, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.; Yeole, U.; Singh, V.; Moiyadi, A. Navigated ultrasound-based image guidance during resection of gliomas: Practical utility in intraoperative decision-making and outcomes. Neurosurg. Focus 2021, 50, E14. [Google Scholar] [CrossRef]
- Moiyadi, A.V.; Shetty, P.; John, R. Non-enhancing gliomas: Does intraoperative ultrasonography improve resections? Ultrasonography 2019, 38, 156–165. [Google Scholar] [CrossRef]
- Hu, X.; Xu, R.; Ding, H.; Lv, R.; Yang, L.; Wang, Y.; Xie, R. The total resection rate of glioma can be improved by the application of US-MRI fusion combined with contrast-enhanced ultrasound. Clin. Neurol. Neurosurg. 2021, 208, 106892. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.A.O.; Chang, S.M.; Berger, M.S. Current and future strategies for treatment of glioma. Neurosurg. Rev. 2017, 40, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Bi, W.L.; Griffith, B.; Kaufmann, T.J.; la Fougère, C.; Schmidt, N.O.; Tonn, J.C.; A Vogelbaum, M.; Wen, P.Y.; Aldape, K.; et al. Imaging and diagnostic advances for intracranial meningiomas. Neuro-Oncol. 2019, 21, i44–i61. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J.; Ruzevick, J.; Malayeri, A.A.; Rigamonti, D.; Lim, M.; Redmond, K.J.; Kleinberg, L. Postradiation imaging changes in the CNS: How can we differentiate between treatment effect and disease progression? Future Oncol. 2014, 10, 1277–1297. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, D.; Stalpers, L.; Taal, W.; Sminia, P.; van den Bent, M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Rane, N.; Quaghebeur, G. CNS effects following the treatment of malignancy. Clin. Radiol. 2012, 67, 61–68. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Chung, C.; Pope, W.B.; Boxerman, J.L.; Kaufmann, T.J. Pseudoprogression, radionecrosis, inflammation or true tumor progression? challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J. Neuro-Oncol. 2017, 134, 495–504. [Google Scholar] [CrossRef]
- Wang, Y.X.; King, A.D.; Zhou, H.; Leung, S.F.; Abrigo, J.; Chan, Y.L.; Hu, C.W.; Yeung, D.K.W.; Ahuja, A.T. Evolution of radiation-induced brain injury: MR imaging-based study. Radiology 2010, 254, 210–218. [Google Scholar] [CrossRef]
- Shah, R.; Vattoth, S.; Jacob, R.; Manzil, F.F.P.; O’malley, J.P.; Borghei, P.; Patel, B.N.; Curé, J.K. adiation necrosis in the brain: Imaging features and differentiation from tumor recurrence. RadioGraphics 2012, 32, 1343–1359. [Google Scholar] [CrossRef]
- Mullins, M.E.; Barest, G.D.; Schaefer, P.W.; Hochberg, F.H.; Gonzalez, R.G.; Lev, M. HRadiation necrosis versus glioma recurrence: Conventional MR imaging clues to diagnosis. AJNR Am. J. Neuroradiol. 2005, 26, 1967–1972. [Google Scholar]
- Kazda, T.; Bulik, M.; Pospisil, P.; Lakomy, R.; Smrcka, M.; Slampa, P.; Jancalek, R. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: Single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. NeuroImage Clin. 2016, 11, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Bernstock, J.D.; E Gary, S.; Klinger, N.; A Valdes, P.; Ibn Essayed, W.; E Olsen, H.; Chagoya, G.; Elsayed, G.; Yamashita, D.; Schuss, P.; et al. Standard clinical approaches and emerging modalities for glioblastoma imaging. Neuro-Oncol. Adv. 2022, 4, vdac080. [Google Scholar] [CrossRef] [PubMed]
- Boothe, D.; Young, R.; Yamada, Y.; Prager, A.; Chan, T.; Beal, K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro-Oncol. 2013, 15, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.R.; Cascino, T.L.; Schold, S.C.; Cairncross, J.G., Jr. Response criteria for phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990, 8, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Macdonald, D.R.; Reardon, D.A.; Cloughesy, T.F.; Sorensen, A.G.; Galanis, E.; DeGroot, J.; Wick, W.; Gilbert, M.R.; Lassman, A.B.; et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J. Clin. Oncol. 2010, 28, 1963–1972. [Google Scholar] [CrossRef]
- Erker, C.; Tamrazi, B.; Poussaint, T.Y.; Mueller, S.; Mata-Mbemba, D.; Franceschi, E.; A Brandes, A.; Rao, A.; Haworth, K.B.; Wen, P.Y.; et al. Response assessment in paediatric high-grade glioma: Recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol. 2020, 21, e317–e329. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Weinberg, B.D.; Hu, R.; Saindane, A.; Mullins, M.; Allen, J.; Hoch, M.J. Quantitative Improvement in Brain Tumor MRI Through Structured Reporting (BT-RADS). Acad. Radiol. 2020, 27, 780–784. [Google Scholar] [CrossRef]
- Kim, S.; Hoch, M.J.; Peng, L.; Somasundaram, A.; Chen, Z.; Weinberg, B.D. A brain tumor reporting and data system to optimize imaging surveillance and prognostication in high-grade gliomas. J. Neuroimaging 2022, 32, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Bent, M.v.D.; Youssef, G.; Cloughesy, T.F.; Ellingson, B.M.; Weller, M.; Galanis, E.; Barboriak, D.P.; de Groot, J.; Gilbert, M.R.; et al. RANO 2.0: Update to the Response Assessment in Neuro-Oncology Criteria for High- and Low-Grade Gliomas in Adults. J. Clin. Oncol. 2023, 41, 5187–5199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raverot, G.; Burman, P.; McCormack, A.; Heaney, A.; Petersenn, S.; Popovic, V.; Trouillas, J.; Dekkers, O.M.; European Society of Endocrinology. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur. J. Endocrinol. 2018, 178, G1–G24. [Google Scholar] [CrossRef]
- Galldiks, N.; Niyazi, M.; Grosu, A.L.; Kocher, M.; Langen, K.-J.; Law, I.; Minniti, G.; Kim, M.M.; Tsien, C.; Dhermain, F.; et al. Contribution of PET imaging to radiotherapy planning and monitoring in glioma patients—A report of the PET/RANO group. Neuro-Oncol. 2021, 23, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Kas, A.; Darcourt, J.; Guedj, E. PET Imaging in Neuro-Oncology: An Update and Overview of a Rapidly Growing Area. Cancers 2022, 14, 1103. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.R.; Muzi, M.; Peck, M.; Krohn, K.A. Multimodality Brain Tumor Imaging: MR Imaging, PET, and PET/MR Imaging. J. Nucl. Med. 2015, 56, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Muoio, B.; Trevisi, G.; Mattoli, M.V.; Albano, D.; Bertagna, F.; Giovanella, L. Diagnostic Performance and Prognostic Value of PET/CT with Different Tracers for Brain Tumors: A Systematic Review of Published Meta-Analyses. Int. J. Mol. Sci. 2019, 20, 4669. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Lohmann, P.; Albert, N.L.; Tonn, J.C.; Langen, K.-J. Current status of PET imaging in neuro-oncology. Neuro-Oncol. Adv. 2019, 1, vdz010. [Google Scholar] [CrossRef]
- Lewington, V.; Hughes, S.J. Nuclear medicine functional imaging of the brain. Clin. Med. 2012, 12, 364–368. [Google Scholar] [CrossRef]
- Giovacchini, G.; Riondato, M.; Giovannini, E.; Ciarmiello, A. Diagnostic Applications of Nuclear Medicine: Brain Tumors. In Nuclear Oncology: From Pathophysiology to Clinical Applications; Strauss, H.W., Mariani, G., Volterrani, D., Larson, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 467–505. [Google Scholar]
- Zhang, J.; Traylor, K.S.; Mountz, J.M. PET and SPECT Imaging of Brain Tumors. Semin. Ultrasound CT MRI 2020, 41, 530–540. [Google Scholar] [CrossRef]
- Shooli, H.; Dadgar, H.; Wáng, Y.-X.J.; Vafaee, M.S.; Kashuk, S.R.; Nemati, R.; Jafari, E.; Nabipour, I.; Gholamrezanezhad, A.; Assadi, M.; et al. An update on PET-based molecular imaging in neuro-oncology: Challenges and implementation for a precision medicine approach in cancer care. Quant. Imaging Med. Surg. 2019, 9, 1597–1610. [Google Scholar] [CrossRef]
- Valotassiou, V.; Leondi, A.; Angelidis, G.; Psimadas, D.; Georgoulias, P. SPECT and PET imaging of meningiomas. Sci. World J. 2012, 2012, 412580. [Google Scholar] [CrossRef]
- Al-Faham, Z.; Kassir, M.A.; Wood, D.; Balon, H.R. Appearance of Meningioma on 99mTc-HMPAO SPECT: Correlation with MRI. J. Nucl. Med. Technol. 2016, 44, 90–91. [Google Scholar] [CrossRef]
- Jeune, F.P.; Dubois, F.; Blond, S.; Steinling, M. Sestamibi technetium-99m brain single-photon emission computed tomography to identify recurrent glioma in adults: 201 studies. J. Neuro-Oncol. 2006, 77, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Gunawardana, D.H.; Rosenthal, M.A. Differentiation of tumor recurrence from radiation necrosis in high-grade gliomas using 201Tl-SPECT. J. Clin. Neurosci. 2008, 15, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Santra, A.; Sharma, P.; Kumar, R.; Bal, C.; Kumar, A.; Julka, P.K.; Malhotra, A. Comparison of glucoheptonate single photon emission com-puted tomography and contrast-enhanced MRI in detection of recurrent glioma. Nucl. Med. Commun. 2011, 32, 206–211. [Google Scholar] [CrossRef]
- Rani, N.; Singh, B.; Kumar, N.; Singh, P.; Hazari, P.P.; Singh, H. Differentiation of Recurrent/Residual Glioma From Radiation Necrosis Using Semi Quantitative 99mTc MDM (Bis-Methionine-DTPA) Brain SPECT/CT and Dynamic Susceptibility Con-trast-Enhanced MR Perfusion: A Comparative Study. Clin. Nucl. Med. 2018, 43, e74–e81. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Frantellizzi, V.; De Vincentis, G.; Schillaci, O.; Evangelista, L. Clinical Applications of TSPO PET for Glioma Imaging: Current Evidence and Future Perspective—A Systematic Review. Diagnostics 2023, 13, 1813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, D.; Patel, C.B.; Xu, G.; Iagaru, A.; Zhu, Z.; Zhang, L.; Cheng, Z. Visualization of Diagnostic and Therapeutic Targets in Glioma With Molecular Imaging. Front. Immunol. 2020, 11, 592389. [Google Scholar] [CrossRef]
- Mahajan, A.; Sahu, A.; Ashtekar, R.; Kulkarni, T.; Shukla, S.; Agarwal, U.; Bhattacharya, K. Glioma radiogenomics and artificial intelligence: Road to precision cancer medicine. Clin. Radiol. 2022, 78, 137–149. [Google Scholar] [CrossRef]
- Davatzikos, C.; Barnholtz-Sloan, J.S.; Bakas, S.; Colen, R.; Mahajan, A.; Quintero, C.B.; Font, J.C.; Puig, J.; Jain, R.; E Sloan, A.; et al. AI-based prognostic imaging biomarkers for precision neuro-oncology: The ReSPOND consortium. Neuro-Oncol. 2020, 22, 886–888. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, J.; Zhang, J.; Bu, J.; Yu, Y.; Tan, Y.; Feng, Q.; Huang, M. Differentiation of Recurrence from Radiation Necrosis in Gliomas Based on the Radiomics of Combinational Features and Multimodality MRI Images. Comput. Math. Methods Med. 2019, 2019, 2893043. [Google Scholar] [CrossRef]
- Choy, G.; Khalilzadeh, O.; Michalski, M.; Synho, D.; Samir, A.E.; Pianykh, O.S.; Geis, J.R.; Pandharipande, P.V.; Brink, J.A.; Dreyer, K.J. Current Applications and Future Impact of Machine Learning in Radiology. Radiology 2018, 288, 318–328. [Google Scholar] [CrossRef]
- Drabycz, S.; Roldán, G.; de Robles, P.; Adler, D.; McIntyre, J.B.; Magliocco, A.M.; Cairncross, J.G.; Mitchell, J.R. An analysis of image texture, tumor location, and MGMT promoter methylation in glioblastoma using magnetic resonance imaging. NeuroImage 2010, 49, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, Y.; Ye, M.; Li, Y.; Sun, Y.; Liang, J.; Lu, J.; Wang, Z.; Zhu, Z.; Zhang, X.; et al. Predicting MGMT Promoter Methylation in Diffuse Gliomas Using Deep Learning with Radiomics. J. Clin. Med. 2022, 11, 3445. [Google Scholar] [CrossRef] [PubMed]
- Bakas, S.; Akbari, H.; Pisapia, J.; Martinez-Lage, M.; Rozycki, M.; Rathore, S.; Davatzikos, C. In Vivo Detection of EGFRvIII in Glioblastoma via Perfusion Magnetic Resonance Imaging Signature Consistent with Deep Peritumoral Infiltration: The φ-Index. Clin. Cancer Res. 2017, 23, 4724–4734. [Google Scholar] [CrossRef]
- van Kempen, E.J.; Post, M.; Mannil, M.; Witkam, R.L.; ter Laan, M.; Patel, A.; Meijer, F.J.A.; Henssen, D. Performance of machine learning algorithms for glioma segmentation of brain MRI: A systematic literature review and meta-analysis. Eur. Radiol. 2021, 31, 9638–9653. [Google Scholar] [CrossRef]
- Lao, J.; Chen, Y.; Li, Z.C.; Li, Q.; Zhang, J.; Liu, J.; Zhai, G. A Deep Learning-Based Radiomics Model for Prediction of Survival in Glioblastoma Multiforme. Sci. Rep. 2017, 7, 10353. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, C.; Ke, X.; Zhou, J. Treatment Response and Prognosis Evaluation in High-Grade Glioma: An Imaging Review Based on MRI. J. Magn. Reson. Imaging 2022, 56, 325–340. [Google Scholar] [CrossRef]
- Booth, T.C.; Grzeda, M.; Chelliah, A.; Roman, A.; Al Busaidi, A.; Dragos, C.; Shuaib, H.; Luis, A.; Mirchandani, A.; Alparslan, B.; et al. Imaging Biomarkers of Glioblastoma Treatment Response: A Systematic Review and Meta-Analysis of Recent Machine Learning Studies. Front. Oncol. 2022, 12, 799662. [Google Scholar] [CrossRef]
- Bhandari, A.; Marwah, R.; Smith, J.; Nguyen, D.; Bhatti, A.; Lim, C.P.; Lasocki, A. Machine learning imaging applications in the differenti-ation of true tumour progression from treatment-related effects in brain tumours: A systematic review and meta-analysis. J. Med. Imaging Radiat. Oncol. 2022, 66, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, O.M.; Álvarez-Torres, M.d.M.; Figueiredo, P.; Hangel, G.; Keil, V.C.; Nechifor, R.E.; Riemer, F.; Schmainda, K.M.; Warnert, E.A.H.; Wiegers, E.C.; et al. High-Grade Glioma Treatment Response Monitoring Biomarkers: A Position Statement on the Evidence Supporting the Use of Advanced MRI Techniques in the Clinic, and the Latest Bench-to-Bedside Developments. Part 1: Perfusion and Diffusion Techniques. Front. Oncol. 2022, 12, 810263. [Google Scholar] [CrossRef]
- Afridi, M.; Jain, A.; Aboian, M.; Payabvash, S. Brain Tumor Imaging: Applications of Artificial Intelligence. Semin. Ultrasound CT MRI 2022, 43, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Cho, H.-H.; Kim, S.T.; Park, H.; Nam, D.; Kong, D.-S. Radiomics features to distinguish glioblastoma from primary central nervous system lymphoma on multi-parametric MRI. Neuroradiology 2018, 60, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Nakaura, T.; Namimoto, T.; Kitajima, M.; Uetani, H.; Tateishi, M.; Oda, S.; Utsunomiya, D.; Makino, K.; Nakamura, H.; et al. Machine learning based on multi-parametric magnetic resonance imaging to differentiate glioblastoma multiforme from primary cerebral nervous system lymphoma. Eur. J. Radiol. 2018, 108, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhou, X.; Duan, C.; Zhao, J.; Sui, Q.; Liu, X.; Zhang, X.J.Z. Differentiation Researches on the Meningioma Subtypes by Radiomics from Contrast-Enhanced Magnetic Resonance Imaging: A Preliminary Study. World Neurosurg. 2019, 126, e646–e652. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Li, Q.; Xu, D.; Xiu, W.; Zeng, Q.; Zhu, X.; Xu, F.; Jiang, B.; Zhang, M. Differentiation between pilocytic astrocytoma and glioblastoma: A decision tree model using contrast-enhanced magnetic resonance imaging-derived quantitative radiomic features. Eur. Radiol. 2019, 29, 3968–3975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Song, G.; Zang, Y.; Jia, J.; Wang, C.; Li, C.; Tian, J.; Dong, D.; Zhang, Y. Non-invasive radiomics approach potentially predicts non-functioning pituitary adenomas subtypes before surgery. Eur. Radiol. 2018, 28, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Shrot, S.; Salhov, M.; Dvorski, N.; Konen, E.; Averbuch, A.; Hoffmann, C. Application of MR morphologic, diffusion tensor, and perfusion imaging in the classification of brain tumors using machine learning scheme. Neuroradiology 2019, 61, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, S.; Sotiras, A.; Milchenko, M.; LaMontagne, P.; Hileman, M.; Marcus, D. MRI-based Identification and Classification of Major Intracranial Tumor Types by Using a 3D Convolutional Neural Network: A Retrospective Multi-institutional Analysis. Radiol. Artif. Intell. 2021, 3, e200301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Purpose | Importance of Sequences | |
|---|---|---|
| Diagnosis | Benign versus malignant |
|
| Prediction of histopathology and grading |
| |
| Management | Targeting site of biopsy |
|
| Directing path of surgery |
| |
| Post-treatment evaluation | Response assessment |
|
| Localization | Number | Single/Multiple |
|---|---|---|
| Compartment | Intra-axial/extra-axial | |
| Supratentorial/Infratentorial | ||
| Lateralization | Midline-parenchymal/ventricular | |
| Lateralized-white matter/grey matter | ||
| Complications needing emergent management | Eloquent/non-eloquent area of the brain significant midline shift, acute obstructive hydrocephalus, trans-tentorial herniation, and brainstem compression | |
| Sequences used | Clinical Utility | |
| T1 | Evaluation of anatomy
| |
| Characterization | T2/FLAIR | Evaluation of pathology
|
| T2*/SWI |
| |
| DWI |
| |
| Post-contrast T1 | Postcontrast enhancement reflects the breakdown of the blood–brain barrier. | |
| MR spectroscopy |
Myoinositol: Low-grade diffuse gliomas, Ependymomas Taurine: Medulloblastoma Alanine: Meningioma | |
| Pre-surgical planning | PWI | Dynamic Susceptibility Contrast (DSC)—The main metric for tumor evaluation is relative cerebral blood volume (rCBV), the most commonly used technique.
Arterial Spin Labelling (ASL)—the main metric is cerebral blood flow (CBF).
|
| DTI |
| |
| fMRI |
| |
| Special sequences | MRA and MRV |
|
| 3D-FIESTA/CISS |
|
| Tumor | Management |
|---|---|
| Diffusely infiltrating glioma |
O6-methylguanine-DNAmethyl-transferase (MGMT) methylation status determines the management of elderly who are not candidates for combined radio-chemotherapy. |
| Meningioma |
|
| Pituitary adenoma |
|
| Criteria | Comment |
|---|---|
| MacDonald criteria (1990) [67] |
|
| RANO criteria (2010) [68] |
|
| Response Assessment in Pediatric Neuro-Oncology (RAPNO) (2020) [69] |
|
| Brain Tumor—Reporting and Data System (BT-RADS) (2020) [70,71] |
|
| |
| RANO 2.0 [72] |
|
| Modality | Tracers Available | Utility |
|---|---|---|
| Role of PET |
| Initial characterization—high SUV in lymphoma (FDG)Screening for primary (FDG)Glioma diagnosis and surveillance—in case of equivocal findings in MRI for treatment-related changes versus progression (FET-, F-DOPA)Somatostatin receptor analogs—specific for meningiomas (DOTATATE)TSPO-PET is a newer agent that is showing promising results in the diagnosis and follow-up of gliomas. |
| Role of SPECT |
| Multiple studies in follow-up imaging of glioma show variable sensitivity and specificity of these techniques (80–95%) as compared with DSC-PWI and MRS. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, K.; Mahajan, A. Imaging Recommendations for Diagnosis, Staging, and Management of Primary Central Nervous System Neoplasms in Adults. Neuroglia 2024, 5, 370-390. https://doi.org/10.3390/neuroglia5040025
Bhattacharya K, Mahajan A. Imaging Recommendations for Diagnosis, Staging, and Management of Primary Central Nervous System Neoplasms in Adults. Neuroglia. 2024; 5(4):370-390. https://doi.org/10.3390/neuroglia5040025
Chicago/Turabian StyleBhattacharya, Kajari, and Abhishek Mahajan. 2024. "Imaging Recommendations for Diagnosis, Staging, and Management of Primary Central Nervous System Neoplasms in Adults" Neuroglia 5, no. 4: 370-390. https://doi.org/10.3390/neuroglia5040025
APA StyleBhattacharya, K., & Mahajan, A. (2024). Imaging Recommendations for Diagnosis, Staging, and Management of Primary Central Nervous System Neoplasms in Adults. Neuroglia, 5(4), 370-390. https://doi.org/10.3390/neuroglia5040025







