Rosenfeld’s Staining: A Valuable Tool for In Vitro Assessment of Astrocyte and Microglia Morphology
Abstract
:1. Introduction
| Reference | Title | Aim of the Study | Tissue/Cell Culture |
|---|---|---|---|
| Dourado et al. [7] | Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated With Alzheimer’s Disease. | To evaluate the neuroprotective and neuroimmunomodulatory potential of apigenin using in vitro models of neuroinflammation associated with AD. | Primary co-cultures of neurons and glial cells from brain neonates and embryos of Wistar rats. |
| Cholich et al. [12] | Cytotoxic activity induced by the alkaloid extract from Ipomoea carnea on primary murine mixed glial cultures. | To verify the cytotoxicity of the alkaloids swainsonine and calistheins B1, B2, B3 and C1 from Ipomoea carnea. | Mixed glial primary cultures from neonatal mice (CF-1). |
| Nascimento et al. [13] | Involvement of astrocytic CYP1A1 isoform in the metabolism and toxicity of the alkaloid pyrrolizidine monocrotaline. | To elucidate the metabolism and the toxicity of monocrotaline in C6 astrocyte cell line and primary cultures of astrocytes by investigating metabolic enzymatic mechanisms of the cytochrome P450 (CYP) system and conjugation with glutathione. | C6 cell line derived from rat glioma and primary cultures of astrocytes from brain Wistar rat. |
| Zuo et al. [14] | Baicalin Attenuates Ketamine-Induced Neurotoxicity in the Developing Rats: Involvement of PI3K/Akt and CREB/BDNF/Bcl-2 Pathways. | Investigate the neuroprotective effects of baicalin against ketamine-induced apoptotic neurotoxicity. | Neurons and glia in the mixed cultures from the cerebral cortex of neonatal Sprague–Dawley rats. |
| Coelho et al. [17] | Flavonoids from the Brazilian plant Croton betulaster inhibit the growth of human glioblastoma cells and induce apoptosis. | To investigate the effects of the flavonoids 5-hydroxy-7,4′-dimethoxyflavone, casticin, and pendulletin on the growth and viability of the human glioblastoma cell line GL-15. | GL-15 cell line derived from human glioblastoma. |
| Zuo et al. [15] | Existence of glia mitigated ketamine-induced neurotoxicity in neuron–glia mixed cultures of neonatal rat cortex and the glia-mediated protective effect of 2-PMPA. | Compare ketamine-induced neurotoxicity and explored the neuroprotective effect of the NAAG peptidase inhibitor 2-(phosphonomethyl) pentanedioic acid (2-PMPA). | Neuron–glia mixed cultures and neuronal cultures of neonatal Sprague Dawley rats. |
| Wang et al. [16] | Primary co-culture of cortical neurons and astrocytes of new-born SD rats. | Establish a simple and practical co-culture method. | Co-culture of cortical neurons and astrocytes from new-born SD rats. |
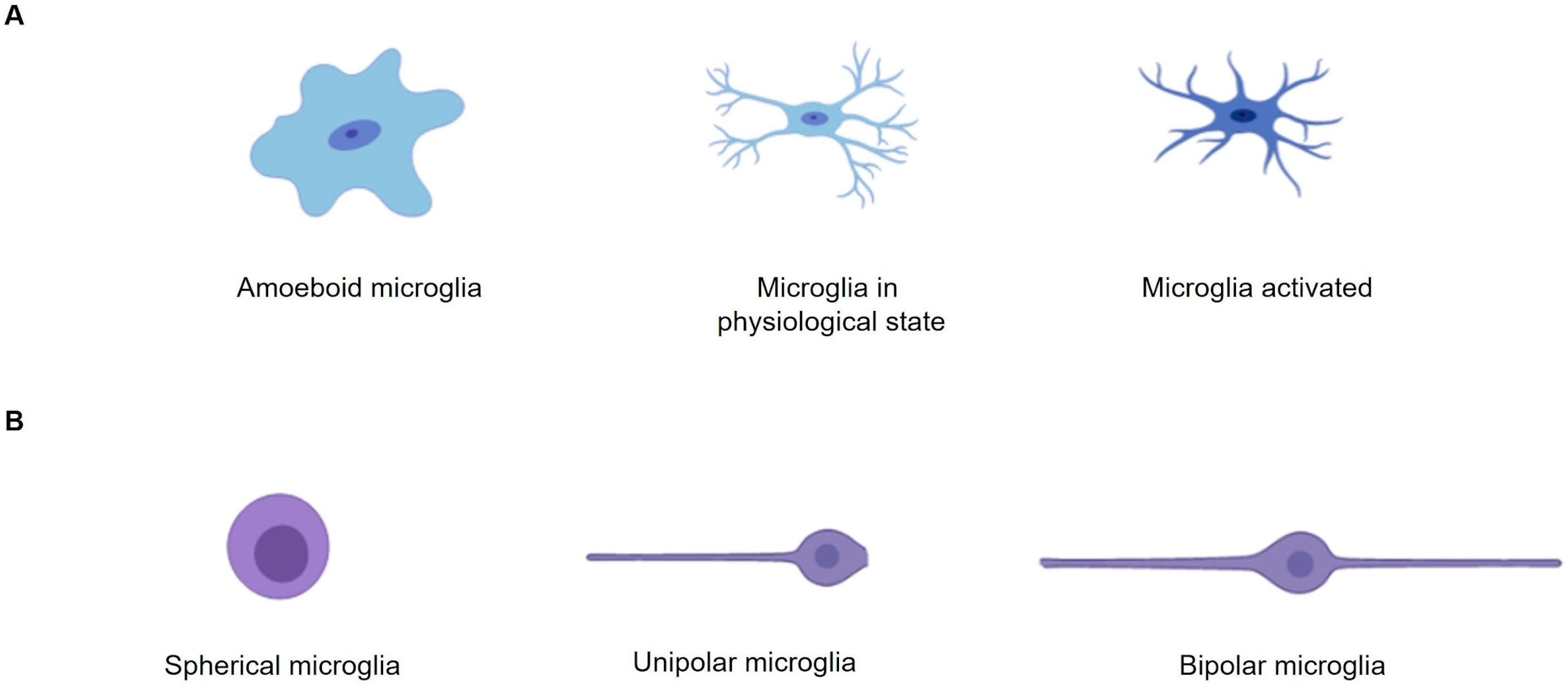
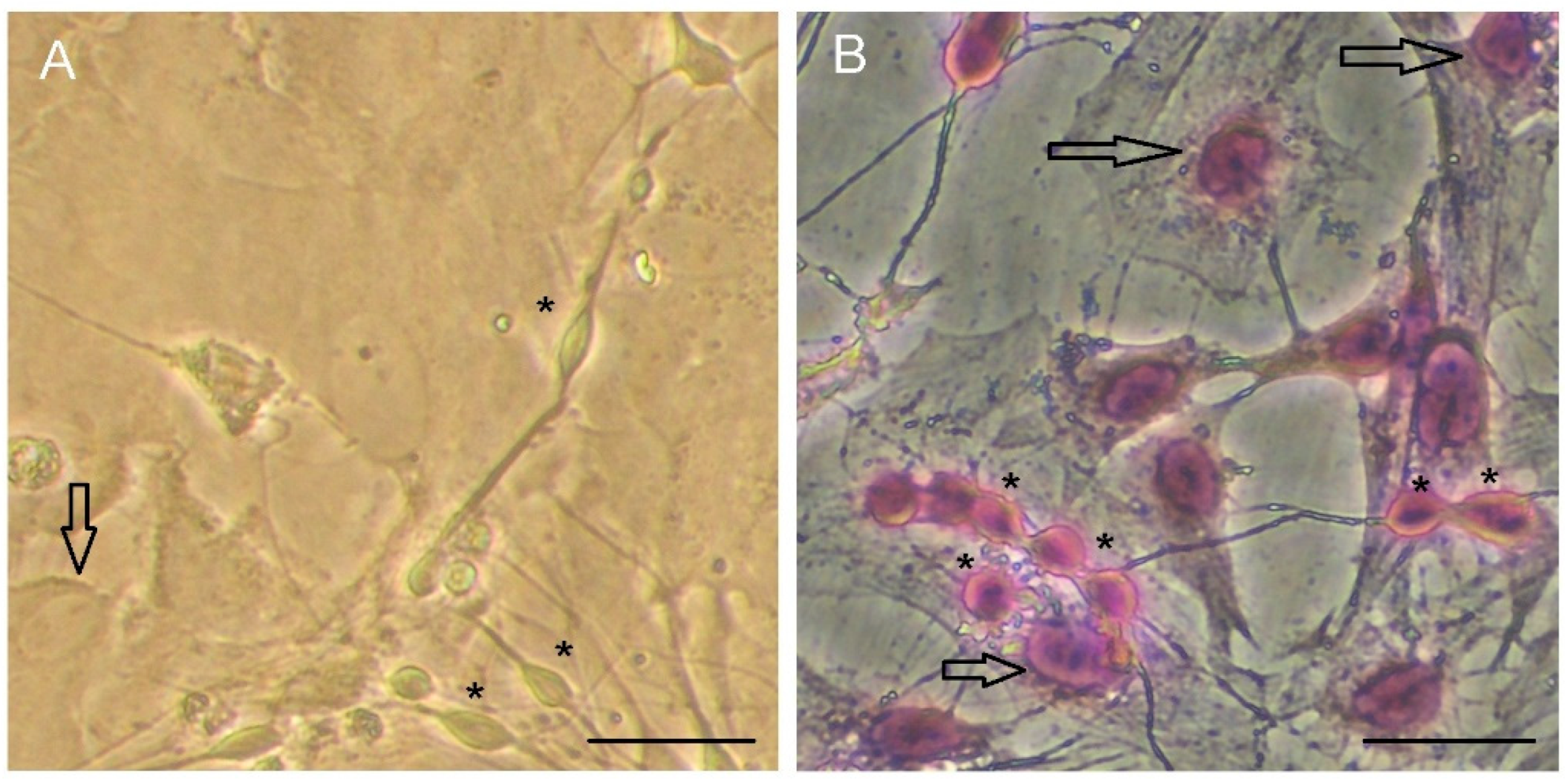
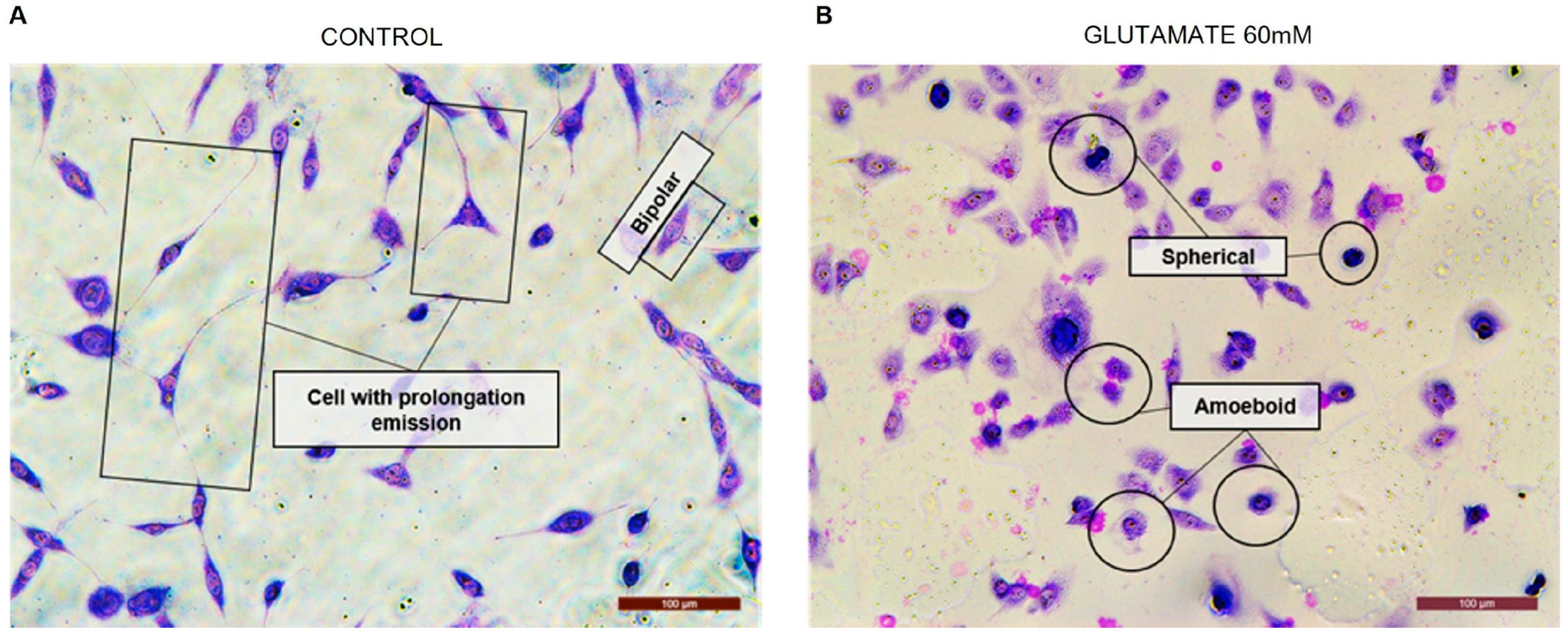
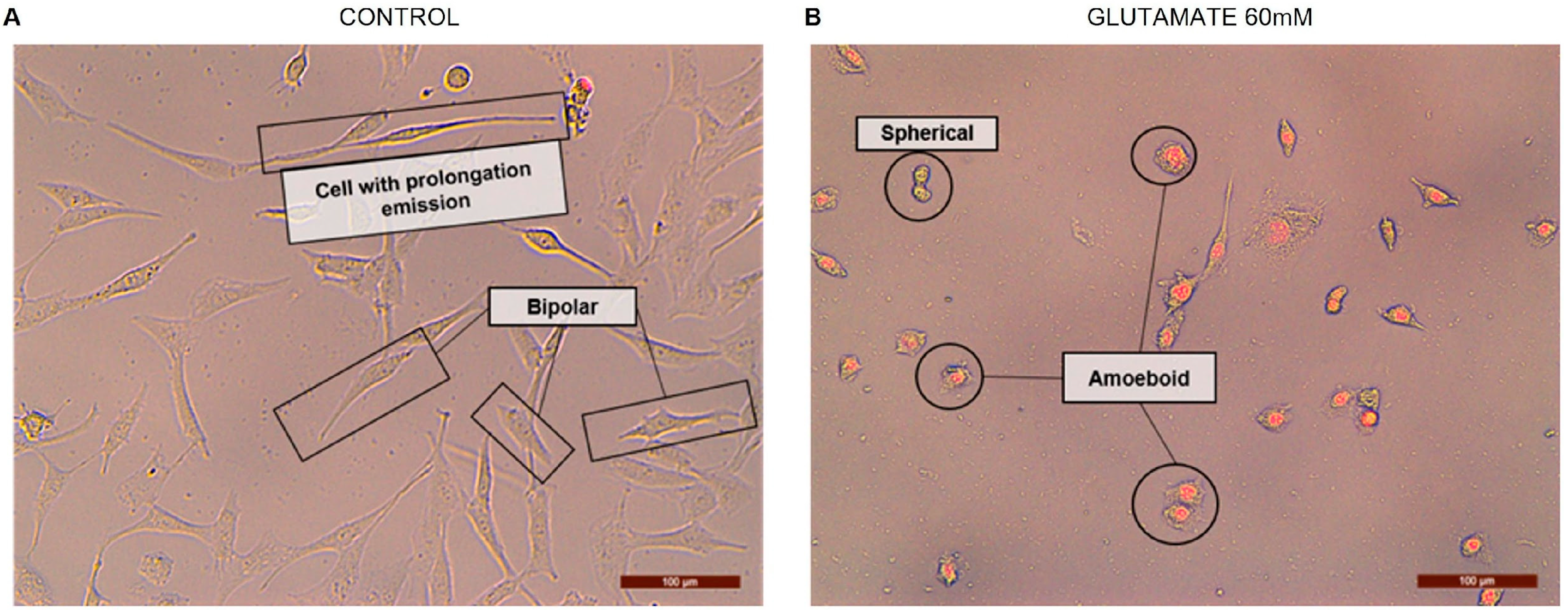
2. How Important Is Rosenfeld’s Staining to Study the Microglia Morphology in Cultures?
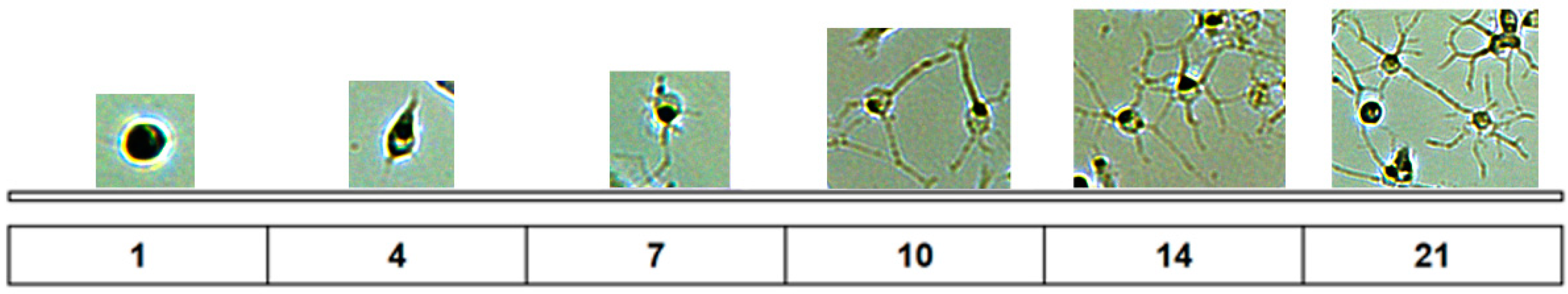
3. How Important Is Studying Astrocyte Morphology in Cultures?
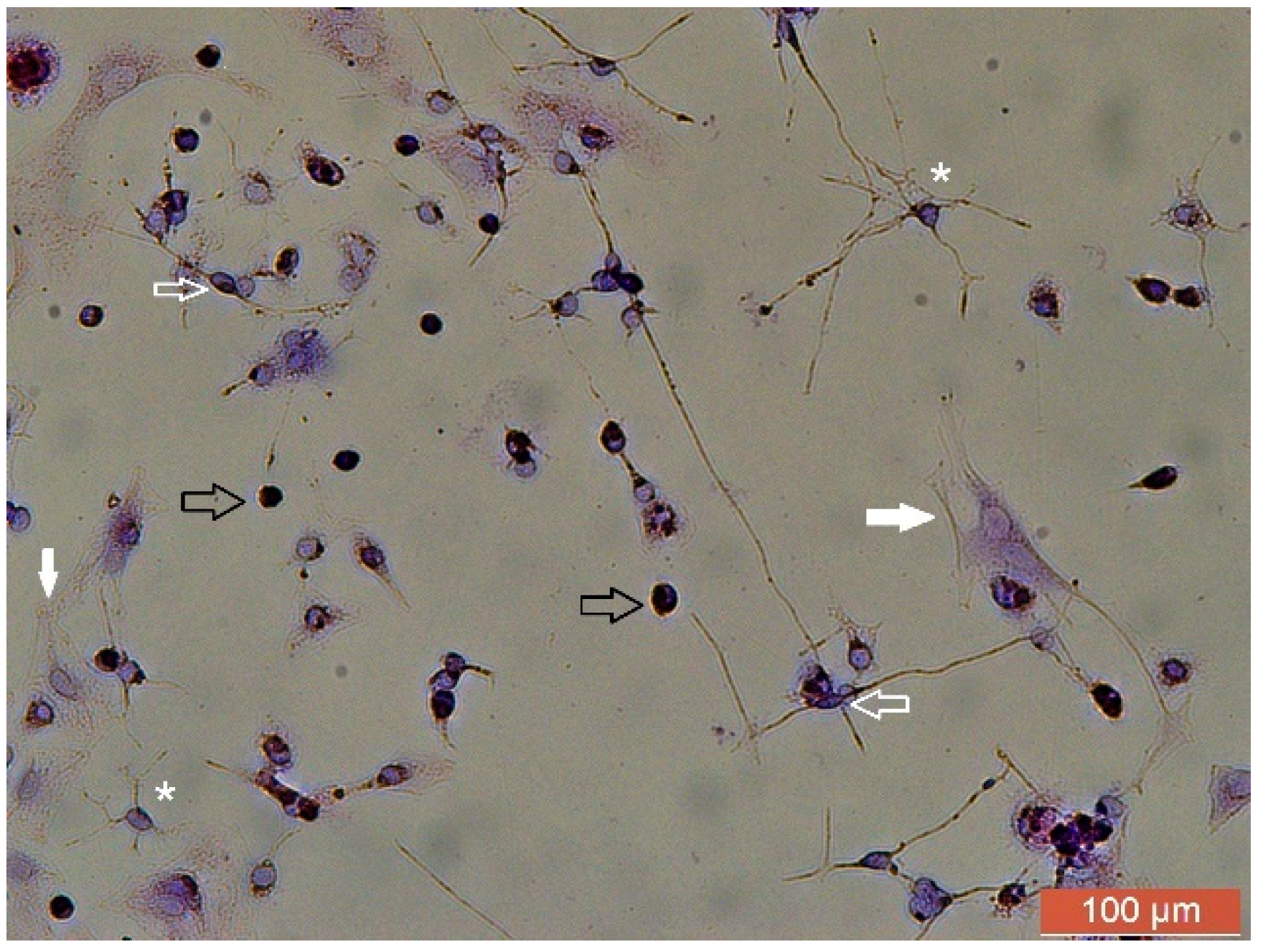
4. Rosenfeld’s Staining: An Alternative Technique to Evaluate Cell Morphology in Glial Cell Culture
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasband, M.N. Glial Contributions to Neural Function and Disease. Mol. Cell Proteom. 2016, 15, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Boullerne, A.I.; Feinstein, D.L. History of Neuroscience I. Pío del Río-Hortega (1882–1945): The Discoverer of Microglia and Oligodendroglia. ASN Neuro. 2020, 12, 1759091420953259. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Goshi, N.; Morgan, R.K.; Lein, P.J.; Seker, E. A primary neural cell culture model to study neuron, astrocyte, and microglia interactions in neuroinflammation. J. Neuroinflammation 2020, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.C.; Araújo, F.M.; Ferreira, R.S.; Silva, V.B.; Silva, J.H.C.; Grangeiro, M.S.; Soares, É.N.; Pereira, É.P.L.; Souza, C.S.; Costa, S.L.; et al. Aminochrome induces microglia and astrocyte activation. Toxicol. Vitr. 2017, 42, 54–60. [Google Scholar] [CrossRef]
- Dourado, N.S.; Souza, C.D.S.; de Almeida, M.M.A.; Bispo da Silva, A.; Dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; da Silva, J.S.; Souza, D.O.; Costa, M.F.D.; et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated with Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 119. [Google Scholar] [CrossRef]
- Maric, D.; Jahanipour, J.; Li, X.R.; Singh, A.; Mobiny, A.; Van Nguyen, H.; Sedlock, A.; Grama, K.; Roysam, B. Whole-brain tissue mapping toolkit using large-scale highly multiplexed immunofluorescence imaging and deep neural networks. Nat. Commun. 2021, 12, 1550. [Google Scholar] [CrossRef]
- Pothayee, N.; Maric, D.; Sharer, K.; Tao-Cheng, J.H.; Calac, A.; Bouraoud, N.; Pickel, J.; Dodd, S.; Koretsky, A. Neural precursor cells form integrated brain-like tissue when implanted into rat cerebrospinal fluid. Commun. Biol. 2018, 1, 114. [Google Scholar] [CrossRef]
- Swanson, M.E.V.; Murray, H.C.; Ryan, B.; Faull, R.L.M.; Dragunow, M.; Curtis, M.A. Quantitative immunohistochemical analysis of myeloid cell marker expression in human cortex captures microglia heterogeneity with anatomical context. Sci. Rep. 2020, 10, 11693. [Google Scholar] [CrossRef]
- Rosenfeld, G. Método rápido de coloração de esfregaços de sangue. Noções práticas sobre corantes pancrômicos e estudo de diversos fatores. Mem. Inst. Butantan 1947, 20, 315–328. [Google Scholar]
- Cholich, L.A.; Pistán, M.E.; Torres, A.M.; Ortega, H.H.; Gardner, D.R.; Bustillo, S. Cytotoxic activity induced by the alkaloid extract from Ipomoea carnea on primary murine mixed glial cultures. Toxicon 2020, 188, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.P.; Oliveira, J.L.; Carvalho, J.L.C.; Santos, W.A.; Pires, T.R.C.; Batatinha, M.J.M.; El-Bachá, R.S.; Silva, V.D.A.; Costa, S.L. Involvement of astrocytic CYP1A1 isoform in the metabolism and toxicity of the alkaloid pyrrolizidine monocrotaline. Toxicon 2017, 134, 41–49. [Google Scholar] [CrossRef]
- Zuo, D.; Lin, L.; Liu, Y.; Wang, C.; Xu, J.; Sun, F.; Li, L.; Li, Z.; Wu, Y. Baicalin Attenuates Ketamine-Induced Neurotoxicity in the Developing Rats: Involvement of PI3K/Akt and CREB/BDNF/Bcl-2 Pathways. Neurotox. Res. 2016, 2, 159–172. [Google Scholar] [CrossRef]
- Zuo, D.; Wang, C.; Li, Z.; Lin, L.; Duan, Z.; Qi, H.; Li, L.; Sun, F.; Wu, Y. Existence of glia mitigated ketamine-induced neurotoxicity in neuron-glia mixed cultures of neonatal rat cortex and the glia-mediated protective effect of 2-PMPA. Neurotoxicology 2014, 44, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.N.; Lin, L.; Duan, Z.F.; Zhong, F.; Zuo, D.Y.; Wu, Y.L. Primary co-culture of cortical neurons and astrocytes of new-born SD rats. Yao Xue Xue Bao 2013, 11, 1729–1732. [Google Scholar] [PubMed]
- Pitanga, B.P.; Silva, V.D.; Souza, C.S.; Junqueira, H.A.; Fragmeni, B.O.N.; Nascimento, R.P.; Silva, A.R.; Costa, M.F.D.; El-Bachá, R.S.; Costa, S.L. Assessment of neurotoxicity of monocrotaline, an alkaloid extracted from Crotalaria retusa in astrocyte/neuron co-culture system. Neurotoxicology 2011, 32, 776–784. [Google Scholar] [CrossRef]
- Savage, J.C.; Carrier, M.; Tremblay, M.È. Morphology of Microglia Across Contexts of Health and Disease. Methods Mol. Biol. 2019, 2034, 13–26. [Google Scholar] [CrossRef]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, D.; Li, G. The role of microglia in viral encephalitis: A review. J. Neuroinflammation 2019, 16, 76. [Google Scholar] [CrossRef]
- de Araújo, F.M.; Cuenca-Bermejo, L.; Fernández-Villalba, E.; Costa, S.L.; Silva, V.D.A.; Herrero, M.T. Role of Microgliosis and NLRP3 Inflammasome in Parkinson’s Disease Pathogenesis and Therapy. Cell Mol. Neurobiol. 2022, 42, 1283–1300. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-inflammatory Stimuli (LPS, IFNγ+TNFα) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Au, N.P.B.; Ma, C.H.E. Recent Advances in the Study of Bipolar/Rod-Shaped Microglia and their Roles in Neurodegeneration. Front. Aging Neurosci. 2017, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.; Gulya, K. Development of the microglial phenotype in culture. Neuroscience 2013, 241, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Ransohoff, R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016, 19, 987–991. [Google Scholar] [CrossRef]
- Mecha, M.; Iñigo, P.M.; Mestre, L.; Hernangómez, M.; Borrell, J.; Guaza, C. An easy and fast way to obtain a high number of glial cells from rat cerebral tissue: A beginners approach. Protoc. Exch. 2011. [Google Scholar] [CrossRef]
- Laffer, B.; Bauer, D.; Wasmuth, S.; Busch, M.; Jalilvand, T.V.; Thanos, S.; Meyer Zu Hörste, G.; Loser, K.; Langmann, T.; Heiligenhaus, A.; et al. Loss of IL-10 Promotes Differentiation of Microglia to a M1 Phenotype. Front. Cell Neurosci. 2019, 13, 430. [Google Scholar] [CrossRef]
- Peggion, C.; Stella, R.; Lorenzon, P.; Spisni, E.; Bertoli, A.; Massimino, M.L. Microglia in Prion Diseases: Angels or Demons? Int. J. Mol. Sci. 2020, 21, 7765. [Google Scholar] [CrossRef]
- Cherry, J.D.; Olschowka, J.A.; O’Banion, M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflammation 2014, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.C.; Ma, L.S.; Chu, Z.H.; Xu, H.; Wu, W.Q.; Liu, F. Regulation of microglial activation in stroke. Acta Pharmacol. Sin. 2017, 38, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Augusto-Oliveira, M.; Arrifano, G.P.; Lopes-Araújo, A.; Santos-Sacramento, L.; Takeda, P.Y.; Anthony, D.C.; Malva, J.O.; Crespo-Lopez, M.E. What Do Microglia Really Do in Healthy Adult Brain? Cells 2019, 8, 1293. [Google Scholar] [CrossRef]
- Lin, L.; Desai, R.; Wang, X.; Lo, E.H.; Xing, C. Characteristics of primary rat microglia isolated from mixed cultures using two different methods. J. Neuroinflammation 2017, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Kou, D.; Li, T.; Liu, H.; Liu, C.; Yin, Y.; Wu, X.; Yu, T. Transplantation of rat-derived microglial cells promotes functional recovery in a rat model of spinal cord injury. Braz. J. Med. Biol. Res. 2018, 51, 7076. [Google Scholar] [CrossRef]
- He, Y.; Taylor, N.; Yao, X.; Bhattacharya, A. Mouse primary microglia respond differently to LPS and poly(I:C) in vitro. Sci. Rep. 2021, 11, 10447. [Google Scholar] [CrossRef]
- Rustenhoven, J.; Park, T.I.; Schweder, P.; Scotter, J.; Correia, J.; Smith, A.M.; Gibbons, H.M.; Oldfield, R.L.; Bergin, P.S.; Mee, E.W.; et al. Isolation of highly enriched primary human microglia for functional studies. Sci. Rep. 2016, 6, 19371. [Google Scholar] [CrossRef]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia morphophysiological diversity and its implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef]
- Angelova, D.M.; Brown, D.R. Microglia and the aging brain: Are senescent microglia the key to neurodegeneration? J. Neurochem. 2019, 151, 676–688. [Google Scholar] [CrossRef]
- Bermúdez-Muñoz, O.M. Sonic Hedgehog (SHH) pathway in the adult brain: Key signaling for astrocyte reactivation and brain repair. Actual. Biológicas 2016, 38, 197–209. [Google Scholar]
- Zuchero, J.B.; Barres, B.A. Glia in mammalian development and disease. Development 2015, 142, 3805–3809. [Google Scholar] [CrossRef] [PubMed]
- Vogels, T.; Murgoci, A.N.; Hromádka, T. Intersection of pathological tau and microglia at the synapse. Acta Neuropathol. Commun. 2019, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 3, 312–325. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2014, 7, a020420. [Google Scholar] [CrossRef] [PubMed]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef]
- Tabata, H. Diverse subtypes of astrocytes and their development during corticogenesis. Front. Neurosci. 2015, 9, 114. [Google Scholar] [CrossRef]
- Guillamón-Vivancos, T.; Gómez-Pinedo, U.; Matías-Guiu, J. Astrocytes in neurodegenerative diseases (I): Function and molecular description. Neurologia 2015, 30, 119–129. [Google Scholar] [CrossRef]
- Sica, R.E.; Caccuri, R.; Quarracino, C.; Capani, F. Are astrocytes executive cells within the central nervous system? Arq. Neuro-Psiquiatr. 2016, 74, 671–678. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Butt, A. Glial Neurobiology: A Textbook, 1st ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007; pp. 93–123. [Google Scholar]
- Oliveira-Junior, M.S.; Pereira, E.P.; de Amorim, V.C.M.; Reis, L.T.C.; do Nascimento, R.P.; da Silva, V.D.A.; Costa, S.L. Lupeol inhibits LPS-induced neuroinflammation in cerebellar cultures and induces neuroprotection associated to the modulation of astrocyte response and expression of neurotrophic and inflammatory factors. Int. Immunopharmacol. 2019, 70, 302–312. [Google Scholar] [CrossRef]
- Michalovicz, L.T.; Kelly, K.A.; Vashishtha, S.; Ben-Hamo, R.; Efroni, S.; Miller, J.V.; Locker, A.R.; Sullivan, K.; Broderick, G.; Miller, D.B.; et al. Astrocyte-specific transcriptome analysis using the ALDH1L1 bacTRAP mouse reveals novel biomarkers of astrogliosis in response to neurotoxicity. J. Neurochem. 2019, 150, 420–440. [Google Scholar] [CrossRef]
- Silva, A.M.; Silva, A.R.; Pinheiro, A.M.; Freitas, S.R.; Silva, V.D.; Souza, C.S.; Hughes, J.B.; El-Bachá, R.S.; Costa, M.F.; Velozo, E.S.; et al. Alkaloids from Prosopis juliflora leaves induce glial activation, cytotoxicity and stimulate NO production. Toxicon 2007, 49, 601–614. [Google Scholar] [CrossRef]
- de Almeida, M.M.A.; Souza, C.D.S.; Dourado, N.S.; da Silva, A.B.; Ferreira, R.S.; David, J.M.; David, J.P.; Costa, M.F.D.; da Silva, V.D.A.; Butt, A.M.; et al. Phytoestrogen Agathisflavone Ameliorates Neuroinflammation-Induced by LPS and IL-1β and Protects Neurons in Cocultures of Glia/Neurons. Biomolecules 2020, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Bastos, E.M.S.; da Silva, A.B.; Coelho, P.L.C.; Borges, J.M.P.; da Silva, V.D.A.; da Cunha, V.H.M.; Costa, S.L. Anti-inflammatory activity of Jatropha curcas L. in brain glial cells primary cultures. J. Ethnopharmacol. 2021, 264, 113201. [Google Scholar] [CrossRef]
- Silva, V.D.; Pitanga, B.P.; Nascimento, R.P.; Souza, C.S.; Coelho, P.L.; Menezes-Filho, N.; Silva, A.M.; Costa, M.d.F.; El-Bachá, R.S.; Velozo, E.S.; et al. Juliprosopine and juliprosine from prosopis juliflora leaves induce mitochondrial damage and cytoplasmic vacuolation on cocultured glial cells and neurons. Chem. Res. Toxicol. 2013, 26, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, D. On the nature of Romanowsky dyes and the Romanowsky-Giemsa effect. Clin. Lab. Haematol. 1979, 1, 247–262. [Google Scholar] [CrossRef]
- Ozzetti, P.A.; Cavlac, C.L.; Sano-Martins, I.S. Hematological reference values of the snakes Oxyrhopus guibei and Xenodon neuwiedii (Serpentes: Dipsadidae). Comp. Clin. Pathol. 2015, 24, 101–108. [Google Scholar] [CrossRef]
- Pereira, D.S.P.; Guerra-Santos, B.; Medeiros, S.D.C.D.; Albinati, R.C.B.; Ayres, M.C.C. Comparison of methodologies used in the analysis of parameters of blood and total protein of Nile tilapia (“Oreochromis niloticus”). Rev. Bras. Saúde Produção Anim. 2015, 16, 893–904. [Google Scholar] [CrossRef]
- Coelho, P.L.C.; Freitas, S.R.V.B.D.; Pitanga, B.P.S.; Silva, V.D.A.D.; Oliveira, M.N.; Grangeiro, M.S.; Costa, S.L. Flavonoids from the Brazilian plant Croton betulaster inhibit the growth of human glioblastoma cells and induce apoptosis. Rev. Bras. Farmacogn. 2016, 26, 34–43. [Google Scholar] [CrossRef]
- da Silva, A.B.; Cerqueira Coelho, P.L.; das Neves Oliveira, M.; Oliveira, J.L.; Oliveira Amparo, J.A.; da Silva, K.C.; Soares, J.R.P.; Pitanga, B.P.S.; Dos Santos Souza, C.; de Faria Lopes, G.P.; et al. The flavonoid rutin and its aglycone quercetin modulate the microglia inflammatory profile improving antiglioma activity. Brain Behav. Immun. 2020, 85, 170–185. [Google Scholar] [CrossRef]
- Zuo, D.; Wang, C.; Li, Z.; Lin, L.; Duan, Z.; Qi, H.; Li, L.; Sun, F.; Wu, Y. Corrigendum to “Existence of glia mitigated ketamine-induced neurotoxicity in neuron–glia mixed cultures of neonatal rat cortex and the glia-mediated protective effect of 2-PMPA” [Neurotoxicology 44 (2014) 218–230]. Neurotoxicology 2022, 91, 370–372. [Google Scholar] [CrossRef]

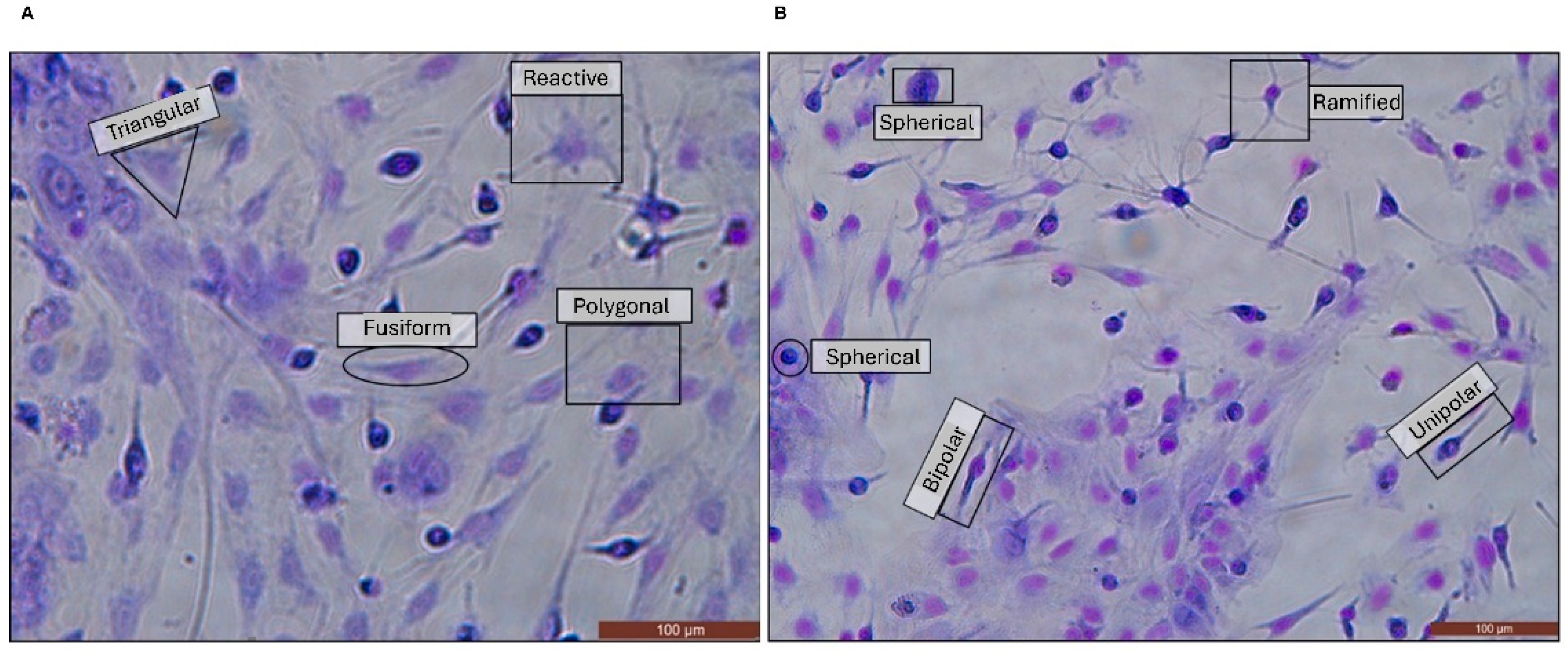
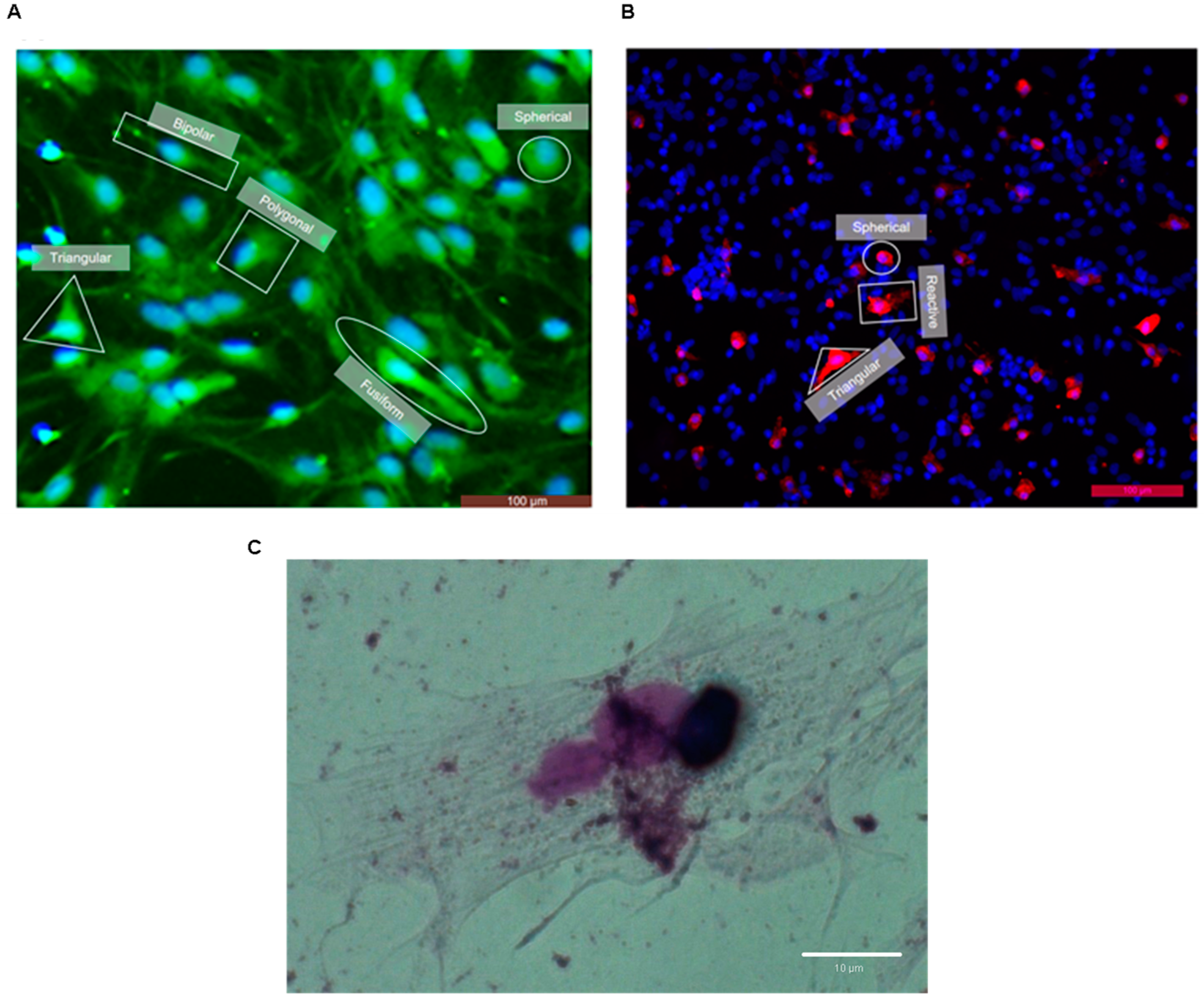
| Morphology | GFAP+ | Rosenfeld |
|---|---|---|
| Bipolar |  | 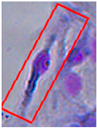 |
| Spherical |  |  |
| Fusiform |  |  |
| Polygonal |  |  |
| Triangular |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farias, A.A.; Costa, A.C.d.S.; Souza, J.T.; Soares, É.N.; Santos Costa, C.C.d.O.; Nascimento, R.P.d.; Costa, S.L.; da Silva, V.D.A.; Costa, M.d.F.D. Rosenfeld’s Staining: A Valuable Tool for In Vitro Assessment of Astrocyte and Microglia Morphology. Neuroglia 2025, 6, 16. https://doi.org/10.3390/neuroglia6020016
Farias AA, Costa ACdS, Souza JT, Soares ÉN, Santos Costa CCdO, Nascimento RPd, Costa SL, da Silva VDA, Costa MdFD. Rosenfeld’s Staining: A Valuable Tool for In Vitro Assessment of Astrocyte and Microglia Morphology. Neuroglia. 2025; 6(2):16. https://doi.org/10.3390/neuroglia6020016
Chicago/Turabian StyleFarias, Alana Alves, Ana Carla dos Santos Costa, Jéssica Teles Souza, Érica Novaes Soares, Cinthia Cristina de Oliveira Santos Costa, Ravena Pereira do Nascimento, Silvia Lima Costa, Victor Diogenes Amaral da Silva, and Maria de Fátima Dias Costa. 2025. "Rosenfeld’s Staining: A Valuable Tool for In Vitro Assessment of Astrocyte and Microglia Morphology" Neuroglia 6, no. 2: 16. https://doi.org/10.3390/neuroglia6020016
APA StyleFarias, A. A., Costa, A. C. d. S., Souza, J. T., Soares, É. N., Santos Costa, C. C. d. O., Nascimento, R. P. d., Costa, S. L., da Silva, V. D. A., & Costa, M. d. F. D. (2025). Rosenfeld’s Staining: A Valuable Tool for In Vitro Assessment of Astrocyte and Microglia Morphology. Neuroglia, 6(2), 16. https://doi.org/10.3390/neuroglia6020016






