Surgical Treatment of Multiple Bone Cysts Using a Platelet-Rich Fibrin and BoneAlbumin Composite Graft: A Case Report
Abstract
1. Introduction
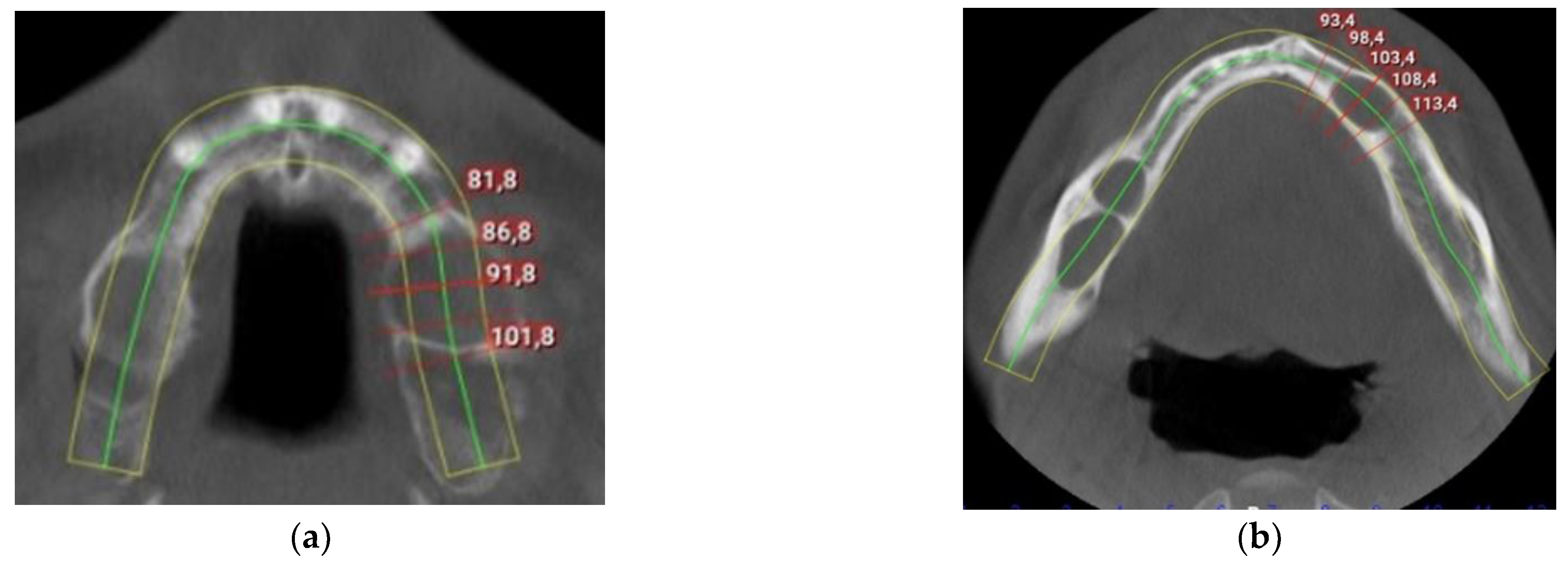
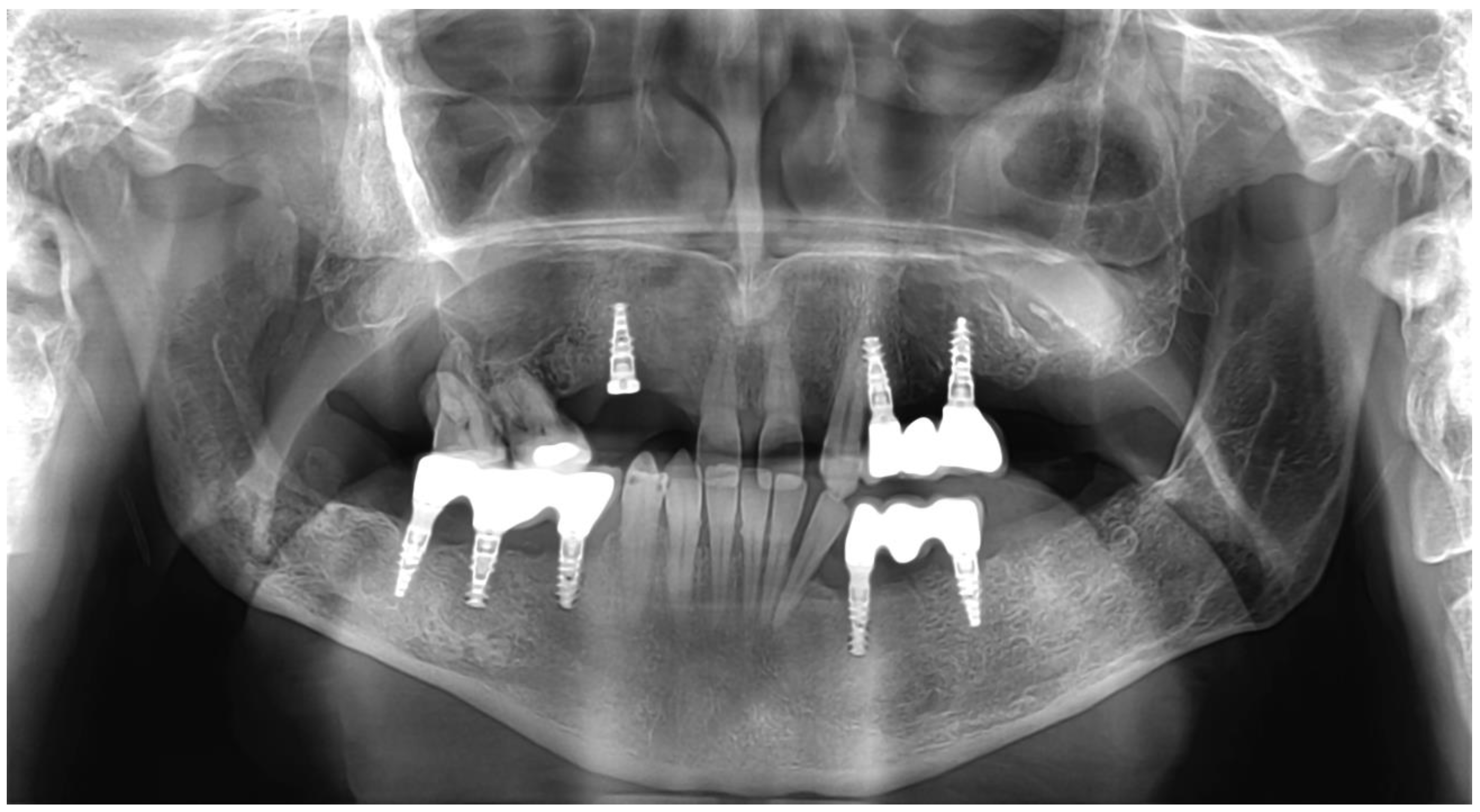
2. Detailed Case Description
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonffy, L.; Minya, F.; Trimmel, B.; Lacza, Z.; Dobo-Nagy, C. Albumin-Impregnated Allograft Filling of Surgical Extraction Sockets Achieves Better Bone Remodeling Than Filling with Either Blood Clot or Bovine Xenograft. Int. J. Oral Maxillofac. Implant. 2020, 35, 297–304. [Google Scholar] [CrossRef]
- Kaposvari, I.; Kormoczi, K.; Csurgay, K.; Horvath, F.; Ashourioun, A.H.; Buglyo, A.; Turai, A.R.; Joob-Fancsaly, A. Delayed-onset infections after lower third molar surgery: A Hungarian case-control study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 132, 641–647. [Google Scholar] [CrossRef]
- Buchbender, M.; Neukam, F.W.; Lutz, R.; Schmitt, C.M. Treatment of enucleated odontogenic jaw cysts: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 399–406. [Google Scholar] [CrossRef]
- Khan, A.A.; Qahtani, S.A.; Dawasaz, A.A.; Saquib, S.A.; Asif, S.M.; Ishfaq, M.; Kota, M.Z.; Ibrahim, M. Management of an extensive odontogenic keratocyst: A rare case report with 10-year follow-up. Medicine 2019, 98, e17987. [Google Scholar] [CrossRef]
- Johnson, N.R.; Gannon, O.M.; Savage, N.W.; Batstone, M.D. Frequency of odontogenic cysts and tumors: A systematic review. J. Investig. Clin. Dent. 2014, 5, 9–14. [Google Scholar] [CrossRef]
- Koca, H.; Esin, A.; Aycan, K. Outcome of dentigerous cysts treated with marsupialization. J. Clin. Pediatr. Dent. 2009, 34, 165–168. [Google Scholar] [CrossRef]
- Chima, K.K.; Seldin, E.B.; Dodson, T.B. Comparison of wound management methods after removal of maxillofacial osseous lesions. J. Oral Maxillofac. Surg. 2006, 64, 1398–1403. [Google Scholar] [CrossRef]
- Ogle, O.E.; Santosh, A.B. Medication Management of Jaw Lesions for Dental Patients. Dent. Clin. N. Am. 2016, 60, 483–495. [Google Scholar] [CrossRef]
- Santamaria, J.; Garcia, A.M.; de Vicente, J.C.; Landa, S.; Lopez-Arranz, J.S. Bone regeneration after radicular cyst removal with and without guided bone regeneration. Int. J. Oral Maxillofac. Surg. 1998, 27, 118–120. [Google Scholar] [CrossRef]
- Szabo, G.S.Z.; Barabas, J.; Németh, Z.; Hrabák, K. Filling of triple mandibular cyst with different bone substitutional materilas. Quintessenz 2003, 2, 123–125. [Google Scholar]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma. 2018, 32 (Suppl. 1), S7–S11. [Google Scholar] [CrossRef]
- Major, M.T.B.; Polyák, M.; Kovács, D.; Szabó, G. A Platelet Rich Fibrin szerepe a fogászatban és a maxillofaciális sebészetben: Irodalmi áttekintés. Fogorvosi Szle. 2022, 115, 202–206. [Google Scholar] [CrossRef]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Lucarelli, E.; Beccheroni, A.; Donati, D.; Sangiorgi, L.; Cenacchi, A.; Del Vento, A.M.; Meotti, C.; Bertoja, A.Z.; Giardino, R.; Fornasari, P.M.; et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials 2003, 24, 3095–3100. [Google Scholar] [CrossRef]
- Yu, J.; Ustach, C.; Kim, H.R. Platelet-derived growth factor signaling and human cancer. J. Biochem. Mol. Biol. 2003, 36, 49–59. [Google Scholar] [CrossRef]
- Winkler, R.; Pasleau, F.; Boussif, N.; Hodzic, D. The IGF system: Summary and recent data. Rev. Med. Liege 2000, 55, 725–739. [Google Scholar]
- Evans, T.W. Review article: Albumin as a drug--biological effects of albumin unrelated to oncotic pressure. Aliment. Pharmacol. Ther. 2002, 16 (Suppl. S5), 6–11. [Google Scholar] [CrossRef]
- Kuten Pella, O.; Hornyak, I.; Horvathy, D.; Fodor, E.; Nehrer, S.; Lacza, Z. Albumin as a Biomaterial and Therapeutic Agent in Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 557. [Google Scholar] [CrossRef]
- Bartos, V.; Kullova, M.; Adamicova, K.; Paucinova, I. Gorlin-Goltz syndrome. Klin. Onkol. 2019, 32, 124–128. [Google Scholar] [CrossRef]
- Pogrel, M.A. Decompression and marsupialization as a treatment for the odontogenic keratocyst. Oral Maxillofac. Surg. Clin. N. Am. 2003, 15, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Kormoczi, K.; Komlos, G.; Papocsi, P.; Horvath, F.; Joob-Fancsaly, A. The early loading of different surface-modified implants: A randomized clinical trial. BMC Oral Health 2021, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Riley, D.S.; Barber, M.S.; Kienle, G.S.; Aronson, J.K.; von Schoen-Angerer, T.; Tugwell, P.; Kiene, H.; Helfand, M.; Altman, D.G.; Sox, H.; et al. CARE guidelines for case reports: Explanation and elaboration document. J. Clin. Epidemiol. 2017, 89, 218–235. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Gundersen, H.J.; Boyce, R.W.; Nyengaard, J.R.; Odgaard, A. The Conneulor: Unbiased estimation of connectivity using physical disectors under projection. Bone 1993, 14, 217–222. [Google Scholar] [CrossRef]
- Starz, F.; Valdec, S.; Lotz, M. Alveolar ridge preservation with autologous dentin following cystectomy. Swiss Dent. J. 2022, 132, 113–116. [Google Scholar]
- Hollinger, J.O.; Kleinschmidt, J.C. The critical size defect as an experimental model to test bone repair materials. J. Craniofac. Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef]
- Schlegel, K.A.; Lang, F.J.; Donath, K.; Kulow, J.T.; Wiltfang, J. The monocortical critical size bone defect as an alternative experimental model in testing bone substitute materials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 7–13. [Google Scholar] [CrossRef]
- Neukam, F.W.S.H.; Schmelzeisen, R. Curriculum Zahnärztliche Chirurgie; Quintessenz: Berlin, Germany, 2001; Volume 434–458. [Google Scholar]
- Wiltfang, J.; Kloss, F.R.; Kessler, P.; Nkenke, E.; Schultze-Mosgau, S.; Zimmermann, R.; Schlegel, K.A. Effects of platelet-rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical-size defects. An animal experiment. Clin. Oral Implant. Res. 2004, 15, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Trimmel, B.; Gyulai-Gaal, S.; Kivovics, M.; Jakob, N.P.; Hegedus, C.; Szabo, B.T.; Dobo-Nagy, C.; Szabo, G. Evaluation of the Histomorphometric and Micromorphometric Performance of a Serum Albumin-Coated Bone Allograft Combined with A-PRF for Early and Conventional Healing Protocols after Maxillary Sinus Augmentation: A Randomized Clinical Trial. Materials 2021, 14, 1810. [Google Scholar] [CrossRef]
- Guillaume, B.; Gaudin, C.; Georgeault, S.; Mallet, R.; Basle, M.F.; Chappard, D. Viability of osteocytes in bone autografts harvested for dental implantology. Biomed. Mater. 2009, 4, 015012. [Google Scholar] [CrossRef]
- Nkenke, E.; Neukam, F.W. Autogenous bone harvesting and grafting in advanced jaw resorption: Morbidity, resorption and implant survival. Eur. J. Oral Implantol. 2014, 7 (Suppl. 2), S203–S217. [Google Scholar]
- Szabo, G.; Huys, L.; Coulthard, P.; Maiorana, C.; Garagiola, U.; Barabas, J.; Nemeth, Z.; Hrabak, K.; Suba, Z. A prospective multicenter randomized clinical trial of autogenous bone versus beta-tricalcium phosphate graft alone for bilateral sinus elevation: Histologic and histomorphometric evaluation. Int. J. Oral Maxillofac. Implant. 2005, 20, 371–381. [Google Scholar]
- Monje, A.; Monje, F.; Hernandez-Alfaro, F.; Gonzalez-Garcia, R.; Suarez-Lopez del Amo, F.; Galindo-Moreno, P.; Montanero-Fernandez, J.; Wang, H.L. Horizontal Bone Augmentation Using Autogenous Block Grafts and Particulate Xenograft in the Severe Atrophic Maxillary Anterior Ridges: A Cone-Beam Computerized Tomography Case Series. J. Oral Implantol. 2015, 41, 366–371. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Chakravarthi, S.P.; Neelima Devi, K.N.; Sridhar, M.S.; Prasad, L.K. Comparative evaluation of bovine derived hydroxyapatite and synthetic hydroxyapatite graft in bone regeneration of human maxillary cystic defects: A clinico-radiological study. Indian. J. Dent. Res. 2014, 25, 594–601. [Google Scholar] [CrossRef]
- Horvathy, D.B.; Vacz, G.; Szabo, T.; Szigyarto, I.C.; Toro, I.; Vamos, B.; Hornyak, I.; Renner, K.; Klara, T.; Szabo, B.T.; et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 126–132. [Google Scholar] [CrossRef]
- Horvathy, D.B.; Schandl, K.; Schwarz, C.M.; Renner, K.; Hornyak, I.; Szabo, B.T.; Niculescu-Morzsa, E.; Nehrer, S.; Dobo-Nagy, C.; Doros, A.; et al. Serum albumin-coated bone allograft (BoneAlbumin) results in faster bone formation and mechanically stronger bone in aging rats. J. Tissue Eng. Regen. Med. 2019, 13, 416–422. [Google Scholar] [CrossRef]
- Marton, K.; Tamas, S.B.; Orsolya, N.; Bela, C.; Ferenc, D.; Peter, N.; Csaba, D.N.; Lajos, C.; Zsombor, L.; Eitan, M.; et al. Microarchitecture of the Augmented Bone Following Sinus Elevation with an Albumin Impregnated Demineralized Freeze-Dried Bone Allograft (BoneAlbumin) versus Anorganic Bovine Bone Mineral: A Randomized Prospective Clinical, Histomorphometric, and Micro-Computed Tomography Study. Materials 2018, 11, 202. [Google Scholar] [CrossRef]
- Gyulay, K.K.; Karaszi, P.; Redei, M.; Solymos, P.; Schandl, K.; Lacza, Z.; Horvathy, D.B. Evaluation of Serum Albumin-Coated Bone Allograft for Bone Regeneration: A Seven-Year Follow-Up Study of 26 Cases. Int. J. Mol. Sci. 2023, 24, 9232. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Chou, T.M.; Chang, H.P.; Wang, J.C. Autologous platelet concentrates in maxillofacial regenerative therapy. Kaohsiung J. Med. Sci. 2020, 36, 305–310. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Pichotano, E.C.; de Molon, R.S.; de Souza, R.V.; Austin, R.S.; Marcantonio, E.; Zandim-Barcelos, D.L. Evaluation of L-PRF combined with deproteinized bovine bone mineral for early implant placement after maxillary sinus augmentation: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 253–262. [Google Scholar] [CrossRef]
- Kobayashi, E.; Fluckiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]


| Abbreviation | Variable | Description | Standard Unit |
|---|---|---|---|
| BV/TV | Bone volume fraction | Ratio of the segmented bone volume to the total volume of the region of interest | % |
| BS/TV | Bone surface density | Ratio of the segmented bone surface to the total volume of the region of interest | mm−1 |
| BS/BV | Specific bone surface | Ratio of the segmented bone surface to the segmented bone volume | mm−1 |
| Tb.Th | Trabecular thickness | Mean thickness of trabeculae assessed using direct 3D methods | mm |
| Tb.Sp | Trabecular separation | Mean distance between trabeculae assessed using direct 3D methods | mm |
| Tb.Pf | Trabecular bone-pattern factor | Index of connectivity of trabecular bone that calculates an index of the relative convexity or concavity of the total bone surface on the principle that concavity indicates connectivity (and the presence of ‘nodes’) and convexity indicates isolated disconnected structures (struts) | 1/mm |
| DA | Degree of anisotropy | DA is 1 ¼ isotropic and >1 ¼ anisotropic by definition; DA is ¼ the length of the longest divided by the shortest mean intercept length vector | None |
| SMI | Structure model index | An indicator of the structure of trabeculae; SMI is 0 for parallel plates and 3 for cylindrical rods | None |
| Po(op) | Open porosity | Percent open porosity is the volume of open pores as a percent of the total VOI volume | % |
| Po(tot) | Total porosity | Total porosity is the volume of all open plus closed pores as a percent of the total volume of the region of interest | % |
| Conn | Connectivity | An effective and efficient method to compute the Euler connectivity in three dimensions is the Conneulor. This algorithm gauges a property termed ‘redundant connectivity’, which is the extent to which different parts of the object are interconnected. It quantifies the maximum number of connections in a structure that can be cut before the entire structure splits into two disjointed segments | None |
| Abbreviation | Sample | n | Mean |
|---|---|---|---|
| BV/TV | 3 months of healing | 2 | 25.7886 |
| 6 months of healing | 2 | 39.7210 | |
| Native bone | 4 | 41.0630 | |
| BS/BV | 3 months of healing | 2 | 0.0317 |
| 6 months of healing | 2 | 0.018.6 | |
| Native bone | 4 | 0.0146 | |
| BS/TV | 3 months of healing | 2 | 0.0069 |
| 6 months of healing | 2 | 0.0067 | |
| Native bone | 4 | 0.0059 | |
| Tb.Th | 3 months of healing | 2 | 151.8090 |
| 6 months of healing | 2 | 210.2448 | |
| Native bone | 4 | 216.2362 | |
| Tb.Sp | 3 months of healing | 2 | 316.6615 |
| 6 months of healing | 2 | 300.3770 | |
| Native bone | 4 | 256.2631 | |
| Tb.Pf | 3 months of healing | 2 | 0.0080 |
| 6 months of healing | 2 | 0.0055 | |
| Native bone | 4 | 0.0017 | |
| DA | 3 months of healing | 2 | 1.1206 |
| 6 months of healing | 2 | 1.1882 | |
| Native bone | 4 | 1.5026 | |
| SMI | 3 months of healing | 2 | 1.4071 |
| 6 months of healing | 2 | 1.9613 | |
| Native bone | 4 | 0.7452 | |
| Po(tot) | 3 months of healing | 2 | 74.2112 |
| 6 months of healing | 2 | 60.2789 | |
| Native bone | 4 | 58.9067 | |
| Po(op) | 3 months of healing | 2 | 74.2022 |
| 6 months of healing | 2 | 60.2427 | |
| Native bone | 4 | 58.9067 | |
| Conn | 3 months of healing | 2 | 1641.5 |
| 6 months of healing | 2 | 2160.5 | |
| Native bone | 4 | 1131.4 |
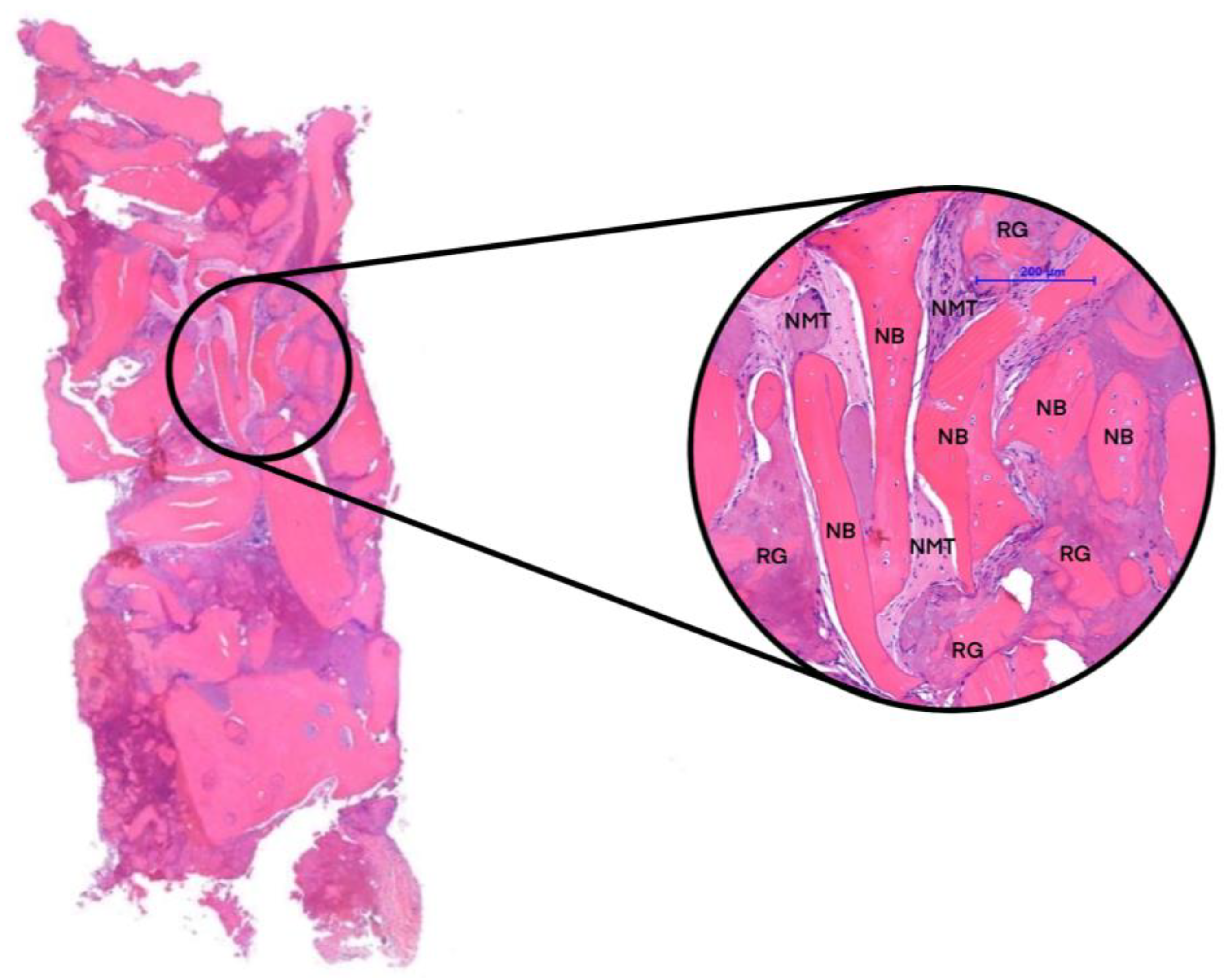
| Groups | n | NFB | NMT | RG |
|---|---|---|---|---|
| 3 months of healing | 2 | 40.59% | 30.82% | 28.59% |
| 6 months of healing | 2 | 55.92% | 27.95% | 16.13% |
| Native bone (NB) | 4 | 60.78% | 39.22% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Major, M.; Kivovics, M.; Szabó, B.T.; Déri, T.; Polyák, M.; Jákob, N.P.; Csete, D.; Mócsai, A.; Németh, Z.; Szabó, G. Surgical Treatment of Multiple Bone Cysts Using a Platelet-Rich Fibrin and BoneAlbumin Composite Graft: A Case Report. Reports 2024, 7, 7. https://doi.org/10.3390/reports7010007
Major M, Kivovics M, Szabó BT, Déri T, Polyák M, Jákob NP, Csete D, Mócsai A, Németh Z, Szabó G. Surgical Treatment of Multiple Bone Cysts Using a Platelet-Rich Fibrin and BoneAlbumin Composite Graft: A Case Report. Reports. 2024; 7(1):7. https://doi.org/10.3390/reports7010007
Chicago/Turabian StyleMajor, Martin, Márton Kivovics, Bence Tamás Szabó, Tamás Déri, Melinda Polyák, Noémi Piroska Jákob, Dániel Csete, Attila Mócsai, Zsolt Németh, and György Szabó. 2024. "Surgical Treatment of Multiple Bone Cysts Using a Platelet-Rich Fibrin and BoneAlbumin Composite Graft: A Case Report" Reports 7, no. 1: 7. https://doi.org/10.3390/reports7010007
APA StyleMajor, M., Kivovics, M., Szabó, B. T., Déri, T., Polyák, M., Jákob, N. P., Csete, D., Mócsai, A., Németh, Z., & Szabó, G. (2024). Surgical Treatment of Multiple Bone Cysts Using a Platelet-Rich Fibrin and BoneAlbumin Composite Graft: A Case Report. Reports, 7(1), 7. https://doi.org/10.3390/reports7010007






