Pulmonologist-Performed Ultrasound-Guided Fine-Needle Aspiration of Lung Lesions
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. Diagnostic Category
2.3. Study Design
Statistical Analysis
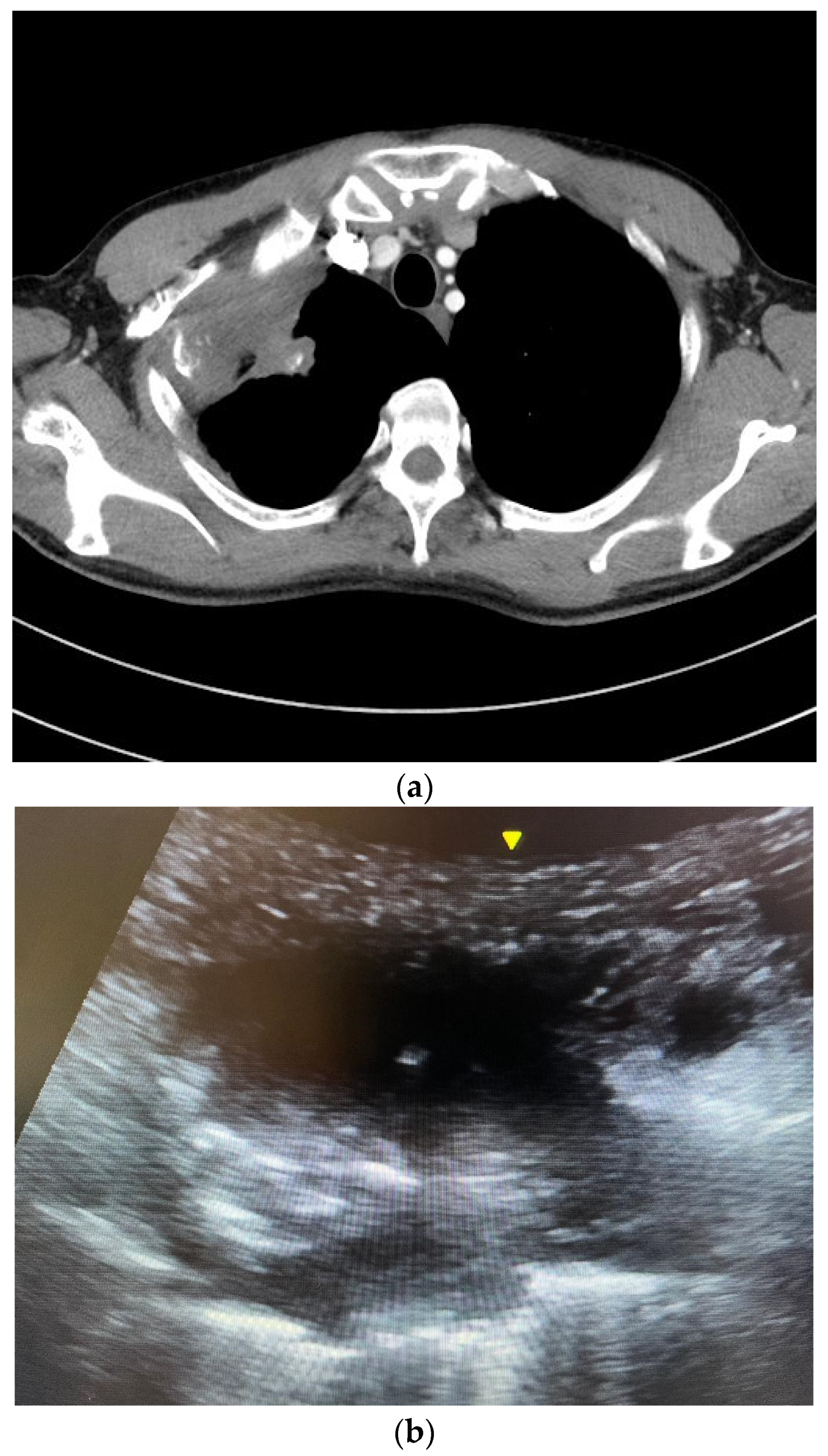
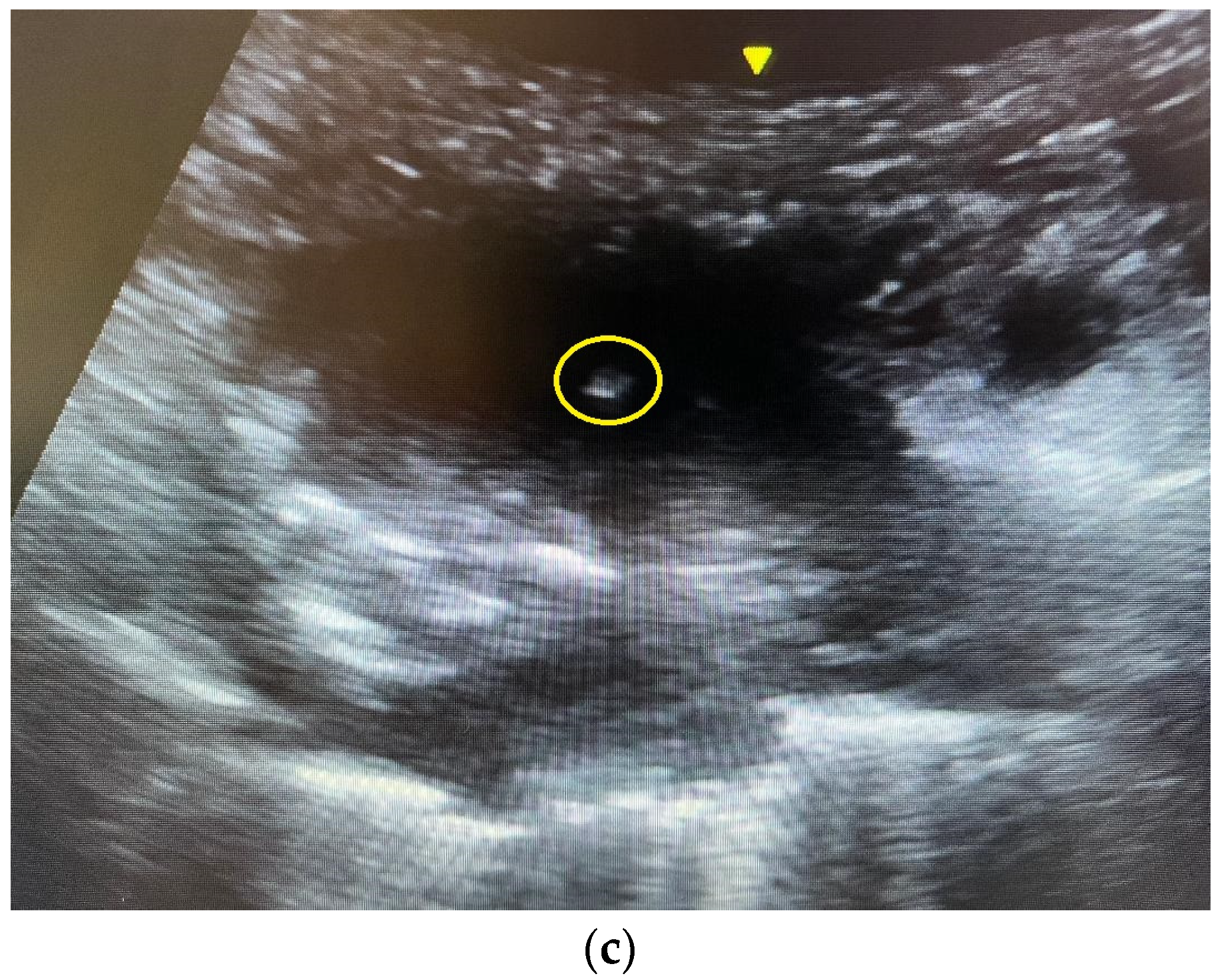
2.4. The Ultrasound-Guided Fine-Needle Aspiration
3. Results
3.1. Baseline Characteristics and Lung Lesion Profile
3.2. Diagnostic Accuracy and Yield
3.3. Complication Rates
3.4. The Diagnoses
3.5. Predictive Factors
3.6. Genetic Mutation Analysis
4. Discussions
4.1. Diagnostic Accuracy in the Literature
4.2. The Complication Rate in the Literature
4.3. Predictive Factors of Diagnostic Yield
4.4. Strengths
4.5. Limitations
4.6. Further Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALK | Anaplastic Lymphoma Kinase |
| CDARS | Clinical Data Analysis and Reporting System |
| CI | Confidence Interval |
| CNB | Core Needle Biopsy |
| CT | Computed Tomography |
| EGFR | Epidermal Growth Factor Receptor |
| FNA | Fine-Needle Aspiration |
| ICD | International Code of Disease |
| OR | Odds Ratio |
| PDL1 | Programmed Cell Death Ligand 1 |
References
- Gould, M.K.; Tang, T.; Liu, I.L.; Lee, J.; Zheng, C.; Danforth, K.N.; Kosco, A.E.; Di Fiore, J.L.; Suh, D.E. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am. J. Respir. Crit. Care Med. 2015, 192, 1208–1214. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Lewis, S.Z.; Diekemper, R.; Addrizzo-Harris, D.; Alberts, W.M. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. S5), 7S–37S. [Google Scholar] [CrossRef]
- Yung, R.C. Tissue diagnosis of suspected lung cancer: Selecting between bronchoscopy, transthoracic needle aspiration, and resectional biopsy. Respir. Care Clin. N. Am. 2003, 9, 51–76. [Google Scholar] [CrossRef]
- Koh, D.M.; Burke, S.; Davies, N.; Padley, S.P. Transthoracic US of the chest: Clinical uses and applications. Radiographics 2002, 22, e1. [Google Scholar] [CrossRef]
- Targhetta, R.; Bourgeois, J.M.; Marty-Double, C.; Coste, E.; Proust, A.; Balmes, P.; Pourcelot, L. Peripheral pulmonary lesions: Ultrasonic features and ultrasonically guided fine needle aspiration biopsy. J. Ultrasound Med. 1993, 12, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; Schuurmans, M.M.; Theron, J.; Schubert, P.T.; Wright, C.A.; Bolliger, C.T. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respiration 2004, 71, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.F.; Pastorello, R.; Osmani, L.; Hopkins, M.; Kryatova, M.; Kawamoto, S.; Maleki, Z. Ultrasound-Guided Transthoracic Fine-Needle Aspiration: A Reliable Tool in Diagnosis and Molecular Profiling of Lung Masses. Acta Cytol. 2020, 64, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Koegelenberg, C.F.; Bolliger, C.T.; Plekker, D.; Wright, C.A.; Brundyn, K.; Louw, M.; Schubert, P.; van den Heuvel, M.M.; Diacon, A.H. Diagnostic yield and safety of ultrasound-assisted biopsies in superior vena cava syndrome. Eur. Respir. J. 2009, 33, 1389–1395. [Google Scholar] [CrossRef]
- Knox, D.; Halligan, K. Case series of trans-thoracic nodule aspirate performed by interventional pulmonologists. Respir. Med. Case Rep. 2021, 32, 101362. [Google Scholar] [CrossRef]
- Cozzolino, I.; Ronchi, A.; Messina, G.; Montella, M.; Morgillo, F.; Vicidomini, G.; Tirino, V.; Grimaldi, A.; Marino, F.Z.; Santini, M.; et al. Adequacy of Cytologic Samples by Ultrasound-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology of Peripheral Pulmonary Nodules for Morphologic Diagnosis and Molecular Evaluations: Comparison with Computed Tomography-Guided Percutaneous Transthoracic Fine-Needle Aspiration Cytology. Arch. Pathol. Lab. Med. 2020, 144, 361–369. [Google Scholar] [CrossRef]
- Meena, N.; Bartter, T. Ultrasound-guided Percutaneous Needle Aspiration by Pulmonologists: A Study of Factors with Impact on Procedural Yield and Complications. J. Bronchol. Interv. Pulmonol. 2015, 22, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Portela-Oliveira, E.; Souza, C.A.; Gupta, A.; Bayanati, H.; Inacio, J.; Rakhra, K. Ultrasound-guided percutaneous biopsy of thoracic lesions: High diagnostic yield and low complication rate. Clin. Radiol. 2021, 76, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ye, J.; Qiu, Y.; Peng, W.; Lan, N.; Huang, T.; Ou, Y.; Deng, X.; Li, Y. Ultrasound-Guided Percutaneous Core Needle Biopsy of Peripheral Pulmonary Nodules </ 2 cm: Diagnostic Performance, Safety and Influence Factors. Front. Oncol. 2021, 11, 671884. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Watanabe, T.; Yamada, K.; Nakai, T.; Suzumura, T.; Sakagami, K.; Yoshimoto, N.; Sato, K.; Tanaka, H.; Mitsuoka, S.; et al. Efficacy and safety of ultrasound (US) guided percutaneous needle biopsy for peripheral lung or pleural lesion: Comparison with computed tomography (CT) guided needle biopsy. J. Thorac. Dis. 2019, 11, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.E.; Suresh, P.K.; Sridevi, H.B.; Sahu, K.K.; Adiga, D.; Minal, J.; Rai, S.; Acharya, V. Image-guided Fine Needle Aspiration Cytology of Intrathoracic Lesions. J. Cytol. 2019, 36, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lim, K.Y.; Suh, Y.J.; Hur, J.; Han, D.H.; Kang, M.J.; Choo, J.Y.; Kim, C.; Kim, J.I.; Yoon, S.H.; et al. Diagnostic Accuracy of Percutaneous Transthoracic Needle Lung Biopsies: A Multicenter Study. Korean J. Radiol. 2019, 20, 1300–1310. [Google Scholar] [CrossRef]
- Knybel, L.; Cvek, J.; Molenda, L.; Stieberova, N.; Feltl, D. Analysis of Lung Tumor Motion in a Large Sample: Patterns and Factors Influencing Precise Delineation of Internal Target Volume. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 751–758. [Google Scholar] [CrossRef]
- Shimizu, S.; Shirato, H.; Kagei, K.; Nishioka, T.; Bo, X.; Dosaka-Akita, H.; Hashimoto, S.; Aoyama, H.; Tsuchiya, K.; Miyasaka, K. Impact of respiratory movement on the computed tomographic images of small lung tumors in three-dimensional (3D) radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Swensen, S.J.; Silverstein, M.D.; Ilstrup, D.M.; Schleck, C.D.; Edell, E.S. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch. Intern. Med. 1997, 157, 849–855. [Google Scholar] [CrossRef]
- McWilliams, A.; Tammemagi, M.C.; Mayo, J.R.; Roberts, H.; Liu, G.; Soghrati, K.; Yasufuku, K.; Martel, S.; Laberge, F.; Gingras, M.; et al. Probability of cancer in pulmonary nodules detected on first screening CT. N. Engl. J. Med. 2013, 369, 910–919. [Google Scholar] [CrossRef]
- Greif, J.; Marmor, S.; Schwarz, Y.; Staroselsky, A.N. Percutaneous core needle biopsy vs. fine needle aspiration in diagnosing benign lung lesions. Acta Cytol. 1999, 43, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Gorg, C. Transcutaneous contrast-enhanced sonography of pleural-based pulmonary lesions. Eur. J. Radiol. 2007, 64, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Lou, J.; Bao, L.; Lv, Z. Contrast-enhanced ultrasound for needle biopsy of central lung cancer with atelectasis. J. Med. Ultrason. 2018, 45, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.T.; Yeung, Y.C.; Chan, Y.H.; Ho, M.Y. Pulmonologist performed ultrasound-guided fine-needle aspiration of lung lesions. Chest 2023, 164, A5255. [Google Scholar] [CrossRef]
| Needle Type | Count |
|---|---|
| Temno | 40.5 |
| Vac-Cut | 20.5 |
| Magnum | 3 |
| Chiba | 2 |
| Ultracore | 1 |
| Spinal | 0.5 |
| Coaxial | 0.5 |
| Needle Size | Count |
|---|---|
| 17 G | 1 |
| 18 G | 45.5 |
| 19.5 G | 6 |
| 20 G | 10 |
| 21 G | 2.5 |
| 22 G | 3 |
| Pulmonologist Group (n = 113) | Radiologist Group (n = 68) | p-Value | |
|---|---|---|---|
| Age, year, mean ± SD [range] | 68.7 ± 12 [26–94] | 64.4 ± 12.6 [31–84] | 0.020 |
| Gender (male/female) | 85:28 | 55:13 | 0.378 |
| Smoking status, n (%) | 0.558 | ||
| Non-smoker | 35 (31) | 22 (32.5) | |
| Ex-smoker | 33 (29) | 24 (35) | |
| Smoker | 45 (40) | 22 (32.5) | |
| Lung lesion size, centimeter, mean ± SD [range] | 6.5 ± 2.9 [1.7–19] | 6.7 ± 3.2 [2–17] | 0.687 |
| Lung lesion location, n (%) | 0.049 | ||
| Right upper lobe | 40 (35) | 20 (29.4) | |
| Right middle lobe | 7 (7) | 3 (4.4) | |
| Right lower lobe | 26 (23) | 16 (23.5) | |
| Left upper lobe | 17 (15) | 17 (25) | |
| Left lingula lobe | 0 | 4 (5.9) | |
| Left lower lobe | 23 (20) | 8 (11.8) |
| Pulmonologist Group (n = 112) | Radiologist Group (n = 68) | p-Value | |
|---|---|---|---|
| Accuracy (95% CI) | 80.4% (72.0%−86.7%) | 86.8% (76.7%−92.9%) | 0.270 |
| Sensitivity (95% CI) | 80% (71.6%−86.4%) | 86.4% (76.1%−92.7%) | 0.283 |
| Specificity (95% CI) | 100% (34.2%−100%) | 100% (34.2%−100%) | 1 |
| Positive predictive value (95% CI) | 100% (95.8%−100%) | 100% (93.7%−100%) | 1 |
| Negative predictive value (95% CI) | 8.3% (2.3%−25.8%) | 18.2% (5.1%−47.7%) | 0.575 |
| Pulmonologist Group (n = 113) | Radiologist Group (n = 68) | p-Value | |
|---|---|---|---|
| Any complication, n (%) | 6 (5.3%) | 5 (7.4%) | 0.749 |
| Pneumothorax, n (%) | 6 (5.3%) | 4 (5.9%) | 1 |
| Hemoptysis, n (%) | 0 | 1 (1.5%) | 0.376 |
| Pulmonologist Group (n = 113) | Radiologist Group (n = 68) | |
|---|---|---|
| Lung cancer | ||
| Adenocarcinoma | 44 (39%) | 18 (26%) |
| Squamous cell carcinoma | 14 (12%) | 14 (21%) |
| Non-small-cell carcinoma | 10 (9%) | 3 (4%) |
| Small cell carcinoma | 6 (5%) | 3 (4%) |
| SMARCA4-deficient malignant tumor | 1 (1%) | 0 |
| Sarcomatoid carcinoma | 0 | 2 (3%) |
| Lymphoepithelioma-like carcinoma | 2 (2%) | 1 (1%) |
| Adenosquamous carcinoma | 1 (1%) | 0 |
| Metastasis | 1 (1%) | 5 (7%) |
| Hematological malignancies | ||
| Lymphoma | 3 (3%) | 2 (3%) |
| Leukemic infiltrate | 1 (1%) | 1 (1%) |
| Malignancy not otherwise specified | ||
| Malignant cells | 1 (1%) | 0 |
| Carcinoma | 1 (1%) | 0 |
| Spindle cell lesion | 0 | 1 (1%) |
| Infection | ||
| Aspergillosis | 1 (1%) | 2 (3%) |
| Cryptococcal infection | 0 | 1 (1%) |
| Tuberculosis | 3 (3%) | 1 (1%) |
| Benign lesions | ||
| Fibrous tumor | 0 | 2 (3%) |
| Neurilemmoma | 0 | 1 (1%) |
| Non-diagnostic | ||
| Acute inflammation | 2 (2%) | 0 |
| Necrosis | 3 (3%) | 1 (1%) |
| Suspicious cells | 3 (3%) | 1 (1%) |
| Atypical cells | 2 (2%) | 1 (1%) |
| Quantity insufficiency | 7 (6%) | 1 (1%) |
| Negative | 7 (6%) | 7 (10%) |
| Univariable Regression | |||
|---|---|---|---|
| ORunadj (95% CI) | p | ||
| Lesion size | 1.12 | (0.93–1.34) | 0.230 |
| Lesion location a | |||
| Lower (ref.) | 1 | — | |
| Middle/lingular | 44.67 | (0.18–11,240.83) | 0.173 |
| Upper | 5.32 | (1.78–15.87) | 0.002 |
| Age | 1.01 | (0.98–1.05) | 0.475 |
| Male | 1.53 | (0.55–4.26) | 0.412 |
| Smoking status | |||
| Never (ref.) | 1 | — | |
| Ex | 1.28 | (0.39–4.21) | 0.680 |
| Current | 1.37 | (0.46–4.11) | 0.574 |
| Univariable Regression | Multivariable Regression | |||||
|---|---|---|---|---|---|---|
| ORunadj (95% CI) | p | ORadj (95% CI) | p | |||
| Lesion size, cm | 1.17 | (1.00–1.38) | 0.048 | 1.12 | (0.95–1.31) | 0.176 |
| Lesion location | ||||||
| Lower (ref.) | 1 | — | 1 | — | ||
| Middle/lingular | 1.48 | (0.38–5.85) | 0.575 | 1.39 | (0.35–5.56) | 0.638 |
| Upper | 4.96 | (1.97–12.48) | <0.001 | 4.42 | (1.73–11.28) | 0.002 |
| Age, year | 1.01 | (0.98–1.04) | 0.475 | — | — | |
| Male | 1.81 | (0.77–4.24) | 0.171 | — | — | |
| Smoking status | ||||||
| Never (ref.) | 1 | — | ||||
| Ex | 1.09 | (0.44–2.73) | 0.852 | — | — | |
| Current | 1.97 | (0.74–5.21) | 0.174 | — | — | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwok, C.-T.; Yeung, Y.-C.; Chan, Y.-H.; Ho, M.-Y. Pulmonologist-Performed Ultrasound-Guided Fine-Needle Aspiration of Lung Lesions. Reports 2024, 7, 26. https://doi.org/10.3390/reports7020026
Kwok C-T, Yeung Y-C, Chan Y-H, Ho M-Y. Pulmonologist-Performed Ultrasound-Guided Fine-Needle Aspiration of Lung Lesions. Reports. 2024; 7(2):26. https://doi.org/10.3390/reports7020026
Chicago/Turabian StyleKwok, Chin-Tong, Yiu-Cheong Yeung, Yu-Hong Chan, and Man-Ying Ho. 2024. "Pulmonologist-Performed Ultrasound-Guided Fine-Needle Aspiration of Lung Lesions" Reports 7, no. 2: 26. https://doi.org/10.3390/reports7020026
APA StyleKwok, C.-T., Yeung, Y.-C., Chan, Y.-H., & Ho, M.-Y. (2024). Pulmonologist-Performed Ultrasound-Guided Fine-Needle Aspiration of Lung Lesions. Reports, 7(2), 26. https://doi.org/10.3390/reports7020026






