Mobility of Metals in Sediments Contaminated with Historical Mining Wastes: Example from the Tri-State Mining District, USA
Abstract
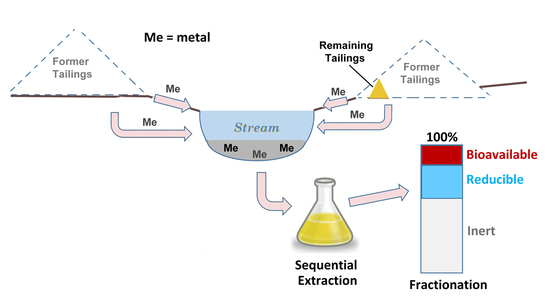
:1. Introduction
2. Materials and Methods
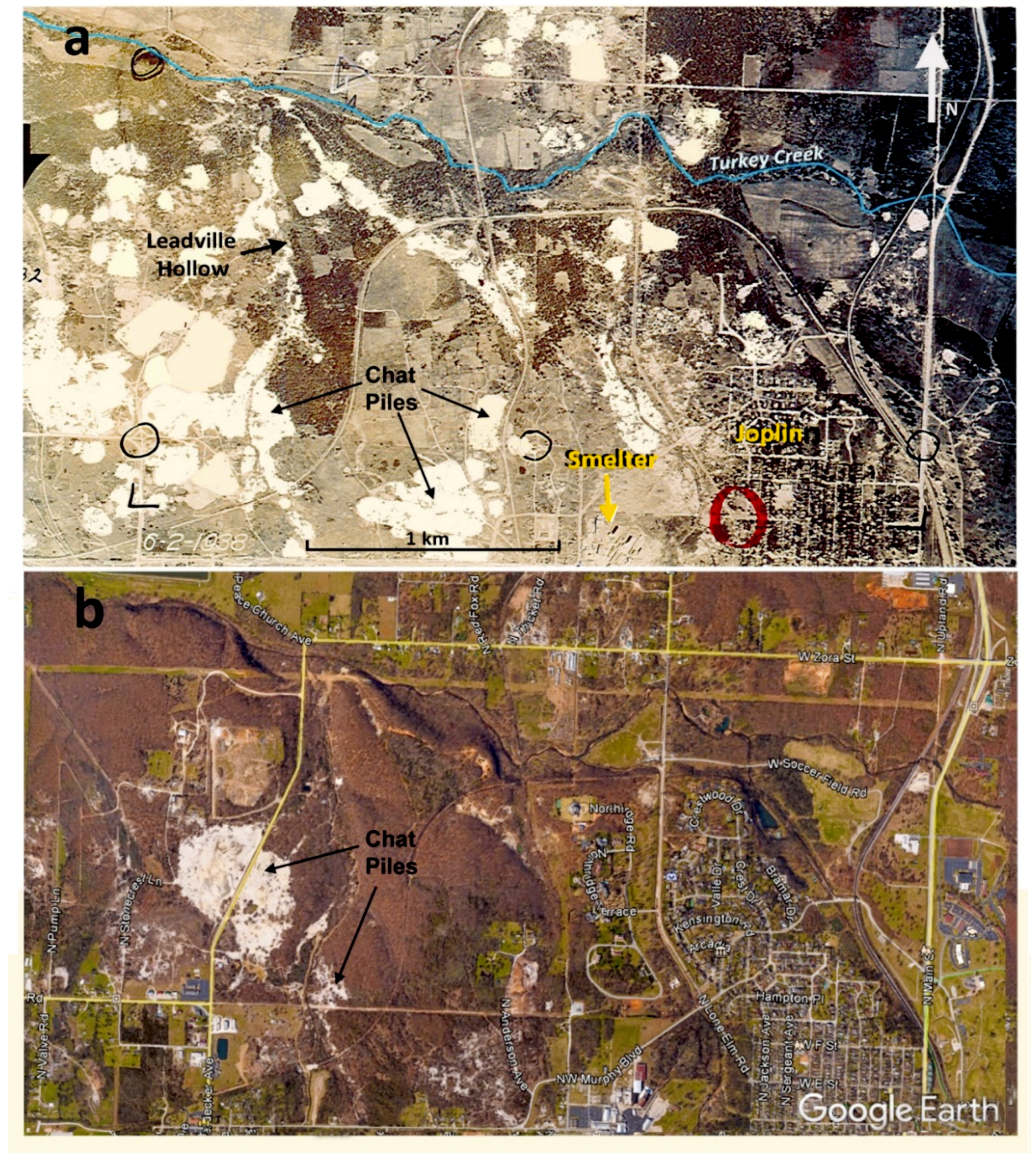
2.1. Study Area
2.2. Remediation of the Study Area from the 1980s to Present Time
2.3. Sampling and Data Collection
2.4. Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fields, S. The Earth’s open wounds: Abandoned and orphaned mines. Environ. Health Perspect. 2003, 111, A154–A161. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, M.; Mickus, K.; Camacho, L.M. Abandoned Pb Zn mining wastes and their mobility as proxy to toxicity: A review. Sci. Total Environ. 2016, 565, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, A.; Woch, M.W.; Kapusta, P. Inconspicuous waste heaps left by historical Zn-Pb mining are hot spots of soil contamination. Geoderma 2014, 235–236, 1–8. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Wu, S.; Rodriguez, J.; Jones, A.D.; Lockwood, B. Assessing the state of contamination for a historic mining town using sediment chemistry. Arch. Environ. Contam. Toxicol. 2016, 70, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.; Gutiérrez, M.; Gouzie, D.; McAlily, R. State of remediation and metal toxicity in the Tri-State Mining District, USA. Chemosphere 2016, 144, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Pope, L.M. Assessment of Contaminated Streambed Sediment in the Kansas Part of the Historic Tri-State Lead and Zinc Mining District, Cherokee County, 2004; U.S. Geological Survey Scientific Investigations Report 2005-5251; U.S. Geological Survey: Reston, VA, USA, 2005; p. 61.
- Favas, J.C.; Kumar Sankar, S.; Rakshit, D. Geochemical fractionation of trace elements in stream sediments contaminated by mining activity. Clean Soil Air Water 2014, 43, 446–455. [Google Scholar] [CrossRef]
- Schaider, L.A.; Senn, D.B.; Brabander, D.J.; McCarthy, K.D.; Shine, J.S. Characterization of zinc, lead, and cadmium in mine waste: Implications for transport, exposure, and bioavailability. Environ. Sci. Technol. 2007, 41, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Concas, A.; Ardau, C.; Cristini, A.; Zuddas, P.; Cao, G. Mobility of heavy metals from tailings to stream waters in a mining activity contaminated site. Chemosphere 2006, 63, 244–253. [Google Scholar] [CrossRef] [PubMed]
- O’Day, P.A.; Carroll, S.A.; Waychunas, G.A. Rock-water interactions controlling zinc, cadmium, and lead concentrations in surface waters and sediments, U.S. Tri-State Mining District. 1. Molecular identification using X-ray absorption spectroscopy. Environ. Sci. Technol. 1998, 32, 943–955. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 1, 844–851. [Google Scholar] [CrossRef]
- Pearson, M.A. Geochemical Fractionation, Speciation, and Bioavailability of Heavy Metals in Stream Sediments in Aurora, MO. Master’s Thesis, Missouri State University, Springfield, MO, USA, 2017. Available online: https://bearworks.missouristate.edu/theses/3119 (accessed on 16 February 2019).
- Roig, N.; Sierra, J.; Moreno-Garrido, I.; Nieto, E.; Perez-Gallego, E.; Schumacher, M.; Blasco, J. Metal bioavailability in freshwater sediment samples and their influence on ecological status of river basins. Sci. Total Environ. 2016, 540, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, D.D.; Ingersoll, C.G.; Berger, T.A. Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch. Environ. Contam. Toxicol. 2000, 39, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, C.G.; Ivey, C.D.; Brumbaugh, W.G.; Besser, J.M.; Kemble, N.E. Toxicity Assessment of Sediments from the Grand Lake O’ the Cherokees with the Amphipod Hyalella Azteca. U.S. Geological Survey Administrative Report CERC-8335-FY09-20-01; 2009; 97p. Available online: https://www.fws.gov/southwest/es/oklahoma/nrdar.htm (accessed on 18 February 2019).
- NOAA (National Oceanic and Atmospheric Administration). Climate Data. Available online: http://www.ncdc.noaa.gov/cdo-web/ (accessed on 15 March 2019).
- Gibson, A.M. Wilderness Bonanza: The Tri-State District of Missouri, Kansas, and Oklahoma; University of Oklahoma Press: Norman, OK, USA, 1972; 362p. [Google Scholar]
- Besser, J.M.; Brumbaugh, W.G.; Ingersoll, C.G. Characterizing toxicity of metal-contaminated sediments from mining areas. Appl. Geochem. 2015, 57, 73–84. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xiao, T.; Bao, Z.; Lippold, H.; Luo, X.; Yin, M.; Ren, J.; Chen, Y.; Linghu, W. Geochemical dispersal of thallium and accompanying metals in sediment profiles from a smelter-impacted area in South China. Appl. Geochem. 2017, 88, 239–246. [Google Scholar] [CrossRef]
- Brockie, D.C.; Hare, E.H., Jr.; Dingess, P.R. The geology and ore deposits of the TriState district of Missouri, Kansas, and Oklahoma. In Ore Deposits of the United States, 1933–1967 (Graton-Sales Volumes); Ridge, J.D., Ed.; The American Institute of Mining, Metalmlurgical and Petroleum Engineers: New York, NY, USA, 1968; Volume 1, pp. 400–430. [Google Scholar]
- Cosatt, M.; Gibson, A.M. Wilderness Bonanza: The Tri-State District of Missouri, Kansas, and Oklahoma. Master’s Thesis, Missouri State University, Springfield, MO, USA, 2011; 77p. [Google Scholar]
- Brown, S.; Mahoney, M.; Sprenger, M. A comparison of the efficacy and ecosystem impact of residual-based and topsoil-based amendments for restoring historic mine tailings in the Tri-State Mining District. Sci. Total Environ. 2014, 485–486, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Peebles, J. Spatial Analysis of Mining Related Sediment Contamination of Turkey Creek Watershed in the Tri-State Mining District. Master’s Thesis, Missouri State University, Joplin, MO, USA, 2013. [Google Scholar]
- Carroll, S.A.; O’Day, P.A.; Piechowski, M. Rock-water interactions controlling zinc, cadmium, and lead concentrations in surface waters and sediments, U.S. Tri-State Mining District. 2. Geochemical Interpretation. Environ. Sci. Technol. 1998, 32, 956–965. [Google Scholar] [CrossRef]
- Schaider, L.A.; Senn, D.B.; Estes, E.R.; Brabander, D.J.; Shine, J.S. Sources and fates of heavy metals in a mining-impacted stream: Temporal variability and the role of iron oxides. Sci. Total Environ. 2014, 490, 456–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, A.; Held, A.; Linsinger, T.P.J.; Perez, A.; Ricci, M. Comparison of total and aqua regia extractability of heavy metals in sewage sludge: The case study of a certified reference material. Trends Anal. Chem. 2017, 89, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Ščančar, J.; Milačič, R.; Horvat, M. Comparison of various digestion and extraction procedures in analysis of heavy metals in sediments. Water Air Soil Pollut. 2000, 118, 87–99. [Google Scholar] [CrossRef]
- Rauret, G.; Lopez-Sanchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Extraction Procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Juracek, K.E. Sedimentation and Occurrence and Trends of Selected Chemical Constituents in Bottom Sediment, Empire Lake, Cherokee County, Kansas, 1905–2005; U.S. Geological Survey Scientific Investigations Report 2006-5307; U.S. Geological Survey: Reston, VA, USA, 2006; p. 79.
- Juracek, K.E. Occurrence and Variability of Mining-Related Lead and Zinc in the Spring River Flood Plain and Tributary Flood Plains, Cherokee County, Kansas, 2009–11; USGS Scientific Investigations Report 2013-5028; U.S. Geological Survey: Reston, VA, USA, 2013; p. 70.
- Pope, L.M.; Mehl, H.E.; Coiner, R.L. Quality Characteristics of Ground Water in the Ozark Aquifer of Northwestern Arkansas, Southeastern Kansas, Southwestern Missouri, and Northeastern Oklahoma, 2006–07; USGS Scientific Investigations Report 2009-5093; U.S. Geological Survey: Reston, VA, USA, 2009; p. 72.



| Cd, mg/kg | Pb, mg/kg | Zn, mg/kg | Source | |
|---|---|---|---|---|
| PEC | 4.98 | 128 | 459 | [14] |
| PEC-TSMD | 11.1 | 150 | 2083 | [15] |
| Empire Lake, Kansas N = 428 | 29 | 270 | 4900 | [29] |
| Turkey Creek, Kansas N = 1 | 50 | 900 | 8000 | [6] |
| *Turkey Creek, Missouri N = 27 | 14 | 238 | 2850 | [23] |
| *Chat Creek, Missouri N = 33 | 35 | 190 | 5791 | [12] |
| Tar Creek, Oklahoma N = 3 | 45 | 110 | 5800 | [30] |
| %Bioavailable | %Fe-oxides | %Org/Residual | |
|---|---|---|---|
| Turkey Creek, N = 19 | |||
| Cd | 5.0 (5.2) | 35.1 (26.6) | 59.8 (29.1) |
| Pb | 0.4 (0.4) | 21.3 (25.7) | 78.3 (25.7) |
| Zn | 7.7 (5.8) | 25.1 (20.0) | 66.7 (24.1) |
| Chat Creek, N = 33 | |||
| Cd | 9.9 (20.8) | 32.4 (15.8) | 58.9 (5.0) |
| Pb | 13.4 (11.8) | 26.9 (2.05) | 59.8 (13.9) |
| Zn | 17.1 (12.8) | 35.0 (15.0) | 48.0 (2.2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, M.; Collette, Z.J.; McClanahan, A.M.; Mickus, K. Mobility of Metals in Sediments Contaminated with Historical Mining Wastes: Example from the Tri-State Mining District, USA. Soil Syst. 2019, 3, 22. https://doi.org/10.3390/soilsystems3010022
Gutiérrez M, Collette ZJ, McClanahan AM, Mickus K. Mobility of Metals in Sediments Contaminated with Historical Mining Wastes: Example from the Tri-State Mining District, USA. Soil Systems. 2019; 3(1):22. https://doi.org/10.3390/soilsystems3010022
Chicago/Turabian StyleGutiérrez, Mélida, Zachary J. Collette, Anastasia M. McClanahan, and Kevin Mickus. 2019. "Mobility of Metals in Sediments Contaminated with Historical Mining Wastes: Example from the Tri-State Mining District, USA" Soil Systems 3, no. 1: 22. https://doi.org/10.3390/soilsystems3010022