Abstract
This study aimed to identify the characteristics of Andisols under tea plantations affected by different Oldeman’s agro-climatic zones, of different ages, and containing different types of volcanic ash material. For this study, three tea plantation estates were chosen, the Ciater Site (CTR), Sinumbra Site (SNR), and Sedep Site (SDP), having Oldeman’s agro-climatic zones of A, B1, and B2, respectively. Three profiles (CTR-A, CTR-B, and SNR-A) were created from andesitic volcanic ash, and three profiles (SNR-B, SDP-A, and SDP-B) were created from basaltic volcanic ash materials. The CTR-A, SNR-B, and SDP-B profiles were obtained from Holocene parent materials, while the CTR-B, SNR-A, and SDP-A profiles were derived from Pleistocene parent materials. Soil samples were taken from the soil profiles from depths of 0 to 153 cm incrementally, dependent on each soil horizon thickness. The findings of the study reveal that the age of parent materials and the variance in agro-climatic zones result in considerable differences in soil chemical characteristics, such as pH (H2O), base saturation (BS), and organic C, while the qualities of the basaltic and andesitic volcanic ash parent materials were also shown to be unaffected. All Andisol profiles went through cambic weathering processes. Moreover, the key pedogenetic strategies were the production of short-range-order minerals through the leaching of easily dissolved elements and the coprecipitation of SiO2 and Al2O3 gels. Halloysite was formed by the resilication of short-range-order minerals, while gibbsite was formed by desilication. The XRD analysis indicated that amorphous materials predominated with some HIV and kaolinite minerals were also present.
1. Introduction
Indonesia has the most active volcanoes in the world, with 127 volcanoes [1]. Volcanic eruptions regularly produce significant volumes of pyroclastic materials, which serve as parent materials for soil formation or the rejuvenation of existing soils. Andisols or Andosols are the most common volcanic ash soils. In Indonesia, such soils cover around 5.395 million ha, or 2.9 percent of the total land area [2]. Andisols are common as the native tropical rainforest land area, and are utilized for productive agricultural soil, particularly for horticultural crops, tea plantations, and coffee plantations [3,4]. Tan [5] stated that in Indonesia, these soils were developed from various parent materials, leading to various development processes and characteristics. According to Anda et al. [3], horticultural Andisols have a higher pH, exchangeable cations, and greater micronutrient levels than plantations.
Andisols are soils formed from pyroclastic materials with an exchanged complex dominated by short-range-order minerals, with Al, Si, and humus being the primary components [6,7], or soils with acidic properties [8]. These soils have large organic components, a high water-holding capacity, P-retention, and a low bulk density. Although their colloidal charge is dependent on the soil pH [9,10,11], the variable charge component of Andisols is formed from allophanes, Al- and Fe-humus complexes, kaolinite, and organic matter. pH0 refers to the point when a variable charge component’s negative and positive charges are equal [10]. Its value indicates the total contribution to protonated/deprotonated processes. It determines the point on the pH scale where equal numbers of protonated and deprotonated sites exist on the soil surfaces [12]. The key elements that influence the pedogenesis of Andisols are the climate and parent material [13,14].
Soil formation is driven by climate (precipitation and temperature), vegetation, and terrain [15,16] and is more reliant on the parent material’s composition [17]. In tropical and temperate climate regions, the weathering intensity of Andisols will alter metastable non-crystalline to stable crystalline minerals [18]. Tsai’s [16] investigation of Taiwan’s Andisols with andesitic parent material suggested that the formation of andic soils is due to the leaching process with high precipitation rates, whereas the presence of kaolinite and the absence of andic properties was observed in low precipitation areas. This study was in line with the results of Taylor et al. [19] who studied New Zealand’s Andisols with rhyolitic parent material derived from the Taupō volcanic eruptions and found that crystalline minerals were formed along with increased silica activity in soil solution, whereas amorphous clay minerals tend to form when silica is leached [19,20,21,22,23,24,25]. Moreover, both studies were conducted in bamboo forest, pasture farmland, and pine forest with a temperate climate.
However, the pedogenetic processes of Andisols formation with basaltic and andesitic parent materials in tropical climate regions with persistent annual rainfall, such as in Indonesia, remain unclear. Oldeman [26] classified several agro-climatic zones in Java based on the ratio of wet, moist, and dry seasons. It is important to emphasize whether the formation of Andisols in Indonesia would follow similar pedogenetic patterns as those in temperate climate regions. We hypothesize that climatic factors (e.g., precipitation, temperature), eruption age, and the type of parent material will strongly influence the different characteristics of Andisols in tropical zones. The main objective of this study was to examine which factors—climate, age, and types of parent material—influence the differences in pedogenetic processes in basaltic and andesitic tropical Andisols. We expect that this study will provide important information for the development of good soil-management practices for tropical areas.
2. Materials and Methods
2.1. Site Description
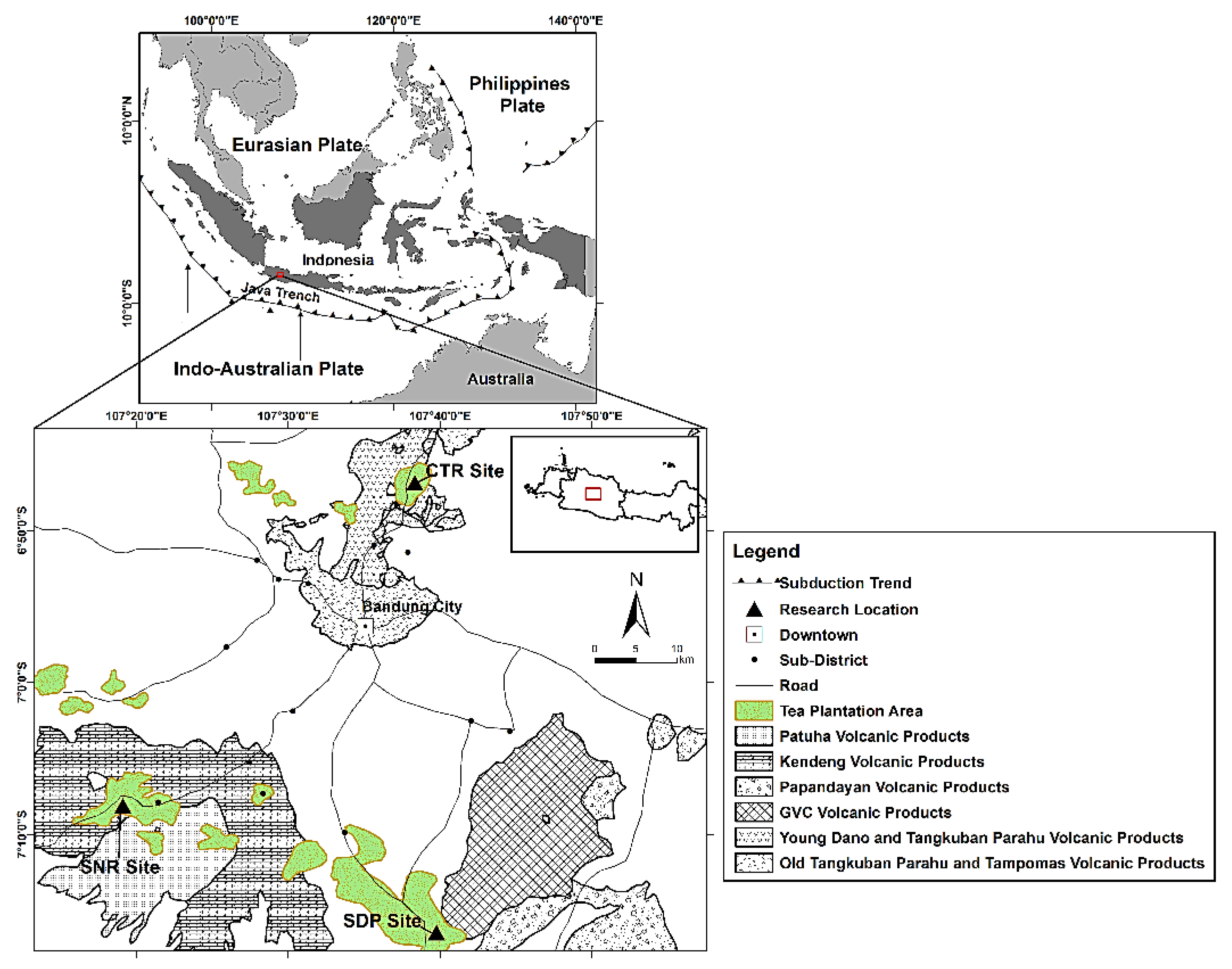
The research was conducted in three estate tea plantations in West Java Province, Indonesia: Ciater (CTR), Sinumbra (SNR), and Sedep (SDP) (Figure 1). These sites are part of the Java trench system, which extends 1200 km from Java in the west to Flores Island in the east [27]. The Java trench system was formed when the Indo-Australian plate subducted beneath the Eurasian plate, resulting in a mixed ocean–continental volcanic ring along the trench [28]. This arc–trench system is primarily responsible for the formation of basaltic to rhyolitic volcanic rocks in the southwestern part of Indonesia. However, basaltic and andesitic rocks are abundant in Java Island due to its thinner crustal thickness than that in Sumatera [29].

Figure 1.
Study location of three estate tea plantations under six different parent materials in West Java, Indonesia.
Geological formations in the studied location were dominated by volcanic products from several distinct eruption periods. Volcanic materials ejected by the Dano, Tangkuban Parahu, and Tampomas volcanic eruptions in the late Pleistocene to late Holocene, comprising of andesitic sandy tuff, pyroclastic breccia with pumiceous fragment, and vesiculated lava [30], were found at the CTR. The Patuha volcano, which erupted during the Holocene [31], yielded volcanic rocks consisting of pyroxene andesitic lava and laharic deposit with greyish sandy tuff groundmass at the SNR site. Older Pleistocene volcanic products accumulated in the SNR following the Kendeng volcanic eruption, which erupted andesitic lava flows interbedded with laharic breccia. At the SDP site, volcanic materials were generated by two different eruption periods: the Pleistocene Guntur Volcanic Complex (GVC) and the Holocene Papandayan volcano. The GVC produced undifferentiated basaltic lava flow, whereas the Papandayan volcanic eruption produced efflata intercalated with basaltic lava [32].
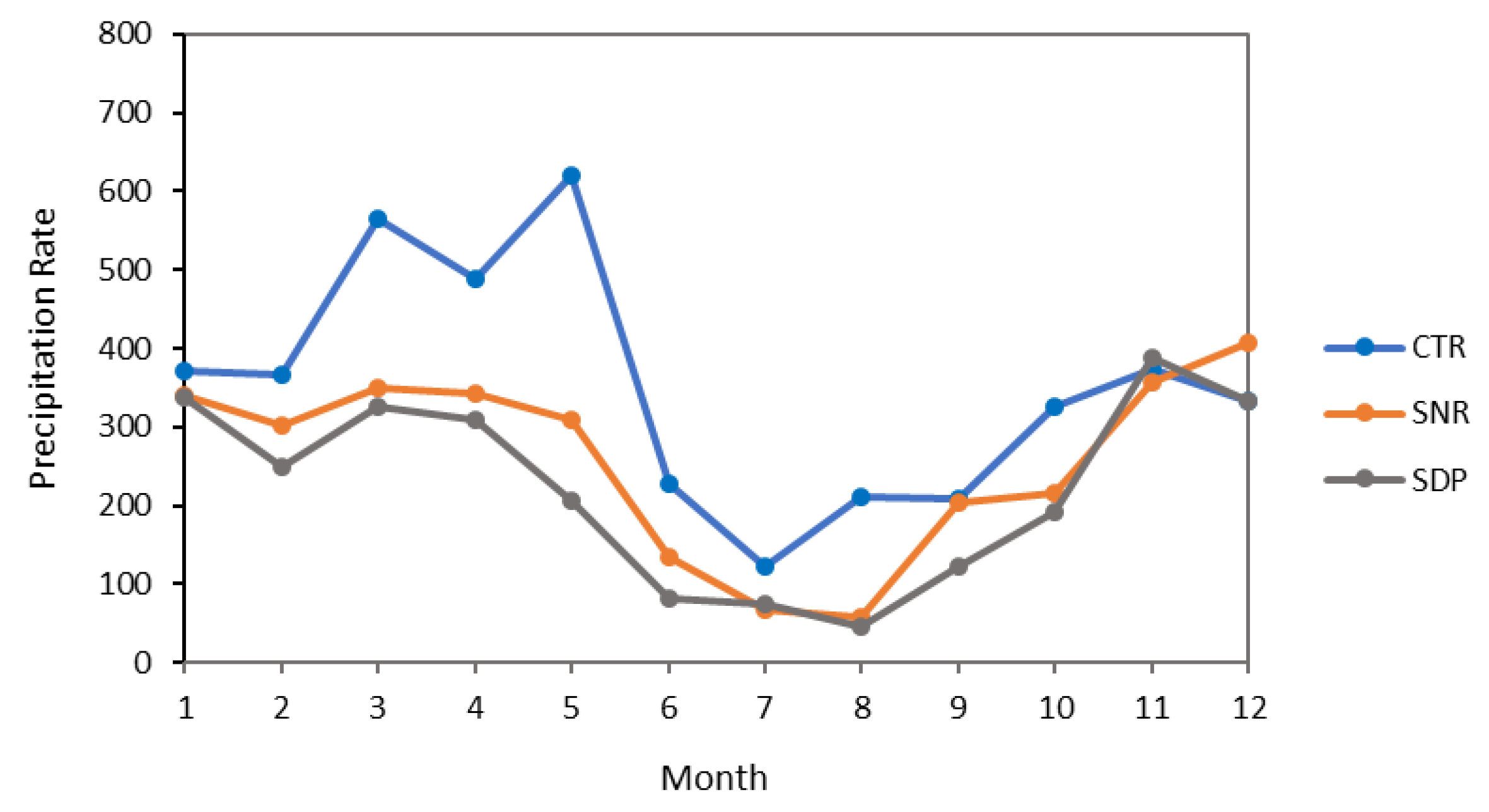
The study was carried out in three agro-climatic zones, A, B1, and B2, according to Oldeman’s [26] classification system with annual rainfall quantities of 4215 mm, 3087 mm, and 2868 mm at the CTR, SNR, and SDP sites, respectively (Figure 2). Other climate data were gathered from the Margahayu Climatological Station for the CTR site at 1250 m-above-sea-level (masl) and from the Cinchona Climatological Station for the SNR and SDP sites at 1430 masl. The mean annual air temperatures are 20.25 °C at the CTR (monthly min. = 19.53 °C, monthly max. = 20.56 °C), 15.59 °C at the SNR (monthly min. = 15.6 °C, monthly max. = 16.6 °C), and 15 °C at the SDP (monthly min. = 19.53 °C, monthly max. = 20.56 °C).

Figure 2.
The monthly precipitation rates for the studied areas show a trend for higher precipitation at the CTR site than at the two other sites (SNR and SDP).
2.2. Soil Sampling Technique
Soil samples were collected from 6 profiles of Andisols, originating from six periodic volcanic eruptions, differing in age from Pleistocene to Holocene, and with different types of parent materials, namely andesitic and basaltic volcanic ash (Figure 1 and Table 1). Two profiles with 4–10 horizons were made at each site, depending on the depth of parent materials (0 to 153 cm), yielding a total of 51 disturbed and undisturbed soil samples. Three profiles (CTR-A, CTR-B, SNR-A) were developed from andesitic volcanic ash, and three profiles were generated from basaltic volcanic ash (SNR-B, SDP-A, SDP-B). The CTR-A, SNR-B, and SDP-B profiles were obtained from Holocene parent material. Meanwhile, the SNR-A, SDP-A, and CTR-B profiles were derived from Pleistocene parent material. According to Oldeman’s agro-climatic zones, the CTR-A and CTR-B profiles were found in the A zone, the SNR-A and SNR-B profiles were found in the B1 zone, and the SDP-A and SDP-B profiles were found in the B2 zone. In other words, the CTR-A profile had andesitic volcanic ash, was of Holocene age, and was an A agro-climatic zone. Andesitic volcanic ash, Pleistocene age, and A agro-climatic zone were characteristics of the CTR-B profile. SNR-A had andesitic volcanic ash, was of Pleistocene age, and was a B1 agro-climatic zone. Basaltic volcanic ash, Holocene age, and B1 agro-climatic zone were all characteristics of the SNR-B profile. Basaltic volcanic ash, Pleistocene age, and the B2 agro-climatic zone were characteristics of the SDP-A profile. Basaltic volcanic ash, Holocene age, and B2 agro-climatic zone were characteristics of the SDP-B profile.

Table 1.
Characteristics of each studied pedon in this research.
2.3. Soil Physico-Chemical Analyses
The soil bulk density and permeability were measured using the core method in accordance with Baver and Gardner [33]. The volume percentage of water retention at 1500 kPa was used to calculate the plant-available water holding capacity, as described by Anda et al. [3]. A 2 mm (sieved) sample of air-dry soil was mounted on a porous ceramic plate and moistened by capillary force. The gravimetric water content was determined when the equilibrium was reached at 1500 kPa.
H2O2 (0.3 M) was utilized in the destruction process of organic matter in soil samples for soil textural determination. Wet sieving (50 µm) was performed to separate the sand fraction. Köhn’s pipette technique was used to determine the silt and clay fractions following dispersion with Na4P2O7 (0.04 M). The percentages for each fraction were estimated on a dry weight basis.
Determination of the pH0 was carried out as described by Gillman and Summer [12]. Six sections of 2 g of 2 mm air-dried samples were weighed into 50 mL centrifuge tubes, saturated with 0.1 M CaCl2, washed, and equilibrated with 0.002 M CaCl2. For the first determination, it was considered equivalent to the ionic strength of the natural soil solution. The pH of the suspension was changed to six different values in the range of 3.5 to 6.5 using HCl or Ca (OH)2. When 0.002 M CaCl2 reached equilibrium, the pH was recorded as 0.002. For equilibration, 0.5 mL of 2 M CaCl2 was added, and then the pH was recorded as 0.05. Interpolation was used to determine the pH0’s location from the zero difference between pH (0.05) and pH (0.002). This parameter is usually used to assess the charge characteristics of the variable charge component of pH0, determined by potentiometric titration. The measurement involved the net absorption of H+ or OH- at different pH values and ionic strengths [34]. The pH0 of the soil is the pH at which the concentrations of H+ and OH on colloidal surfaces are equal [10,35,36,37,38].
The organic carbon content was quantified using the Walkey and Black technique. The content of H2SO4 was determined by oxidation with K2Cr2O7 and titration with Fe2(SO4)3 as an indicator of the organic matter content. The Cation Exchange Capacity (CEC) was calculated using a soil leaching technique with 1 M NH4O at pH 7. Excess NH4O was extracted using 0.95 M ethyl alcohol. The magnitude of the CEC was evaluated by direct distillation and the total quantity of NH4+ retained by the soil. After that, 0.05 M of HCl solution was used for titrating the distillate. Ca2+, Mg2+, Na+, and K+ are the basic cations that can be exchanged in the NH4O extract, as determined by the Atomic Absorption Spectroscopy (AAS) instrument. The CEC and the exchangeable base cation values are reported in cmolc kg−1 soil. The base saturation was calculated from the values of CEC and exchangeable cations. Free sesquioxide determination was conducted in accordance with Nagatsuka [39]. Extractable organo-metals such as Fe and Al were obtained using ammonium oxalate (C2H8N2O4) and Na-pyrophosphate.
2.4. Mineralogical Analyses
To separate the light and heavy minerals, the sand fraction (50–500 µm) from soil samples was poured with bromoform at a density of 2.89 g/cm3. Light minerals floated on the surface of the solution, while heavy minerals settled at the bottom of the separating funnel. After completion of the separation procedure, the mineral grains were placed on the glass slide. Mineral observations were undertaken using a polarizing microscope, as described by Mange and Maurer [40]. The percentage of each type of mineral was estimated for the 100 translucent and opaque minerals.
Clay minerals in the crystalline structure were quantified and identified using X-ray Diffraction (XRD) analysis [41]. Wet sieving, sedimentation, and centrifugation were used to obtain the clay fraction [42]. Soil samples were pre-treated with 0.3 M of H2O2 solution to yield a fine clay fraction (<0.2 µm). H2O2 was used as a decomposer of organic materials [43]. The soil aggregate dispersion process was then performed in a 1000 mL beaker glass with distilled water and a 0.04 M Na4P2O7 solution. After being incubated for 6.5 h in the beaker glass, the suspended fine clay fraction was separated. The iron oxide concentration of the fine clay fraction samples was removed using a sodium dithionite solution with a citrate-bicarbonate buffer [44]. The fine clay fraction samples were centrifuged for 20 min at 5000 rotations per minute (rpm). For the XRD scanning stage, Phillips Automatic Power Diffraction (APD) with a Cu tube at 40 Kv was used. The fine clay fraction samples were subjected to four treatments: Mg2+, K+, glycerol (Mg2+ saturated treatment), and heating to 550 °C (K+ saturated treatment). The identification of clay mineral types after XRD analyses was performed in accordance with Volzone and Ortiga [45]. Since XRD can only be used for determining crystalline clay minerals, amorphous clays were identified using the Differential Thermal Analysis (DTA) method with the SHIMADZU DTA DTG-60 H instrument (manufactured by Shimadzu Corporation, Kyoto, Japan). For the analysis of SEM, we used JEOL JSM-6360LA (manufactured by JEOL Ltd., Tokyo, Japan) for clay micromorphological examination. To determine the micromorphological description, we followed the method of Bullock [46]. Laboratory analyses were controlled based on the pedogenic assessment processes and weathering level assessments.
3. Results
3.1. Soil Morphological Characteristics
Due to the periodic accumulation of volcanic eruptions, each profile has varied stratification of volcanic elements based on the soil morphological characteristics. It was thought that the basaltic parent material qualities along with low rainfall locations (SDP) resulted in a more reddish color. Meanwhile, soil in high rainfall locations (CTR) tended to be yellowish-brown in color (Table 2). Due to the deposition of substantial organic matter, the surface horizon (Ap) was often dark in hue. Changes in rainfall, age, and the parent materials showed modest color differences. In general, it was observed that most Ap horizons of Andisols had a silty loam and loam texture except for the SDP-A profile, which showed a sandy loam texture. This phenomenon is thought to have been caused by contamination produced by adjacent volcanoes in recent volcanic eruptions. The surface of the lower layers was usually fine, ranging from silty clay to silty clay loam to clay. There was no noticeable variance in the soil texture due to differences in the nature of the parent material.

Table 2.
Morphological properties of soils with different agro-climatic zones, types, and ages of parent materials.
Most horizons showed fine to very fine angular blocky and subangular blocky characteristics with moderate to strong developed structures, except for the BC horizon in profile CTR-A, which was found to have a massive structure. The surface horizons were usually crumbs to prisms. Changes in rainfall and the source material’s composition revealed minor differences in the soil structure.
Pedons with older parent material generally had a loose to firm consistency, while pedons with younger parent material were loose and smeary when pressed between the fingers. Differences in the consistency of andesitic and basaltic parent materials were unclear, except for the pedon CTR-A, which had a duripan due to the extensive weathering and leaching processes of aluminum and iron oxides collected in the BC horizon. The topography of the horizon’s boundary was found to vary from flat to wavy with abrupt to diffuse distinctness boundaries. The transition from the surface horizon to the underlying horizon was usually clear to abrupt. Horizons formed by previous eruptions and buried afterwards typically have specific boundary transition horizon. Dark-colored horizons reveal the stratification of different age materials at the bottom of the profile with a significant proportion of organic materials (thaptic). On the horizon boundary, differences in agro-climatic zones and the nature of the parent materials exhibited fewer evident distinctions. Meanwhile, pedons with older parent material had more diffuse horizon boundaries than pedons with younger parent material. The number and size of pores were not affected by changes in agro-climatic zones or the parent materials. However, there were generally fewer macro-pores in pedons with older parent material, notably in the lower horizon, than in those developed from younger parent material. This phenomenon occurred due to the filling of some soil plasma from the upper horizon, resulting in a denser soil matrix. Coarse fractions were typically found in considerable numbers in profiles created from immature parent materials. Coarse fractions can represent magnetic rock pieces (lapilli) or caldera wall rock fragments thrown at the eruption. The content of coarse materials in all studied profiles was not significantly altered by changes in agro-climatic zones or the type of parent materials. In each profile, there were repeated accumulations of volcanic materials with the same or distinct mineral associations. These occurrences were common in the Andisols [7,47,48].
3.2. Mineralogical Compositions of Soil Sand Fractions
The mineralogical composition of the sand fractions (50–2000 μm) of six profiles varied (Table 3). Andisols formed from andesitic volcanic ash and basaltic volcanic ash had varied mineral parent material compositions. Andisols formed from andesitic volcanic ash contained the mineral andesine, whereas Andisols formed from basaltic volcanic ash contained the minerals bytownite and olivine. Pleistocene andesitic or basaltic volcanic ash had more weathered minerals than their Holocene counterparts, indicating that more soils developed with Pleistocene age elements. When comparing the CTR-A and CTR-B profiles created from andesitic volcanic ash materials in the same A agro-climatic zone, the CTR-A profile of Holocene age had greater contents of volcanic glass, labradorite, hypersthene, and augite minerals, but significantly lower contents of hornblende and no andesine minerals. The CTR-A profile had more augite and hypersthene but less hornblende in the heavy sand fraction than the CTR-B profile. The effect of the agro-climatic zone on the mineral compositions of soils from andesitic volcanic ash materials was assessed by comparing SNR-A and CTR-B profiles derived from similar materials (andesitic volcanic ash) and of similar ages (Pleistocene age). The CTR-B profile with an A agro-climatic zone contained more andesine (feldspar), hornblende, and augite but less volcanic glass. It was discovered that soils in the B1 agro-climatic zone had advanced weathering stages, as evidenced by the depletion of andesine (feldspar), hornblende, and augite minerals, leaving behind high contents of hypersthene and worn minerals. The mineralogy of soils made from basaltic volcanic ash under identical B2 agro-climatic conditions (SPD-A profile) with Pleistocene age contained more weathered minerals but less labradorite hornblende, hypersthene, and augite than those with the SDP-B profile and of Holocene age. The SNR-B and SPD-B profiles produced from Pleistocene basaltic volcanic ash revealed that the SNR-B profile with a B1 agro-climatic zone included more volcanic glass, labradorite, and olivine than the SPD-B profile with a B2 agro-climatic zone. The weathering of minerals decreased in the order B2 > B1 > A as shown by andesite of Holocene age and basaltic volcanic ash of Pleistocene age under different agro-climatic zones.

Table 3.
Mineralogical compositions of the sand fractions of soils from different agro-climatic zones, of different types, and with different ages of parent materials.
The CTR-B, SNR A, and SDP A profiles of the Pleistocene age exhibited a more advanced weathering stage than the CTR A, SNR B, and SDP B profiles of the Holocene age for source materials based on the sand fraction mineralogical composition. The crushed elements were more prevalent in weathered profiles, whereas the rock fragment materials were more prevalent in younger profiles. Furthermore, except for the old profile SDP A, which had been contaminated by a recent volcanic eruption from adjacent volcanoes, the old profiles exhibited larger ratios of weathered and easily weatherable minerals than the young profiles.
The results of the weathering level assessment based on the criteria detailed in [49] criteria showed that all profiles had the viril weathering stage. This result was supported by the presence of high, easily weatherable minerals. In addition, it physically and morphologically showed a clay material content of 27.14–40.36%. The classification of parent materials based on the criteria detailed by Blatt and Tracy [50] showed that the SNR-B, SDP-A, and SDP-B profiles developed from the basaltic volcanic ash, while the CTR-A, CTR-B, and SNR-A profiles developed from andesitic volcanic ash. The weathering stage was characterized by the decomposition of most weatherable minerals and an increased clay content. The kind and quantity of plagioclase and the percentages of mafic and quartz minerals were used to distinguish between andesitic and basaltic volcanic ash. The type of plagioclase found in basaltic volcanic ash was bytownite (An70–90), while that found in andesitic volcanic ash was andesine (An30–50). The SDP-A weathering profile was a more advanced version of the SDP. Its topsoil, on the other hand, was expected to be contaminated by new eruption products from adjacent volcanoes. Micromorphological investigations in thin sections revealed that the topsoil contained euhedral minerals that had not been weathered for a long time, combined with anhedral minerals. In the meantime, the clay minerals of short-range order (allophane and imogolite) had been converted into halloysite.
3.3. Mineralogical Compositions of the Clay Fraction of the Studied Soils
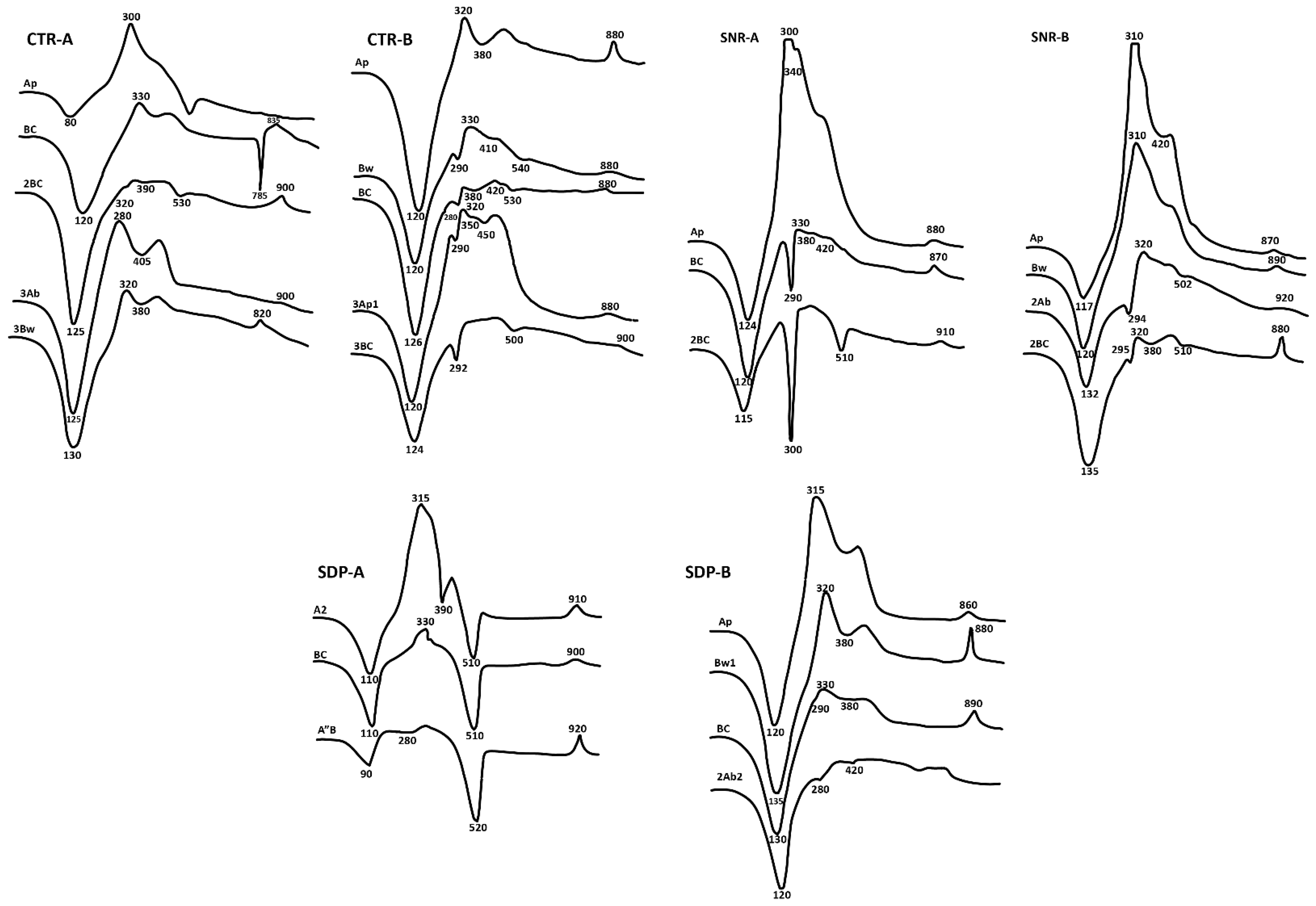
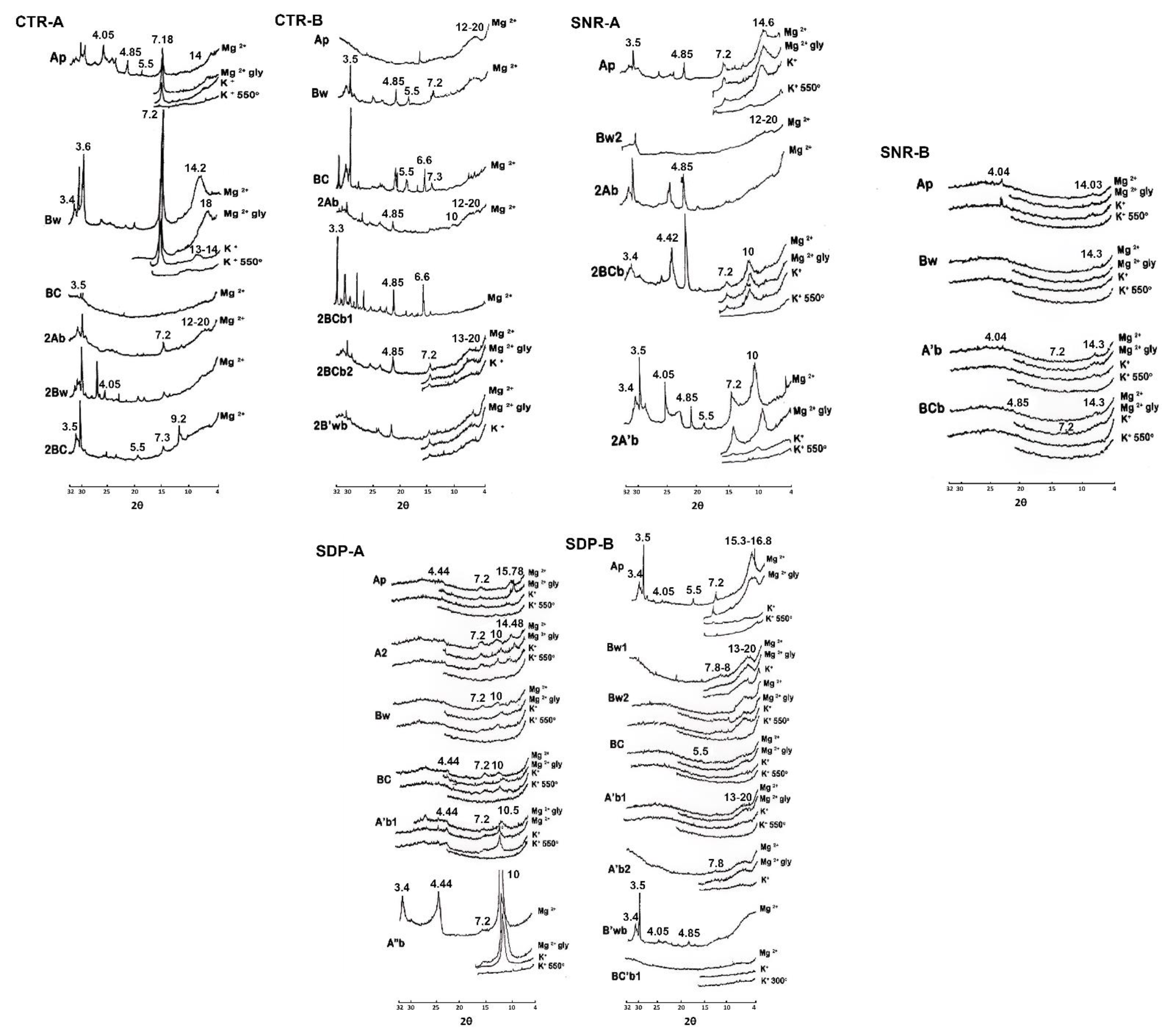
The results of the Differential Thermal Analysis (DTA) and X-Ray Diffraction (XRD) analysis of the clay fractions of selected horizons are given in Table 4, Figure 3 and Figure 4. The mineralogical compositions of the clay fractions of three profiles (CTR-A, CTR-B, and SNR-A) derived from andesitic volcanic ash parent materials differed from those of profiles (SNR-B, SDP-A, and SDP-B) developed from basaltic volcanic ash parent materials. Profiles derived from andesitic volcanic ash were found to contain gibbsite (endothermic peaks at 280, 292, 294, 295, 300 °C and XRD peaks at 4.85 Å). Vermiculites were dominant in soils from basaltic materials but were not found in profiles derived from andesitic volcanic materials. Due to differences in the materials’ ages and agro-climatic zones, significant differences were revealed. When comparing the CTR-A and CTR-B profiles, which were created from andesitic volcanic ash in the same A agro-climatic zone but with differed parent material ages (CTR-A Holocene and CTR-B Pleistocene), the CTR-A profile was found to contain greater volcanic glass, labradorite, hypersthene, and augite mineral contents, but much less hornblende and no andesine minerals.

Table 4.
Differential Thermal and X-Ray Diffraction Analyses of soils from different agro-climatic zones, of different types, and with different ages of parent materials.

Figure 3.
Differential thermal analysis (DTA) curves of clay fractions of sampled Andisol profiles.

Figure 4.
X-ray diffractograms of clay fractions of studied soils.
The composition of clay minerals in the CTR-A, CTR-B, SNR-B, and SDP-B profiles was dominated by short-range-order minerals. In contrast, the SNR-A profile was discovered, especially in the bottom layer, to have a high gibbsite content. A high content of halloysite was found in the SDP-A profile. The allophane mineral was characterized by a low temperature of endothermal reaction peaks (80–135 °C) and a high temperature of exothermal reaction peaks (880–920 °C). A pattern of XRD peaks supported these findings.
The characteristics of gibbsite were an intermediate temperature of endothermal reaction peaks (280–340 °C) and the XRD peak at 4.85Å [51], while halloysite occurred at an intermediate temperature of endothermal reaction peaks (510–540 °C) and X-ray diffraction peaks at 10 Å, 7.2–7.3 Å, and 4.42 Å [52]. The weak endothermal reaction peak at 350–390 °C characterized the occurrence of a low content of iron oxide minerals (goethite). Imogolite occurred in the CTR-B, SNR-A, SNR-B, and SDP-B profiles, characterized by an endothermal reaction peak at 420 °C and an XRD peak at 5.50 Å with wide peaks at 7.00–8.00 Å and 13–20 Å [53,54]. The X-ray diffraction analysis also confirmed the dominance of amorphous materials, with a tendency to use some hydroxyl-interlayered-vermiculite (HIV) minerals, as detected in some of the samples. Hydroxyl-interlayered-vermiculite was found in profiles SDP-A and SDP-B and developed from andesitic volcanic ash in more developed profiles. This finding is in line with that of Allen and Hajek [55], who mentioned that HIV has been found in relatively acidic and leached soils.
The decomposed organic matter was characterized by an endothermal reaction of the DTA curve at around 320 °C [56]. Generally, these peaks represented soils with high contents of organic matter (Ap horizon). Organic matter in Andisols usually forms complex substances with Al and Fe ions [18,57,58,59]; hence, Al and Si coprecipitation, as a result of parent materials weathering into allophane and imogolite, was obstructed. Consequently, the short-range-order mineral content of the surface horizon was lower than that of the underlying horizons. The contents of Al- and Fe-humus complexes in the surface horizon or buried horizon were greater than those in the underlying horizons. These phenomena were also shown by Delmelle et al. [17] and were attributed to the presence of organic matter. Al- and Fe humus complexes were shown to impact the stability of organic matter and low secondary crystalline mineral formation. The gibbsite’s profile content developed from andesitic volcanic ash in a relatively dry area and originated from old parent material with a relatively high halloysite content.
The profile was dominated by short-range-order minerals in wetter areas (rainfall > 4000 mm/year). The gibbsite content was more significant when creating profiles from older parent materials (CTR-B) than in profiles developed from younger parent materials. The gibbsite content was more evident in profiles formed from old parent material with andesitic characteristics in heavy rainfall areas (CTR-B and SNR-A) than in other profiles. Because of the extensive Si leaching process (desilication) and gibbsite crystallization, this phenomenon was predicted. Gibbsite is commonly discovered in worn acid soil. According to Hsu [51], the gibbsite in the SNR-A profile increased with an increasing depth. It was found that, as the pH rose, Al-humus from the top layer was mobilized and collected in the lower layer, forming a coating on the pores. Desilication was inhibited in the somewhat dry area and in parent material of older age (SDP-A). Thus, the soil solution was high in Si and generated halloysite mineral [48].
In the DTA (520 °C), the surface horizon (Ap horizon) of CTR-A had a mid-endothermal reaction peak and an X-ray diffraction peak of 7.18. This was one of the kaolinite clay mineral properties. The SEM micrograph (Figure 5e), which showed the kaolinite clay mineral, corroborated this. The same kaolinite mineral characteristics were also identified by [60]. The formation of kaolinite in this layer was predicted due to the formation of the Al-humus complex; therefore, the top layer was rich in Si (silicon) and disordered kaolinite formed.

Figure 5.
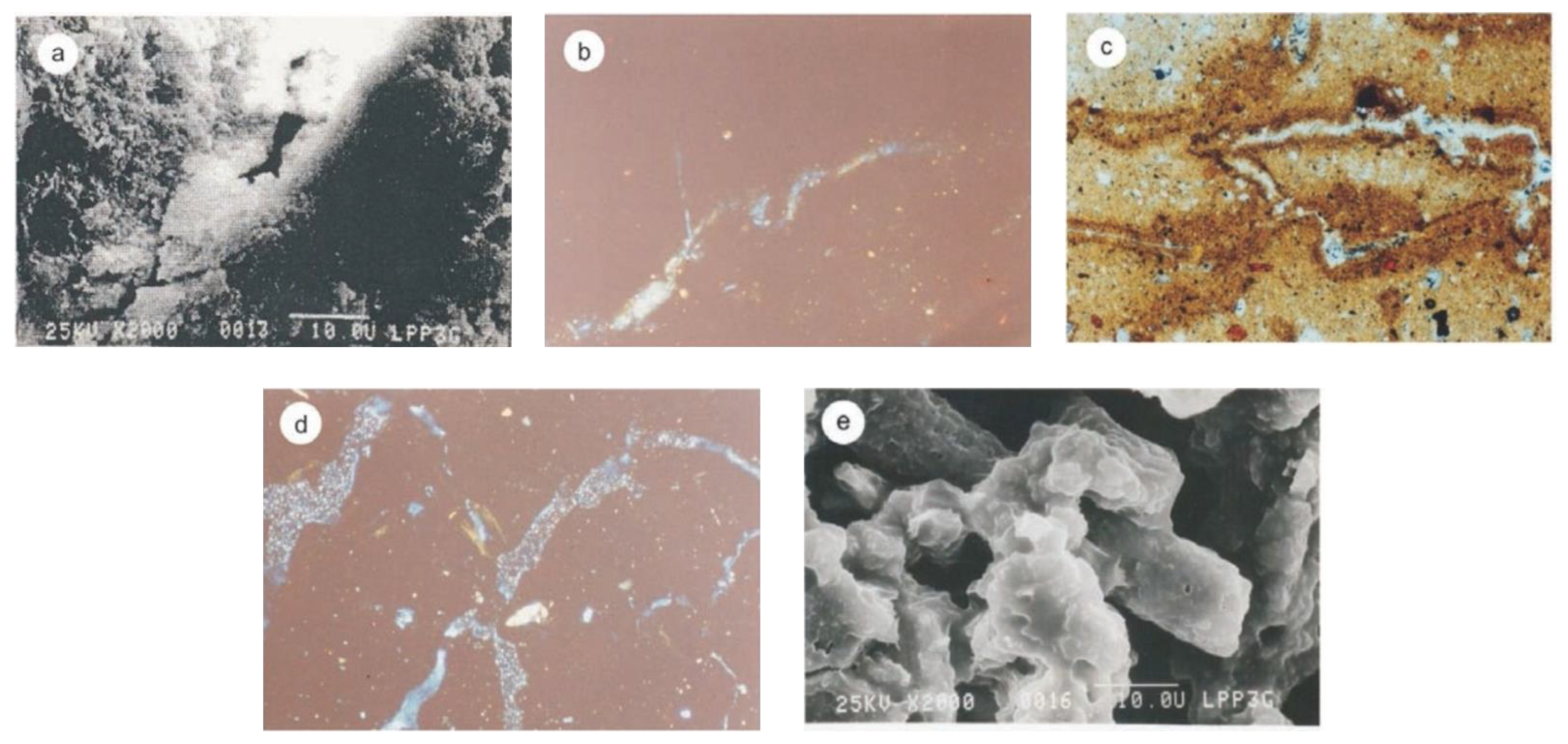
Photomicrograph obtained by scanning electron microscopy. Allophane (A) in the B’wb horizon. SDP-B (a); imogolite (Im) in the Ap horizon, SNR-B (b); halloysite (H) in the BC horizon, SDP-A (c); gibbsite (Gb) in the BC’b horizon, SNR-A (d); kaolinite in the Ap horizon, CTR-A (e); diatom (D) in the Ap horizon, SNR-B (f).
The scanning electron microscopy (SEM) results are shown in Figure 5. In the 2Bw horizon (SDP-B), allophane was recognized as round-shaped, smooth clustered, or scattered aggregates (Figure 5a). A single aggregate’s structure (0.25 µm) turned into a larger aggregate (2–20 µm) with no regular structure pattern. This result was matched with those of Eswaran [61], Wada [62], and Goenadi [63]. The imogolite found on the Ap horizon of the SDP-B profile was identified as a long-grouped particle (Figure 5b). In contrast, the imogolite found on the Ap horizon of the SNR-B profile was identified as a tubular form with a diameter of 0.5 m and length of 5–10 m (10,000 × zoom) scattered between the amorphous globular particles in the SDP-B profile (Figure 5b). A similar finding was achieved by Eswaran [61]. Additionally, in the SNR-B profile, a diatom fossil was found to be a characteristic of water organisms (limnic matter) (Figure 5f). This finding is in line with that of Dahlgren et al. [64].
Halloysite in the BC horizon (SDP-A) looked like tubular-shaped particles with diameters of 0.2 µm and lengths of 0.5–1.0 µm (Figure 5c). This finding is in line with those of previous studies by Dixon [60], Eswaran [61], and Papoulis et al. [65]. Meanwhile, the gibbsite mineral in SNR-A (2BC’b) was identified as round-shaped discrete crystalline particles with diameters of 1–2 µm (Figure 5d). This result is similar to that obtained through photomicrography by Schoen and Roberson (1970) in [51]. Kaolinite occurred in the Ap horizon in CTR-A (Figure 5e). Photomicrographs obtained by scanning electron microscopy or SEM (a and e) and microscopic pictures of thin slices are shown in Figure 6b–d. The gibbsite mineral in the SNR-A (horizon 2BC’b) was examined by SEM and found to be discretely rounded crystalline particles with diameters of 1–2 μm (Figure 6a). Hsu [51] reported a photomicrograph with a similar result. The weakening indication of clay illuviation in the vacuum and on the ped surface with a cutaneous shape on the B’wb horizon in SDP-A (Figure 6b) resulted from old parent material and was a unique pedological phenomenon. An organic material coating was also discovered on the B’wb horizon in SDP-A (Figure 6c). Furthermore, SNR-A infilled pores with coarser fractions in horizon 2BCb (Figure 6d), while a clay coating was identified on sand grains in horizon Bw, SNR-B (Figure 6e).

Figure 6.
Photomicrographs obtained by scanning electron microscopy (a,e) and microscopic images of thin sections (b–d). (a). Gibbsite coating in Hor. 2BC’b, SNR A; (b). clay coating in Hor. B’wb, SDP A, XPL; (c). coatings of organic material in Hor. B’wb, SDP A, PPL; (d). pores with infilling in Hor.2BCb, SNR A; (e). clay coating on sand grains, Hor. Bw, SNR B.
3.4. Chemical Properties of Soils Developed from Different Parent Materials and in Different Agro-Climatic Zones under Tea Plantations
The chemical characteristics of the soil vary depending on the profile (Table 5 and Table 6). The pH (H2O) of three profiles (CTR-A, CTR-B, and SNR-A) derived from andesitic volcanic ash parent materials was found to range from 3.6 to 5.4, while the pH (H2O) of profiles SNR-B, SDP-A, and SDP-B derived from basaltic volcanic ash ranged from 4.4 to 6.2, depending on the soil depth, agro-climatic zone, and the age of the parent material. The pH (H2O) was lower in the top two layers (part of current soils) and then increased in the buried subsoils in any profile (paleosol). Furthermore, for profiles developed from andesitic parent materials, pH (H2O) values were lower in Holocene parent materials than in Pleistocene parent materials within a similar agro-climatic zone, but there was no apparent effect of the parent material’s age on the profile development from basaltic parent materials. As a result of the varied parent materials, the ΔpH (pH (KCl)-pH (H2O)) values were noticeably different within and between profiles. Positive ΔpH values were found in soils formed from andesitic volcanic ash, while negative ΔpH values were found in basaltic volcanic ash. Some higher layers of SNR-A had small negative ΔpH values (−0.1 unit), probably due to the high organic matter content. In contrast, some originally buried soils (CTR-B and SNR-A profiles) had significant negative ΔpH values. Positive ΔpH values indicated a positive charge on the colloidal surfaces of the CTR-A and CTR-B profiles. The measurement of soil pH in NaF solution after two minutes of shaking showed high values, varying from 9.0 to 11, but mostly from 9.4 to 11. This indicated the high content of amorphous materials and dominated the soil exchange complex as the characteristic of Andisols. The soil pH (NaF) was lower in the SDP-A profile, among others, irrespective of the parent materials’ types and ages. This occurred in parallel to the findings of lower ferrihydrite and allophane content in this profile. By measuring the point of zero charges (pH0) on colloidal surfaces, the presence of a net positive surface charge for a changeable charge component was proven. As expected, the pH0 values of three profiles (CTR-A, CTR-B, and SNR-A) derived from andesitic parent materials varied from 3.4 to 5.4, higher than the pH of natural soil (H2O), indicating the presence of a net positive charge on a variable charge component of colloidal surfaces.

Table 5.
The pH, point zero charge, exchangeable cations, and cation exchange capacity of soils with different agro-climatic zones, of different types, and with different ages of parent materials.

Table 6.
P retention and amorphous material, organometal, ferrihydrite, and allophane contents of soils with different agro-climatic zones, of different types, and with different ages of parent materials.
The pH0 values of the SDP-A, SDP-B, and SNR-B profiles formed from basaltic ash volcanic materials were lower than the pH (H2O). This is in contrast to the results of soils derived from andesitic volcanic ash materials. The pH0 values ranged from 2.9 to 5.7 (depending on the depth of the soil and the age of the parent materials), whereas the pH (H2O) values ranged from 4.0 to 6.2. That the pH (H2O) of the soil was higher than the pH0 suggests that the colloidal soil surfaces were negatively charged. The pH0 values of the SDP-A profile with Holocene basaltic volcanic ash were substantially lower (2.9–3.5, with the greatest values in the uppermost three layers) than those of the SDP-B and SNR-B profiles with Pleistocene basaltic volcanic ash (4.2–5.7). The pH0 values in the topsoils of the SDP-B and SNR-B profiles were lower, and they increased by roughly 1.5 units in the lower sections of the profiles. The lower pH0 values of the SDP-A profile compared with those of the SDP-B and SNR-B profiles were attributed to the lower allophane concentration of the former (6% vs. 11–70%). The pH0 values of amorphous materials have long been known to be high [7,10].
In general, the sum of exchangeable cations in soil profiles obtained from basaltic volcanic ash was substantially higher than that in soil profiles derived from andesitic volcanic ash (Table 5). The sum of exchangeable cations in soil profiles derived mainly from andesitic volcanic ash was less than 2 cmolc kg−1. In contrast, the sum of exchangeable cations in soil profiles (SNR-B, SDP-A, and SDP-B) derived from basaltic volcanic ash was 3.3–9.2 cmolc kg−1, except for in SNR-B and in the upper layer (0–54 cm) of the SDP-B profile, where this low quantity of cations was linked to the lower capacity of soils derived from andesitic volcanic ash components to hold cations as well as the low pH of these soils. It is clear from the results of this study that the magnitude of soil CEC for Andisols is governed by the soil’s pH and organic C, where the higher the CEC of soils is, the higher the soil organic C, pH, or both are. Topsoils with a low soil pH but higher organic C were found to have a high CEC, revealing the function of soil organic C in increasing CEC. Subsoil with reduced organic C was shown to play a role in determining the soil’s pH, although a high soil pH was associated with an increased CEC. Na dominated the exchangeable cations, followed by Ca and then Mg or K for soils derived from andesitic volcanic ash, while the order of dominance was Ca > Mg > Na > K for soils derived from basaltic volcanic ash, except for in the upper parts of the SNR-B and SDP-B profiles which were found to have a more acidic soil pH and a cation order of Na~Ca > Mg > K.
Soils formed from andesitic volcanic ash materials were found to have a lower cation exchange capacity (CEC) than soils derived from basaltic materials. The low CEC of soils made from andesitic volcanic ash was linked to colloidal surfaces with a positive charge, as evidenced by the pH0 being lower than the pH (H2O). The CEC of soils developed from andesitic volcanic ash (CTR-A, CTR-B, and SNR-A) ranged from 11 to 15 cmolc kg−1 in the topsoil and 3–13 cmolc kg−1 in the subsoil. For soils formed from basaltic volcanic ash (SNR-B, SDP-A, and SDP-B), the corresponding CEC ranged from 12 to 18 cmolc kg−1 in the topsoil and 6 to 36 cmolc kg−1 in the subsoil. For all soils, the CEC in the topsoil was higher than that in the surrounding subsoil due to the higher concentration of organic matter in the former. The CEC of soils generated from basaltic volcanic ash of Pleistocene age was higher in the subsoils than the adjacent overlying topsoils, except for in soils derived from basaltic volcanic ash of Pleistocene age, which had a higher CEC in the subsoils than in the adjacent overlying topsoils. Although topsoils were found to have more organic C than subsoils, subsoils had a greater CEC due to their higher clay content. Since the CEC was measured at pH 7, while the natural soil pH was 5.9 and the pH0 was 5.5–5.7, this was most likely due to high concentrations of ferrihydrite and allophane, which contributed to the high CEC deprotonation of colloidal surfaces. There were also different CEC values within and between soil profiles of similar parent materials. A much higher CEC in the subsoils (45–152cm) than in the overlying layers (0–45 cm) occurred in the SDP-B profile, which was associated with the high pH values in the former (5.1–5.9 vs. 4.4–4.8) and the high vermiculite content. Due to the deprotonation of hydroxyl groups in allophane materials, elevating the normal soil pH (5.1–5.9) to pH 7 during CEC measurements could cause a negative charge. The uneven decrease in the CEC with the soil depth was also linked to variations in the buried layer nature in terms of the soil pH and organic C content. Soil base saturation did not demonstrate a clear relationship with the soil depth. Instead, changes in magnitude (decreased or raised) were linked to the soil pH and different buried layers. The high pH of the soil caused high base saturation in the subsurface soil layers.
All soil profiles exhibited strong P-retention, ranging from 88 to 99% (but mainly 94–99%), which shows that all soils had excellent P-retention. Different soil parent materials and agro-climatic zones had little effect on P-retention. For any given profile, the P-retention in topsoils was somewhat lower than that in the underlying horizons. This was linked to the increased organic C, which limited P retention by competing for colloidal surface positive interface sites. The significant P-retention for profiles generated from andesitic volcanic ash materials was not unexpected, as the pH0 results suggest that the colloidal surfaces had a positive charge under natural conditions, resulting in a strong affinity for P with a negative charge. Surprisingly, the results for the three profiles produced from basaltic ash volcanic materials show that the colloidal surfaces had a negative charge (the pH0 was lower than the pH (H2O) of the soil), yet their P-retention was still high. This could be because the ligand exchange process on colloidal surfaces favors P-retention over the retention of organic C functional groups. P-retention was 97–99% in the SDP-B and SNR-B profiles derived from older parent material (Pleistocene age), whereas P-retention was mostly less than 89% in the SDP-A profiles from the Holocene period, representing the lowest pH0 values (natural soil pH was much higher than pH0 values) among other soil profiles due to the lower content of allophane.
Amorphous materials, which were extracted by ammonium oxalate acid, showed the dominance of Alo followed by Feo and Sio, except for the SDP-A profile in which Feo was dominant, followed by Alo and Sio. Except for the SDP-A profile, where the ferrihydrite content was greater than that of allophane, the allophane concentration was generally higher than that of ferrihydrite. The amount of allophane in the buried horizon was higher than that in the overlaying materials in general. The type and age of parent materials had no discernible effect on the amount of amorphous material present.
3.5. The Relationship of the Pedogenic Environment with Several Chemical, Physical, and Mineralogical Characteristics of Andisols
The relationships of three soil-forming factors (climate, the age of parent material, the characteristic of parent material) with some chemical, physical, and mineralogical characteristics of profiles were determined (Table 7). According to Table 6, the climate was the main soil-forming factor affecting the Andisols characteristics. The second was the age of the soil’s parent materials. The third was the characteristics of the soil’s parent materials. The soil’s parent materials revealed a link to the mineral composition of sand. The olivine mineral was found in low to medium concentrations in basaltic volcanic ash. Bytownite and labradorite were the types of plagioclase minerals found. Andesitic volcanic ash, on the other hand, tends to have more hornblende than basaltic volcanic ash. The difference in the compositions of other minerals in basaltic compared with andesitic volcanic ash remains unclear. This phenomenon could be due to the volcanic material having a specific mineral composition despite coming from the same rock type (Shoji (1985) in Yoshinaga [48]). Because the studied soils were weathered at various ages and under different pedogenic environmental conditions, many minerals could not weather faster than others. The age of the parent material shows the impact of environmental factors on the sand mineral composition (including the WM/EWM ratio). The age of the parent material can be viewed as a result of environmental factors that altered the sand mineral composition, such as the ratio of weathered minerals (WM) to easily weatherable minerals (EWM) or the WM/EWM ratio.

Table 7.
The Relationship of Soil Forming Factors with Some Chemical, Physical, and Mineralogical Characteristics of Andisols.
The composition of clay minerals was slightly affected by the parent material characteristics and mainly affected by the climate and the age of the soil’s parent material. The effects of climate and the age of the parent material on the composition of short-range-order minerals were associated with the impacts on organic matter. In a dry environment with old parent material, the composition of organic carbon was lower than that in a wet climate with young parent material. In dry areas, profiles created from old parent material contained less short-range-order minerals (allophane plus imogolite and ferrihydrite) than in profiles formed from young parent material. On the other hand, the quantity of short-range-order minerals in profiles with high contents of organic carbon was lower in wet areas.
In the exact location, the pH0 of profiles with old parent material was lower than that in young parent material, except in Ciater (CTR). The change of allophane and imogolite into halloysite in old parent material profiles were predicted to decrease the pH0. Sakurai et al. [66] stated that the pH0 values of short-range-order minerals were higher than those of layered silicate minerals. This theory is supported by the findings of this investigation, which found a positive association between the pH0 and all acidic soil parameters. The presence of kaolinite in young parent material profiles (CTR-A) in the Ciater region lowered the pH0 when compared to that of old parent material profiles (CTR-B).
An intensive leaching and weathering process in the Ciater area caused the colloid fraction to have a positive net charge, while relatively dry places had a negative net charge. Uehara and Gillman [10] stated that soil with an acidic characteristics is commonly found in the easily weathered basaltic rocks in tropical areas.
The pH (H2O), pH (KCl), and pH (NaF) values increased in areas with less rainfall. The old parent material profiles commonly had lower pH values than those originating from the young parent material. The number of bases (Ca, Mg, K, Na) increased along with a decrease in rainfall. In areas with high rainfall (Profile CTR A), Andisols developed from andesitic parent material had the least total bases. The proportion of exchangeable aluminum was found to be better associated with the weathering stage of the profile (the age of the parent material), and Al was usually found to increase as the weathering stage rose. Exchangeable H ions were found to be more prevalent in areas with heavy rainfall (Ciater), while the exchangeable H ion concentration was lower in dry areas. Furthermore, compared with younger parent material, the profile of exchangeable H ions generated from old parent material tended to be higher. In profiles located in wet areas (Ciater), extractable acidity (H+, BaCl2 TEA, pH 8.2) and pH-dependent charge (pH0) predominated—the drier the site, the lower the extractable acidity and pH0. While the extractable acidity (H+, BaCl2 TEA, pH 8.2) and pH-dependent charge (pH0) were lower, the opposite was true for base saturation (pH 7) and CEC (pH 7): the drier the area, the higher the extractable acidity and pH0. Although the amount of (Al + ½ Fe) oxalate varied, it was considerably influenced by the age of the parent material in generally dry locations (Sedep/SDP). The content of (Al + ½Fe) oxalate in parent material decreases with age. In contrast to the age and properties of parent material, rainfall had a more significant impact on the Al- and Fe-humus complexes—the more rain, the more (Al + ½Fe) oxalate was present. The bulk density of Andisols and the concentrations of the sand and clay fractions were more closely related to the age of the parent material, the low fraction content of sand, and the higher weathering profile.
4. Discussion
The weathering of primary alumino-silicate minerals and the formation of secondary minerals, particularly short-range-order minerals such as allophane, imogolite, and ferrihydrite, and the transformation of short-range-order minerals into crystallized minerals such as halloysite, gibbsite, and kaolinite were the main processes of primary soil formation on Andisols in all studied locations. Translocation and accumulation of those materials in the solum of profiles developed from young parent materials decreased and increased along with an increase in the age of the parent materials. In addition, organic matter was accumulated, weathered, and transformed into humus (humification) before being mixed with the soil fraction to produce granular and crumb soil structures.
Andisols are known for their high levels of organic matter accumulated on the surface horizon [17,67]. Such organic materials frequently form complexes with Al and Fe; the surface layer of Andisols typically has higher Al- and Fe-humus contents than the underlying horizons. Furthermore, there is base and silicon leaching; Al- and Fe-humus translocation and immobilization; migration of clay, fine silt (lessivage), and organic matter; iron release from primary minerals (braunification); iron accumulation (nodule); and element or leached material accumulation in the underlying horizons. The ochric epipedon, cambic horizon, duripan, and microbiological features, such as infilling, voids, or coating on pores or peds by clay fractions (allophane/imogolite, halloysite, gibbsite), organic matter, and micro pads remain diagnostic traits. Allophane has been converted into metahalloysite and hydrous halloysite on profiles obtained from old source materials in relatively dry locations (SDP A). The silication process, meaning chemical Al migration was predictive of the formation of such minerals; therefore, Si was pushed to the left, and its concentration rose. Meanwhile, short-range-order minerals were found to predominate in somewhat damp areas (Ciater), with a few occurrences of gibbsite in the underlying horizon. In the profile generated from andesitic volcanic ash in the area of rather high rainfall, allophane and/or imogolite had crystallized gibbsite (SNR A). Its content grew in correlation with the depth of the soil. Gibbsite mineral formation in this profile is thought to be due to Al translocation in the form of Al-humus, which accumulated in the lower layer due to an increase in pH and sufficient time for gibbsite crystallization. Based on the sequential transformation of short-range-order minerals (amorphous) stated by Aomine and Wada [68], it was assumed that SNR-A profiles had undergone advanced weathering. The allophane on the surface horizon of all profiles had a lower content than that in the underlying horizon. On the contrary, the Al- and Fe-humus contents were higher on the surface horizon. These phenomena were due to the increased accumulation of organic matter on the surface horizon. The formation of Al- and Fe-humus complexes was assumed to retard allophane formation due to the detained process of coprecipitation of Al and Si, resulting in the weathering of volcanic ash [57]. The decrease in the Al-humus complex and the increasing soil depth always accompanied allophane and gibbsite contents. Due to the heavy rainfall and high temperature in Ciater, intensive weathering and leaching processes caused Si and Al elements to be leached and collected in the lower horizon (2AC). Both materials later cemented sand and silt fractions, forming cemented horizons (duripan). Furthermore, this cementing process was characterized by a change in the drainage conditions. The underlying horizon’s drainage was slightly preserved due to a particle size distribution containing more finer particles than the higher horizon. The various compositions from earlier eruptions caused this phenomenon to arise.
Andisols formed in areas with an annual rainfall of above 3000 mm (Ciater and Sinumbra) have an acrudoxic quality, resulting in intense leaching due to a lack of bases. In addition, the colloidal component was regularly detected in positive net charged areas with rainfall > 4000 mm per year (Ciater). The acidic pH of the soil was changed by intensive leaching. Short-range-order minerals with changeable charges (allophane, imogolite, and ferrihydrite) would have positive charges under acidic conditions [69]. The abundance of short-range-order minerals was discovered in all agro-climatic zones and with all parent material types and ages in Andisols and in association with the hydric feature. In the Soil Taxonomic classification system, the hydric characteristic was found in the great group category in areas with high rainfall (Ciater), whereas it was located in the subgroup category in dry regions [8,70].
5. Conclusions
Oldeman’s agro-climatic zones A (4215 mm/year), B1 (3067 mm/year), and B2 (2686 mm/year) as well as the age of parent materials (Pleistocene and Holocene) were shown to have significant impacts on various pedogenesis processes and profile weathering stages. As a result, Andisols exhibit a wide range of physical, chemical, and clay mineralogical characteristics. Meanwhile, the parent materials of andesitic and basaltic volcanic ash do not affect such attributes.
Cambic weathering stages were seen in all Andisols profiles. The key pedogenetic processes involved the creation of short-range-order minerals (allophane, imogolite, and ferrihydrite) through the leaching of easily dissolved elements and the coprecipitation of SiO2 and Al2O3 gels. Halloysite was formed by resilication of short-range-order minerals, while gibbsite was formed by desilication.
Author Contributions
Conceptualization, M.A. (Mahfud Arifin) and R.D.; methodology, M.A. (Mahfud Arifin) and M.A. (Markus Anda); software, D.H.G. and A.N.; validation, M.A. (Mahfud Arifin), R.D. and M.A. (Markus Anda); formal analysis, M.A. (Markus Anda), D.H.G. and R.D.; investigation, A.N. and M.A. (Markus Anda); resources, M.A. (Mahfud Arifin) and R.D.; data curation, A.N. and M.A. (Mahfud Arifin); writing—original draft preparation, M.A. (Mahfud Arifin), R.D. and A.N.; writing—review and editing, A.N.; visualization, M.A. (Markus Anda), R.D. and A.N.; supervision, M.A. (Mahfud Arifin), D.H.G. and M.A. (Markus Anda); project administration, R.D.; funding acquisition, M.A. (Mahfud Arifin). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to their many colleagues who provided thoughtful criticism during the prolonged preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saing, U.B.; Bani, P.; Haerani, N.; Aiuppa, A.; Primulyana, S.; Alfianti, H.; Syahbana, D.K. First characterization of Gamkonora gas emission, North Maluku, East Indonesia. Bull. Volcanol. 2020, 82, 37. [Google Scholar] [CrossRef]
- Kartawisastra, S.; Dariah, A. Tanah Andisol di Indonesia: Karakteristik, Potensi, Kendala, dan Pengelolaannya untuk Pertanian, 1st ed.; Balai Besar Penelitian dan Pengembangan Sumber Daya Lahan Pertanian: Bogor, Indonesia, 2014.
- Anda, M.; Dahlgren, R.A. Long-term response of tropical Andisol properties to conversion from rainforest to agriculture. CATENA 2020, 194, 104679. [Google Scholar] [CrossRef]
- Fiantis, D.; Hakim, N.; van Ranst, E. Properties and utilization of Andisols in Indonesia. J. Integr. Field Sci. 2005, 2, 29–37. [Google Scholar]
- Tan, K.H. The Andosol in Indonesia. Soil Sci. 1965, 99, 375–378. [Google Scholar] [CrossRef]
- Smith, G.D. A preliminary proposal for reclassification of Andepts and some andic subgroups (The Andisol proposal, 1978). N. Z. Soil Bur. Rec. 1978, 96. [Google Scholar] [CrossRef]
- Van Ranst, E.; Qafoku, N.P.; Noble, A.; Xu, R.-k. Variable charge soils: Mineralogy and chemistry. In Encyclopedia of Soil Science; Lal, R., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 2432–2439. [Google Scholar]
- Staff, S.S. Keys to Soil Taxonomy, 12th ed.; U.S. Department of Agriculture (USDA): Washington, DC, USA, 2014.
- Nanzyo, M.; Dahlgren, R.; Shoji, S. Chemical Characteristics of Volcanic Ash Soils. In Developments in Soil Science; Shoji, S., Nanzyo, M., Dahlgren, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 21, pp. 145–187. [Google Scholar]
- Uehara, G.; Gillman, G.P. The Mineralogy, Chemistry, and Physics of Tropical Soils with Variable Charge Clays; Westview Press: Boulder, CO, USA, 1981. [Google Scholar]
- Xu, R.-k.; Qafoku, N.P.; van Ranst, E.; Li, J.-y.; Jiang, J. Adsorption Properties of Subtropical and Tropical Variable Charge Soils: Implications from Climate Change and Biochar Amendment. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 135, pp. 1–58. [Google Scholar]
- Gillman, G.P.; Sumner, M.E. Surface Charge Characterization and Soil Solution Composition of Four Soils from the Southern Piedmont in Georgia. Soil Sci. Soc. Am. J. 1987, 51, 589–594. [Google Scholar] [CrossRef]
- FitzPatrick, E.A. Soils, Their Formation, Classification and Distribution; Longman: London, UK, 1980. [Google Scholar]
- Tan, K.H. Soils in the Humid Tropics and Monsoon Region of Indonesia, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 584. [Google Scholar]
- Egli, M.; Alioth, L.; Mirabella, A.; Raimondi, S.; Nater, M.; Verel, R. Effect of climate and vegetation on soil organic carbon, humus fractions, allophanes, imogolite, kaolinite, and oxyhydroxides in volcanic soils of Etna (Sicily). Soil Sci. 2007, 172, 673–691. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chen, Z.S.; Kao, C.I.; Ottner, F.; Kao, S.J.; Zehetner, F. Pedogenic development of volcanic ash soils along a climosequence in Northern Taiwan. Geoderma 2010, 156, 48–59. [Google Scholar] [CrossRef]
- Delmelle, P.; Opfergelt, S.; Cornelis, J.-T.; Ping, C.-L. Volcanic Soils. In The Encyclopedia of Volcanoes, 2nd ed.; Sigurdsson, H., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 1253–1264. [Google Scholar]
- Ugolini, F.; Dahlgren, R. Soil development in volcanic ash. Global Environ. Res. Engl. Ed. 2002, 6, 69–81. [Google Scholar]
- Taylor, M.D.; Lowe, D.J.; Hardi, P.; Smidt, G.A.; Schnug, E. Comparing volcanic glass shards in unfertilised and fertilised Andisols derived from rhyolitic tephras, New Zealand: Evidence for accelerated weathering and implications for land management. Geoderma 2016, 271, 91–98. [Google Scholar] [CrossRef]
- Churchman, J.; Lowe, D. Alteration, Formation, and Occurrence of Minerals in Soils, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; Volume 1, pp. 33.29–33.48. [Google Scholar]
- Parfitt, R.L. Allophane in new zealand—A review. Aust. J. Soil Res. 1990, 28, 343–360. [Google Scholar] [CrossRef]
- Parfitt, R.L. Allophane and imogolite: Role in soil biogeochemical processes. Clay Miner. 2009, 44, 135–155. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Russell, M.; Orbell, G.E. Weathering sequence of soils from volcanic ash involving allophane and halloysite, New Zealand. Geoderma 1983, 29, 41–57. [Google Scholar] [CrossRef]
- Parfitt, R.L.; Saigusa, M.; Cowie, J.D. Allophane and halloysite formation in a volcanic ash bed under different moisture conditions. Soil Sci. 1984, 138, 360–364. [Google Scholar] [CrossRef]
- Singleton, P.L.; McLeod, M.; Percival, H.J. Allophane and halloysite content and soil solution silicon in soils fromrhyolitic volcanic material, new zealand. Aust. J. Soil Res. 1989, 27, 67–77. [Google Scholar] [CrossRef]
- Oldeman, L.R. An Agro-Climatic Map of Java; Central Research Institute for Agriculture: Bogor, Indonesia, 1975. [Google Scholar]
- Vigouroux, N.; Wallace, P.J.; Williams-Jones, G.; Kelley, K.; Kent, A.J.R.; Williams-Jones, A.E. The sources of volatile and fluid-mobile elements in the Sunda arc: A melt inclusion study from Kawah Ijen and Tambora volcanoes, Indonesia. Geochem. Geophys. Geosystems 2012, 13. [Google Scholar] [CrossRef]
- Deegan, F.M.; Whitehouse, M.J.; Troll, V.R.; Geiger, H.; Jeon, H.; le Roux, P.; Harris, C.; van Helden, M.; González-Maurel, O. Sunda arc mantle source δ18O value revealed by intracrystal isotope analysis. Nat. Commun. 2021, 12, 3930. [Google Scholar] [CrossRef]
- Gasparon, M.; Varne, R. Crustal assimilation versus subducted sediment input in west Sunda arc volcanics: An evaluation. Mineral. Petrol. 1998, 64, 89–117. [Google Scholar] [CrossRef]
- Silitonga, P.H. Geological Map of Bandung Quadrangle, Java; Geological Survey of Indonesia; Ministry of Mines: Bandung, Indonesia, 1973.
- Koesmono, M.; Kusnama; Suwarna, N. Geological Map of Sindangbarang Quadrangle, Java; Geological Survey of Indonesia; Ministry of Mines: Bandung, Indonesia, 1996.
- Alzwar, M.; Akbar, N.; Bachri, S. Geological Map of Garut-Pamempeuk Quadrangle, Java; Geological Survey of Indonesia; Ministry of Mines: Bandung, Indonesia, 1975.
- Baver, L.D.; Gardner, W.H. Soil Physics, 4th ed.; John Wiley & Sons: New York, NY, USA, 1972; p. 498. [Google Scholar]
- Marcano-Martinez, E.; McBride, M.B. Calcium and Sulfate Retention by Two Oxisols of the Brazilian Cerrado. Soil Sci. Soc. Am. J. 1989, 53, 63–69. [Google Scholar] [CrossRef]
- Hendershot, W.H.; Lavkulich, L.M. Effect of Sesquioxide Coatings on Surface Charge of Standard Mineral and Soil Samples. Soil Sci. Soc. Am. J. 1983, 47, 1252–1260. [Google Scholar] [CrossRef]
- Schwertmann, U.; Taylor, R.M. Iron Oxides. In Minerals in Soil Environments; Soil Science Society of America, Inc.: Madison, WI, USA, 1989; pp. 379–438. [Google Scholar]
- Sharpley, A.N.; Singh, U.; Uehara, G.; Kimble, J. Modeling Soil and Plant Phosphorus Dynamics in Calcareous and Highly Weathered Soils. Soil Sci. Soc. Am. J. 1989, 53, 153–158. [Google Scholar] [CrossRef]
- Van Raij, B.; Peech, M. Electrochemical Properties of Some Oxisols and Alfisols of the Tropics. Soil Sci. Soc. Am. J. 1972, 36, 587–593. [Google Scholar] [CrossRef]
- Nagatsuka, S. Methods of type-analysis of iron. In A Handbook of Quartenary Research; Japan Association for Quartenary Research, Ed.; University of Tokyo Press: Tokyo, Japan, 1993; pp. 22–32. [Google Scholar]
- Mange, M.A.; Maurer, H.F.W. Heavy Minerals in Colour; Springer: Berlin/Heidelberg, Germany, 1992; p. 147. [Google Scholar]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Xing, B.; Dudas, M.J. Characterization of Clay Minerals in White Clay Soils, People’s Republic of China. Soil Sci. Soc. Am. J. 1994, 58, 1253–1259. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Imoto, H.; Kurihara, H. Clay mineralogy of a podzol developed under a Sciadopitys verticillata forest on Mt. Irazu, Shikoku mountains, Southwest Japan. Soil Sci. Plant Nutr. 2004, 50, 331–338. [Google Scholar] [CrossRef][Green Version]
- Uzarowicz, Ł.; Skiba, S.; Skiba, M.; Šegvić, B. Clay-Mineral Formation in Soils Developed in the Weathering Zone of Pyrite-Bearing Schists: A Case Study from The Abandoned Pyrite Mine in Wieściszowice, Lower Silesia, SW Poland. Clays Clay Miner. 2011, 59, 581–594. [Google Scholar] [CrossRef]
- Volzone, C.; Ortiga, J. SO2 gas adsorption by modified kaolin clays: Influence of previous heating and time acid treatments. J. Environ. Manag. 2011, 92, 2590–2595. [Google Scholar] [CrossRef]
- Bullock, P. Handbook for Soil Thin Section Description; Waine Research: Wolverhampton, UK, 1985. [Google Scholar]
- Nanzyo, M.; Shoji, S.; Dahlgren, R. Physical Characteristics of Volcanic Ash Soils. In Developments in Soil Science; Shoji, S., Nanzyo, M., Dahlgren, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 21, pp. 189–207. [Google Scholar]
- Yoshinaga, N. Mineralogical characteristics, II. In Ando Soils in Japan, Wada, K., Ed.; Kyushu University Press: Fukuoka, Japan, 1986; pp. 41–56. [Google Scholar]
- Hardjowigeno, S. Klasifikasi Tanah dan Pedogenesis; Akademika Pressindo: Jakarta, Indonesia, 1993; Volume 320, p. 274. [Google Scholar]
- Blatt, H.; Tracy, R. Petrology, Second Edition: Igneous, Sedimentary, and Metamorphic; W. H. Freeman: Basingstoke, UK, 1996; p. 529. [Google Scholar]
- Hsu, P.H. Aluminum Hydroxides and Oxyhydroxides. In Minerals in Soil Environments; Soil Science Society of America, Inc.: Madison, WI, USA, 1989; pp. 331–378. [Google Scholar]
- Tan, K.H. Principles of Soil Chemistry, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 390. [Google Scholar]
- Brown, G.; Brindley, G.W. X-ray Diffraction Procedures for Clay Mineral Identification. In Crystal Structures of Clay Minerals and Their X-ray Identification; Brindley, G.W., Brown, G., Eds.; Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 1980; Volume 5. [Google Scholar]
- Otsuka, H. Characteristics and Genesis of Volcanic Ash Soil in the Philippines. II. Some Physical and Chemical Properties; Tropical Agriculture Research Center: Rome, Italy, 1985. [Google Scholar]
- Allen, B.L.; Hajek, B.F. Mineral Occurrence in Soil Environments. In Minerals in Soil Environments; Soil Science Society of America, Inc.: Madison, WI, USA, 1989; pp. 199–278. [Google Scholar]
- Taylor, R.M. Amorphous Iron Oxides in Soils. J. Soil Sci. 1959, 10, 309–315. [Google Scholar] [CrossRef]
- Inoue, K.; Higashi, T. Al-and Fe-humus Complexes in Andisols. In Proceedings of the Ninth International Soil Classfication Workshop, Tokyo, Japan, 20 July–1 August 1987; pp. 81–96. [Google Scholar]
- Qafoku, N.P.; Ranst, E.V.; Noble, A.; Baert, G. Variable Charge Soils: Their Mineralogy, Chemistry and Management. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2004; Volume 84, pp. 159–215. [Google Scholar]
- Qafoku, N.P.; Sumner, M.E.; West, L.T. Mineralogy and chemistry of some variable charge subsoils. Commun. Soil Sci. Plant Anal. 2000, 31, 1051–1070. [Google Scholar] [CrossRef]
- Dixon, J.B. Kaolin and Serpentine Group Minerals. In Minerals in Soil Environments; Soil Science Society of America, Inc.: Madison, WI, USA, 1989; pp. 467–525. [Google Scholar]
- Eswaran, H. Morphology of allophane, imogolite and halloysite. Clay Miner. 1972, 9, 281–285. [Google Scholar] [CrossRef]
- Wada, K. Allophane and imogolite. In Developments in Sedimentology; Sudo, T., Shimoda, S., Eds.; Elsevier: Amsterdam, The Netherlands, 1978; Volume 26, pp. 147–187. [Google Scholar]
- Goenadi, D.H.; Tan, K.H. Mineralogy and Micromorphology of Soils from Volcanic Tuffs in the Humid Tropics. Soil Sci. Soc. Am. J. 1989, 53, 1907–1911. [Google Scholar] [CrossRef]
- Dahlgren, R.; Shoji, S.; Nanzyo, M. Mineralogical Characteristics of Volcanic Ash Soils. In Developments in Soil Science; Shoji, S., Nanzyo, M., Dahlgren, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; Volume 21, pp. 101–143. [Google Scholar]
- Papoulis, D.; Tsolis-Katagas, P.; Katagas, C. Progressive Stages in the Formation of Kaolin Minerals of Different Morphologies in the Weathering of Plagioclase. Clays Clay Miner. 2004, 52, 275–286. [Google Scholar] [CrossRef]
- Sakurai, K.; Teshima, A.; Kyuma, K. Changes in Zero Point of Charge (ZPC), Specific Surface Area (SSA), and Cation Exchange Capacity (CEC) of kaolinite and montmorillonite, and strongly weathered soils caused by Fe and Al coatings. Soil Sci. Plant Nutr. 1990, 36, 73–81. [Google Scholar] [CrossRef]
- Wada, K. The Distinctive Properties of Andosols. In Advances in Soil Science; Springer: New York, NY, USA, 1985; pp. 173–229. [Google Scholar]
- Aomine, S.; Wada, K. Differential weathering of volcanic ash and pumice, resulting in formation of hydrated halloysite1. Am. Mineral. 1962, 47, 1024–1048. [Google Scholar]
- Parfitt, R.L.; Theng, B.K.G.; Whitton, J.S.; Shepherd, T.G. Effects of clay minerals and land use on organic matter pools. Geoderma 1997, 75, 1–12. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy. In A Basic System of Soil Classification for Making and Interpreting Soil Surveys; US Department of Agriculture: Washington, DC, USA, 1999. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).