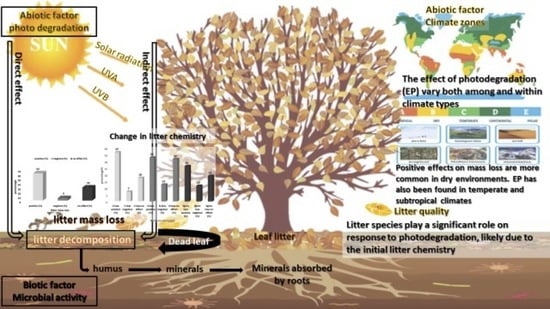
Photodegradation and Its Effect on Plant Litter Decomposition in Terrestrial Ecosystems: A Systematic Review
Abstract
:1. Introduction
2. Methods
Literature Review
3. Results and Discussion
3.1. Synthesis of Results
3.2. Distribution (Country and Habitat), Duration, Plant Species, and Functional Groups Included in Photodegradation Studies
3.3. Litter Quality and Photodegradation
3.4. Climate and Photodegradation
3.5. Effect of Photodegradation on Litter Mass Loss
3.6. Soil Biota and Photodegradation
3.7. Effect of Photodegradation on Carbon and Nutrient Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borneman, J.; Skroch, P.W.; O’Sullivan, K.M.; Palus, J.A.; Rumjanek, N.G.; Jansen, J.L.; Nienhuis, J.; Triplett, E.W. Molecular Microbial Diversity of an Agricultural Soil in Wisconsin. Appl. Environ. Microbiol. 1996, 62, 1935–1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, M. Nutrient Storage and Stoichiometry of the Forest Floor Organic Matter in Japanese Forests. Soil Syst. 2021, 5, 51. [Google Scholar] [CrossRef]
- Falconer, J.G.; Wright, J.W.; Beall, H.W. The Decomposition of Certain Types of Forest Litter Under Field Conditions. Am. J. Bot. 1933, 20, 196–203. [Google Scholar] [CrossRef]
- Gholz, H.L.; Wedin, D.A.; Smitherman, S.M.; Harmon, M.E.; Parton, W.J. Long-term Dynamics of Pine and Hardwood Litter in Contrasting Environments: Toward a Global Model of Decomposition. Glob. Chang. Biol. 2000, 6, 751–765. [Google Scholar] [CrossRef]
- Grosskopf, W. Wie Verandern Sich Stofflich und Morphologisch Die Fichtennadeln Bei der Bildung von Auflagehumus in Geschlossenen Fichtenreinbestanden. Tharandter Forstl. 1928, 79, 343–362. [Google Scholar]
- Melin, E. Biological Decomposition of Some Types of Litter from North American Forests. Ecology 1930, 11, 72–101. [Google Scholar] [CrossRef]
- Barnes, P.W.; Throop, H.L.; Archer, S.R.; Breshears, D.D.; McCulley, R.L.; Tobler, M.A. Sunlight and Soil–Litter Mixing: Drivers of Litter Decomposition in Drylands. In Progress in Botany: Volume 76; Lüttge, U., Beyschlag, W., Eds.; Springer International Publishing: Cham, Swizarland, 2015; pp. 273–302. ISBN 978-3-319-08807-5. [Google Scholar]
- King, J.Y.; Brandt, L.A.; Adair, E.C. Shedding Light on Plant Litter Decomposition: Advances, Implications and New Directions in Understanding the Role of Photodegradation. Biogeochemistry 2012, 111, 57–81. [Google Scholar] [CrossRef] [Green Version]
- Throop, H.L.; Archer, S.R. Resolving the Dryland Decomposition Conundrum: Some New Perspectives on Potential Drivers. In Progress in Botany; Lüttge, U., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 171–194. ISBN 978-3-540-68421-3. [Google Scholar]
- Bradford, M.A.; Veen, G.F.; Bonis, A.; Bradford, E.M.; Classen, A.T.; Cornelissen, J.H.C.; Crowther, T.W.; De Long, J.R.; Freschet, G.T.; Kardol, P.; et al. A Test of the Hierarchical Model of Litter Decomposition. Nat. Ecol. Evol. 2017, 1, 1836–1845. [Google Scholar] [CrossRef]
- Coûteaux, M.-M.; Bottner, P.; Berg, B. Litter Decomposition, Climate and Liter Quality. Trends Ecol. Evol. 1995, 10, 63–66. [Google Scholar] [CrossRef]
- García-Palacios, P.; Maestre, F.T.; Kattge, J.; Wall, D.H. Climate and Litter Quality Differently Modulate the Effects of Soil Fauna on Litter Decomposition across Biomes. Ecol. Lett. 2013, 16, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Hartig, R. Die Zersetzungserscheinungen des Holzes der Nadelholzbäume und der Eiche in Forstlicher, Botanischer und Chemischer Richtung; J. Springer: Berlin/Heidelberg, Germany, 1878. [Google Scholar]
- Lindquist, B. Undersökningar Över Några Skandinaviska Daggmaskarters Betydelse För Lövförnans Omvandling Och För Mulljordens Struktur i Svensk Skogsmark. Sven. Skogsv-Foren. Tidskr. 1941, 39, 179–242. [Google Scholar]
- Waksman, S.A.; Tenney, F.G.; Stevens, K.R. The Role of Microorganisms in the Transformation of Organic Matter in Forest Soils. Ecology 1928, 9, 126–144. [Google Scholar] [CrossRef]
- Parton, W.; Silver, W.L.; Burke, I.C.; Grassens, L.; Harmon, M.E.; Currie, W.S.; King, J.Y.; Adair, E.C.; Brandt, L.A.; Hart, S.C.; et al. Global-Scale Similarities in Nitrogen Release Patterns During Long-Term Decomposition. Science 2007, 315, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.A.; Welbourn, P. Environmental Toxicology; Cambridge Environmental Chemistry Series; Cambridge University Press: New York, NY, USA, 2002; ISBN 978-0-521-58151-6. [Google Scholar]
- Almagro, M.; Martínez-Mena, M. Exploring Short-Term Leaf-Litter Decomposition Dynamics in a Mediterranean Ecosystem: Dependence on Litter Type and Site Conditions. Plant Soil 2012, 358, 323–335. [Google Scholar] [CrossRef]
- Brandt, L.A.; King, J.Y.; Milchunas, D.G. Effects of Ultraviolet Radiation on Litter Decomposition Depend on Precipitation and Litter Chemistry in a Shortgrass Steppe Ecosystem. Glob. Chang. Biol. 2007, 13, 2193–2205. [Google Scholar] [CrossRef]
- Lee, H.; Rahn, T.; Throop, H. An Accounting of C-based Trace Gas Release during Abiotic Plant Litter Degradation. Glob. Chang. Biol. 2012, 18, 1185–1195. [Google Scholar] [CrossRef]
- Austin, A.T.; Vivanco, L. Plant Litter Decomposition in a Semi-Arid Ecosystem Controlled by Photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.E.; Sinsabaugh, R.L.; Cabaniss, S.E. The Role of Ultraviolet Radiation in Litter Decomposition in Arid Ecosystems. Appl. Soil Ecol. 2006, 34, 82–91. [Google Scholar] [CrossRef]
- Gehrke, C.; Johanson, U.; Callaghan, T.V.; Chadwick, D.; Robinson, C.H. The Impact of Enhanced Ultraviolet-B Radiation on Litter Quality and Decomposition Processes in Vaccinium Leaves from the Subarctic. Oikos 1995, 72, 213. [Google Scholar] [CrossRef]
- Austin, A.T.; Ballaré, C.L. Dual Role of Lignin in Plant Litter Decomposition in Terrestrial Ecosystems. Proc. Natl. Acad. Sci. USA 2010, 107, 4618–4622. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Pieristè, M.; Liu, C.; Kenta, T.; Robson, T.M.; Kurokawa, H. The Contribution of Photodegradation to Litter Decomposition in a Temperate Forest Gap and Understorey. New Phytol. 2021, 229, 2625–2636. [Google Scholar] [CrossRef]
- Berenstecher, P.; Vivanco, L.; Pérez, L.I.; Ballaré, C.L.; Austin, A.T. Sunlight Doubles Aboveground Carbon Loss in a Seasonally Dry Woodland in Patagonia. Curr. Biol. 2020, 30, 3243–3251.e3. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Karlen, S.D.; Ralph, J.; King, J.Y. Short-Term Facilitation of Microbial Litter Decomposition by Ultraviolet Radiation. Sci. Total Environ. 2018, 615, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.A.; Bohnet, C.; King, J.Y. Photochemically Induced Carbon Dioxide Production as a Mechanism for Carbon Loss from Plant Litter in Arid Ecosystems. J. Geophys. Res. Biogeosci. 2009, 114. [Google Scholar] [CrossRef] [Green Version]
- Day, T.A.; Bliss, M.S. Solar Photochemical Emission of CO2 from Leaf Litter: Sources and Significance to C Loss. Ecosystems 2020, 23, 1344–1361. [Google Scholar] [CrossRef]
- Almagro, M.; Maestre, F.T.; Martínez-López, J.; Valencia, E.; Rey, A. Climate Change May Reduce Litter Decomposition while Enhancing the Contribution of Photodegradation in Dry Perennial Mediterranean Grasslands. Soil Biol. Biochem. 2015, 90, 214–223. [Google Scholar] [CrossRef]
- Smith, W.K.; Gao, W.; Steltzer, H.; Wallenstein, M.D.; Tree, R. Moisture Availability Influences the Effect of Ultraviolet-B Radiation on Leaf Litter Decomposition. Glob. Chang. Biol. 2010, 16, 484–495. [Google Scholar] [CrossRef]
- Austin, A.T.; Méndez, M.S.; Ballaré, C.L. Photodegradation Alleviates the Lignin Bottleneck for Carbon Turnover in Terrestrial Ecosystems. Proc. Natl. Acad. Sci. USA 2016, 113, 4392–4397. [Google Scholar] [CrossRef] [Green Version]
- Pancotto, V.A.; Sala, O.E.; Cabello, M.; Lopez, N.I.; Matthew Robson, T.; Ballare, C.L.; Caldwell, M.M.; Scopel, A.L. Solar UV-B Decreases Decomposition in Herbaceous Plant Litter in Tierra Del Fuego, Argentina: Potential Role of an Altered Decomposer Community. Glob. Chang. Biol 2003, 9, 1465–1474. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, S.; Zhang, B.; Liu, W.; Deng, M.; Chen, S.; Liu, L. Temporal Dynamics of Ultraviolet Radiation Impacts on Litter Decomposition in a Semi-Arid Ecosystem. Plant Soil 2017, 419, 71–81. [Google Scholar] [CrossRef]
- Moran, M.A.; Zepp, R.G. Role of Photoreactions in the Formation of Biologically Labile Compounds from Dissolved Organic Matter. Limnol. Oceanogr. 1997, 42, 1307–1316. [Google Scholar] [CrossRef] [Green Version]
- Foereid, B.; Bellarby, J.; Meier-Augenstein, W.; Kemp, H. Does Light Exposure Make Plant Litter More Degradable? Plant Soil 2010, 333, 275–285. [Google Scholar] [CrossRef]
- Johnson, D. Response of Terrestrial Microorganisms to Ultraviolet-B Radiation in Ecosystems. Res. Microbiol. 2003, 154, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zeng, L.; Xiao, W.; Huang, Z.; Geng, X.; Tan, B. Effect of Litter Substrate Quality and Soil Nutrients on Forest Litter Decomposition: A Review. Acta Ecol. Sin. 2013, 33, 102–108. [Google Scholar] [CrossRef]
- Giweta, M. Role of Litter Production and Its Decomposition, and Factors Affecting the Processes in a Tropical Forest Ecosystem: A Review. J. Ecol. Environ. 2020, 44, 11. [Google Scholar] [CrossRef]
- Hui, W.; Jiangming, M.; Jinghua, X.; Yunting, F.; Jiong, L. Effects of Elevated Nitrogen Deposition on the Activities of Enzymes in Forest Litter Decomposition: A Review. J. Trop. Subtrop. Bot. 2006, 14, 539–546. [Google Scholar]
- Krishna, M.P.; Mohan, M. Litter Decomposition in Forest Ecosystems: A Review. Energ. Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tsukamoto, J.; Rahman, M.M.; Yoneyama, A.; Mostafa, K.M. Lignin and Its Effects on Litter Decomposition in Forest Ecosystems. Chem. Ecol. 2013, 29, 540–553. [Google Scholar] [CrossRef]
- Trofymow, J.; Moore, T.; Titus, B.; Prescott, C.; Morrison, I.; Siltanen, M.; Smith, S.; Fyles, J.; Wein, R.; Camiré, C. Rates of Litter Decomposition over 6 Years in Canadian Forests: Influence of Litter Quality and Climate. Can. J. For. Res. 2002, 32, 789–804. [Google Scholar] [CrossRef] [Green Version]
- Xiaojing, X.; ZHANG, K.; Bo, L. Review on Litter Decomposition in Forest Ecosystems. Sci. Soil Water Conserv. 2007, 5, 108–114. [Google Scholar]
- Yan, H.; Gu, X.; Shen, H. Microbial Decomposition of Forest Litter: A Review. Chin. J. Ecol. 2010, 29, 1827–1835. [Google Scholar]
- Wang, X.-Y.; Zhao, X.-Y.; Li, Y.-L.; Lian, J.; Qu, H.; Yue, X.-F. Effects of environmental factors on litter decomposition in arid and semi-arid regions: A review. Ying Yong Sheng Tai Xue Bao 2013, 24, 3300–3310. [Google Scholar] [PubMed]
- Aerts, R. Climate, Leaf Litter Chemistry and Leaf Litter Decomposition in Terrestrial Ecosystems: A Triangular Relationship. Oikos 1997, 79, 439–449. [Google Scholar] [CrossRef]
- Zhang, D.; Hui, D.; Luo, Y.; Zhou, G. Rates of Litter Decomposition in Terrestrial Ecosystems: Global Patterns and Controlling Factors. J. Plant Ecol. 2008, 1, 85–93. [Google Scholar] [CrossRef] [Green Version]
- David, J.-F. The Role of Litter-Feeding Macroarthropods in Decomposition Processes: A Reappraisal of Common Views. Soil Biol. Biochem. 2014, 76, 109–118. [Google Scholar] [CrossRef]
- Frouz, J. Effects of Soil Macro-and Mesofauna on Litter Decomposition and Soil Organic Matter Stabilization. Geoderma 2018, 332, 161–172. [Google Scholar] [CrossRef]
- Graça, M.A. The Role of Invertebrates on Leaf Litter Decomposition in Streams–a Review. Int. Rev. Hydrobiol. A J. Cover. All Asp. Limnol. Mar. Biol. 2001, 86, 383–393. [Google Scholar] [CrossRef]
- Zhang, H.; Song, X.; Ai, J.; Jiang, H.; Yu, S. A Review of UV-B Radiation and Its Influence on Litter Decomposition. J. Zhejiang For. Coll. 2010, 27, 134–142. [Google Scholar]
- Ferreira, V.; Castagneyrol, B.; Koricheva, J.; Gulis, V.; Chauvet, E.; Graça, M.A. A Meta-analysis of the Effects of Nutrient Enrichment on Litter Decomposition in Streams. Biol. Rev. 2015, 90, 669–688. [Google Scholar] [CrossRef] [Green Version]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen Additions and Litter Decomposition: A Meta-Analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Peng, C.; Jiang, H.; Zhu, Q.; Wang, W. Direct and Indirect Effects of UV-B Exposure on Litter Decomposition: A Meta-Analysis. PLoS ONE 2013, 8, e68858. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, L.; Wang, X.; Chen, Y. The Interaction between Abiotic Photodegradation and Microbial Decomposition under Ultraviolet Radiation. Glob. Chang. Biol. 2015, 21, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozema, J.; Tosserams, M.; Nelissen, H.J.M.; van Heerwaarden, L.; Broekman, R.A.; Flierman, N. Stratospheric Ozone Reduction and Ecosystem Processes: Enhanced UV-B Radiation Affects Chemical Quality and Decomposition of Leaves of the Dune Grassland Species Calamagrostis Epigeios. Plant Ecol. 1997, 128, 285–294. [Google Scholar] [CrossRef]
- Marinho, O.A.; Martinelli, L.A.; Duarte-Neto, P.J.; Mazzi, E.A.; King, J.Y. Photodegradation Influences Litter Decomposition Rate in a Humid Tropical Ecosystem, Brazil. Sci. Total Environ. 2020, 715, 136601. [Google Scholar] [CrossRef]
- Predick, K.I.; Archer, S.R.; Aguillon, S.M.; Keller, D.A.; Throop, H.L.; Barnes, P.W. UV-B Radiation and Shrub Canopy Effects on Surface Litter Decomposition in a Shrub-Invaded Dry Grassland. J. Arid Environ. 2018, 157, 13–21. [Google Scholar] [CrossRef]
- Day, T.A.; Zhang, E.T.; Ruhland, C.T. Exposure to Solar UV-B Radiation Accelerates Mass and Lignin Loss of Larrea tridentata Litter in the Sonoran Desert. Plant Ecol. 2007, 193, 185–194. [Google Scholar] [CrossRef]
- Pancotto, V.A.; Sala, O.E.; Robson, T.M.; Caldwell, M.M.; Scopel, A.L. Direct and Indirect Effects of Solar Ultraviolet-B Radiation on Long-Term Decomposition. Glob. Chang. Biol. 2005, 11, 1982–1989. [Google Scholar] [CrossRef]
- Henry, G.H.R.; Molau, U. Tundra Plants and Climate Change: The International Tundra Experiment (ITEX). Glob. Chang. Biol. 1997, 3, 1–9. [Google Scholar] [CrossRef]
- Day, T.A.; Bliss, M.S.; Tomes, A.R.; Ruhland, C.T.; Guénon, R. Desert Leaf Litter Decay: Coupling of Microbial Respiration, Water-Soluble Fractions and Photodegradation. Glob. Chang. Biol. 2018, 24, 5454–5470. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.A.; Christman, M.P.; Hall, S.J. Nutrient Dynamics during Photodegradation of Plant Litter in the Sonoran Desert. J. Arid Environ. 2019, 160, 1–10. [Google Scholar] [CrossRef]
- Brandt, L.A.; King, J.Y.; Hobbie, S.E.; Milchunas, D.G.; Sinsabaugh, R.L. The Role of Photodegradation in Surface Litter Decomposition Across a Grassland Ecosystem Precipitation Gradient. Ecosystems 2010, 13, 765–781. [Google Scholar] [CrossRef] [Green Version]
- Esch, E.H.; King, J.Y.; Cleland, E.E. Foliar Litter Chemistry Mediates Susceptibility to UV Degradation in Two Dominant Species from a Semi-Arid Ecosystem. Plant Soil 2019, 440, 265–276. [Google Scholar] [CrossRef]
- Gallo, M.E.; Porras-Alfaro, A.; Odenbach, K.J.; Sinsabaugh, R.L. Photoacceleration of Plant Litter Decomposition in an Arid Environment. Soil Biol. Biochem. 2009, 41, 1433–1441. [Google Scholar] [CrossRef]
- Hewins, D.B.; Throop, H.L. Leaf Litter Decomposition Is Rapidly Enhanced by the Co-Occurrence of Monsoon Rainfall and Soil-Litter Mixing across a Gradient of Coppice Dune Development in the Chihuahuan Desert. J. Arid Environ. 2016, 129, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Hewins, D.B.; Lee, H.; Barnes, P.W.; McDowell, N.G.; Pockman, W.T.; Rahn, T.; Throop, H.L. Early Exposure to UV Radiation Overshadowed by Precipitation and Litter Quality as Drivers of Decomposition in the Northern Chihuahuan Desert. PLoS ONE 2019, 14, e0210470. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhao, H.; Li, Y. Litter Decomposition in Hyper-Arid Deserts: Photodegradation Is Still Important. Sci. Total Environ. 2017, 601, 784–792. [Google Scholar] [CrossRef]
- Huang, G.; Li, Y. Photodegradation Effects Are Related to Precipitation Amount, Precipitation Frequency and Litter Traits in a Desert Ecosystem. Soil Biol. Biochem. 2017, 115, 383–392. [Google Scholar] [CrossRef]
- Vanderbilt, K.L.; White, C.S.; Hopkins, O.; Craig, J.A. Aboveground Decomposition in Arid Environments: Results of a Long-Term Study in Central New Mexico. J. Arid. Environ. 2008, 72, 696–709. [Google Scholar] [CrossRef]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecol. 1963, 44, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Almagro, M.; Martínez-López, J.; Maestre, F.T.; Rey, A. The Contribution of Photodegradation to Litter Decomposition in Semiarid Mediterranean Grasslands Depends on Its Interaction with Local Humidity Conditions, Litter Quality and Position. Ecosystems 2017, 20, 527–542. [Google Scholar] [CrossRef]
- Barnes, P.W.; Throop, H.L.; Hewins, D.B.; Abbene, M.L.; Archer, S.R. Soil Coverage Reduces Photodegradation and Promotes the Development of Soil-Microbial Films on Dryland Leaf Litter. Ecosystems 2012, 15, 311–321. [Google Scholar] [CrossRef]
- Bosco, T.; Bertiller, M.B.; Carrera, A.L. Combined Effects of Litter Features, UV Radiation, and Soil Water on Litter Decomposition in Denuded Areas of the Arid Patagonian Monte. Plant Soil 2016, 406, 71–82. [Google Scholar] [CrossRef]
- Chen, M.; Parton, W.J.; Adair, E.C.; Asao, S.; Hartman, M.D.; Gao, W. Simulation of the Effects of Photodecay on Long-Term Litter Decay Using DayCent. Ecosphere 2016, 7, e01631. [Google Scholar] [CrossRef]
- Asao, S.; Parton, W.J.; Chen, M.; Gao, W. Photodegradation Accelerates Ecosystem N Cycling in a Simulated California Grassland. Ecosphere 2018, 9, e02370. [Google Scholar] [CrossRef] [Green Version]
- Erdenebileg, E.; Ye, X.; Wang, C.; Huang, Z.; Liu, G.; Cornelissen, J.H.C. Positive and Negative Effects of UV Irradiance Explain Interaction of Litter Position and UV Exposure on Litter Decomposition and Nutrient Dynamics in a Semi-Arid Dune Ecosystem. Soil Biol. Biochem. 2018, 124, 245–254. [Google Scholar] [CrossRef]
- Erdenebileg, E.; Wang, C.; Ye, X.; Cui, Q.; Du, J.; Huang, Z.; Liu, G.; Cornelissen, J.H.C. Multiple Abiotic and Biotic Drivers of Long-Term Wood Decomposition within and among Species in the Semi-Arid Inland Dunes: A Dual Role for Stem Diameter. Funct. Ecol. 2020, 34, 1472–1484. [Google Scholar] [CrossRef]
- Gaxiola, A.; Armesto, J.J. Understanding Litter Decomposition in Semiarid Ecosystems: Linking Leaf Traits, UV Exposure and Rainfall Variability. Front. Plant Sci. 2015, 6, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliksman, D.; Rey, A.; Seligmann, R.; Dumbur, R.; Sperling, O.; Navon, Y.; Haenel, S.; Angelis, P.D.; Arnone, J.A.; Grünzweig, J.M. Biotic Degradation at Night, Abiotic Degradation at Day: Positive Feedbacks on Litter Decomposition in Drylands. Glob. Chang. Biol. 2017, 23, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Gliksman, D.; Navon, Y.; Dumbur, R.; Haenel, S.; Grünzweig, J.M. Higher Rates of Decomposition in Standing vs. Surface Litter in a Mediterranean Ecosystem during the Dry and the Wet Seasons. Plant Soil 2018, 428, 427–439. [Google Scholar] [CrossRef]
- Mao, B.; Zhao, L.; Zhao, Q.; Zeng, D. Effects of Ultraviolet (UV) Radiation and Litter Layer Thickness on Litter Decomposition of Two Tree Species in a Semi-Arid Site of Northeast China. J. Arid Land 2018, 10, 416–428. [Google Scholar] [CrossRef] [Green Version]
- Méndez, M.S.; Martinez, M.L.; Araujo, P.I.; Austin, A.T. Solar Radiation Exposure Accelerates Decomposition and Biotic Activity in Surface Litter but Not Soil in a Semiarid Woodland Ecosystem in Patagonia, Argentina. Plant Soil 2019, 445, 483–496. [Google Scholar] [CrossRef]
- Uselman, S.M.; Snyder, K.A.; Blank, R.R.; Jones, T.J. UVB Exposure Does Not Accelerate Rates of Litter Decomposition in a Semi-Arid Riparian Ecosystem. Soil Biol. Biochem. 2011, 43, 1254–1265. [Google Scholar] [CrossRef]
- Yanni, S.F.; Suddick, E.C.; Six, J. Photodegradation Effects on CO2 Emissions from Litter and SOM and Photo-Facilitation of Microbial Decomposition in a California Grassland. Soil Biol. Biochem. 2015, 91, 40–49. [Google Scholar] [CrossRef]
- Henry, H.A.L.; Brizgys, K.; Field, C.B. Litter Decomposition in a California Annual Grassland: Interactions Between Photodegradation and Litter Layer Thickness. Ecosystems 2008, 11, 545–554. [Google Scholar] [CrossRef]
- Hoorens, B.; Aerts, R.; Stroetenga, M. Elevated UV-B Radiation Has No Effect on Litter Quality and Decomposition of Two Dune Grassland Species: Evidence from a Long-term Field Experiment. Glob. Chang. Biol. 2004, 10, 200–208. [Google Scholar] [CrossRef]
- Keiser, A.D.; Warren, R.; Filley, T.; Bradford, M.A. Signatures of an Abiotic Decomposition Pathway in Temperate Forest Leaf Litter. Biogeochemistry 2021, 153, 177–190. [Google Scholar] [CrossRef]
- Moody, S.A.; Paul, N.D.; Björn, L.O.; Callaghan, T.V.; Lee, J.A.; Manetas, Y.; Rozema, J.; Gwynn-Jones, D.; Johanson, U.; Kyparissis, A. The Direct Effects of UV-B Radiation on Betula Pubescens Litter Decomposing at Four European Field Sites. Plant Ecol. 2001, 154, 27–36. [Google Scholar] [CrossRef]
- Pieristè, M.; Chauvat, M.; Kotilainen, T.K.; Jones, A.G.; Aubert, M.; Robson, T.M.; Forey, E. Solar UV-A Radiation and Blue Light Enhance Tree Leaf Litter Decomposition in a Temperate Forest. Oecologia 2019, 191, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Pieristè, M.; Forey, E.; Lounès-Hadj Sahraoui, A.; Meglouli, H.; Laruelle, F.; Delporte, P.; Robson, T.M.; Chauvat, M. Spectral Composition of Sunlight Affects the Microbial Functional Structure of Beech Leaf Litter During the Initial Phase of Decomposition. Plant Soil 2020, 451, 515–530. [Google Scholar] [CrossRef]
- Messenger, D.J.; Fry, S.C.; Yamulki, S.; McLeod, A.R. Effects of UV-B Filtration on the Chemistry and Decomposition of Fraxinus Excelsior Leaves. Soil Biol. Biochem. 2012, 47, 133–141. [Google Scholar] [CrossRef]
- Pieristè, M.; Neimane, S.; Solanki, T.; Nybakken, L.; Jones, A.G.; Forey, E.; Chauvat, M.; Ņečajeva, J.; Robson, T.M. Ultraviolet Radiation Accelerates Photodegradation under Controlled Conditions but Slows the Decomposition of Senescent Leaves from Forest Stands in Southern Finland. Plant Physiol. Biochem. 2020, 146, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Fellman, J.B.; Petrone, K.C.; Grierson, P.F. Leaf Litter Age, Chemical Quality, and Photodegradation Control the Fate of Leachate Dissolved Organic Matter in a Dryland River. J. Arid Environ. 2013, 89, 30–37. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, W.; Wu, F.; Tan, B. Effects of Light Intensity on Litter Decomposition in a Subtropical Region. Ecosphere 2017, 8, e01770. [Google Scholar] [CrossRef]
- Song, X.; Jiang, H.; Zhang, H.; Peng, C.; Yu, S. Elevated UV-B Radiation Did Not Affect Decomposition Rates of Needles of Two Coniferous Species in Subtropical China. Eur. J. Soil Biol. 2011, 47, 343–348. [Google Scholar] [CrossRef]
- Song, X.; Jiang, H.; Zhang, Z.; Zhou, G.; Zhang, S.; Peng, C. Interactive Effects of Elevated UV-B Radiation and N Deposition on Decomposition of Moso Bamboo Litter. Soil Biol. Biochem. 2014, 69, 11–16. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Wang, H.; Li, C.; Mo, Q.; Liu, Y. Photodegradation Accelerates Coarse Woody Debris Decomposition in Subtropical Chinese Forests. For. Ecol. Manag. 2018, 409, 225–232. [Google Scholar] [CrossRef]
- Agrawal, S.B.; Kumari, R. Assessment of Litter Degradation in Medicinal Plants Subjected to Ultraviolet-B Radiation. J. Environ. Biol. 2013, 34, 739. [Google Scholar]
- Day, T.A.; Bliss, M.S. A Spectral Weighting Function for Abiotic Photodegradation Based on Photochemical Emission of CO2 from Leaf Litter in Sunlight. Biogeochemistry 2019, 146, 173–190. [Google Scholar] [CrossRef]
- Foereid, B.; Zarov, E.A.; Latysh, I.M.; Filippov, I.V.; Lapshina, E.D. Photo-Exposure Affects Subsequent Peat Litter Decomposition. Geoderma 2018, 315, 104–110. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F.; Lambie, S.M.; Zhou, H. No UV Enhancement of Litter Decomposition Observed on Dry Samples under Controlled Laboratory Conditions. Soil Biol. Biochem. 2011, 43, 1300–1307. [Google Scholar] [CrossRef]
- Lambie, S.M.; Kirschbaum, M.U.F.; Dando, J. No Photodegradation of Litter and Humus Exposed to UV-B Radiation under Laboratory Conditions: No Effect of Leaf Senescence or Drying Temperature. Soil Biol. Biochem. 2014, 69, 46–53. [Google Scholar] [CrossRef]
- Lin, Y.; Scarlett, R.D.; King, J.Y. Effects of UV Photodegradation on Subsequent Microbial Decomposition of Bromus Diandrus Litter. Plant Soil 2015, 395, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Hu, R.; Cai, G.; Lin, S.; Zhao, J.; Li, Y. The Role of UV-B Radiation and Precipitation on Straw Decomposition and Topsoil C Turnover. Soil Biol. Biochem. 2014, 77, 197–202. [Google Scholar] [CrossRef]
- Newsham, K.K.; McLeod, A.R.; Roberts, J.D.; Greenslade, P.D.; Emmett, B.A. Direct Effects of Elevated UV-B Radiation on the Decomposition of Quercus Robur Leaf Litter. Oikos 1997, 79, 592–602. [Google Scholar] [CrossRef]
- Newsham, K.K.; Greenslade, P.D.; Kennedy, V.H.; McLeod, A.R. Elevated UV-B Radiation Incident on Quercus Robur Leaf Canopies Enhances Decomposition of Resulting Leaf Litter in Soil. Glob. Chang. Biol. 1999, 5, 403–409. [Google Scholar] [CrossRef]
- Ruhland, C.T.; Niere, J.A. The Effects of Surface Albedo and Initial Lignin Concentration on Photodegradation of Two Varieties of Sorghum Bicolor Litter. Sci. Rep. 2019, 9, 18748. [Google Scholar] [CrossRef]
- Song, X.Z.; Zhang, H.L.; Chang, S.X.; Jiang, H.; Peng, C.H.; Yu, S.Q. Elevated UV-B Radiation Increased the Decomposition of Cinnamomum Camphora and Cyclobalanopsis Glauca Leaf Litter in Subtropical China. J. Soils Sediments 2012, 12, 307–311. [Google Scholar] [CrossRef]
- Zhou, G.; Wei, F.; Qiu, X.; Xu, X.; Zhang, J.; Guo, X. Influence of Enhanced Ultraviolet-B Radiation during Rice Plant Growth on Rice Straw Decomposition with Nitrogen Deposition. Sci. Rep. 2018, 8, 14512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornelissen, J.H.C. An Experimental Comparison of Leaf Decomposition Rates in a Wide Range of Temperate Plant Species and Types. J. Ecol. 1996, 84, 573–582. [Google Scholar] [CrossRef]
- Martinez-Yrizar, A.; Nuñez, S.; Burquez, A. Leaf Litter Decomposition in a Southern Sonoran Desert Ecosystem, Northwestern Mexico: Effects of Habitat and Litter Quality. Acta Oecologica 2007, 32, 291–300. [Google Scholar] [CrossRef]
- Kotilainen, T.; Haimi, J.; Tegelberg, R.; Julkunen-Tiitto, R.; Vapaavuori, E.; Aphalo, P.J. Solar Ultraviolet Radiation Alters Alder and Birch Litter Chemistry That in Turn Affects Decomposers and Soil Respiration. Oecologia 2009, 161, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.T.; Yahdjian, L.; Stark, J.M.; Belnap, J.; Porporato, A.; Norton, U.; Ravetta, D.A.; Schaeffer, S.M. Water Pulses and Biogeochemical Cycles in Arid and Semiarid Ecosystems. Oecologia 2004, 141, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.T. Has Water Limited Our Imagination for Aridland Biogeochemistry? Trends Ecol. Evol. 2011, 26, 229–235. [Google Scholar] [CrossRef]
- MacKay, W.P.; Loring, S.J.; Zak, J.C.; Silva, S.I.; Fisher, F.M.; Whitford, W.G. Factors Affecting Loss in Mass of Creosotebush Leaf-Litter on the Soil Surface in the Northern Chihuahuan Desert. Southwest. Nat. 1994, 39, 78–82. [Google Scholar] [CrossRef]
- Bornman, J.F.; Barnes, P.W.; Robinson, S.A.; Ballaré, C.L.; Flint, S.D.; Caldwell, M.M. Solar Ultraviolet Radiation and Ozone Depletion-Driven Climate Change: Effects on Terrestrial Ecosystems. Photochem. Photobiol. Sci. 2015, 14, 88–107. [Google Scholar] [CrossRef] [Green Version]
- Madronich, S.; McKenzie, R.L.; Björn, L.O.; Caldwell, M.M. Changes in Biologically Active Ultraviolet Radiation Reaching the Earth’s Surface. J. Photochem. Photobiol. B Biol. 1998, 46, 5–19. [Google Scholar] [CrossRef]
- Sercu, B.K.; Baeten, L.; van Coillie, F.; Martel, A.; Lens, L.; Verheyen, K.; Bonte, D. How Tree Species Identity and Diversity Affect Light Transmittance to the Understory in Mature Temperate Forests. Ecol. Evol. 2017, 7, 10861–10870. [Google Scholar] [CrossRef]
- Duguay, K.J.; Klironomos, J.N. Direct and Indirect Effects of Enhanced UV-B Radiation on the Decomposing and Competitive Abilities of Saprobic Fungi. Appl. Soil Ecol. 2000, 14, 157–164. [Google Scholar] [CrossRef]
- Verhoef, H.A.; Verspagen, J.M.; Zoomer, H.R. Direct and Indirect Effects of Ultraviolet-B Radiation on Soil Biota, Decomposition and Nutrient Fluxes in Dune Grassland Soil Systems. Biol. Fertil. Soils 2000, 31, 366–371. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Huang, J.; Yu, H.; Guan, X.; Wang, G.; Guo, R. Accelerated Dryland Expansion under Climate Change. Nat. Clim. Chang. 2016, 6, 166–171. [Google Scholar] [CrossRef]
- Williamson, C.E.; Zepp, R.G.; Lucas, R.M.; Madronich, S.; Austin, A.T.; Ballaré, C.L.; Norval, M.; Sulzberger, B.; Bais, A.F.; McKenzie, R.L. Solar Ultraviolet Radiation in a Changing Climate. Nat. Clim. Chang. 2014, 4, 434–441. [Google Scholar] [CrossRef]
- Zepp, R.; Erickson Iii, D.; Paul, N.; Sulzberger, B. Effects of Solar UV Radiation and Climate Change on Biogeochemical Cycling: Interactions and Feedbacks. Photochem. Photobiol. Sci. 2011, 10, 261–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, N.R.; Allison, S.D. Ultraviolet Photodegradation Facilitates Microbial Litter Decomposition in a Mediterranean Climate. Ecology 2015, 96, 1994–2003. [Google Scholar] [CrossRef]
- Feng, X.; Hills, K.M.; Simpson, A.J.; Whalen, J.K.; Simpson, M.J. The Role of Biodegradation and Photo-Oxidation in the Transformation of Terrigenous Organic Matter. Org. Geochem. 2011, 42, 262–274. [Google Scholar] [CrossRef]
- Lin, Y.; King, J.Y.; Karlen, S.D.; Ralph, J. Using 2D NMR Spectroscopy to Assess Effects of UV Radiation on Cell Wall Chemistry during Litter Decomposition. Biogeochemistry 2015, 125, 427–436. [Google Scholar] [CrossRef]
- Arriaga, L.; Maya, Y. Spatial Variability in Decomposition Rates in a Desert Scrub of Northwestern Mexico. Plant Ecol. 2007, 189, 213–225. [Google Scholar] [CrossRef]
- Vossbrinck, C.R.; Coleman, D.C.; Woolley, T.A. Abiotic and Biotic Factors in Litter Decomposition in a Sermiarid Grassland. Ecology 1979, 60, 265–271. [Google Scholar] [CrossRef]
- Bornman, J.F.; Barnes, P.W.; Robson, T.M.; Robinson, S.A.; Jansen, M.A.; Ballaré, C.L.; Flint, S.D. Linkages between Stratospheric Ozone, UV Radiation and Climate Change and Their Implications for Terrestrial Ecosystems. Photochem. Photobiol. Sci. 2019, 18, 681–716. [Google Scholar] [PubMed] [Green Version]
- Rutledge, S.; Campbell, D.I.; Baldocchi, D.; Schipper, L.A. Photodegradation Leads to Increased Carbon Dioxide Losses from Terrestrial Organic Matter. Glob. Chang. Biol. 2010, 16, 3065–3074. [Google Scholar] [CrossRef]
- Berg, B. Initial Rates and Limit Values for Decomposition of Scots Pine and Norway Spruce Needle Litter: A Synthesis for N-Fertilized Forest Stands. Can. J. For. Res. 2000, 30, 122–135. [Google Scholar] [CrossRef]
- Dyer, M.L.; Meentemeyer, V.; Berg, B. Apparent Controls of Mass Loss Rate of Leaf Litter on a Regional Scale: Litter Quality vs. Climate. Scand. J. For. Res. 1990, 5, 311–323. [Google Scholar] [CrossRef]
- O’neill, E.; Johnson, D.; Ledford, J.; Todd, D. Acute Seasonal Drought Does Not Permanently Alter Mass Loss and Nitrogen Dynamics during Decomposition of Red Maple (Acer rubrum L.) Litter. Glob. Chang. Biol. 2003, 9, 117–123. [Google Scholar] [CrossRef]
- Edmonds, R.L. Litter Decomposition and Nutrient Release in Douglas-Fir, Red Alder, Western Hemlock, and Pacific Silver Fir Ecosystems in Western Washington. Can. J. For. Res. 1980, 10, 327–337. [Google Scholar] [CrossRef]
- Gholz, H.; Fisher, R.; Prichett, W. Nutrient Dynamics in Slash Pine Plantation Ecosystems: Ecological Archives. Ecology 1985, 66, 647–659. [Google Scholar] [CrossRef]
- Waring, R.H.; Schlesinger, W. Forest Ecosystems: Analysis at Multiple Scales; Academic Press Inc.: Orlando, FL, USA; San Diego, CA, USA, 1985; Volume 55. [Google Scholar]
- Yavitt, J.B.; Fahey, T.J. Litter Decay and Leaching from the Forest Floor in Pinus Contorta (Lodgepole Pine) Ecosystems. J. Ecol. 1986, 74, 525–545. [Google Scholar] [CrossRef]
- Prescott, C.; Zabek, L.; Staley, C.; Kabzems, R. Decomposition of Broadleaf and Needle Litter in Forests of British Columbia: Influences of Litter Type, Forest Type, and Litter Mixtures. Can. J. For. Res. 2000, 30, 1742–1750. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global Patterns in Root Decomposition: Comparisons of Climate and Litter Quality Effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H. Structure and Function of the Global Topsoil Microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Crowther, T.W.; van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The Global Soil Community and Its Influence on Biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.R.P.; Guerra, C.A.; Bartz, M.L.C.; Briones, M.J.I.; Brown, G.; Crowther, T.W.; Ferlian, O.; Gongalsky, K.B.; van den Hoogen, J.; Krebs, J.; et al. Global Distribution of Earthworm Diversity. Science 2019, 366, 480–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potapov, A.M.; Guerra, C.A.; van den Hoogen, J.; Babenko, A.; Bellini, B.C.; Berg, M.P.; Chown, S.L.; Deharveng, L.; Kováč, Ľ.; Kuznetsova, N.A.; et al. Globally Invariant Metabolism but Density-Diversity Mismatch in Springtails. bioRxiv 2022, 2022.01.07.475345. [Google Scholar] [CrossRef]
- Van Den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; De Goede, R.G.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S. Soil Nematode Abundance and Functional Group Composition at a Global Scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Adenan, S.; Oja, J.; Alatalo, J.M.; Shraim, A.M.; Alsafran, M.; Tedersoo, L.; Zobel, M.; Ahmed, T. Diversity of Arbuscular Mycorrhizal Fungi and Its Chemical Drivers across Dryland Habitats. Mycorrhiza 2021, 31, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A Global Atlas of the Dominant Bacteria Found in Soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Tedersoo, L.; Mikryukov, V.; Anslan, S.; Bahram, M.; Khalid, A.N.; Corrales, A.; Agan, A.; Vasco-Palacios, A.-M.; Saitta, A.; Antonelli, A. The Global Soil Mycobiome Consortium Dataset for Boosting Fungal Diversity Research. Fungal Divers. 2021, 111, 573–588. [Google Scholar] [CrossRef]
- Griffiths, H.M.; Ashton, L.A.; Parr, C.L.; Eggleton, P. The Impact of Invertebrate Decomposers on Plants and Soil. New Phytol. 2021, 231, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Rinnan, R.; Gehrke, C.; Michelsen, A. Two Mire Species Respond Differently to Enhanced Ultraviolet-B Radiation: Effects on Biomass Allocation and Root Exudation. New Phytol. 2006, 169, 809–818. [Google Scholar] [CrossRef]
- Sanders, R.W.; Cooke, S.L.; Fischer, J.M.; Fey, S.B.; Heinze, A.W.; Jeffrey, W.H.; Macaluso, A.L.; Moeller, R.E.; Morris, D.P.; Neale, P.J.; et al. Shifts in Microbial Food Web Structure and Productivity after Additions of Naturally Occurring Dissolved Organic Matter: Results from Large-Scale Lacustrine Mesocosms. Limnol. Oceanogr. 2015, 60, 2130–2144. [Google Scholar] [CrossRef] [Green Version]
- Geisen, S.; Lara, E.; Mitchell, E.A.; Völcker, E.; Krashevska, V. Soil Protist Life Matters! Soil Org. 2020, 92, 189–196. [Google Scholar]
- Araujo, P.I.; Yahdjian, L.; Austin, A.T. Do Soil Organisms Affect Aboveground Litter Decomposition in the Semiarid Patagonian Steppe, Argentina? Oecologia 2012, 168, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.R.; Parkinson, D.; Parsons, W.F. Nitrogen and Lignin Content as Predictors of Litter Decay Rates: A Microcosm Test. Ecology 1989, 70, 97–104. [Google Scholar] [CrossRef]
- Aber, J.D.; Melillo, J.M. Nitrogen Immobilization in Decaying Hardwood Leaf Litter as a Function of Initial Nitrogen and Lignin Content. Can. J. Bot. 1982, 60, 2263–2269. [Google Scholar] [CrossRef]
- Melillo, J.; Naiman, R.; Aber, J.; Linkins, A. Factors Controlling Mass Loss and Nitrogen Dynamics of Plant Litter Decaying in Northern Streams. Bull. Mar. Sci. 1984, 35, 341–356. [Google Scholar]
- Melillo, J.M.; Aber, J.D.; Muratore, J.F. Nitrogen and Lignin Control of Hardwood Leaf Litter Decomposition Dynamics. Ecology 1982, 63, 621–626. [Google Scholar] [CrossRef]
- Aber, J.D.; Melillo, J.M.; McClaugherty, C.A. Predicting Long-Term Patterns of Mass Loss, Nitrogen Dynamics, and Soil Organic Matter Formation from Initial Fine Litter Chemistry in Temperate Forest Ecosystems. Can. J. Bot. 1990, 68, 2201–2208. [Google Scholar] [CrossRef] [Green Version]
- Berg, B.; McClaugherty, C. Nitrogen Release from Litter in Relation to the Disappearance of Lignin. Biogeochemistry 1987, 4, 219–224. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Cornelissen, J.H.; Amatangelo, K.; Dorrepaal, E.; Eviner, V.T.; Godoy, O.; Hobbie, S.E.; Hoorens, B.; Kurokawa, H.; Pérez-Harguindeguy, N. Plant Species Traits Are the Predominant Control on Litter Decomposition Rates within Biomes Worldwide. Ecol. Lett. 2008, 11, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; King, J.Y. Effects of UV Exposure and Litter Position on Decomposition in a California Grassland. Ecosystems 2014, 17, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Adair, E.C.; Parton, W.J.; King, J.Y.; Brandt, L.A.; Lin, Y. Accounting for Photodegradation Dramatically Improves Prediction of Carbon Losses in Dryland Systems. Ecosphere 2017, 8, e01892. [Google Scholar] [CrossRef] [Green Version]
- George, B.; Suttie, E.; Merlin, A.; Deglise, X. Photodegradation and Photostabilisation of Wood–the State of the Art. Polym. Degrad. Stab. 2005, 88, 268–274. [Google Scholar] [CrossRef]
- Ruhland, C.T.; Xiong, F.S.; Clark, W.D.; Day, T.A. The Influence of Ultraviolet-B Radiation on Growth, Hydroxycinnamic Acids and Flavonoids of Deschampsia Antarctica during Springtime Ozone Depletion in Antarctica. Photochem. Photobiol. 2005, 81, 1086–1093. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Callaghan, T. Effects of Increasing Ultraviolet B Radiation on Decomposition and Soil Organic Matter Dynamics: A Synthesis and Modelling Study. Biol. Fertil. Soils 1994, 18, 19–26. [Google Scholar] [CrossRef]
- Qu, H.; Zhao, X.; Wang, S.; Lian, J.; Tang, X.; Wang, X.; Zhang, R.; Medina-Roldán, E. Abiotic Factors Affect Leaf Litter Mass Loss More Strongly than Initial Litter Traits under Sand Burial Conditions. CATENA 2021, 196, 104900. [Google Scholar] [CrossRef]
- Davidson, R.S. The Photodegradation of Some Naturally Occurring Polymers. J. Photochem. Photobiol. B Biol. 1996, 33, 3–25. [Google Scholar] [CrossRef]
- Xiang, S.-R.; Doyle, A.; Holden, P.A.; Schimel, J.P. Drying and Rewetting Effects on C and N Mineralization and Microbial Activity in Surface and Subsurface California Grassland Soils. Soil Biol. Biochem. 2008, 40, 2281–2289. [Google Scholar] [CrossRef]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric Controls on Carbon, Nitrogen, and Phosphorus Dynamics in Decomposing Litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef] [Green Version]
- Keuskamp, J.A.; Hefting, M.M.; Dingemans, B.J.; Verhoeven, J.T.; Feller, I.C. Effects of Nutrient Enrichment on Mangrove Leaf Litter Decomposition. Sci. Total Environ. 2015, 508, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, S.; Jackson, R.B.; Trofymow, J.A.; Porporato, A. The Global Stoichiometry of Litter Nitrogen Mineralization. Science 2008, 321, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Michopoulos, P.; Baloutsos, G.; Economou, A.; Nikolis, N. Effects of Nitrogen Deposition on Nitrogen Cycling in an Aleppo Pine Stand in Athens, Greece. Sci. Total Environ. 2004, 323, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Rustad, L.E. The Response of Terrestrial Ecosystems to Global Climate Change: Towards an Integrated Approach. Sci. Total Environ. 2008, 404, 222–235. [Google Scholar] [CrossRef] [PubMed]

| Climate Types | Country | Experiment Duration (Days) | Litter Types | Number of Species | Litter (Plant) Species | Effect of Photodegradation (EP) | Methods | References | |||||||
| 1. Litter Mass Loss | 2. Effect on Nutrient Breakdown (ENB) | Evaluating Litter Decomposition | Evaluating Radiation Effect | Nutrient Analyses (C, N, P, Lignin, and Phenolic) | |||||||||||
| C% Loss | N% Loss | P% Loss | Lignin Loss | Phenolic Content | |||||||||||
| Arid | USA | 280 | Leaves | 1 | A. deltoidea | + | NA | + | + | NA | NA | Litter bag technique. | UV transparent plastic film, UV opaque blocking plastic film. Ash-free dry mass ANOVA. | Elemental analyzer | [65] |
| USA | 730 | Grass | 2 | A. gerardi, B. gracilis | (+,0) * | NA | (+,0) * | NA | (+,0) * | NA | Litter bag technique. | UV transparent acrylic plastic screen, UV blocking polycarbonate plastic screen. Ash-free dry mass ANOVA. | Elemental analyzer, fiber analyzer, spectroscopy | [66] | |
| USA | 120–150 | Leaves, twigs, seeds | 1 | L. tridentata | + | 0 | 0 | NA | + | NA | Litter bag technique. | Aclar UV transparent plastic film and mylar UV block plastic film ANOVA. | Elemental analyzer | [61] | |
| USA | 757 | Senescent leaf | 2 | A. fatua, S. mellifera | (+,0) * | NA | NA | NA | + | NA | Litter bag technique. Ash-free dry mass. | UV pass, UV block. Ash-free dry mass ANOVA. | Fiber analyzer | [67] | |
| USA | 486–639 | senescent | 3 | J. monosperma, P. edulis, P. deltoides | + | + | 0 | 0 | NA | NA | Litter bag technique ash-free dry mass. Decay rate coefficients, K: Mt = Moe-kt using linear regression. | Sunlight exposure (shade or ambient) ANOVA. | Elemental analyzer | [68] | |
| USA | 731 | Honey mesquite leaflets | 1 | P. glandulosa | 0 | 0 | 0 | NA | NA | NA | Litter bag technique modification with litter cages. Ash-free dry mass. Decay rate coefficients, K: Mt = M0e−kt using a 1) split-plot generalized linear model (GLM) and 2) ANCOVA model. | Step-wise multiple regression and Akaike information criterion corrected (AICc) model. | Elemental analyzer | [69] | |
| USA | 365 | Filter paper, thick sheets of basswood, leaves | 3 | Cellulosic filter paper, Tilia sp., P. glandulosa | 0 | 0 | 0 | NA | NA | NA | Litter bag technique. Ash-free dry mass. Decay rate coefficients, K: Mt = M0e−kt. | ANOVA | Elemental analyzer | [70] | |
| China | 913 | Leaves, stem | 3 | H. ammodendron, P. australis, T. aestivum | + | + | (+) * | NA | (+) * | NA | Litter bag technique. Ash-free dry mass. | UV-transparent aclar fluoropolymer films, UV filtering acrylic sheets. ANOVA and ANCOVA. | Elemental analyzer, colorimetric analysis, fiber analysis | [71] | |
| China | 875 | Leaves, stem | 5 | S. arabicus, E. oxyrrhynchum, S. passerine, S. santolinum, H. ammodendron | + | NA | NA | NA | + | NA | Litter bag technique. | UV block acrylic film, solar radiation transparent frame without film. Paired Student’s t-test was used to test UV treatment effects on those parameters across the entire dataset. The UV photodegradation effect (UVE) was calculated as the ratio of ((sunlight—UV block)/sunlight) decomposition rate (k), with data based on subplot. | Elemental analyzer, colorimetric analysis, fiber analysis | [72] | |
| USA | 365 * 10 | Senescent leaves, grass | 5 | B. eriopoda, O. hymenoides, J. monosperma, L.tridentata, B. gracilis, | + | + | + | NA | + | NA | Litter bag technique. Ash-free dry mass. | NA | Elemental analyzer | [73] | |
| Habitat | Country | Experiment Duration (Days) | Litter Types | Number of Litter Species | Litter (Plant) Species | Effect of Photodegradation (EP) | Methods | References | |||||||
| 1. Litter Mass Loss | 2. Effect on Nutrient Breakdown (ENB) | Evaluating Litter Decomposition | Evaluating Radiation Effect | Nutrient Analyses (C, N, P, Lignin and Phenolic) | |||||||||||
| C% Loss | N% Loss | P% Loss | Lignin Loss | Phenolic Content | |||||||||||
| Semi-Arid | Spain | 457 | Senescent leaves | 1 | S. tenacissima | + | NA | NA | NA | + | NA | Litter bag technique. A single exponential decay model ([74] was used for the decomposition constant k (yr−1) | ANOVA | Elemental analyzer, Fiber Analyzer | [30] |
| Spain | 457 | Senescent leaves | 2 | S. tenacissima, R. sphaerocarpa | + | NA | (0,+) * | NA | + | Litter bag technique. | Ash-free dry mass basis at the end of the experiment. ANOVA | Elemental analyzer, fiber analyzer | [75] | ||
| Argentina | 540 | Senescent leaves | 3 | S. speciosa, P. ligularis, S. humilis | + | + | NA | NA | NA | NA | Litter bag technique. ANOVA | NA | NA | [21] | |
| USA | 224 | Senesced leaflets | 2 | P. glandulosa, Prosopis velutina | + | + | ± | NA | NA | NA | Leaflets. Ash content was statically compared. Decomposition decay constants were estimated using linear regressions of intrans formed mass loss data and compared statically with Student’s t-test | Film ANOVA | Elemental analyzer | [76] | |
| Argentina | 420 | Leaves and fine woody stems | 2 | L. divaricata, N. tenuis | + | NA | 0 | NA | 0 | + | Litter bag technique | 2 mm nylon mesh blocked approximately 7%, 13%, and 10% of the PAR, UVB and UVA, respectively. | C concentration: dry digestion at 550 °C, total N: semi-micro Kjeldahl | [77] | |
| USA | 1095 | Senesced tissue | 3 | B. gracilis, S. comata, A. longiseta | + | NA | (0,+) * | NA | 0 | NA | Litter bag technique | Polycarbonate plastic (UV block) and acrylic (UV pass) | Elemental analyzer, fiber analyzer | [19] | |
| USA | 3650 | Leaves, conifer, graminoid | 6 | A. saccharum, D. Glauca, P. resinosa, Q. prinus, T. plicata, T. aestivum | + | + | + | NA | - | NA | DayCent-UV model Litter bag technique | The LIDET mass and nitrogen remaining data from the six different surface litter decay results from CPER site were used to parameterize UV litter decay parameters. We used a model optimization program to calculate the optimal values of the parameters. | NA | [78] | |
| USA | 2190 | Grass | 6 | B. distachyon, H. glabra, T. dubium, T. hirtum, D. volubile, E. botrys | 0 | + | + | NA | NA | NA | DayCent-UV model using a simplified Farquhar model of SIPNET | Modified model | NA | [79] | |
| China | 365 | Senescent leaves | 8 | P. villosa, P. centrasiaticum, C. pseudophragmites, S. psammophila, C. korshinskii, A. pungens, C. chinganicum, A. bracteata | + | + | (0,+,−) * | NA | NA | NA | Litter bag technique | UV transparent acrylic plastic, UV blocking polycarbonate plastic. ANOVA | Element analyzer | [80] | |
| China | 1020 | Senesced (stem, branches and twigs) finer woody | 4 | A. ordosica, C. korshinskii, H. laeve, S. psammophila | 0 * | NA | NA | NA | NA | NA | Litter bag technique the Archimedes’ principle of water displacement were used to measure. Initial and decomposed litter volumes were measured using | UV transparent acrylic plastic, UV blocking polycarbonate plastic. ANOVA | NA | [81] | |
| Chile | 150 | Green leaves | 2 | P. chilensis, P. pungens | + | (+, 0) * | 0 | NA | + | NA | Litter bag technique Biomass loss % = ((initial weight—final weight)/initial weight)∗100. | Plexiglas UV transmitting film, Mylar- type UV blocking film ANOVA | Elemental analyzer | [82] | |
| Israel, Italy, Spain | 276 | Fresh, standing dead material | 3 | A. sterilis, S. prolifera, C. villosa | 0 | NA | NA | NA | NA | NA | No litter bags were used | Multiple linear regression model | NA | [83] | |
| Israel | NA | Standing dead plant material, freshly fallen litter | 6 | A. sterilis, S. prolifera, S. officinale, D. pentaphyllum, H. italicum, C. monspeliensis | + | NA | 0 | NA | + | NA | Litter bag technique | No screens or frames (control), transparent polyethylene screens, solar UV and PAR blocked by 179 chrome or orange filter ANOVA | NA | [84] | |
| China | 180 | Senescent leaves | 2 | P. sylvestris var. mongolica, P. Xiaozhuanica | + | NA | ± * | NA | ± * | NA | Litter boxes | Acrylic UV pass boxes, polycarbonate UV block boxes ANOVA | Continuous-flow auto analyzer | [85] | |
| Argentina | 365 | Senesced | 1 | P. nigra | + | NA | 0 | − | + | NA | Litter bag technique | Polyethylene transparent film filter, orange block filter | Elemental analyzer and fiber analyzer | [86] | |
| USA | 320 | Leaves | 1 | P. velutina | 0 | 0 | + | NA | NA | NA | Litter bag technique. Ash-free dry mass | UV transparent acrylic plastic, UV blocking polycarbonate plastic. ANOVA | Elemental analyzer | [60] | |
| USA | 363 | Senescent leaves | 2 | Tamarix Sp., L. latifolium | 0 | NA | 0 * | + | NA | NA | Litter bag technique | No cover plastic (control), aclar UV transparent plastic film and mylar UV block plastic film. Slopes of the linear regressions. ANOVA | CN analyzer | [87] | |
| USA | 157 | Grass | 1 | E. glaucus | NA | + | + | NA | + | NA | Litter bag technique | Clear plastic film, aluminum foil. | Gas chromatograph | [88] | |
| Habitat | Country | Experiment Duration (Days) | Litter Types | Number of Litter Species | Litter (Plant) Species | Effect of Photodegradation (EP) | Methods | References | |||||||
| 1. Litter Mass Loss | 2. Effect on Nutrient Consumption (ENC) | Evaluating Litter Decomposition | Evaluating Radiation Effect | Nutrient Analyses (C, N, P, Lignin and Phenolic) | |||||||||||
| C% Loss | N% Loss | P% Loss | Lignin Loss | Phenolic Content | |||||||||||
| Temperate | Argentina | 100 | Senescent leaves | 23 | A. auracana, B. pictus, C. acanthoides, C. culeou, D. glomerata, F. americana, G. max, H. annuus, L. multiflorum, M. boaria, M. spinosum, N. obliqua, N. dombeyi, N. nervosa, N. antarctica, P. quadrifarium, P. ponderosa, P. ligularis, P. nigra, S. scoparium, S. speciosa, T. aestivum, Z. mays | + | + | NA | NA | + | NA | Ash-free dry mass. | Three light treatments [(i) >290 nm; (ii) >400 nm; (iii) >550 nm] meta analyses. | Spectrophotometer, fiber analyzer | [32] |
| Sweden | 300–365 | Senescent leaves | 1 | V. myrtillus | + | NA | NA | NA | NA | NA | Litter cups. | Direct and indirect effect of UVB. ANOVA. | NA | [23] | |
| USA | 72 | Leaf, stem of grass, forb | 2 | C. vesicaria, L. multiflorum | + | + | (0, +) * | NA | + | NA | Polyester mesh pulled tight across the top of 10 cm diameter PVC rings. | NA | Elemental analyzer | [89] | |
| The Netherlands | 730 | senesced leaf | 2 | C. arenaria, C. epigejos | 0 | 0 | 0 | 0 | 0 | 0 | Litter bag technique. Student’s t-test, | Bivariate statistical tests. A Hotelling test. | Elemental analyzer, calorimetrically | [90] | |
| USA | 300 | Senescent leaves | 2 | Q. rubra L., A. rubrum L. | 0 | + | ± * | NA | ± * | + | Mesh | UVB-transmit acetate film, UVB-block polyester film a linear mixed effects (LME) model. | Elemental analyzer, gas chromatography–mass spectrometry, thermochemolytic analysis | [91] | |
| Norway, Sweden, the Netherlands, Greece | 420 | Leaves | 1 | B. pubescens | (+, 0) * | NA | (+, 0) * | NA | 0 | NA | Mesh ANOVA | ANOVA. | Elemental analyzer | [92] | |
| Argentina | 306 | Senescent leaves | 1 | G. magellanica | (−) * | NA | 0 | 0 | 0 | NA | Litter bag technique. | Aclar UV transparent plastic film and mylar UV block plastic film. UV-B-absorbing compounds were expressed as absorbance units (UA) per milligram of dry mass diluted in 1 mL of extract. | Chemical analysis | [33] | |
| France | 300 | Leaves | 3 | F. excelsior, F. sylvatica, Q. robur | (+) * | + | - | NA | NA | NA | Litter bag technique. | Five different filters. NA | Elemental analyzer | [93] | |
| France | 180 | Senescent leaves | 1 | Fagus sylvatica L. | (−) * | 0 | + | NA | NA | NA | Litter bag technique. | Six different UV treatments were used. | Elemental analyzer | [94] | |
| UK | 365 | Leaves | 1 | F. excelsior | + | − | − | NA | 0 | NA | Litter bag technique. | Four treatments: all UV, UVA only, no UV and ambient UV (control). ANOVA. | Elemental analyzer | [95] | |
| Finland | 186 | Senescent leaves | 2 | B. pendula, F. sylvatica | + | − | 0 | NA | NA | 0 | NA. | Four filter types: dark, no-UVA/Blue, no-UVA, full-spectrum). A split-plot mixed-model ANOVA. | Element analyzer, HPLC | [96] | |
| Japan | 230 | Senescent leaves | 12 | F. camtschatica, P. trilobata, L. obtusiloba Blume, S. chinensis, H. sieboldiana, F. japonica, V. coignetiae, L. bicolor Turcz. var. bicolor, A. carpinifolium, F. crenata Blume, B. platyphylla, Q. crispula Blume | + | + | NA | NA | NA | NA | Litter bag technique. | Six treatments: full-spectrum, no UVB, no UV, no UV/blue, no UV/blue-green and dark treatment. ANOVA. Benjamini–Hochberg (BH) method used to calculate the p-value. | Elemental analyzer | [25] | |
| Habitat | Country | Experiment Duration (Days) | Litter Types | Number of Litter Species | Litter (Plant) Species | Effect of Photodegradation (EP) | Methods | References | |||||||
| 1. Litter Mass Loss | 2. Effect on Nutrient Consumption (ENC) | Evaluating Litter Decomposition | Evaluating Radiation Effect | Nutrient Analyses (C, N, P, Lignin and Lhenolic) | |||||||||||
| C% Loss | N% Loss | P% Loss | Lignin Loss | Phenolic Content | |||||||||||
| Subtropical | Australia | 180 | Leaves | 3 | E. camaldulensis subsp. refulgens, A. coriacea, M. argentea | NA | + | + | NA | NA | NA | Litter bag technique | NA | Elemental analyzer | [97] |
| China | 730 | Needle and broadleaf foliar litter | 6 | P. massoniana, C.iliate, C. lanceolata, C. camphora, T. iliate, Q. acutissima | + | NA | NA | NA | NA | NA | Litter bag technique. Mass loss calculated as follows: C = (Mt−1− Mt)/(M0−Mt) 100 | NA | NA | [98] | |
| Brazil | 300 | Senescent leaves | 1 | T. sellowiana | + | + | 0 | NA | 0 | NA | Litter bag technique. Ash-free dry mass. Three decomposition models were evaluated: the single evaluation model, the double exponential, and the exponential deceleration. | Three treatments using plastic film: aclar transparent films, Mylar-type blocking film, and Maylar type blocking film under shade. | Elemental analyzer | [59] | |
| China | 365 | Needles | 2 | C. lanceolata, P. massoniana | + | NA | 0/+ | + | 0 | NA | Litter bag technique. Exponential decay model. | Two treatments: ambient and elevated UV-B exposure. ANOVA. | CN analyzer, photometric | [99] | |
| China | 600 | leaves | 1 | P. pubescens | 0 | + | − | 0 | 0 | NA | Litter bag technique. Differences between mass at the start of the experiment and mass at each sampling time were used to calculate the rate of leaf litter decomposition. | Elevated UV-B radiation, elevated UV-B radiation, and elevated N deposition (UV-B + N) and control group. ANOVA. | Analysis | [100] | |
| China | 720 | Coarse woody debris | 2 | C. lanceolata, C. camphora | + | NA | NA | NA | NA | NA | 24 segments of coarse woody debris. | NA | Elemental analyzer | [101] | |
| Habitat | Country | Experiment Duration (Days) | Litter Types | Number of Litter Species | Litter (Plant) Species | Effect of Photodegradation (EP) | Methods | References | |||||||
| 1. Litter Mass Loss | 2. Effect on Nutrient Consumption (ENC) | Evaluating Litter Decomposition | Evaluating Radiation Effect | Nutrient Analyses (C, N, P, Lignin and Phenolic) | |||||||||||
| C% Loss | N% Loss | P% Loss | Lignin Loss | ||||||||||||
| Laboratory Conditions | India | 90 | Stem, seed, grass | 3 | A. calamus, O. tenuiflorum, C. citratus | (+/0 *) | (−,0 *) | (−,0 *) | NA | NA | (−,0 *) | Litter bag technique. | Three treatments: one ambient UVB (control); two supplemental UV-B exposure at sUV1 (±1.8 kJ m−2 d−1) and 3- at sUV2 (±3.6 kJ m−2 d-1). ANOVA. | Different chemical analysis methods | [102] |
| USA | 70 | Leaf and stem of dead litter, freshly fallen oak litter, grass litter | 5 | A. gerardii, B. eriopoda, B. gracilis, Q. ellipsoidalis, Q. macrocarpa | 0 | 0 | 0 | NA | NA | NA | Microcosms. | UV lamp. ANOVA. | Elemental analyzer, fiber analyzer | [28] | |
| USA | 426 | Senescent leaves | 3 | P. velutina, C. dactylon, A. deltoidea | + | + | NA | NA | NA | NA | Incubation tube. | Polychromatic solar radiation broadband radiation sensors were used to monitor irradiance. Nonlinear least-squares regression. | Spectral weighting functions (WFs) for the photochemical emission of CO2 | [103] | |
| UK | 289 | Senescent leaves | 1 | M. giganteus | + | 0 | 0 | NA | NA | NA | Dry plant material was placed in foil trays. | The growth chamber light was supplemented with 4 Arcadia 3D reptile lamp, 90 cm. Student’s t-test. ANOVA. | NA | [36] | |
| Siberia | 14 | Peat | 3 | Scheuchzeria peat, Sph. Fuscum peat, Sph. Papillosum peat | + | + | NA | NA | 0 | NA | Bags with cotton on one side for heat exchange followed by drying and storage in jar. | UV transparent Alcal plastic film. Student’s t-test. | Elemental analyzer | [104] | |
| USA | 180 | Senescent leaf | 2 | P. edulis, J. monosperma | 0 | 0 | 0 | NA | NA | ± * | Microcosms. | Beneath fluorescent lamps containing UV-A and UV-B tubes. ANOVA. | Elemental analyzer | [22] | |
| Sweden | 62 | Senescent leaves | 1 | V. uliginosum | 0 | + | + | + | − | + | Before and after the experiment, the litter in each microcosm was air-dried at room temperature and weighed. The relative mass loss (%) was calculated. | UVB indirect effect: CO2 release using microcosms. ANOVA. | Allen 1989 methods | [23] | |
| New Zealand | 60 | Pine and ryegrass | 2 | P. radiata, L. perenne cv Nui | 0 | NA | NA | NA | − | NA | Petri dishes. | Fluorescent UV lamps direct weight loss. | Acid detergent fiber sulfuric acid procedure followed by gravimetric determination of the residues | [105] | |
| New Zealand | 59 | Senescent, fresh foliage leaves, grass, needles, humus | 3 | P. radiate, L. scopariumm, H. lanatus L | − | NA | NA | NA | NA | NA | Polystyrene Petri dishes. | Six UV-emitting fluorescent lights. Litter materials were exposed to five levels (0, 30, 56, 75, and 100%) of incident UV-B radiation using metal mesh screens that allowed different levels of radiation transmission. ANOVA. | NA | [106] | |
| USA | 365 | Senescent litter lying | 1 | B. diandrus | + | − | + | NA | 0 | NA | NA. | Steel frames with plastic louvers that either pass or block UV radiation. Student’s t-test. | Elemental analyzer | [107] | |
| USA | 128 | Senescent leaves | 3 | A. fatua, B. diandrus, Q. douglasii | + | NA | NA | NA | 0 | NA | Mass loss. | Envelops made of UV transparent polychlorotrifluoroethylene film. Student’s t-test. | Elemental analyzer | [27] | |
| China | 228 | Straw | 1 | O. sativa (rice) | 0 * | 0 * | NA | NA | NA | 0 * | Litter bag technique (aluminum mesh bags). | Four treatments: an ambient UVB under dry and wet condition and reduced UVB under dry and wet conditions. A general linear model. | Elemental analyzer | [108] | |
| UK | 448 | Leaves | 1 | Q. robur | 0 | (− *) | 0 * | 0 * | (+ *) | NA | Mass loss. | UV lamp. Two-way generalized linear model and a Tukey’s multiple comparisons test multiple comparisons test a split-plot. ANOVA. | Flow colorimetry (for N and P), elemental analyzer (for carbon) | [109] | |
| UK | 244 | Leaves | 1 | Q. robur L. | + | + | + | + | + | + | Litter bag technique. | UV lamp. ANOVA and a Tukey’s multiple comparisons test. | NA | [110] | |
| Argentina | 883 | Straw, leaves | 1 | H. vulgare L. (barley) | + | + | + | − | − | NA | Litter bag technique. Ash-free dry mass. | Two treatments: under near-ambient UV-B and under reduced UV-B conditions. ANOVA. | NA | [62] | |
| USA | 200 | Leaves, stems, reproductive | 2 | 2 Sorghum bicolor cultivars | + | + | ± * | NA | − | NA | Litter bag technique. | Aclar UV transparent plastic film. ANOVA. | Elemental analyzer, fiber analyzer | [111] | |
| USA | 24 | Leaves | 1 | P. tremuloides | ± * | NA | − | NA | NA | NA | Container was used in a greenhouse. | Microcosms under three UVB treatments: no UVB, ambient UVB and elevated UVB. A biological spectral weighting function (BSWF) was used. | Elemental analyzer | [31] | |
| China | 365 | Leaves | 2 | C. camphora, C. glauca | + | NA | + | NA | NA | NA | Litter bag technique. Exponential decay model. | ANOVA. | NA | [112] | |
| China | 450 | Straw | 1 | O. sativa (rice) | + | + | + | NA | − | + | Pots were placed in a greenhouse. | Two UVB treatments: enhanced UVB radiation and ambient UV radiation using aclar UV transparent plastic film and fluorescent UV-B lamps. ANOVA. | Elemental analyzer | [113] | |
| Effect on Mass Loss/Climate | Positive (n) | Positive (%) | Negative (n) | Negative (%) | No Effect (n) | No Effect (%) |
|---|---|---|---|---|---|---|
| Arid (n = 15) | 12 | 80 | 0 | 0 | 3 | 20 |
| Semi-arid (n = 19) | 15 | 79 | 0 | 0 | 3 | 16 |
| Temperate (n = 11) 12 | 6 | 55 | 2 | 18 | 3 | 27 |
| Subtropical (n = 9) 6 | 6 | 67 | 0 | 0 | 2 | 22 |
| Laboratory conditions (n = 21) 19 | 12 | 57 | 2 | 10 | 7 | 33 |
| * Total (n = 75) | 51 | 68 | 4 | 5 | 18 | 24 |
| Effect on Mass Loss/Climate | C Loss Positive (n) | C Loss Positive (%) | C Loss Negative (n) | C Loss Negative (%) | C Loss No Effect (n) | C Loss No Effect (%) | C Loss NA |
| Arid | 5 | 45 | 1 | 9 | 1 | 9 | 4 |
| Semi-arid | 6 | 33 | 0 | 0 | 2 | 11 | 10 |
| Temperate | 5 | 50 | 0 | 0 | 2 | 20 | 3 |
| Subtropical | 4 | 44 | 2 | 22 | 0 | 0 | 3 |
| Laboratory conditions | 7 | 35 | 3 | 15 | 5 | 25 | 5 |
| Total | 27 | 40 | 6 | 9 | 10 | 15 | 25 |
| Effect on Mass Loss/Climate | N Loss Positive (n) | N Loss Positive (%) | N Loss Negative (n) | N Loss Negative (%) | N Loss no Effect (n) | N Loss no Effect (%) | N Loss NA |
| Arid | 6 | 50 | 1 | 8 | 3 | 25 | 2 |
| Semi-arid | 8 | 35 | 3 | 13 | 8 | 35 | 4 |
| Temperate | 4 | 31 | 2 | 15 | 4 | 31 | 3 |
| Subtropical | 3 | 30 | 2 | 20 | 3 | 30 | 2 |
| Laboratory conditions | 7 | 33 | 3 | 14 | 5 | 24 | 6 |
| Total | 28 | 35 | 11 | 14 | 23 | 29 | 17 |
| Effect on Mass Loss/Climate | Lignin Loss Positive (n) | Lignin Loss Positive (%) | Lignin Loss Negative (n) | Lignin Loss Negative (%) | Lignin Loss no Effect (n) | Lignin Loss no Effect (%) | Lignin Loss NA |
| Arid | 6 | 55 | 0 | 0 | 1 | 9 | 4 |
| Semi-arid | 7 | 39 | 2 | 11 | 2 | 11 | 7 |
| Temperate | 3 | 27 | 1 | 9 | 3 | 27 | 4 |
| Subtropical | 0 | 0 | 0 | 0 | 4 | 44 | 5 |
| Laboratory conditions | 2 | 11 | 5 | 26 | 3 | 16 | 9 |
| Total | 18 | 26 | 8 | 12 | 13 | 19 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.B.; Al-Hadidi, S.H.; Erfanian, M.B.; Yahia, M.N.D.; Mullungal, M.N.; Alsafran, M.; Bai, Y.; Alatalo, J.M. Photodegradation and Its Effect on Plant Litter Decomposition in Terrestrial Ecosystems: A Systematic Review. Soil Syst. 2023, 7, 6. https://doi.org/10.3390/soilsystems7010006
Hussain MB, Al-Hadidi SH, Erfanian MB, Yahia MND, Mullungal MN, Alsafran M, Bai Y, Alatalo JM. Photodegradation and Its Effect on Plant Litter Decomposition in Terrestrial Ecosystems: A Systematic Review. Soil Systems. 2023; 7(1):6. https://doi.org/10.3390/soilsystems7010006
Chicago/Turabian StyleHussain, Mohammed Bakr, Sara H. Al-Hadidi, Mohammad Bagher Erfanian, Mohamed Nejib Daly Yahia, Muhammed Nayeem Mullungal, Mohammed Alsafran, Yang Bai, and Juha M. Alatalo. 2023. "Photodegradation and Its Effect on Plant Litter Decomposition in Terrestrial Ecosystems: A Systematic Review" Soil Systems 7, no. 1: 6. https://doi.org/10.3390/soilsystems7010006
APA StyleHussain, M. B., Al-Hadidi, S. H., Erfanian, M. B., Yahia, M. N. D., Mullungal, M. N., Alsafran, M., Bai, Y., & Alatalo, J. M. (2023). Photodegradation and Its Effect on Plant Litter Decomposition in Terrestrial Ecosystems: A Systematic Review. Soil Systems, 7(1), 6. https://doi.org/10.3390/soilsystems7010006