Effect of Natural Phytohormones on Growth, Nutritional Status, and Yield of Mung Bean (Vigna radiata L.) and N Availability in Sandy-Loam Soil of Sub-Tropics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Site and Climate Conditions
2.2. Experiment Design and Treatments
2.3. Determination of Growth Traits
2.4. Determination of Agronomic Traits
2.5. Estimation of Photosynthetic Traits
2.6. Determination of Total Carbohydrates, Nitrogen, and Protein Contents
2.7. Estimation of Endogenous Growth Regulators
2.8. Statistical Analysis
3. Results
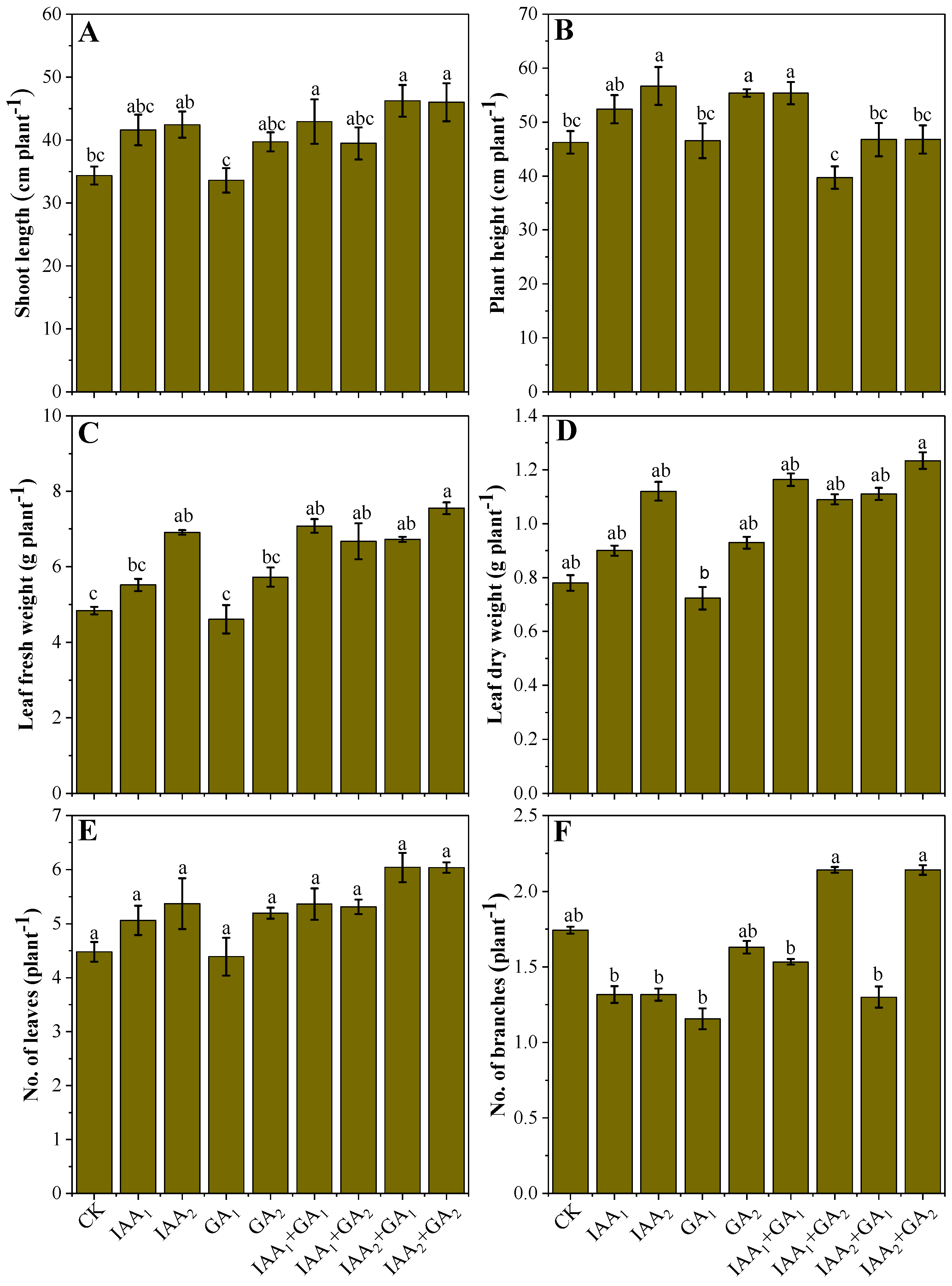
3.1. Influence of Natural Phytohormones on Mung Bean Growth
3.2. Influence of Natural Phytohormones on Yield and Yield Related Parameters
3.3. Influence of Natural Phytohormones on Photosynthetic Pigments
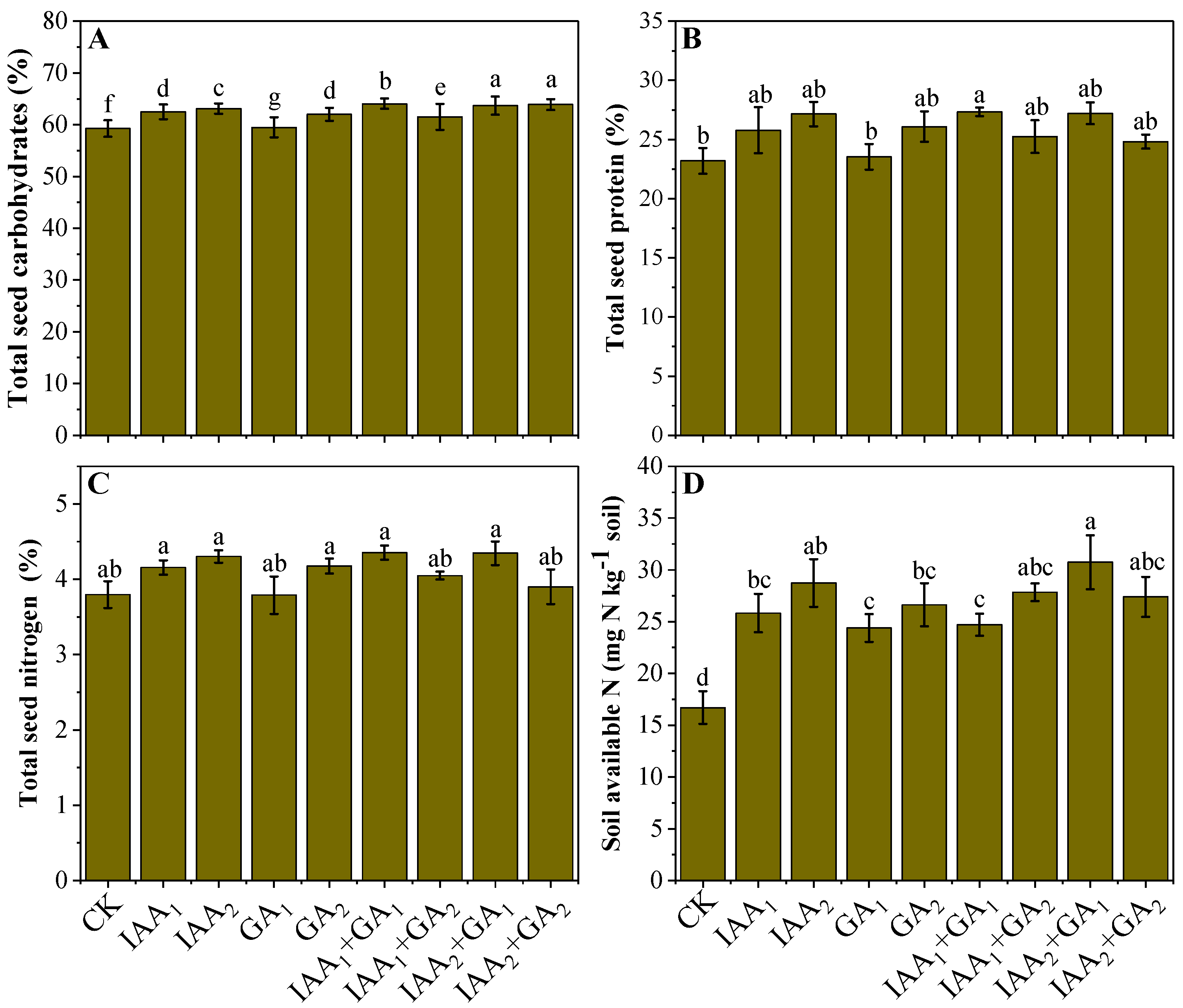
3.4. Influence of Natural Phytohormones on Nutritional Status of Mung Bean Seeds
3.5. Influence of Natural Phytohormones on Soil N Availability
3.6. Influence of Natural Phytohormones on Endogenous Phytohormone Production
3.7. Hierarchical Agglomerative Cluster Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konuma, H. Status and Outlook of Global Food Security and the Role of Underutilized Food Resources: Sago Palm. In Sago Palm: Multiple Contributions to Food Security and Sustainable Livelihoods; Ehara, H., Toyoda, Y., Johnson, D., Eds.; Springer: Singapore, 2018; pp. 3–16. [Google Scholar]
- Hilger, T.; Lewandowski, I.; Winkler, B.; Ramsperger, B.; Kageyama, P.; Colombo, C. Seeds of Change—Plant Genetic Resources and People’s Livelihoods. In Agroecology; IntechOpen: London, UK, 2015. [Google Scholar]
- Sehrawat, N.; Yadav, M.; Sharma, A.K.; Kumar, V.; Bhat, K.V. Salt Stress and Mungbean [Vigna radiata (L.) Wilczek]: Effects, Physiological Perspective and Management Practices for Alleviating Salinity. Arch. Agron. Soil Sci. 2019, 65, 1287–1301. [Google Scholar] [CrossRef]
- Abbas, G.; Ahmed, A.; Amer, M.; Abbas, Z.; Rehman, M.; Hussain, A.; Khan, G.A. Impact of Pre-Emergence Herbicides for the Control of Weeds in Chick Pea (Cicer arietinum L.) under Hot Arid Climate. J. Bioresour. Manag. 2016, 3, 7. [Google Scholar]
- Noble, T.J.; Tao, Y.; Mace, E.S.; Williams, B.; Jordan, D.R.; Douglas, C.A.; Mundree, S.G. Characterization of Linkage Disequilibrium and Population Structure in a Mungbean Diversity Panel. Front. Plant Sci. 2018, 8, 2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mubarak, A.E. Nutritional Composition and Antinutritional Factors of Mung Bean Seeds (Phaseolus aureus) as Affected by Some Home Traditional Processes. Food Chem. 2005, 89, 489–495. [Google Scholar] [CrossRef]
- Rani, S.; Schreinemachers, P.; Kuziyev, B. Mungbean as a Catch Crop for Dryland Systems in Pakistan and Uzbekistan: A Situational Analysis. Cogent Food Agric. 2018, 4, 1499241. [Google Scholar] [CrossRef]
- Raina, S.K.; Govindasamy, V.; Kumar, M.; Singh, A.K.; Rane, J.; Minhas, P.S. Genetic Variation in Physiological Responses of Mungbeans (Vigna radiata (L.) Wilczek) to Drought. Acta Physiol. Plant. 2016, 38, 263. [Google Scholar] [CrossRef]
- FAO; WFP; IFAD; UNICEF; WHO. The State of Food Security and Nutrition in the World 2017. Building Resilience for Peace and Food Security; FAO: Rome, Italy, 2017. [Google Scholar]
- Ogunkunle, A. Management of Nigerian Soil Resources: An Imperative for Sustainable Development; Ibadan University Press: Ibadun, Nigeria, 2016. [Google Scholar]
- Vanzetti, D.; Petersen, E.H.; Rani, S. Economic Review of the Pulses Sector and Pulses-Related Policies in Pakistan. In Proceedings of the Mid-Project Workshop of ACIAR Project ADP/2016/140 “How Can Policy Reform Remove Constraints and Increase Productivity in Pakistan?”, Islamabad, Pakistan, 3 April 2017; Volume 3. [Google Scholar]
- Ullah, A.; Shah, T.M.; Farooq, M. Pulses Production in Pakistan: Status, Constraints and Opportunities. Int. J. Plant Prod. 2020, 14, 549–569. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.K.; El-Mageed, T.A.A.; El-Yazal, M.A.S.; Ali, E.F.; Hassan, F.A.S.; Abdelkhalik, A. Exogenous Gibberellic Acid or Dilute Bee Honey Boosts Drought Stress Tolerance in Vicia Faba by Rebalancing Osmoprotectants, Antioxidants, Nutrients, and Phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef]
- Sadiq, R.; Maqbool, N.; Hussain, M.; Tehseen, S.; Naseer, M.; Rafique, T.; Zikrea, A.; Naqve, M.; Mahmood, A.; Javaid, A.; et al. Boosting Antioxidant Defense Mechanism of Mungbean with Foliar Application of Gibberellic Acid to Alleviate Cadmium Toxicity. Plant Physiol. Rep. 2021, 26, 741–748. [Google Scholar] [CrossRef]
- Malik, Z.; Jamil, M.; Abassi, G.H.; Nafees, M.; Rafey, M.; Kamran, M. Biochar and Fly Ash Role in Improving Mechanical and Physical Properties of Vertisol. Sarhad J. Agric. 2017, 33, 151–161. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Huang, L.; Riaz, M.; Bashir, S.; Mustafa, A.; Abbasi, G.H.; Xue, B.; Ali, U. Ameliorative Effects of Biochar on Rapeseed (Brassica napus L.) Growth and Heavy Metal Immobilization in Soil Irrigated with Untreated Wastewater. J. Plant Growth Regul. 2020, 39, 266–281. [Google Scholar] [CrossRef]
- Kamran, M.; Huang, L.; Nie, J.; Geng, M.; Lu, Y.; Liao, Y.; Zhou, F.; Xu, Y. Effect of Reduced Mineral Fertilization (NPK) Combined with Green Manure on Aggregate Stability and Soil Organic Carbon Fractions in a Fluvo-Aquic Paddy Soil. Soil Tillage Res. 2021, 211, 105005. [Google Scholar] [CrossRef]
- Chen, G.; Shi, L. A Multi-Element Mineral Conditioner: A Supplement of Essential Cations for Red Soil and Crops Growth. Clean 2016, 44, 1690–1699. [Google Scholar] [CrossRef]
- Chen, G.; Shi, L. Removal of Cd(II) and Pb(II) Ions from Natural Water Using a Low-Cost Synthetic Mineral: Behavior and Mechanisms. RSC Adv. 2017, 7, 43445–43454. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Shah, K.J.; Shi, L.; Chiang, P.C.; You, Z. Red Soil Amelioration and Heavy Metal Immobilization by a Multi-Element Mineral Amendment: Performance and Mechanisms. Environ. Pollut. 2019, 254, 112964. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Ding, C.; Guo, N.; Ding, M.; Zhang, H.; Kamran, M.; Zhou, Z.; Zhang, T.; Wang, X. Polymer-Coated Manganese Fertilizer and Its Combination with Lime Reduces Cadmium Accumulation in Brown Rice (Oryza sativa L.). J. Hazard. Mater. 2021, 415, 125597. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duca, D.R.; Glick, B.R. Indole-3-Acetic Acid Biosynthesis and Its Regulation in Plant-Associated Bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.M.; Ortiz-García, P.; Moya-Cuevas, J.; Lehmann, T.; Sánchez-Parra, B.; Björk, R.G.; Karim, S.; Amirjani, M.R.; Aronsson, H.; Wilkinson, M.D.; et al. Endogenous Indole-3-Acetamide Levels Contribute to the Crosstalk between Auxin and Abscisic Acid, and Trigger Plant Stress Responses in Arabidopsis. J. Exp. Bot. 2021, 72, 459–475. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Auxin: The Growth Hormone. Plant Physiol. 2006, 4, 468–507. [Google Scholar]
- Hedden, P. The Current Status of Research on Gibberellin Biosynthesis. Plant Cell Physiol. 2020, 61, 1832–1849. [Google Scholar] [CrossRef] [PubMed]
- Keykha, M.; Ganjali, H.R.; Mobasser, H.R. Effect of Salicylic Acid and Gibberellic Acid on Some Characteristics in Mungbean (Vigna radiata L.). Int. J. Biosci. (IJB) 2014, 5, 70–75. [Google Scholar] [CrossRef]
- Tan, H.; Man, C.; Xie, Y.; Yan, J.; Chu, J.; Huang, J. A Crucial Role of GA-Regulated Flavonol Biosynthesis in Root Growth of Arabidopsis. Mol. Plant 2019, 12, 521–537. [Google Scholar] [CrossRef] [Green Version]
- Camara, M.C.; Vandenberghe, L.P.S.; Rodrigues, C.; de Oliveira, J.; Faulds, C.; Bertrand, E.; Soccol, C.R. Current Advances in Gibberellic Acid (GA3) Production, Patented Technologies and Potential Applications. Planta 2018, 248, 1049–1062. [Google Scholar] [CrossRef]
- Rahman, M.; Khan, A.; Hasan, M.; Banu, L.; Howlader, M. Effect of Foliar Application of Gibberellic Acid on Different Growth Contributing Characters of Mungbean. Progress. Agric. 2018, 29, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Fahad, S.; Adnan, M.; Ali, M.; Rana, M.S.; Kamran, M.; Ali, Q.; Hashem, I.A.; Bhantana, P.; Ali, M.; et al. Foliar Application of Gibberellic Acid Endorsed Phytoextraction of Copper and Alleviates Oxidative Stress in Jute (Corchorus capsularis L.) Plant Grown in Highly Copper-Contaminated Soil of China. Environ. Sci. Pollut. Res. 2020, 27, 37121–37133. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, G.; Chen, Y.; Gao, J.; Sun, Y.R.; Sun, M.F.; Chen, J.P. Exogenous Application of Gibberellic Acid and Ascorbic Acid Improved Tolerance of Okra Seedlings to NaCl Stress. Acta Physiol. Plant 2019, 41, 93. [Google Scholar] [CrossRef]
- Ahmad Dar, T.; Uddin, M.; Khan, M.M.A.; Ali, A.; Hashmi, N.; Idrees, M. Cumulative Effect of Gibberellic Acid and Phosphorus on Crop Productivity, Biochemical Activities and Trigonelline Production in Trigonella foenum-graecum L. Cogent Food Agric. 2015, 1, 995950. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- el Karamany, M.F.; Sadak, M.S.; Bakry, B.A. Improving Quality and Quantity of Mungbean Plant via Foliar Application of Plant Growth Regulators in Sandy Soil Conditions. Bull. Natl. Res. Cent. 2019, 43, 61. [Google Scholar] [CrossRef]
- Sanjida, T.; Alam, M.J.; Rahman, M.M.; Islam, M.S.; Sikdar, M.S.I. Response of Mungbean Growth and Yield to GA3 Rate and Time of Application. Asian J. Crop Soil Sci. Plant Nutr. 2020, 1, 28–36. [Google Scholar] [CrossRef]
- Ghani, M.A.; Mushtaq, A.; Ziaf, K.; Ali, B.; Jahangir, M.M.; Khan, R.W.; Khan, I.; Azam, M.; Noor, A. Exogenously Applied GA3 Promotes Plant Growth in Onion by Reducing Oxidative Stress under Saline Conditions. Tarim Bilim. Derg. 2021, 27, 122–128. [Google Scholar] [CrossRef]
- Ahmad, F.; Kamal, A.; Singh, A.; Ashfaque, F.; Alamri, S.; Siddiqui, M.H.; Khan, M.I.R. Seed Priming with Gibberellic Acid Induces High Salinity Tolerance in Pisum Sativum through Antioxidants, Secondary Metabolites and up-Regulation of Antiporter Genes. Plant Biol. 2021, 23, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Karamany, M.F.E.; Sadak, M.S.; Bakry, B.A. Synergistic Effect of Indole Acetic Acid and Gibberellic Acid on Mung Bean Grown under Sandy Soil Conditions. J. Appl. Sci. 2019, 19, 718–724. [Google Scholar] [CrossRef]
- Nandan, R.; Yadav, R.; Singh, S.P.; Singh, A.K.; Singh, A. Effect of Seed Priming with Plant Growth Regulators on Growth, Biochemical Changes and Yield of Mung Bean (Vigna radiata L.). Int. J. Chem. Stud. 2021, 9, 2922–2927. [Google Scholar] [CrossRef]
- Islam, M.S.; Hasan, M.K.; Islam, B.; Renu, N.A.; Hakim, M.A.; Islam, M.R.; Chowdhury, M.K.; Ueda, A.; Saneoka, H.; Ali Raza, M.; et al. Responses of Water and Pigments Status, Dry Matter Partitioning, Seed Production, and Traits of Yield and Quality to Foliar Application of GA3 in Mungbean (Vigna radiata L.). Front. Agron. 2021, 2, 596850. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophyll and Carotenoid Determination: Pigments of Photosynthetic Biomembranes. Method. Ensymol. 1987, 8, 350–382. [Google Scholar]
- Miller, L.; Houghton, J.A. The MicroKjeldahl Determination of the Nitrogen Content of Amino Acids and Proteins. J. Biol. Chem. 1945, 169, 373–383. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shindy, W.W.; Smith, O.E. Identification of Plant Hormones from Cotton Ovules. Plant Physiol. 1975, 55, 550–554. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Hilgenberg, W. Isomers of Zeatin and Zeatin Riboside in Clubroot Tissue: Evidence for Trans-zeatin Biosynthesis by Plasmodiophora Brassicae. Physiol. Plant 1986, 66, 245–250. [Google Scholar] [CrossRef]
- Chapman, E.J.; Estelle, M. Mechanism of Auxin-Regulated Gene Expression in Plants. Annu. Rev. Genet. 2009, 43, 265–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Su, W.; Kamran, M.; Ahmad, I.; Meng, X.; Wu, X.; Javed, T.; Han, Q. Foliar Application of Melatonin Delay Leaf Senescence in Maize by Improving the Antioxidant Defense System and Enhancing Photosynthetic Capacity under Semi-Arid Regions. Protoplasma 2020, 257, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Ahmar, S.; Kamran, M.; Malik, Z.; Ali, A.; Riaz, M.; Abbasi, G.H.; Khan, M.; Sohail, A.B.; Rizwan, M. Abscisic Acid Signalling Reduced Transpiration Flow, Regulated Na+ Ion Homeostasis and Antioxidant Enzyme Activities to Induce Salinity Tolerance in Wheat (Triticum aestivum L.) Seedlings. Environ. Technol. Innov. 2021, 24, 101808. [Google Scholar] [CrossRef]
- Szalai, G.; Horgosi, S.; Soós, V.; Majláth, I.; Balázs, E.; Janda, T. Salicylic Acid Treatment of Pea Seeds Induces Its de Novo Synthesis. J. Plant Physiol. 2011, 168, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Islam, N.; Ali, M. Growth and Yield of Summer Tomato as Influenced by Plant Growth Regulators. Int. J. Sustain. Agric. 2013, 5, 25–28. [Google Scholar] [CrossRef]
- Hossain, M.E.; Amin, R.; Sani, M.N.H.; Ahamed, K.U.; Hosain, M.T.; Nizam, R. Impact of Exogenous Application of Plant Growth Regulators on Growth and Yield Contributing Attributes of Summer Tomato. Int. J. Plant Soil Sci. 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Naeem, M.; Bhatti, I.; Ahmad, R.H.; Ashraf, M.Y. Effect of Some Growth Hormones (GA3, IAA and Kinetin) on the Morphology and Early or Delayed Initiation of Bud of Lentil (Lens culinaris Medik). Pak. J. Bot. 2004, 36, 801–809. [Google Scholar]
- Sadak, M.S.; Dawood, M.G.; Bakry, B.A.; El-Karamany, M.F. Synergistic Effect of Indole Acetic Acid and Kinetin on Performance, Some Biochemical Constituents and Yield of Faba Bean Plant Grown under Newly Reclaimed Sandy Soil. World J. Agric. Sci. 2013, 9, 335–344. [Google Scholar]
- Atteya, A.K.G.; Genaidy, E.A.E.; Zahran, H.A. Chemical Constituents and Yield of Simmondsia Chinensis Plants as Affected by Foliar Application of Gibberellic Acid and Zinc Sulphate. Biosci. Res. 2018, 15, 1528–1541. [Google Scholar]
- Hanaa, H.; Safaa, A. Foliar Application Foliar Application of IAA at Different Growth Stages and Their Influenced on Growth and Productivity of Bread Wheat (Triticum aestivum L.). J. Phys. Conf. Ser. 2019, 1294, 092029. [Google Scholar] [CrossRef]
- Jalali-Honarmand, S.; Rasaei, A.; Saeidi, M.; Ghobadi, M.E.; Khanizadeh, S. The Effects of Foliar Application of Plant Hormones at Booting Stage Non Wheat Yield Components. Thai J. Agric. Sci. 2015, 48, 35–38. [Google Scholar]
- Cleland, R.E. Auxin-Induced Hydrogen Ion Excretion: Correlation with Growth, and Control by External PH and Water Stress. Planta 1975, 127, 233–242. [Google Scholar] [CrossRef]
- Aldesuquy, H.S.; Gaber, A.M. Effect of Growth Regulators on Vicia Faba Plants Irrigated by Sea Water Leaf Area, Pigment Content and Photosynthetic Activity. Biol. Plant 1993, 35, 519–527. [Google Scholar] [CrossRef]
- Aldesuquy, H.S.; Ibrahim, A.H. Interactive Effect of Seawater and Growth Bioregulators on Water Relations, Abscisic Acid Concentration and Yield of Wheat Plants. J. Agron. Crop Sci. 2001, 187, 185–193. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, D.Q. Stimulation Effect of Gibberellic Acid Short-Term Treatment on Leaf Photosynthesis Related to the Increase in Rubisco Content in Broad Bean and Soybean. Photosynth. Res. 2001, 68, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Miri, M.R.; Ghooshchi, F.; Tohidi-Moghadam, H.R.; Larijani, H.R.; Kasraie, P. Ameliorative Effects of Foliar Spray of Glycine Betaine and Gibberellic Acid on Cowpea (Vigna unguiculata L. Walp.) Yield Affected by Drought Stress. Arab. J. Geosci. 2021, 14, 830. [Google Scholar] [CrossRef]
- Azadi, F.; Hatami, A.; Salek Mearaji, H. The Effect of Cytokinin Foliar on Morpho-Physiological Traits, Yield and Yield Components of Black Cumin (Nigella sativa L.) under Salinity Stress Conditions. Environ. Stress. Crop Sci. 2022, 15, 975–990. [Google Scholar]
- Mostafa, H.A.M.; El-Bassiouny, H.M.S.; Khattab, H.K.I.; Sadak, M.S. Improving the Characteristics of Roselle Seeds as a New Source of Protein and Lipid by Gibberellin and Benzyladenine Application. J. Appl. Sci. Res. 2005, 1, 161–167. [Google Scholar]
- Parida, A.K.; Dagaonkar, V.S.; Phalak, M.S.; Umalkar, G.V.; Aurangabadkar, L.P. Alterations in Photosynthetic Pigments, Protein and Osmotic Components in Cotton Genotypes Subjected to Short-Term Drought Stress Followed by Recovery. Plant Biotechnol. Rep. 2007, 1, 37–48. [Google Scholar] [CrossRef]
- Farhat, F.; Arfan, M.; Wang, X.; Tariq, A.; Kamran, M.; Tabassum, H.N.; Tariq, I.; Mora-Poblete, F.; Iqbal, R.; El-Sabrout, A.M.; et al. The Impact of Bio-Stimulants on Cd-Stressed Wheat (Triticum aestivum L.): Insights Into Growth, Chlorophyll Fluorescence, Cd Accumulation, and Osmolyte Regulation. Front. Plant Sci. 2022, 13, 850567. [Google Scholar] [CrossRef]
- Rashid, N.; Khan, S.; Wahid, A.; Ibrar, D.; Hasnain, Z.; Irshad, S.; Bashir, S.; Al-Hashimi, A.; Elshikh, M.S.; Kamran, M. Exogenous Application of Biostimulants and Synthetic Growth Promoters Improved the Productivity and Grain Quality of Quinoa Linked with Enhanced Photosynthetic Pigments and Metabolomics. Agronomy 2021, 11, 2302. [Google Scholar] [CrossRef]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Foliar Spray of Auxin/IAA Modulates Photosynthesis, Elemental Composition, ROS Localization and Antioxidant Machinery to Promote Growth of Brassica Juncea. Physiol. Mol. Biol. Plants 2020, 26, 2503–2520. [Google Scholar] [CrossRef]
- Pazuki, A.; Sedghi, M.; Aflaki, F. Interaction of Salinity and Phytohormones on Wheat Photosynthetic Traits and Membrane Stability. Agriculture/Pol’nohospodárstvo 2013, 59, 33–41. [Google Scholar] [CrossRef] [Green Version]
- da Costa, W.A.; Ribeiro, V.T.; da Silva, D.C.; Neto, A.; Wanderley Neto, A.d.O.; de Castro Dantas, T.N.; Ferrari, M.; dos Santos, E.S. Low-Energy Nanoemulsified Systems Containing Antioxidant Eutectic Extract from Rhodotorula Mucilaginosa Yeast Cells. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129715. [Google Scholar] [CrossRef]
- Majeed, A.; Kaleem Abbasi, M.; Hameed, S.; Imran, A.; Rahim, N. Isolation and Characterization of Plant Growth-Promoting Rhizobacteria from Wheat Rhizosphere and Their Effect on Plant Growth Promotion. Front. Microbiol. 2015, 6, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashour, N.I.; Neumann, D.; Nieden, U. zur Gibberellic Acid Induced Changes in the Ultrastructure of Chloroplasts and the Content of Chlorophyll in Leaves of Dwarf Maize (Zea mays L.). Biochem. Physiol. Der Pflanz. 1973, 164, 402–413. [Google Scholar] [CrossRef]
- Vichnevetskaia, K.D.; Roy, D.N. Oxidative Stress and Antioxidative Defense with an Emphasis on Plants Antioxidants. Environ. Rev. 1999, 7, 31–51. [Google Scholar] [CrossRef]
- Arteca, R.N. Plant Growth Substances: Principles and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 1475724519. [Google Scholar]
- Sabir, F.; Noreen, S.; Malik, Z.; Kamran, M.; Riaz, M.; Dawood, M.; Parveen, A.; Afzal, S.; Ahmad, I.; Ali, M. Silicon Improves Salinity Tolerance in Crop Plants: Insights into Photosynthesis, Defense System, and Production of Phytohormones. In Silicon and Nano-Silicon in Environmental Stress Management and Crop Quality Improvement; Elsevier: Amsterdam, The Netherlands, 2022; pp. 91–103. [Google Scholar]
- Malik, Z.; Afzal, S.; Dawood, M.; Abbasi, G.H.; Khan, M.I.; Kamran, M.; Zhran, M.; Hayat, M.T.; Aslam, M.N.; Rafay, M. Exogenous Melatonin Mitigates Chromium Toxicity in Maize Seedlings by Modulating Antioxidant System and Suppresses Chromium Uptake and Oxidative Stress. Environ. Geochem. Health 2021, 44, 1451–1469. [Google Scholar] [CrossRef]
- Parveen, A.; Hamzah Saleem, M.; Kamran, M.; Zulqurnain Haider, M.; Chen, J.T.; Malik, Z.; Shoaib Rana, M.; Hassan, A.; Hur, G.; Tariq Javed, M.; et al. Effect of Citric Acid on Growth, Ecophysiology, Chloroplast Ultrastructure, and Phytoremediation Potential of Jute (Corchorus capsularis L.) Seedlings Exposed to Copper Stress. Biomolecules 2020, 10, 592. [Google Scholar] [CrossRef] [Green Version]
- Kamran, M.; Danish, M.; Saleem, M.H.; Malik, Z.; Parveen, A.; Abbasi, G.H.; Jamil, M.; Ali, S.; Afzal, S.; Riaz, M.; et al. Application of Abscisic Acid and 6-Benzylaminopurine Modulated Morpho-Physiological and Antioxidative Defense Responses of Tomato (Solanum lycopersicum L.) by Minimizing Cobalt Uptake. Chemosphere 2021, 263, 128169. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kamran, M.; Abbasi, G.H.; Saleem, M.H.; Ahmad, S.; Parveen, A.; Malik, Z.; Afzal, S.; Ahmar, S.; Dawar, K.M. Melatonin-Induced Salinity Tolerance by Ameliorating Osmotic and Oxidative Stress in the Seedlings of Two Tomato (Solanum lycopersicum L.) Cultivars. J. Plant Growth Regul. 2020, 40, 2236–2248. [Google Scholar] [CrossRef]
- Hashem, M.; Abo-Elyousr, K.A. Management of the Root-Knot Nematode Meloidogyne Incognita on Tomato with Combinations of Different Biocontrol Organisms. Crop Prot. 2011, 30, 285–292. [Google Scholar] [CrossRef]
- Moravcová, Š.; Tůma, J.; Dučaiová, Z.K.; Waligórski, P.; Kula, M.; Saja, D.; Słomka, A.; Bąba, W.; Libik-Konieczny, M. Influence of Salicylic Acid Pretreatment on Seeds Germination and Some Defence Mechanisms of Zea mays L. Plants under Copper Stress. Plant Physiol. Biochem. 2018, 122, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Son, T.K.; Park, S.Y.; Lee, I.J.; Lee, B.H.; Kim, H.Y.; Lee, S.C. Influences of Gibberellin and Auxin on Endogenous Plant Hormone and Starch Mobilization during Rice Seed Germination under Salt Stress. J. Environ. Biol. 2006, 27, 181. [Google Scholar]
- Ali, B.; Hayat, S.; Aiman Hasan, S.; Ahmad, A. A Comparative Effect of IAA and 4-Cl-IAA on Growth, Nodulation and Nitrogen Fixation in Vigna radiata (L.) Wilczek. Acta Physiol. Plant 2008, 30, 35–41. [Google Scholar] [CrossRef]
- Beevers, L. Nitrogen Metabolism in Plants; Springer: Berlin/Heidelberg, Germany, 1981; ISBN 3642679218. [Google Scholar]
- Hopkins G, W.; A, H.N.P. Introduction to Plant Physiology, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]




| Month | Tmax °C | Tmin °C | Total Rain Fall (mm) | R.H (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| October | 36.1 | 37.4 | 21.6 | 20.5 | 0 | 0 | 58 | 54 |
| November | 31.6 | 32.20 | 13.9 | 11.6 | 0 | 0 | 53 | 52 |
| December | 27.5 | 26.6 | 12.2 | 9.0 | 0 | 0 | 57 | 53 |
| January | 26.4 | 22.1 | 11.3 | 8.3 | 0 | 2.5 | 61 | 62 |
| February | 22.4 | 24.2 | 10.2 | 11.0 | 0 | 0 | 45 | 50 |
| March | 35.1 | 34.4 | 18.1 | 15.5 | 0 | 0 | 48 | 48 |
| April | 38.0 | 40.2 | 21.9 | 18.9 | 0 | 0 | 44 | 43 |
| May | 39.5 | 46.0 | 29.0 | 28.0 | 0 | 0 | 58 | 56 |
| June | 41.0 | 42.0 | 28.0 | 27.0 | 0 | 0 | 47 | 45 |
| July | 45.0 | 47.0 | 27.0 | 25.0 | 0.7 | 0 | 56 | 53 |
| August | 42.0 | 43.0 | 28.0 | 23.0 | 0 | 2.6 | 47 | 49 |
| September | 38.0 | 41.0 | 26.0 | 24.5 | 0 | 0 | 50 | 52 |
| Soil Properties | Units | Values |
|---|---|---|
| Texture pH | - - | Sandy loam 7.2 |
| SOM | % | 1.43 |
| Total N | % | 0.07 |
| Na | meq/60 g soil | 0.07 |
| K | meq/60 g soil | 0.12 |
| Mg | meq/60 g soil | 2.1 |
| P | ug/g soil | 12.3 |
| S | ug/g soil | 16.09 |
| Ca | ug/g soil | 3.02 |
| Zn | ug/g soil | 1.43 |
| B | ug/g soil | 0.38 |
| Treatment Number | Treatment Labels | IAA Conc. (mg L−1) | GA3 Conc. (mg L−1) |
|---|---|---|---|
| T1 | CK | 00.0 | 00.0 |
| T2 | IAA1 | 30.0 | 00.0 |
| T3 | IAA2 | 60.0 | 00.0 |
| T4 | GA1 | 00.0 | 30.0 |
| T5 | GA2 | 00.0 | 60.0 |
| T6 | IAA1 + GA1 | 30.0 | 30.0 |
| T7 | IAA1 + GA2 | 30.0 | 60.0 |
| T8 | IAA2 + GA1 | 60.0 | 30.0 |
| T9 | IAA2 + GA2 | 60.0 | 60.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parveen, A.; Aslam, M.M.; Iqbal, R.; Ali, M.; Kamran, M.; Alwahibi, M.S.; Akram, M.; Elshikh, M.S. Effect of Natural Phytohormones on Growth, Nutritional Status, and Yield of Mung Bean (Vigna radiata L.) and N Availability in Sandy-Loam Soil of Sub-Tropics. Soil Syst. 2023, 7, 34. https://doi.org/10.3390/soilsystems7020034
Parveen A, Aslam MM, Iqbal R, Ali M, Kamran M, Alwahibi MS, Akram M, Elshikh MS. Effect of Natural Phytohormones on Growth, Nutritional Status, and Yield of Mung Bean (Vigna radiata L.) and N Availability in Sandy-Loam Soil of Sub-Tropics. Soil Systems. 2023; 7(2):34. https://doi.org/10.3390/soilsystems7020034
Chicago/Turabian StyleParveen, Aasma, Muhammad Mahran Aslam, Rashid Iqbal, Muhammad Ali, Muhammad Kamran, Mona S. Alwahibi, Muhammad Akram, and Mohamed S. Elshikh. 2023. "Effect of Natural Phytohormones on Growth, Nutritional Status, and Yield of Mung Bean (Vigna radiata L.) and N Availability in Sandy-Loam Soil of Sub-Tropics" Soil Systems 7, no. 2: 34. https://doi.org/10.3390/soilsystems7020034
APA StyleParveen, A., Aslam, M. M., Iqbal, R., Ali, M., Kamran, M., Alwahibi, M. S., Akram, M., & Elshikh, M. S. (2023). Effect of Natural Phytohormones on Growth, Nutritional Status, and Yield of Mung Bean (Vigna radiata L.) and N Availability in Sandy-Loam Soil of Sub-Tropics. Soil Systems, 7(2), 34. https://doi.org/10.3390/soilsystems7020034








