Long-Term Integrated Systems of Green Manure and Pasture Significantly Recover the Macrofauna of Degraded Soil in the Brazilian Savannah
Abstract
1. Introduction
2. Materials and Methods
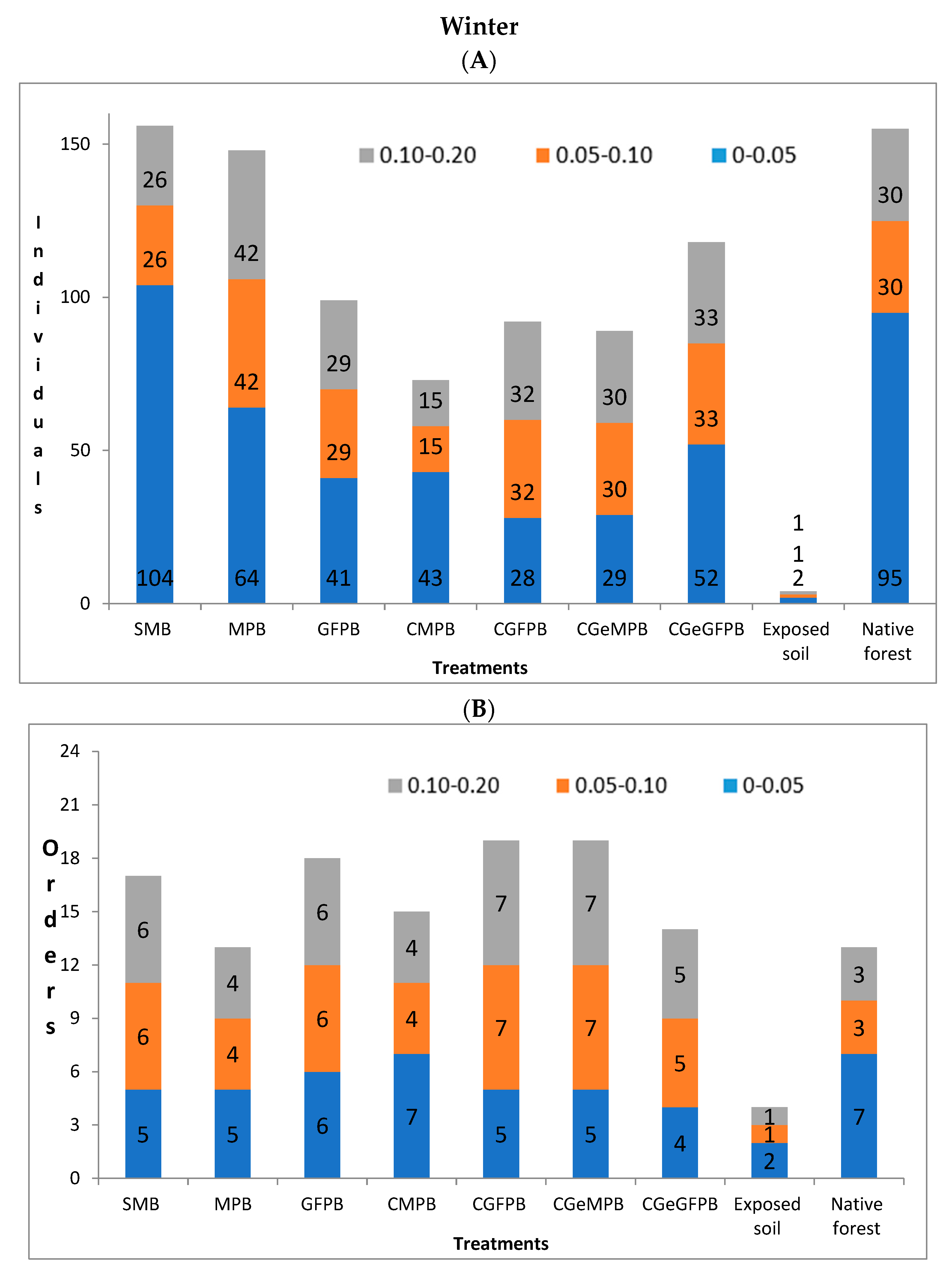
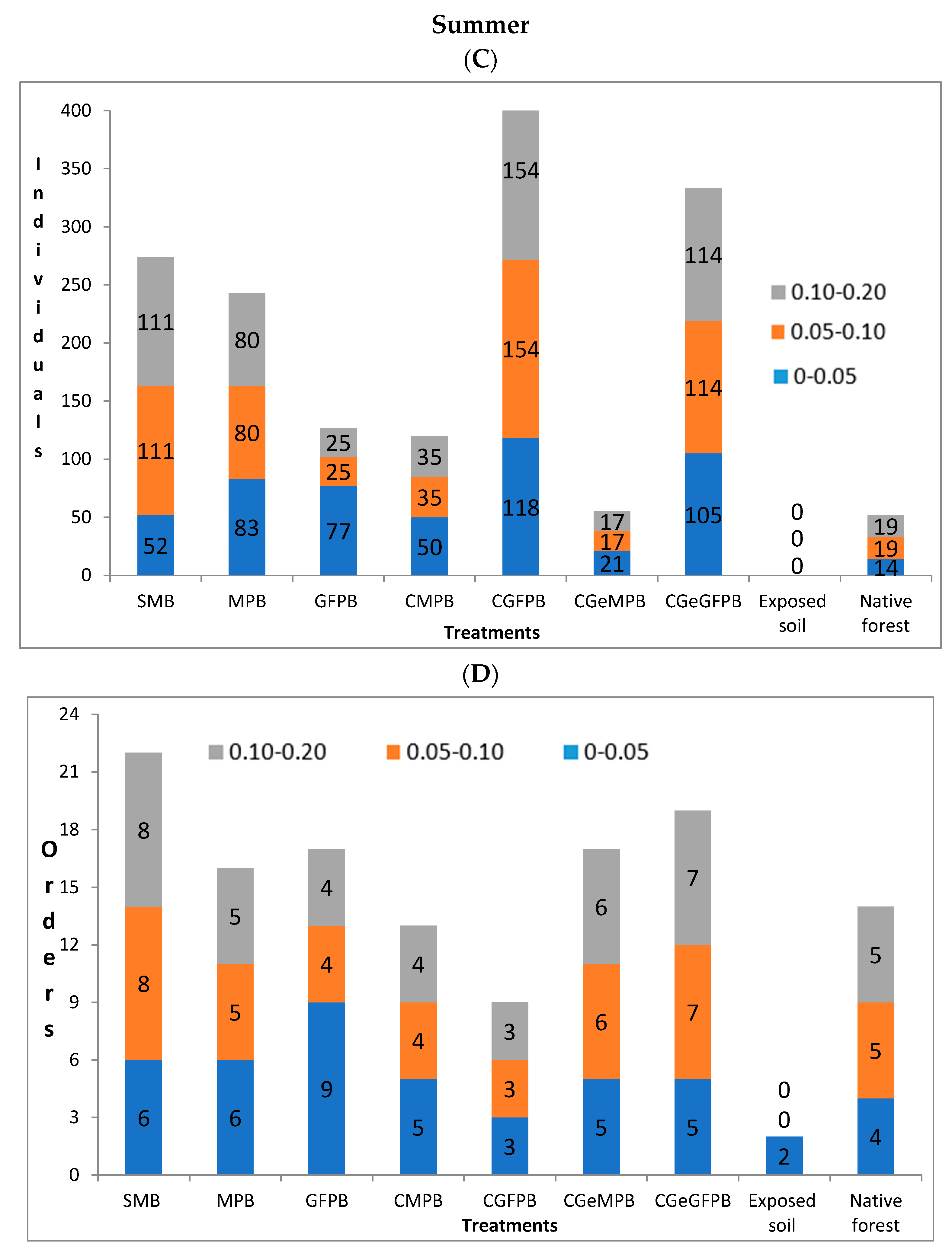
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes-Sánchez, L.B.; Horn, R.; Costantini, E.A.C. (Eds.) Sustainable Soil Management as a Key to Preserving Soil Biodiversity and Stopping Its Degradation; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Kamau, S.; Barrios, E.; Karanja, N.K.; Ayuke, F.O.; Lehmann, J. Soil macrofauna abundance under dominant tree species increases along a soil degradation gradient. Soil Biol. Biochem 2017, 112, 35–46. [Google Scholar] [CrossRef]
- Lavelle, P.; Decaëns, T.; Aubert, M.; Barot, S.; Blouin, M.; Bureau, F.; Margerie, P.; Mora, P.; Rossi, J.-P. Soil invertebrates and ecosystem services. Eur. J. Soil Biol. 2006, 42, S3–S15. [Google Scholar] [CrossRef]
- Obrycki, J.F.; Karlen, D.L. Is Corn Stover Harvest Predictable Using Farm Operation, Technology, and Management Variables? J. Agron. 2018, 110, 749–757. [Google Scholar] [CrossRef]
- Ranaivoson, L.; Naudin, K.; Ripoche, A.; Affholder, F.; Rabeharisoa, L.; Corbeels, M. Agro-ecological functions of crop residues under conservation agriculture. A review. Agron. Sustain. Dev. 2017, 37, 26. [Google Scholar] [CrossRef]
- Sawyer, J.E.; Woli, K.P.; Barker, D.W.; Pantoja, J.L. Stover Removal Impact on Corn Plant Biomass, Nitrogen, and Use Efficiency. J. Agron. 2017, 109, 802–810. [Google Scholar] [CrossRef]
- Sithole, N.J.; Magwaza, L.S.; Mafongoya, P.L.; Thibaud, G.R. Long-term impact of no-till conservation agriculture on abundance and order diversity of soil macrofauna in continuous maize monocropping system. Acta Agric. Scand. B Soil Plant Sci. 2018, 68, 220–229. [Google Scholar] [CrossRef]
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F.; Rajput, V.D.; Minkina, T.; Mandzhieva, S. Plant Nutrition under Climate Change and Soil Carbon Sequestration. Sustainability 2022, 14, 914. [Google Scholar] [CrossRef]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Jouquet, P.; Chintakunta, S.; Bottinelli, N.; Subramanian, S.; Caner, L. The influence of fungus-growing termites on soil macro and micro-aggregates stability varies with soil type. Appl. Soil Ecol. 2016, 101, 117–123. [Google Scholar] [CrossRef]
- Kaiser, D.; Lepage, M.; Konaté, S.; Linsenmair, K.E. Ecosystem services of termites (Blattoidea: Termitoidae) in the traditional soil restoration and cropping system Zaï in northern Burkina Faso (West Africa). Agric. Ecosyst. Environ. 2017, 236, 198–211. [Google Scholar] [CrossRef]
- Lima, S.S.; Pereira, M.G.; Pereira, R.N.; de Pontes, R.M.; Rossi, C.Q.; de Lima, S.S.; Pereira, M.G.; Pereira, R.N.; de Pontes, R.M.; Rossi, C.Q. Termite Mounds Effects on Soil Properties in the Atlantic Forest Biome. Rev. Bras. Cienc. Solo 2018, 42. [Google Scholar] [CrossRef]
- Sarker, J.R.; Singh, B.P.; Dougherty, W.J.; Fang, Y.; Badgery, W.; Hoyle, F.C.; Dalal, R.C.; Cowie, A.L. Impact of agricultural management practices on the nutrient supply potential of soil organic matter under long-term farming systems. Soil Tillage Res. 2018, 175, 71–81. [Google Scholar] [CrossRef]
- Kitamura, A.E.; Tavares, R.L.M.; Alves, M.C.; Souza, Z.M.; Siqueira, D.S. Soil macrofauna as bioindicator of the restoration of degraded Cerrado soil. Cienc. Rural 2020, 50, 8. [Google Scholar] [CrossRef]
- Harit, A.; Moger, H.; Duprey, J.-L.; Gajalakshmi, S.; Abbasi, S.A.; Subramanian, S.; Jouquet, P. Termites can have greater influence on soil properties through the construction of soil sheetings than the production of above-ground mounds. Insect. Soc. 2017, 64, 247–253. [Google Scholar] [CrossRef]
- Oliveira, C.C.; Alves, F.V.; Almeida, R.G.; Gamarra, É.L.; Villela, S.D.J.; Almeida Martins, P.G.M. Thermal comfort indexes assessed in integrated production systems in the Brazilian savannah. Agrofor. Syst. 2018, 92, 1659–1672. [Google Scholar] [CrossRef]
- Sone, J.S.; Oliveira, P.T.S.; Zamboni, P.A.P.; Vieira, N.O.M.; Carvalho, G.A.; Macedo, M.C.M.; Araujo, A.R.; Montagner, D.B.; Alves Sobrinho, T. Effects of long-term crop-livestock-forestry systems on soil erosion and water infiltration in a Brazilian Cerrado site. Sustainability 2019, 11, 5339. [Google Scholar] [CrossRef]
- Alves, M.C.; Suzuki, L.G.A.S.; Suzuki, L.E.A.S. Densidade do solo e infiltração de água como indicadores da qualidade física de um Latossolo Vermelho distrófico em recuperação. Rev. Bras. Cienc. Solo 2007, 31, 617–625. [Google Scholar] [CrossRef]
- Campos, F.S.; Alves, M.C. Uso de lodo de esgoto na reestruturação de solo degradado. Rev. Bras. Cienc. Solo 2008, 32, 1389–1397. [Google Scholar] [CrossRef]
- Bonini, C.B.S.; Alves, M.C. Aggregate stability of a degraded Oxisol in restoration with green manure, lime and gypsum. Rev. Bras. Cienc. Solo 2011, 35, 1263–1270. [Google Scholar] [CrossRef]
- Bonini, C.D.S.B.; Alves, M.C.; Montanari, R. Recuperação da estrutura de um Latossolo vermelho degradado utilizando lodo de esgoto. Rev. Ciênc. Agron 2015, 10, 34–42. [Google Scholar] [CrossRef]
- Monreal, C.M.; Alves, M.C.; Schnitzer, M.; Filho, S.N.S.; Batista Bonini, C.D.S. Mass spectrometry of organic matter influenced by long-term pedogenesis and a short-term reclamation practice in an Oxisol of Brazil. Can. J. Soil Sci. 2016, 96, 64–85. [Google Scholar] [CrossRef]
- Giácomo, R.G.; Souza, R.C.; Alves, M.C.; Pereira, M.G.; Garcia de Arruda, O.; Paz González, A. Soil fauna: Bioindicator of soil restoration in Brazilian savannah. Rev. Ciênc. Agron. 2017, 12, 236–243. [Google Scholar]
- Neto, A.B.; Bonini, C.D.S.B.; Bisi, B.S.; dos Reis, A.R.; Coletta, L.F.S. Artificial neural network for classification and analysis of degraded soils. IEEE Lat. Am. Trans. 2017, 15, 503–509. [Google Scholar] [CrossRef]
- Tseng, C.L.; Alves, M.C.; Crestana, S. Quantifying physical and structural soil properties using X-ray microtomography. Geoderma 2018, 318, 78–87. [Google Scholar] [CrossRef]
- Santos, H.G.; Jocomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbrearas, J.F.; Coelho, M.R.; Almeida, J.Á.; Filho, J.C.A.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Rio de Janeiro, Brasil, 2018; p. 286. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy|NRCS, 12th ed.; EUA: Washington, DC, USA, 2014. [Google Scholar]
- Quaggio, J.A.; Raij, B.; Malavolta, E. Alternative use of the SMP-buffer solution to determine lime requirement of soils. Commun. Soil Sci. Plant Anal. 1985, 16, 245–260. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasilia, Brazil, 2017; p. 557. [Google Scholar]
- Velasquez, E.; Lavelle, P. Soil macrofauna as an indicator for evaluating soil-based ecosystem services in agricultural landscapes. Acta Oecol. 2019, 100, 103446. [Google Scholar] [CrossRef]
- Melman, D.A.; Kelly, C.; Schneekloth, J.; Calderón, F.; Fonte, S.J. Tillage and residue management drive rapid changes in soil macrofauna communities and soil properties in a semiarid cropping system of Eastern Colorado. Appl. Soil Ecol. 2019, 143, 98–106. [Google Scholar] [CrossRef]
- Webster, E.; Gaudin, A.C.M.; Pulleman, M.; Siles, P.; Fonte, S.J. Improved Pastures Support Early Indicators of Soil Restoration in Low-input Agroecosystems of Nicaragua. Environ. Manag. 2019, 64, 201–212. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 8 February 2023).
- Marchão, R.L.; Lavelle, P.; Celini, L.; Balbino, L.C.; Vilela, L.; Becquer, T. Soil macrofauna under integrated crop-livestock systems in a Brazilian Cerrado Ferralsol. Pesqui Agropecu Bras. 2009, 44, 1011–1020. [Google Scholar] [CrossRef]
- Franchini, J.C.; Hoffmann-Campo, C.B.; Torres, E.; Miyazawa, M.; Pavan, M.A. Organic composition of green manure during growth and its effect on cation mobilization in an acid oxisol. Comm. Soil Sci. Plant Anal. 2003, 34, 2045–2058. [Google Scholar] [CrossRef]
- Fonseca, W.S.; Martins, S.V.; Villa, P.M. Green Manure as an Alternative for Soil Restoration in a Bauxite Mining Environment in Southeast Brazil. Floresta E Ambiente 2023, 30, e20220041. [Google Scholar] [CrossRef]
- Raij, B.; Cantarella, H.; Quaggio, J.A.; Hiroce, R.; Furlani, M.C. Recomendações de Adubação e Calagem para o Estado de São Paulo; IAC: São Paulo, Brasil, 1986. [Google Scholar]
- Bowles, T.M.; Jackson, L.E.; Loeher, M.; Cavagnaro, T.R. Ecological intensification and arbuscular mycorrhizas: A meta-analysis of tillage and cover crop effects. J. Appl. Ecol. 2017, 54, 1785–1793. [Google Scholar] [CrossRef]
- Fox, J.T.; Zook, A.N.; Freiss, J.; Appel, B.; Appel, J.; Ozsuer, C.; Sarac, M. Thermal conversion of blended food production waste and municipal sewage sludge to restoreable products. J. Clean. Prod. 2019, 220, 57–64. [Google Scholar] [CrossRef]
- Murphy, B.W. Impact of soil organic matter on soil properties—A review with emphasis on Australian soils. Soil Res. 2015, 53, 605–635. [Google Scholar] [CrossRef]
- Abail, Z.; Whalen, J.K. Corn residue inputs influence earthworm population dynamics in a no-till corn-soybean rotation. Appl. Soil Ecol. 2018, 127, 120–128. [Google Scholar] [CrossRef]
- Lammel, D.R.; Azevedo, L.C.B.; Paula, A.M.; Armas, R.D.; Baretta, D.; Cardoso, E.J.B.N.; Lammel, D.R.; Azevedo, L.C.B.; Paula, A.M.; Armas, R.D.; et al. Microbiological and faunal soil attributes of coffee cultivation under different management systems in Brazil. Braz. J. Biol. 2015, 75, 894–905. [Google Scholar] [CrossRef]
- Gholami, S.; Sayad, E.; Gebbers, R.; Schirrmann, M.; Joschko, M.; Timmer, J. Spatial analysis of riparian forest soil macrofauna and its relation to abiotic soil properties. Pedobiologia 2016, 59, 27–36. [Google Scholar] [CrossRef]
- Suárez, L.R.; Josa, Y.T.P.; Samboni, E.J.A.; Cifuentes, K.D.L.; Bautista, E.H.D.; Salazar, J.C.S.; Suárez, L.R.; Josa, Y.T.P.; Samboni, E.J.A.; Cifuentes, K.D.L.; et al. Soil macrofauna under different land uses in the Colombian Amazon. Pesqui Agropecu Bras. 2018, 53, 1383–1391. [Google Scholar] [CrossRef]
- Wang, S.; Pan, K.; Tariq, A.; Zhang, L.; Sun, X.; Li, Z.; Sun, F.; Xiong, Q.; Song, D.; Olatunji, O.A. Combined effects of cropping types and simulated extreme precipitation on the community composition and diversity of soil macrofauna in the eastern Qinghai-Tibet Plateau. J. Soils Sediments 2018, 18, 3215–3227. [Google Scholar] [CrossRef]
- Baretta, D.; Brescovit, A.D.; Knysak, I.; Cardoso, E.J.B.N. Trap and soil monolith sampled edaphic spiders (Arachnida: Araneae) in Araucaria angustifolia forest. Sci. Agric. 2007, 64, 375–383. [Google Scholar] [CrossRef]
- Bartz, M.L.C.; Pasini, A.; Brown, G.G. Earthworms as soil quality indicators in Brazilian no-tillage systems. Appl. Soil Ecol. 2013, 69, 39–48. [Google Scholar] [CrossRef]
- Bottinelli, N.; Jouquet, P.; Capowiez, Y.; Podwojewski, P.; Grimaldi, M.; Peng, X. Why is the influence of soil macrofauna on soil structure only considered by soil ecologists? Soil Tillage Res 2015, 146, 118–124. [Google Scholar] [CrossRef]
- Pauli, N.; Barrios, E.; Conacher, A.J.; Oberthür, T. Soil macrofauna in agricultural landscapes dominated by the Quesungual Slash-and-Mulch Agroforestry System, western Honduras. Appl. Soil Ecol. 2011, 47, 119–132. [Google Scholar] [CrossRef]
- Santos, D.C.; Guimarães Júnior, R.; Vilela, L.; Pulrolnik, K.; Bufon, V.B.; de S. França, A.F. Forage dry mass accumulation and structural characteristics of Piatã grass in silvopastoral systems in the Brazilian savannah. Agric. Ecosyst. Environ. 2016, 233, 16–24. [Google Scholar] [CrossRef]
- Gongalsky, K.B.; Persson, T. Restoration of soil macrofauna after wildfires in boreal forests. Soil Biol. Biochem. 2013, 57, 182–191. [Google Scholar] [CrossRef]
- Mariotte, P.; Le Bayon, R.-C.; Eisenhauer, N.; Guenat, C.; Buttler, A. Subordinate plant species moderate drought effects on earthworm communities in grasslands. Soil Biol. Biochem. 2016, 96, 119–127. [Google Scholar] [CrossRef]
- Potapov, A.M.; Goncharov, A.A.; Semenina, E.E.; Korotkevich, A.Y.; Tsurikov, S.M.; Rozanova, O.L.; Anichkin, A.E.; Zuev, A.G.; Samoylova, E.S.; Semenyuk, I.I.; et al. Arthropods in the subsoil: Abundance and vertical distribution as related to soil organic matter, microbial biomass and plant roots. Eur. J. Soil Biol. 2017, 82, 88–97. [Google Scholar] [CrossRef]
- Rampelotto, P.H.; de Siqueira Ferreira, A.; Barboza, A.D.M.; Roesch, L.F.W. Changes in Diversity, Abundance, and Structure of Soil Bacterial Communities in Brazilian Savanna Under Different Land Use Systems. Microb. Ecol. 2013, 66, 593–607. [Google Scholar] [CrossRef]
- Franco, A.L.C.; Cherubin, M.R.; Cerri, C.E.P.; Guimarães, R.M.L.; Cerri, C.C. Relating the visual soil structure status and the abundance of soil engineering invertebrates across land use change. Soil Tillage Res. 2017, 173, 49–52. [Google Scholar] [CrossRef]
- Geraei, D.S.; Hojati, S.; Landi, A.; Cano, A.F. Total and labile forms of soil organic carbon as affected by land use change in southwestern Iran. Geoderma Reg. 2016, 7, 29–37. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Culman, S.W.; Horwath, W.R.; Wade, J.; Cass, D.; Beniston, J.W.; Bowles, T.M.; Grandy, A.S.; Franzluebbers, A.J.; Schipanski, M.E.; et al. Comparison of Permanganate-Oxidizable Carbon and Mineralizable Carbon for Assessment of Organic Matter Stabilization and Mineralization. J. Soil Sci. Soc. Am. J. 2016, 80, 1352–1364. [Google Scholar] [CrossRef]
- Korboulewsky, N.; Perez, G.; Chauvat, M. How tree diversity affects soil fauna diversity: A review. Soil Biol. Biochem. 2016, 94, 94–106. [Google Scholar] [CrossRef]
- Moura, E.G.; Aguiar, A.C.F.; Piedade, A.R.; Rousseau, G.X. Contribution of legume tree residues and macrofauna to the improvement of abiotic soil properties in the eastern Amazon. Appl. Soil Ecol. 2015, 86, 91–99. [Google Scholar] [CrossRef]
- Sun, F.; Pan, K.; Tariq, A.; Zhang, L.; Sun, X.; Li, Z.; Wang, S.; Xiong, Q.; Song, D.; Olatunji, O.A. The response of the soil microbial food web to extreme rainfall under different plant systems. Sci. Rep. 2016, 6, 37662. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhu, B. Diversity and abundance of soil fauna as influenced by long-term fertilization in cropland of purple soil, China. Soil Tillage Res. 2015, 146, 39–46. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Chang. Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- de Vries, F.T.; Thébault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjørnlund, L.; Jørgensen, H.B.; Brady, M.V.; Christensen, S.; Ruiter, P.C.; et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA 2013, 110, 14296–14301. [Google Scholar] [CrossRef]

| Code | Integrated System * |
|---|---|
| NC | Exposed soil; negative control |
| SMB | Soil under native vegetation with cultivation of Urochloa decumbens |
| MPB | Succession of Stizolobium sp. and U. decumbens |
| GFPB | Succession of Cajanus sp., Canavalia sp. and U. decumbens |
| CMPB | Succession of Stizolobium sp. and U. decumbens with limestone |
| CGFPB | Succession of Cajanus sp., Canavalia sp. and pasture of U. decumbens with limestone |
| CGeMPB | Succession of Stizolobium sp. and U. decumbens with limestone and gypsum |
| CGeGFPB | Succession of Cajanus sp., Canavalia sp. and U. decumbens with limestone and gypsum |
| PC | Forest; positive control |
| Property | Depth (m) | |
|---|---|---|
| 0.00–0.20 | 0.20–0.40 | |
| Presin (mg dm−3) | 1.00 | 0.00 |
| Organic matter (g dm−3) | 7.00 | 4.00 |
| pH | 4.0 | 4.20 |
| K (cmolc dm−3) | 0.20 | 0.20 |
| Ca (cmolc dm−3) | 2.00 | 2.00 |
| Mg (cmolc dm−3) | 1.00 | 1.00 |
| Potential acidity (cmolc dm−3) | 20.00 | 20.00 |
| Sum of exchangeable cations (cmolc dm−3) | 3.20 | 3.20 |
| Cation-exchange capacity (cmolc dm−3) | 23.20 | 23.10 |
| Saturation of exchangeable cations (%) | 14.00 | 14.00 |
| Total porosity (m3 m−3) | 0.34 | 0.33 |
| Macroporosity (m3 m−3) | 0.09 | 0.07 |
| Microporosity (m3 m−3) | 0.25 | 0.26 |
| Soil bulk density (kg m−3) | 1.60 | 1.74 |
| Integrated System | Period of Sampling | |||||||
|---|---|---|---|---|---|---|---|---|
| Winter | Summer | |||||||
| Index | Diversity | Dominance | Evenness | Total Abundance (Ind m−2) | Diversity | Dominance | Evenness | Total Abundance (Ind m−2) |
| Exposed soil | 0.10 e | 1.00 a | 1.00 a | 0.15 c | 0.00 h | 0.00 h | 0.00 f | 0.00 i |
| SMB | 1.61 b | 0.11 b | 0.23 b | 3.58 a | 0.97 c | 0.40 c | 0.54 b | 5.70 c |
| MPB | 1.61 b | 0.15 b | 0.29 b | 2.90 b | 0.85 d | 0.35 d | 0.47 c | 5.35 d |
| GFPB | 0.79 d | 0.67 a | 0.82 a | 2.05 b | 1.20 b | 0.48 b | 0.55 b | 3.65 e |
| CMPB | 1.95 a | 0.23 b | 0.37 b | 1.93 b | 0.65 e | 0.23 e | 0.40 c | 2.60 f |
| CGFPB | 1.61 b | 0.54 a | 0.70 a | 3.18 a | 0.20 g | 0.30 d | 0.18 e | 7.93 a |
| CGeMPB | 1.61 b | 0.68 a | 0.85 a | 1.55 b | 1.41 a | 0.74 a | 0.88 a | 1.68 g |
| CGeGFPB | 1.39 c | 0.15 b | 0.34 b | 2.45 b | 0.39 f | 0.12 f | 0.24 d | 5.95 b |
| Native forest | 1.95 a | 0.33 b | 0.44 b | 3.58 a | 0.60 e | 0.35 d | 0.43 c | 1.08 h |
| F (5%) | 4000 * | 1190 * | 1403 * | 0910 * | 6447 * | 4828 * | 3430 * | 4591 * |
| CV (%) | 2.14 | 7.69 | 5.85 | 14.37 | 6.60 | 12.20 | 7.46 | 0.62 |
| Integrated System | Presin | SOM | pH | K | Ca | Mg | H + Al3+ | Al3+ | SEC | CEC | SEC/CEC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg dm−3) | (g dm−3) | (mmolc dm−3) | % | ||||||||
| Soil Depth (m) | 0.00–0.10 | ||||||||||
| Exposed soil | 3.00 D | 4.75 C | 5.00 A | 0.25 C | 2.00 C | 2.75 A | 19.75 A | 0.75 A | 5.50 A | 25.25 C | 22.75 AB |
| SMB | 6.75 AB | 10.75 AB | 5.00 A | 1.00 AB | 5.00 AB | 4.25 B | 23.75 A | 1.75 A | 10.25 AB | 34.00 BC | 29.50 A |
| MPB | 7.25 AB | 11.00 AB | 5.00 A | 1.25 B | 5.5 AB | 5.25 C | 25.25 A | 1.75 A | 12.00 AB | 37.25 BC | 32.00 A |
| GFPB | 7.75 AB | 10.00 AB | 5.00 A | 0.75 AB | 5.25 AB | 4.25 B | 24.75 A | 1.75 A | 10.25 AB | 35.00 BC | 28.75 A |
| CMPB | 8.00 AB | 11.75 B | 5.00 A | 1.00 AB | 6.25 AB | 5.00 BC | 25.25 A | 1.75 A | 12.25 B | 37.50 BC | 32.50 A |
| CGFPB | 7.00 AB | 11.00 B | 5.00 A | 1.00 AB | 5.50 A | 4.50 B | 23.5 A | 1.25 A | 12.00 AB | 35.50 BC | 33.50 A |
| CGeMPB | 8.75 A | 10.75 AB | 4.75 A | 1.00 AB | 5.50 A | 4.50 B | 25.00 A | 2.00 A | 11.00 AB | 36.00 BC | 30.25 A |
| CGeGFPB | 7.25 AB | 9.00 AB | 5.00 A | 1.00 AB | 4.00 ABC | 3.25 A | 24.50 A | 2.25 A | 8.25 AB | 32.70 AB | 24.50 AB |
| Native forest | 4.25 C | 12.75 B | 4.00 B | 1.00 AB | 2.50 AB | 2.75 A | 36.50 B | 6.75 B | 5.75 AB | 42.25 A | 13.00 C |
| F-value | 2.83 * | 3.78 * | 16.00 * | 2.70 * | 7.33 * | 2.19 * | 14.99 * | 11.70 * | 3.94 * | 6.95 * | 5.49 * |
| CV (%) | 15.17 | 12.00 | 3.43 | 9.90 | 10.58 | 14.28 | 9.19 | 13.97 | 13.27 | 9.87 | 11.89 |
| MSD | 1.01 | 0.95 | 0.40 | 0.33 | 0.59 | 0.77 | 5.60 | 0.58 | 1.04 | 8.31 | 1.37 |
| 0.10–0.20 | |||||||||||
| Exposed soil | 3.00 A | 3.25 B | 4.25 AB | 0.25 | 1.00 B | 1.00 A | 22.50 BC | 2.25 A | 2.25 B | 19.70 A | 9.25 BC |
| SMB | 3.00 A | 5.00 B | 4.50 AB | 0.20 | 4.25 A | 2.00 C | 20.75 AB | 1.50 A | 6.25 A | 23.50 A | 24.00 A |
| MPB | 3.75 A | 6.25 AB | 4.25 AB | 0.25 | 3.75 A | 2.00 C | 24.25 C | 2.75 A | 6.00 AB | 21.7 A | 20.75 AB |
| GFPB | 3.00 A | 6.75 AB | 5.00 B | 0.25 | 3.50 A | 1.50 B | 22.00 BC | 1.75 A | 5.25 AB | 22.70 A | 19.50 ABC |
| CMPB | 4.75 B | 5.75 AB | 4.75 AB | 0.25 | 3.75 A | 2.00 C | 23.00 BC | 2.25 A | 6.00 AB | 22.70 A | 21.25 AB |
| CGFPB | 3.75 A | 5.75 AB | 4.75 AB | 0.25 | 3.75 A | 2.50 D | 21.50 BC | 1.50 A | 6.50 A | 24.5 A | 23.75 A |
| CGeMPB | 3.00 A | 4.75 AB | 5.00 B | 0.25 | 3.50 A | 1.75 BC | 21.25 B | 1.25 A | 5.50 AB | 22.20 A | 20.75 AB |
| CGeGFPB | 5.00 B | 4.50 AB | 4.75 AB | 0.25 | 2.25 AB | 1.25 A | 18.75 A | 2.25 A | 3.75 AB | 21.70 A | 12.50 ABC |
| Native forest | 3.00 A | 8.00 A | 4.00 A | 0.25 | 1.00 B | 1.00 A | 32.75 D | 7.75 B | 2.25 B | 30.20 B | 6.75 C |
| F-value | 0.43 | 4.11 * | 2.84 * | 1.12 | 9.15 * | 2.64 * | 68.16 * | 7.83 * | 6.36 * | 7.79 * | 10.83 * |
| CV (%) | 19.05 | 10.76 | 9.15 | 18.88 | 11.45 | 12.00 | 4.07 | 17.31 | 12.60 | 9.04 | 11.89 |
| MSD | 0.98 | 0.67 | 1.00 | 0.50 | 0.55 | 0.47 | 2.32 | 0.77 | 0.73 | 5.00 | 1.23 |
| 0.20–0.40 | |||||||||||
| Exposed soil | 2.00 A | 3.00 A | 5.00 AB | 0.20 | 1.00 B | 1.00 A | 21.50 A | 2.00 A | 2.00 A | 23.50 A | 9.25 AB |
| SMB | 3.00 AB | 3.75 ABC | 4.50 AB | 0.17 | 2.50 AB | 1.25 A | 21.00 A | 1.50 A | 3.75 AB | 24.75 A | 16.00 ABC |
| MPB | 3.00 AB | 4.50 BC | 4.75 AB | 0.12 | 2.50 AB | 1.50 A | 21.00 A | 1.50 A | 4.00 AB | 25.00 A | 16.75 ABC |
| GFPB | 2.00 A | 3.25 AB | 5.00 AB | 0.17 | 1.75 AB | 1.25 A | 21.75 A | 2.25 A | 3.00 AB | 24.75 A | 12.75 ABC |
| CMPB | 2.00 A | 4.00 ABC | 5.20 AB | 0.20 | 3.25 A | 2.00 B | 21.25 A | 1.75 A | 5.25 AB | 26.50 A | 20.00 BC |
| CGFPB | 3.00 AB | 5.00 C | 5.00 AB | 0.20 | 3.50 A | 2.00 B | 20.75 A | 1.50 A | 5.75 AB | 26.50 A | 22.00 C |
| CGeMPB | 2.00 A | 3.50 AB | 5.00 AB | 0.12 | 2.25 AB | 1.25 A | 21.00 A | 1.75 A | 3.50 AB | 24.50 A | 14.75 ABC |
| CGeGFPB | 2.00 A | 4.00 ABC | 5.00 AB | 0.17 | 2.00 AB | 1.25 A | 21.75 A | 2.25 A | 3.25 AB | 25.00 A | 13.75 ABC |
| Native forest | 4.00 B | 7.00 D | 4.00 A | 0.22 | 1.00 B | 1.00 A | 31.00 B | 7.75 B | 2.00 A | 33.00 B | 7.00 BC |
| F-value | 5.40 * | 22.80 * | 2.67 * | 1.99 | 4.08 * | 1.26 * | 21.38 * | 28.22 * | 3.58 * | 13.45 * | 5.41 * |
| CV (%) | 14.34 | 10.29 | 9.50 | 27.20 | 13.72 | 12.92 | 6.33 | 9.16 | 14.51 | 5.90 | 11.38 |
| MSD | 1.03 | 1.23 | 1.10 | 0.12 | 0.58 | 0.48 | 3.40 | 0.40 | 0.74 | 3.68 | 1.05 |
| Bartlett’s Test of Sphericity | |||||
|---|---|---|---|---|---|
| Chi-square | 8500 | ||||
| Degree of freedom | 325 | ||||
| p-value | <0.05 * | ||||
| Kaiser–Mayer–Olkin Test | |||||
| Index/variable | Principal Component | ||||
| PCI | PCII | PCIII | PCIV | PCV | |
| Eigenvalue | 7.83 | 5.53 | 3.62 | 1.78 | 1.43 |
| Percentage of variance | 30.12 | 21.29 | 13.93 | 6.84 | 5.50 |
| Cumulative percentage of variance | 30.12 | 51.41 | 65.33 | 72.17 | 77.67 |
| Loading | |||||
| Spiders | 0.06 | −0.25 | −0.16 | 0.13 | 0.04 |
| Beetles | 0.35 | 0.63 * | 0.32 | 0.40 * | 0.20 |
| Adult millipedes | 0.24 | −0.08 | −0.22 | 0.32 | 0.41 * |
| Earthworms | −0.10 | 0.78 * | 0.50 * | 0.01 | 0.06 |
| Adult centipedes | 0.32 | −0.06 | −0.17 | −0.06 | −0.76 * |
| Larval stages | 0.14 | 0.09 | −0.46 * | −0.14 | 0.68 * |
| Eggs | 0.16 | 0.80 * | 0.53 * | 0.08 | 0.03 |
| Termites | 0.65 * | 0.37 | 0.18 | −0.48 * | −0.05 |
| Earwigs | 0.16 | 0.80 * | 0.53 * | 0.08 | 0.03 |
| Ants | 0.17 | 0.20 | −0.28 | −0.21 | −0.12 |
| White grubs | 0.15 | −0.01 | −0.52 * | 0.55 * | 0.06 |
| Stinkbugs | 0.27 | 0.66 * | 0.43 * | 0.19 | −0.02 |
| Crickets | 0.01 | 0.10 | −0.16 | −0.77 * | 0.29 |
| Total of individuals | 0.69 * | 0.45 * | 0.09 | −0.42 * | 0.03 |
| Total of orders | 0.49 * | 0.58 * | −0.34 | 0.24 | −0.14 |
| Presin | 0.84 * | −0.35 | 0.12 | 0.06 | 0.03 |
| SOM | 0.72 * | −0.55 * | 0.37 | 0.01 | −0.04 |
| pH | 0.47 * | 0.43 * | −0.48 * | −0.07 | −0.17 |
| K | 0.82 * | −0.42 * | 0.27 | −0.01 | 0.03 |
| Ca | 0.90 * | −0.22 | −0.31 | 0.06 | 0.01 |
| Mg | 0.96 * | −0.18 | 0.11 | 0.03 | 0.04 |
| Potential acidity | −0.02 | −0.69 * | 0.67 * | 0.05 | 0.05 |
| Al3+ | −0.42 * | −0.57 * | 0.63 * | −0.01 | 0.02 |
| SEC | 0.96 * | −0.21 | −0.07 | 0.03 | 0.02 |
| CEC | 0.60 * | −0.55 * | 0.48 * | −0.02 | 0.09 |
| Saturation of exchangeable cations | 0.95 * | 0.01 | −0.23 | 0.04 | 0.03 |
| Percentage of Contribution | |||||
| Spiders | 0.04 | 1.13 | 0.71 | 0.95 | 0.09 |
| Beetles | 1.57 | 7.07 | 2.78 | 9.04 | 2.87 |
| Adult millipedes | 0.75 | 0.11 | 1.33 | 5.90 | 11.53 |
| Earthworms | 0.00 | 10.94 | 6.92 | 0.00 | 0.23 |
| Adult centipedes | 1.33 | 0.06 | 0.82 | 0.21 | 40.64 |
| Larval stages | 0.24 | 0.13 | 5.91 | 1.07 | 32.36 |
| Eggs | 0.32 | 11.53 | 7.65 | 0.39 | 0.07 |
| Termites | 5.35 | 2.43 | 2.11 | 13.13 | 0.18 |
| Earwigs | 0.32 | 11.53 | 7.65 | 0.39 | 0.07 |
| Ants | 0.38 | 0.71 | 2.11 | 2.48 | 0.93 |
| White grubs | 0.28 | 0.00 | 7.44 | 16.92 | 0.24 |
| Stinkbugs | 0.93 | 7.98 | 5.08 | 2.04 | 0.03 |
| Crickets | 0.00 | 0.18 | 0.72 | 33.28 | 5.91 |
| Total of individuals | 6.06 | 3.72 | 0.23 | 9.87 | 0.05 |
| Total of orders | 3.11 | 6.04 | 3.17 | 3.25 | 1.42 |
| Presin | 9.01 | 2.26 | 0.39 | 0.24 | 0.05 |
| SOM | 6.69 | 5.55 | 3.75 | 0.01 | 0.12 |
| pH | 2.79 | 3.33 | 6.31 | 0.24 | 2.12 |
| K | 8.59 | 3.17 | 1.97 | 0.00 | 0.08 |
| Ca | 10.28 | 0.84 | 2.67 | 0.20 | 0.00 |
| Mg | 11.84 | 0.60 | 0.32 | 0.06 | 0.09 |
| Potential acidity | 0.01 | 8.65 | 12.33 | 0.14 | 0.19 |
| Al3+ | 2.27 | 5.83 | 10.88 | 0.01 | 0.03 |
| SEC | 11.87 | 0.78 | 0.13 | 0.05 | 0.02 |
| CEC | 4.57 | 5.45 | 6.29 | 0.02 | 0.60 |
| Saturation of exchangeable cations | 11.41 | 0.00 | 1.51 | 0.09 | 0.08 |
| Chemical neutralization of toxic elements | Diversity of food sources on the soil surface | Biological decomposition of organic matter | Unavailability of aboveground biomass | Predatory activity | |
| Variable | Fitted Regression Model † | AIC | BIC | Radj2 |
|---|---|---|---|---|
| White grubs | White grubs (ind m−2) = 0.25 + 0.45 Ca * | 108.40 | 112.30 | 0.12 |
| Adult centipedes | Adult centipedes (ind m−1) = −0.12 + 0.09 Ca * | 27.50 | 31.38 | 0.10 |
| Termites | Termites (ind m−2) = 24.09 − 11.48 Ca * − 5.61 K + 20.67 Mg * − 4.75 Al | 245.20 | 252.90 | 0.32 |
| Total of individuals | Total of individuals = 21.68 * + 8.98 Mg ** − 4.65 Al * | 246.90 | 252.10 | 0.38 |
| Total of orders | Total of orders = 2.18 − 34.21 SEC + 19.01 Potential acidity * | 94.69 | 99.87 | 0.46 |
| Soil organic matter | SOM (g dm−3) = 2.78 ** + 0.19 P + 0.01 Ca + 6.55 K ** | 97.43 | 103.90 | 0.82 |
| Spiders | Spiders (ind m−2) = 0.69 ** − 0.30 Stinkbugs * | 53.46 | 57.35 | 0.07 |
| Termites | Termites (ind m−2) = 18.81 ** + 10.73 Earthworms * | 250.10 | 254.00 | 0.11 |
| Eggs | Eggs (ind m−2) = −0.01 + 0.19 Earthworms ** | −45.63 | 41.74 | 0.75 |
| Earwigs | Earwigs (ind m−2) = −0.01 + 0.19 Earthworms ** | −45.63 | 41.74 | 0.75 |
| Beetles | Beetles (ind m−2) = 0.90 ** + 1.07 Earthworms ** | 95.64 | 99.53 | 0.31 |
| Stinkbugs | Stinkbugs (ind m−2) = 0.08 + 0.54 Earthworms ** | −46.10 | 49.99 | 0.43 |
| Earwigs | Earwigs (ind m−2) = −0.05 + 0.04 Beetles * + 0.15 Stinkbugs ** | 37.49 | 32.31 | 0.67 |
| Beetles | Beetles (ind m−2) = 0.84 ** + 1.57 Stinkbugs ** | 89.51 | 93.40 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonini, C.d.S.B.; Maciel, T.M.d.S.; Moreira, B.R.d.A.; Chitero, J.G.M.; Henrique, R.L.P.; Alves, M.C. Long-Term Integrated Systems of Green Manure and Pasture Significantly Recover the Macrofauna of Degraded Soil in the Brazilian Savannah. Soil Syst. 2023, 7, 56. https://doi.org/10.3390/soilsystems7020056
Bonini CdSB, Maciel TMdS, Moreira BRdA, Chitero JGM, Henrique RLP, Alves MC. Long-Term Integrated Systems of Green Manure and Pasture Significantly Recover the Macrofauna of Degraded Soil in the Brazilian Savannah. Soil Systems. 2023; 7(2):56. https://doi.org/10.3390/soilsystems7020056
Chicago/Turabian StyleBonini, Carolina dos Santos Batista, Thais Monique de Souza Maciel, Bruno Rafael de Almeida Moreira, José Guilherme Marques Chitero, Rodney Lúcio Pinheiro Henrique, and Marlene Cristina Alves. 2023. "Long-Term Integrated Systems of Green Manure and Pasture Significantly Recover the Macrofauna of Degraded Soil in the Brazilian Savannah" Soil Systems 7, no. 2: 56. https://doi.org/10.3390/soilsystems7020056
APA StyleBonini, C. d. S. B., Maciel, T. M. d. S., Moreira, B. R. d. A., Chitero, J. G. M., Henrique, R. L. P., & Alves, M. C. (2023). Long-Term Integrated Systems of Green Manure and Pasture Significantly Recover the Macrofauna of Degraded Soil in the Brazilian Savannah. Soil Systems, 7(2), 56. https://doi.org/10.3390/soilsystems7020056








