Abstract
Unique worldwide, nitrate/iodine deposits (NIDs) are located along a 700 km geological belt in the Atacama Desert, Chile. They serve as the primary source of mineral ores for the extraction of iodine, sodium, and potassium nitrates. NIDs have been relatively underexplored from a biological perspective. To address this, we collected sixteen soil samples from abandoned mines in Oficinas Pissis and Savona for chemical, mineralogical, and metagenomic analyses. The soils primarily consisted of halite and darapskite, with only one sample being predominantly composed of thenardite. Deliquescence and water activity measurements yielded values ranging from 0.02% to 0.40% and 0.47 to 0.62, respectively. To investigate the presence, identification, relative abundance, and diversity of microbial life in NID soils, we employed MiSeq high-throughput sequencing and bioinformatic tools. The dominant phyla observed were Firmicutes and Proteobacteria, with Actinobacteria and Cyanobacteria being predominant in two soil samples. Furthermore, we detected nitrate/perchlorate-reducing bacterial activity in enriched cultures from the soil samples. This study sheds light on the resilience of microbial life in the Atacama Desert NIDs, providing compelling evidence for its existence and offering insight into factors that could facilitate it within this unique environment.
1. Introduction
Northern Chile is home to extensive reserves totaling one hundred million tons of nitrate/iodine deposits (NIDs). These deposits are composed of common sulfates, chlorides, carbonates, and borates salts, making them unique worldwide due to the inclusion of rare iodate, chromate, dichromate, and perchlorate salts that are seldom found in nature. Remarkably, these deposits have been preserved throughout geological eras [1,2,3]. While the precise genesis of NIDs remains a subject of ongoing research, it is likely to involve multiple mechanisms, factors, and nitrogen sources, such as microbial nitrogen fixation, nitrification, magmatic processes, and atmospheric deposition [1,3,4,5,6].
The Atacama Desert in Chile is globally recognized as the driest region on Earth, known for its rich nitrate deposits. It serves as a valuable Mars analog, as the nitrate found in its sediments parallels compositions discovered in Martian sediments. This resemblance is attributed to prolonged atmospheric photolysis, oxidation, and possibly impact-induced heating on Mars [1]. Nitrate was identified in mudstone deposits at Gale Crater on Mars at concentrations ranging from 70 to 1100 parts per million (ppm) by the Mars Science Laboratory [2]. Similar nitrate levels have been observed in Martian meteorites, such as EETA79001 and Tissint [3]. These concentrations closely resemble the nitrate levels found in Atacama sediments [4]. It is also conceivable that some of Martian nitrate may have originated from carbon dioxide–nitrogen reactions in the ancient Martian atmosphere, likely stimulated by impact shock heating. Furthermore, thermal decomposition, likely induced by the late heavy bombardment, could have potentially converted Martian nitrate back into atmospheric nitrogen. This process would have redistributed the sedimentary nitrate content, ultimately contributing to the equilibrium between nitrate and atmospheric nitrogen [5,6].
Elevated nitrate levels that accumulate under hyperarid conditions, as observed in environments like the Atacama Desert and the recent Martian surface, have the potential to serve as the primary source of bioavailable nitrogen (N) for local subsurface microbial communities. Nitrate is a significant nitrogen source for a wide range of microorganisms. It not only serves as a nitrogen source for biomass production but also functions as an energy source in chemotrophic metabolisms. As an oxidizing agent, nitrate plays a crucial role as an electron acceptor in natural processes. Nitrate (or nitrite) can be employed in chemotrophic metabolisms, including denitrification, dissimilatory reduction of nitrate to ammonium (DNRA), and anaerobic ammonium oxidation [7].
Deliquescence (Dw) enables the condensation of water vapor within pores in halite salts at a minimum relative humidity of 75% or even at lower humidity levels in perchlorate salts [8,9,10]. This means that local and transient liquid water becomes available to microbial life when the appropriate humidity levels and salt-dependent Dw occur in the Atacama region. This phenomenon has been demonstrated in various Atacama habitats, where lithobiontic microbial communities, including members from genera such as Chroococcidiopsis and Halothece, act as primary producers within these microbial consortia [8,9,11,12,13,14,15,16,17,18,19].
The microbiology of the Atacama Desert has been extensively investigated over the past two decades in this polyextreme hyperarid region characterized by high solar radiation and a lack of regular liquid water, both of which are significant natural constraints for life [7,8,9,11,12,13,14,15]. However, the presence and activity of microbial life within Atacama NIDs have been largely overlooked as a potential microbial habitat.
Metagenomic studies and culture-dependent approaches have provided insights into the colonization of halite nodules from Salar Grande by a diverse and complex microbial community, representing members from the Bacteria, Archaea, and Eukarya domains, including various viruses [10,13]. Within the driest core of the Atacama Desert, Yungay, the microbial community was found to include Proteobacteria, Actinobacteria, Firmicutes, and other phylogenetic groups [7]. Additionally, when examining microbial composition along a soil column down to a depth of 3.4 m, the predominant phyla were Proteobacteria, Actinobacteria, Bacteroidetes, and Firmicutes. These composition variations were observed in response to changes in the physical and chemical soil characteristics [19].
Highly hygroscopic salts, such as halite, perchlorates, and anhydrite, can provide liquid water to NID microorganisms if microbial life exists. Nonetheless, the relationship between nitrate and perchlorate salts and their impact on microbial diversity within NIDs remains an unexplored area of study. This study aims to investigate microbial life within nitrogen/iodide deposits in the arid core of the Atacama Desert. Specific objectives include assessing the presence, abundance, and diversity of microorganisms using metagenomic and culturing techniques and examining their relationship with the physicochemical characteristics of the samples, such as mineralogical composition and water activity.
2. Materials and Methods
2.1. Research Site and Sampling Collection
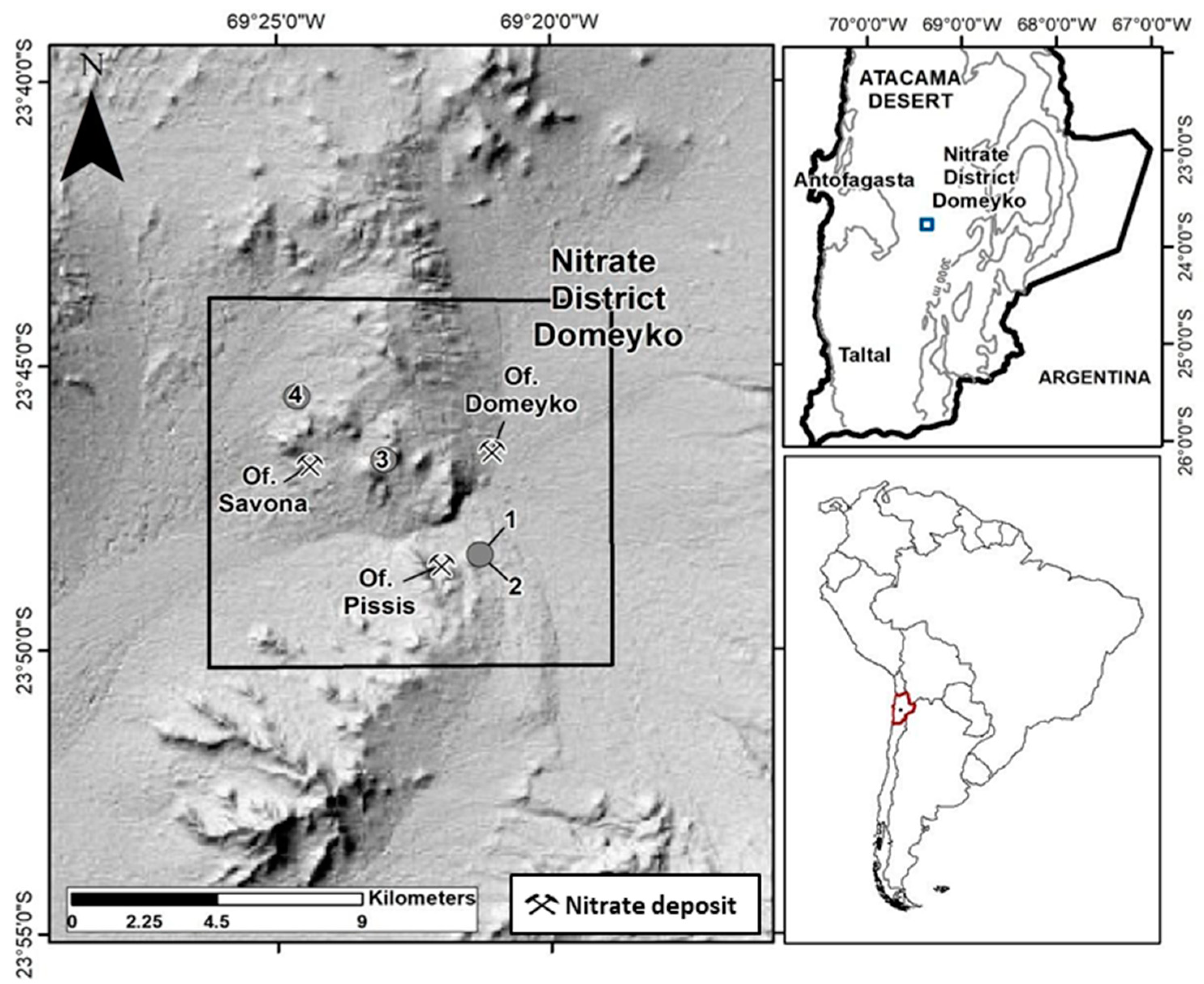
The study area is situated approximately 110 km east of the city of Antofagasta, spanning between UTM 19K coordinates 7,376,000–7,355,000 m N and 454,500–476,600 m E (see Figure 1). The Domeyko District encompasses Cerro El Plomo, characterized by sedimentary deposits, rocks, and salt flats [2]. It is an uninhabited region featuring the remnants of the former Oficinas Salitreras Domeyko, Pissis, Savona, and Cochrane, which were operational in the early 20th century.
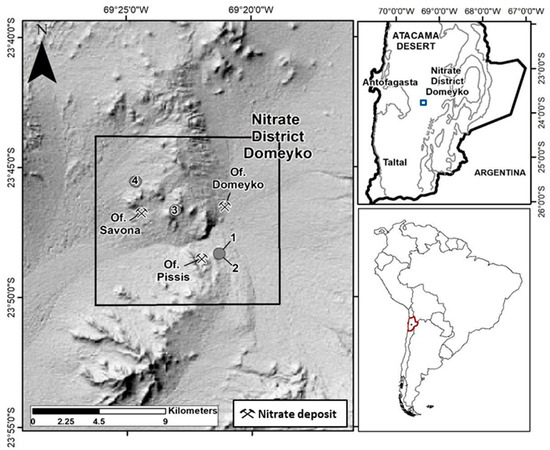
Figure 1.
Location of Domeyko District and sampling sites at Oficina Pissis (sites 1 and 2) and Oficina Savona (sites 3 and 4).
The regional geological framework reveals a complex geology marked by overlapping structures of varying ages. The Caracoles–Punta Negra segment of the Domeyko fault system, formed during the Eocene era, is prominent, along with kilometer-scale faults and folds that divide the area into western, central, and eastern structural domains [3,4,20,21]. These subdivisions consider various factors, including primary and secondary origins, morphology, and ages. These considerations guided our survey campaign planning.
Sixteen mineral soil samples were collected, with ten taken from Oficinas Pissis (OP) and six from Oficina Savona (OS), utilizing sterilized implements. At each sampling site, the surface mineral salts were removed, 30 cm holes were excavated, and soil samples were retrieved from a depth of 15 to 25 cm. Each sample was homogenized using the cone and quarter technique [22] and then divided into three portions for physical, chemical, microbiological, and molecular analyses. The samples were stored at 4 °C in the dark until analysis.
2.2. Physical, Chemical, and Mineralogical Analysis of NIDs
NID soil samples (5–10 g) were suspended and sonicated in 10–20 mL water. Insoluble materials were separated through centrifugation, and the resulting supernatants were subjected to analysis for chloride, nitrate, nitrite, and sulfate concentrations. This analysis used a Dionex ion-chromatography system (Thermo Fisher Sci., Waltham, MA, USA) equipped with an IonPac AS11-HC column. Perchlorate measurements were carried out using an IonPac AS20 column with an eluent composed of 1.9 mM Na2CO3, 1.9 mM NaHCO3, and 25% (w/v) acetonitrile. The detection limit for perchlorate is 5.0 mg/kg.
Salinity, pH, and conductivity were measured in a soil-distilled water suspension at a 1:2.5 ratio using a handheld 340i multimeter (WTW, Weilheim, Germany).
X-ray diffraction analyses of pulverized samples were carried out in Lindemann glass tubes with a diameter of 0.5 mm using a Bruker D8 Advance diffractometer from Germany. Continuous-scan X-ray powder diffraction data were collected in the 2θ angle range of 3–80°, utilizing CoKα radiation. The X-ray diffractograms were analyzed with the International Centre for Diffraction Database PDF-4 (https://www.icdd.com/ accessed on 10 September 2023).
Total organic carbon (TOC) was determined by measuring the weight difference of samples after drying at 110 °C for 24 h and subsequent incubation at 550 °C for 3 h. The Dw capabilities of the collected samples were assessed following the procedure outlined in Parro et al. [10]. In brief, dried samples (dried at 110 °C for 24 h) were placed in glass beakers at 4 °C below 75% relative humidity and sealed in aluminum foil. Their weight was recorded daily to track weight gain. Additionally, the samples were visually examined for the accumulation of liquid water [23].
As previously outlined, water activity (Aw) was assessed using a LabMaster-aw system® from Novasina Instruments, Lachen, Switzerland.
2.3. DNA Extraction, PCR Amplification, and Sequencing
Samples weighing 25–50 g were suspended in a PBS buffer containing 1% v/v Tween 20 and mixed for 2 h at 120 rpm. After centrifugation for 5 min at 3500 rpm using an Eppendorf centrifuge 5417R from Hamburg, Germany, the resulting pellets were discarded, and the supernatants were filtered through 0.22 μm pore size nitrocellulose membranes. The filters were then recovered, suspended in 1 mL of lysis buffer (composed of 50 mM Tris–HCl, pH 8.3, 40 mM EDTA, and 0.75 M sucrose), and stored overnight at −20 °C. Upon thawing, the filters were subjected to incubation with lysozyme (1 mg/mL) at 37 °C for 45 min. Subsequently, proteinase K (0.2 mg/mL) was added and incubated at 55 °C for 1 h. The resulting supernatant was used for genomic DNA extraction, utilizing a High Pure Template Preparation Kit (Qiagen, Germantown, MD, USA). The yield and DNA purity were evaluated spectrophotometrically (using a Nanodrop from Thermo, Dreieich, Germany) and by electrophoresis on a 1% agarose gel, respectively.
Amplification of the 16S rRNA gene was conducted with the bacteria-specific primer set 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) [24] and 519R (5′-GWATTACCGCGGCKGCTG-3′) [25], flanking variable regions V1–V3 of the 16S rRNA gene. Sequencing was performed at MrDNA Next Generation Sequencing Service Provider on 11 April 2017 (www.mrdnalab.com; Shallowater, TX, USA) with a MiSeq sequencer, following the manufacturer’s guidelines.
2.4. Sequence Analysis and Taxonomic Assignation
The QIIME (Quantitative Insights into Microbial Ecology) pipeline [26] was employed for processing the sequences. Unprocessed sequences underwent filtering based on quality score, average base content per reading, and GC distribution in the reads. Singleton reads (abundance < 2) that did not cluster with other sequences were excluded. Chimeric sequences were also eliminated using the UCHIME program [27].
The pre-processed consensus V3 sequences were subsequently grouped into operational taxonomic units (OTUs) using the clustering program UCLUST, with a similarity threshold of 0.97. All the pre-processed reads were employed for OTU identification through the QIIME program, and representative sequences were aligned using the PyNAST program [28] concerning the Greengenes core set reference database. A representative sequence for each OTU was classified using the RDP classifier and the Greengenes OTU database. Alpha rarefaction was calculated using the “core diversity analysis” command, and the number of sequences was standardized to the smallest sample size by applying the Chao 1 method. Alpha and beta diversity analyses were conducted using Primer-6 (Primer-E) [29] and QIIME 2 software (https://qiime2.org). Krona interactive graphs [30] were utilized for visualization.
2.5. Enrichment Cultures of Nitrate/Perchlorate Reducing Bacteria
The OP and OS site samples were chosen based on their highest nitrate content. These samples, each weighing 2.5 g, were suspended in a modified enrichment mineral salt medium (FTW) [31], which included K2HPO4 (225 mg/L), KH2PO4 (225 mg/L), (NH4)2SO4 (225 mg/L), MgSO4·7H2O (50 mg/L), CaCO3 (5 mg/L), FeCl2·4H2O (5 mg/L), and a mixture of trace metals. Carbon sources in acetate (1.5 g/L) and yeast extract (1.0 g/L) were added, and the cultures were incubated at 37 °C under anaerobic conditions. The cultures were then exposed to nitrate concentrations of 0.34 and 0.68 g/L and perchlorate concentrations of 0.1 and 0.2 g/L. Soil samples from the OP site produced four nitrate and perchlorate-reducing cultures, while the OS site yielded one nitrate-reducing culture.
3. Results and Discussion
The Atacama Desert has been extensively investigated regarding its saline facies, including their composition, origin, age, and association with microbial diversity [8,13,32,33,34]. NID formation can be influenced by the chemosynthetic metabolism of bacteria and archaea, the primary sources of carbon at the Atacama nitrate deposits where reduced forms of chromium, sulfur, and nitrogen are the only chemosynthetic substrate available [35]. Nitrate salts are potential nitrogen sources for microorganisms in desert soils under extreme environmental conditions. Atacama soils contain 70 to 1100 ppm nitrate, near those found in Martian soils [1,2,36]. Nitrogen is also considered a limiting nutrient in arid lands [37]. Nitrate, an electron acceptor, is an energy source in chemotrophic metabolism, especially during dissimilatory nitrate reduction to ammonium and anaerobic ammonium oxidation [1,7]. Consequently, nitrate in Atacama and Martian soils may play a role as a contributing factor to microbial colonization, both in the present and in the past. Nitrogen transformation, which encompasses decomposition and mineralization, is primarily carried out by soil microorganisms. However, conditions optimal for microbial activity, such as suitable temperature and soil moisture, are not always present in arid environments, except following rainfall events [38]. Nitrogen mineralization in arid and semi-arid ecosystems displays significant heterogeneity across the landscape and typically occurs in patches or fertility islands. This heterogeneity can be attributed to unique microclimatic conditions, variations in the richness and abundance of plants, and variations in soil nitrogen content [39].
3.1. Physical, Chemical, and Mineralogical Characterization of NIDs
The physical and chemical characteristics (salt type, temperature, pH, salinity, conductivity, Aw, Dw, humidity, and primary salt content) of sixteen samples collected at OP and OS sites in the Domeyko District are summarized in Table 1 and Table 2. All sample sites showed a similar temperature range as expected for Atacama as a temperate desert [40]. Other parameters showed an ample range of values among the sixteen samples collected.

Table 1.
Physical and chemical characteristics of soil samples collected at Pissis (OP) and Savona (OS) sites, Domeyko District, Atacama Desert.

Table 2.
Chemical analysis of the main salts found in the soil of Domeyko District sampling. Oficinas Pissis (OP) and Savona (OS).
The Aw values in NID soils exhibited a relatively narrow range, falling between 0.60 and 0.47. These values are below the lower limit proposed for microbial cell division, as observed in the case of Xeromyces bisporus, a sugar-tolerant xerophilic fungus, and halophilic archaea and bacteria [41]. Among the samples from the Domeyko District, Atacama OP4 was the sole soil sample with an Aw of 0.617, surpassing this limit.
As an indicator of water vapor trapped as liquid water brines by salt crystals, NID samples displayed a high variability in Dw values, likely attributable to differences in salt content and composition. Notably, the OP1 site, rich in nitrate and sulfate salts, exhibited the lowest Dw value at 0.002%, while the highest Dw was recorded at the sulfate-rich OS12 site at 0.401% (refer to Table 1 and Table 2). In comparison, studies on brine formation on the surface of Mars have shown that metastable brines of calcium perchlorate and magnesium perchlorate can be generated at Aw values ranging from 0.80 to 0.01, typically at temperatures around 225 K [42]. Consequently, salt deliquescence can be considered a crucial mechanism that supports the potential existence of extinct or extant life forms on Mars and here in the Atacama Desert [43,44]. A recent report provides compelling evidence of the presence of hydrated salts on the soil crust of the Martian surface [45].
Organic carbon (OC) in soils represents the organic matter fraction resulting from all types of life forms, whether alive or in the decomposition process. OC plays a vital role in providing nutrients to ecosystems [40,46]. In the collection sites for NID samples, OC levels ranged from 0.019% to 3.657% (see Table 1). Notably, Sample OS16, known as Guano Nitrate and rich in nitrate and sulfate, exhibited the highest OC content at 3.66%. It also had the most acidic pH value at 4.68 and was one of two sites with the lowest conductivity at 8.96 mS/cm (refer to Table 1 and Table 2). These distinctions from other NID sites may be attributed to the fact that OS16 has been identified as a nursery site for migratory birds [47].
The chemical composition of major salts in soils collected from sixteen sites within the Domeyko District displayed significant variations (see Table 2). The approximate minimum and maximum values for nitrate, chloride, and sulfate content ranged from 8 to 560, 2 to 700, and 13 to 297 mg/kg, respectively. The level of perchlorate salts fell below the detection limit (with a detection limit of 5.0 mg/kg).
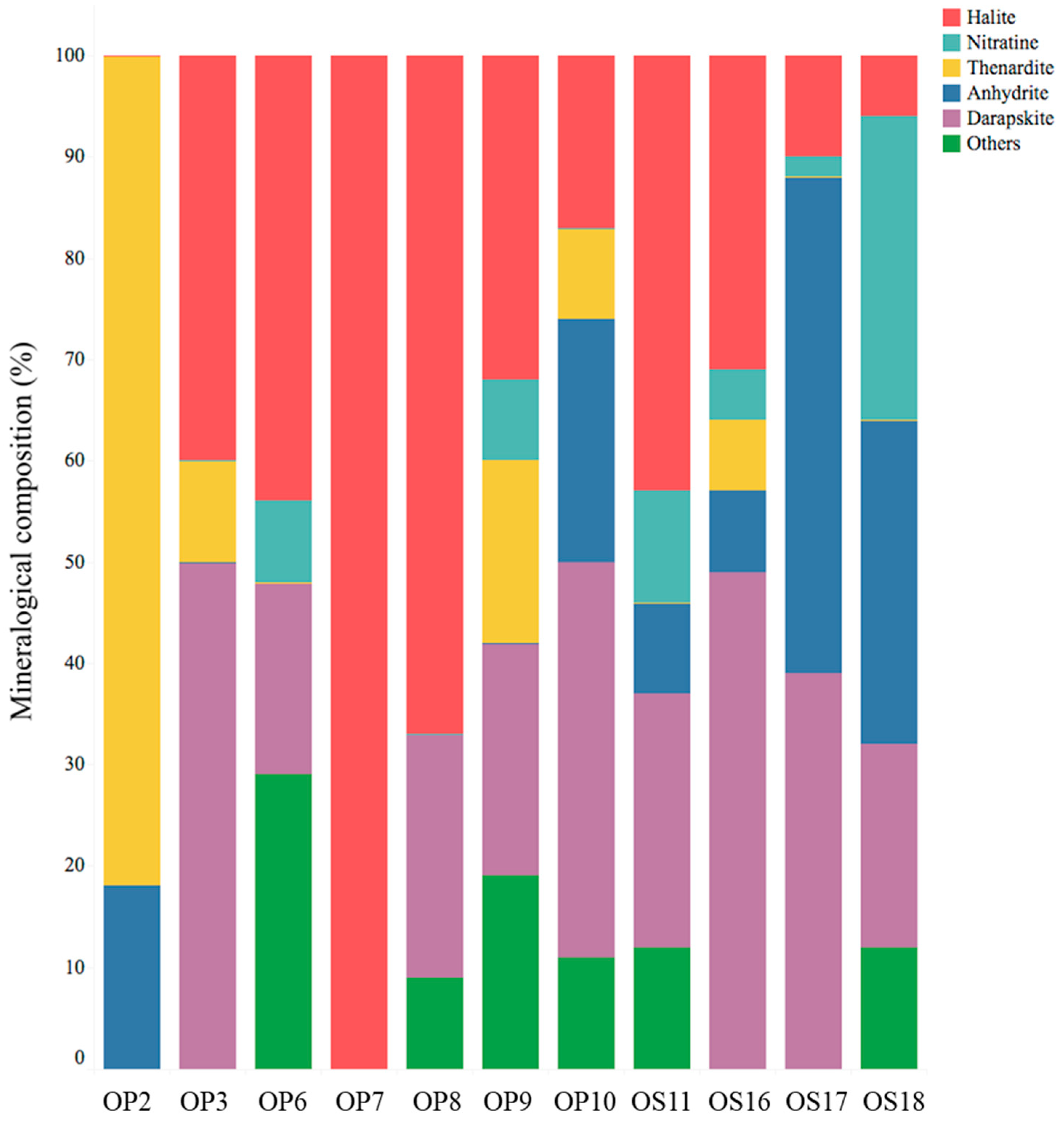
The XRD mineralogical analyses revealed that soils from NID sites consisted of a combination of minerals, including halite (NaCl), darapskite (Na3(SO4) (NO3)·H2O), nitratine (NaNO3), thenardite (Na2SO4), anhydrite (CaSO4), and other salts (see Figure 2). While the salt composition of NID soils was generally similar, variations in their salt contents were observed. However, there were some exceptions to this pattern: the soil at the OP2 site consisted of more than 80% thenardite and less than 20% anhydrite, the soil at the OP7 site was exclusively composed of halite, and neither OP2 nor OP7 contained darapskite. Prior studies on nitrate minerals in the Atacama Desert have identified nitratine (soda niter or Chilean saltpeter), darapskite, and humberstonite (Na7K3Mg2(SO4)6(NO3)2·6H2O) as the most abundant minerals [2,48]. Notably, darapskite is slightly soluble in water [49], and our study only detected it in soil samples that also contained halite.
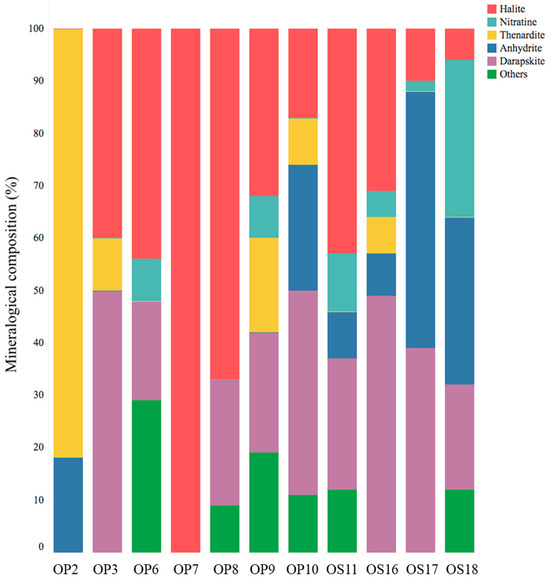
Figure 2.
Mineralogical composition, represented as a percentage, of NID soil samples from the Domeyko District, determined through X-ray diffraction analysis. Samples OP2, OP3, OP6, OP7, OP8, and OP9 were collected from Oficina Pissis, while samples OS11, OS16, OS17, and OS18 were collected from Oficina Savona.
Halite and nitratine salt crystals provide access to liquid water for microbial life through deliquescence in Atacama NIDs. Additionally, they serve as a protective shield against damaging UV photons while allowing the passage of photosynthetically active radiation, as discussed previously in the context of halite crusts as effective lithobiontic microhabitats [8,15,43,49].
3.2. Genomic Analysis of NID Soils
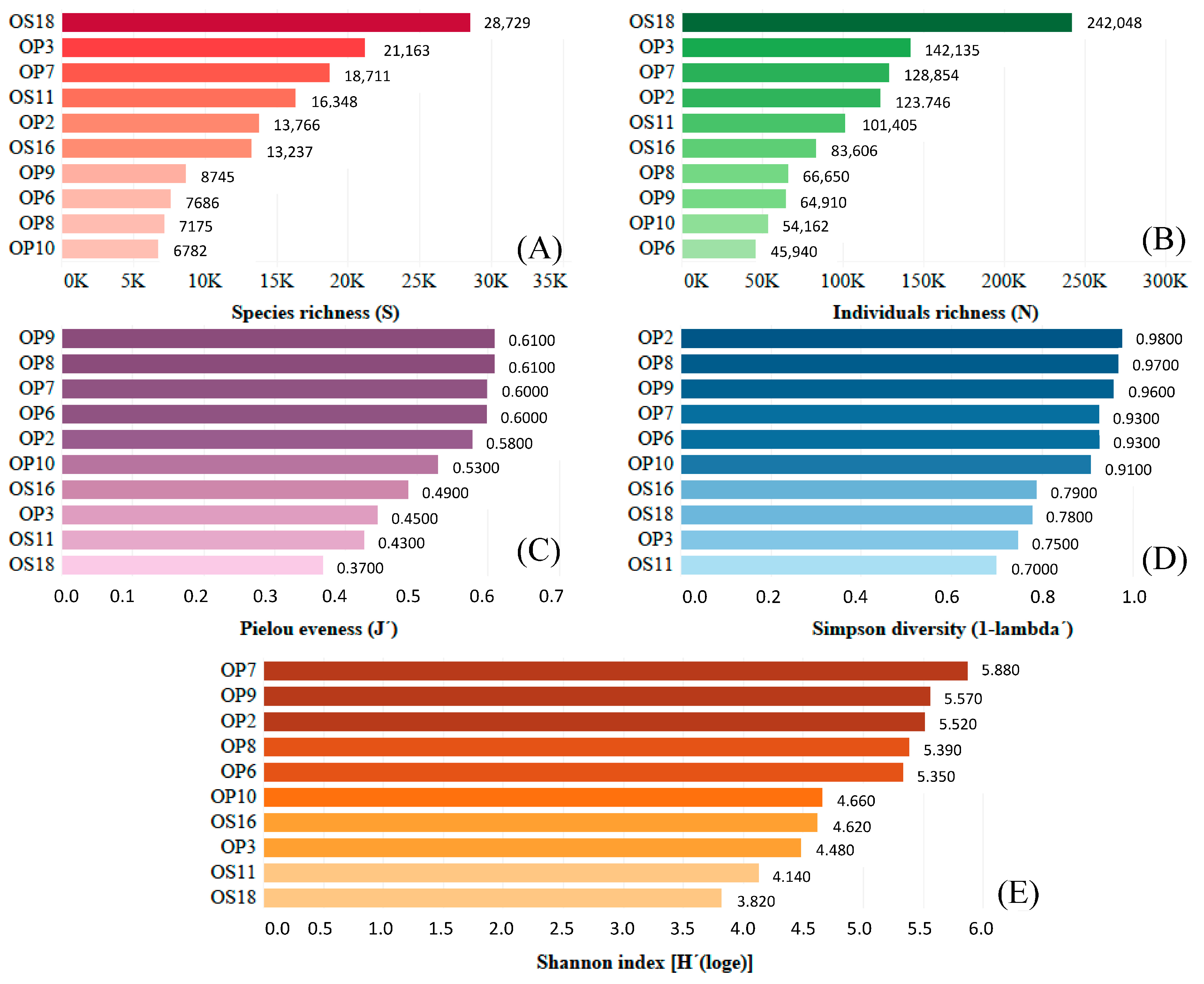
DNA extraction was completed for ten NID soil samples. Quality trimming of the raw sequence data resulted in 1,065,152 reads, with 83,570 sequences remaining after standardization using the Chao1 index for rarefaction. Ultimately, 14,234 operational taxonomic units (OTUs) were identified and defined with a 97% similarity threshold, considering all 10 samples. High levels of alpha diversity, richness, and evenness were observed in bacterial communities across all NID sampling sites studied (see Figure 3). This is evident in the Shannon and Simpson indices, with values ranging from 3.82 to 5.88 and from 0.70 to 0.98, respectively. Furthermore, species and individual richness varied from 6783 to 28,729 and 45,940 to 242,048, respectively. The Pielou evenness index produced values between 0.37 and 0.61. The remarkable microbial diversity discovered in Atacama NID strongly suggests that deliquescent minerals, such as halite, nitrate, and thenardite, provide the necessary moisture to support the development and sustenance of microbial life.
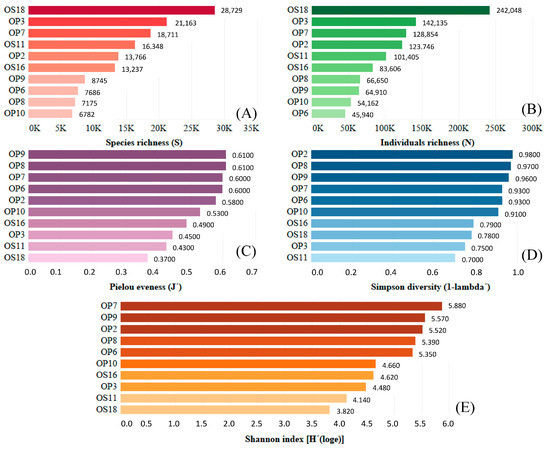
Figure 3.
Microbial diversity analyses from NID soils sampled at the Domeyko District, Atacama Desert. (A) Species richness in red; (B) individual richness in green; (C) Pielou evenness in purple; (D) Simpson diversity in blue; and (E) Shannon index in brown.
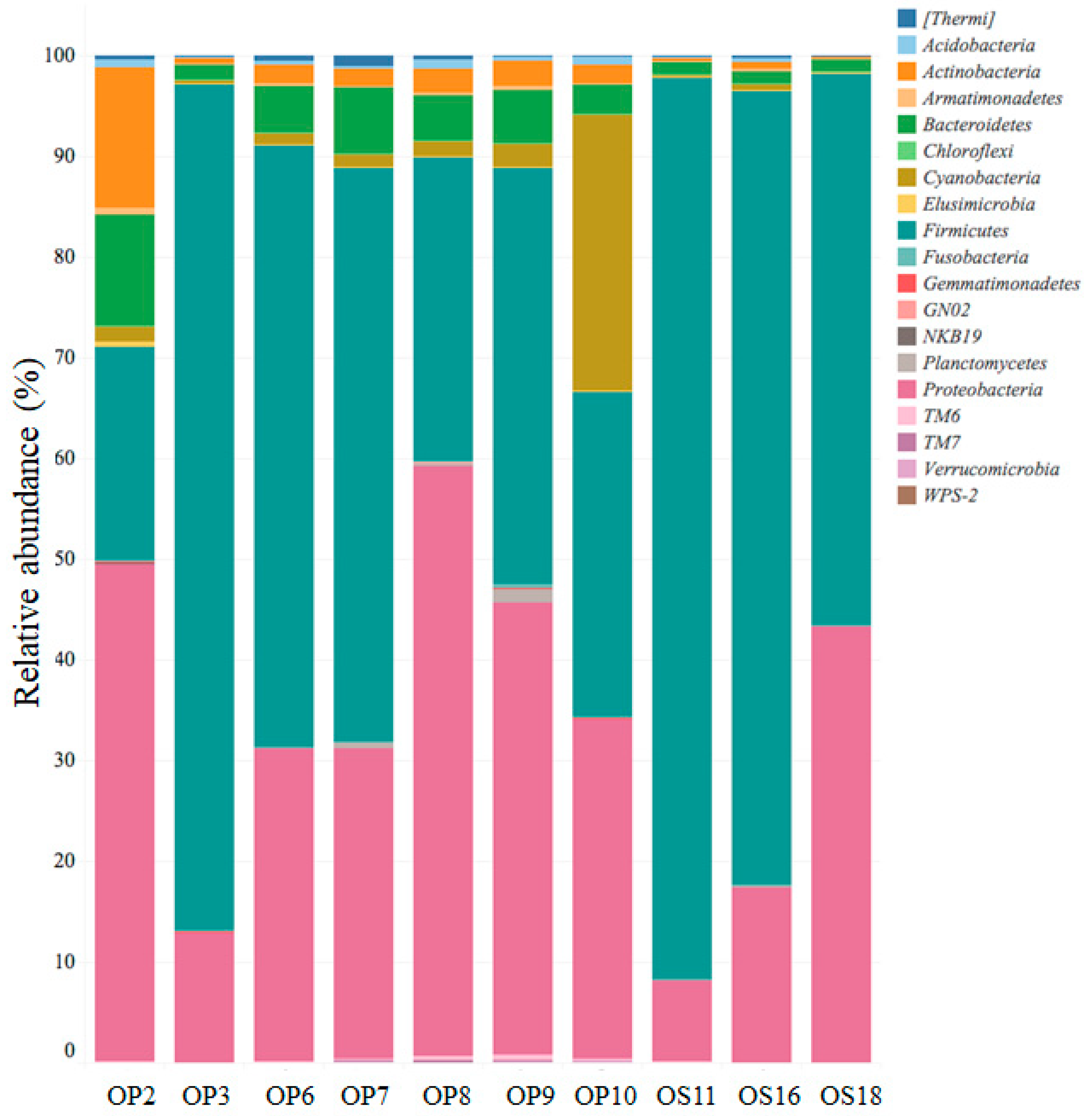
Genomic analyses of microbial life in Atacama NID soil samples revealed the dominance of four phyla: Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes (see Figure 4). A recent study conducted at the Yungay salt flat, located in the core of the Atacama Desert, also reported similar findings regarding microbial diversity, with the presence of less abundant phyla, including Cyanobacteria, Verrucomicrobia, Acidobacteria, and division TM7, among others [19]. The relative abundance of the phyla Bacteroidetes, Cyanobacteria, and Actinobacteria ranged from approximately 1% to 11%, 0.1% to 27%, and 0.2% to 14%, respectively, among the NID soil samples (refer to Figure 4).
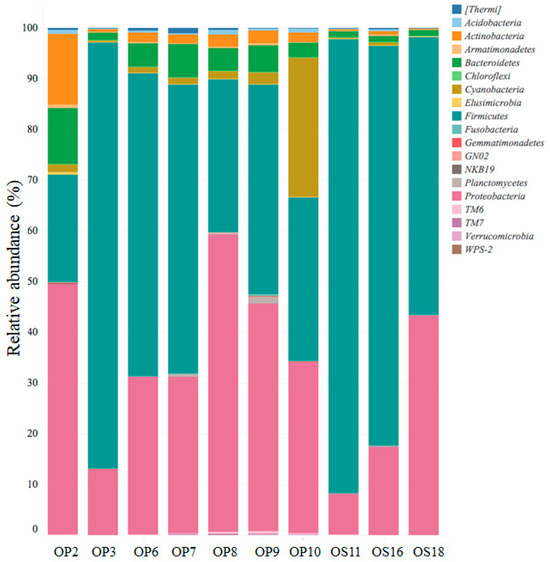
Figure 4.
Relative abundance of major bacterial phyla with 99% similarity.
While Actinobacteria exhibited a low relative abundance in NID soils, it is worth noting that culturable Actinobacteria have been previously reported at various sites in the Atacama Desert, with some strains identified as sources of bioactive molecules [11,50,51]. In comparison, the microbial diversity in soils from Yungay displayed a dominance of the phylum Actinobacteria, followed by Firmicutes, Proteobacteria, and TM7, aligning with findings from soils in other parts of the world [19,52].
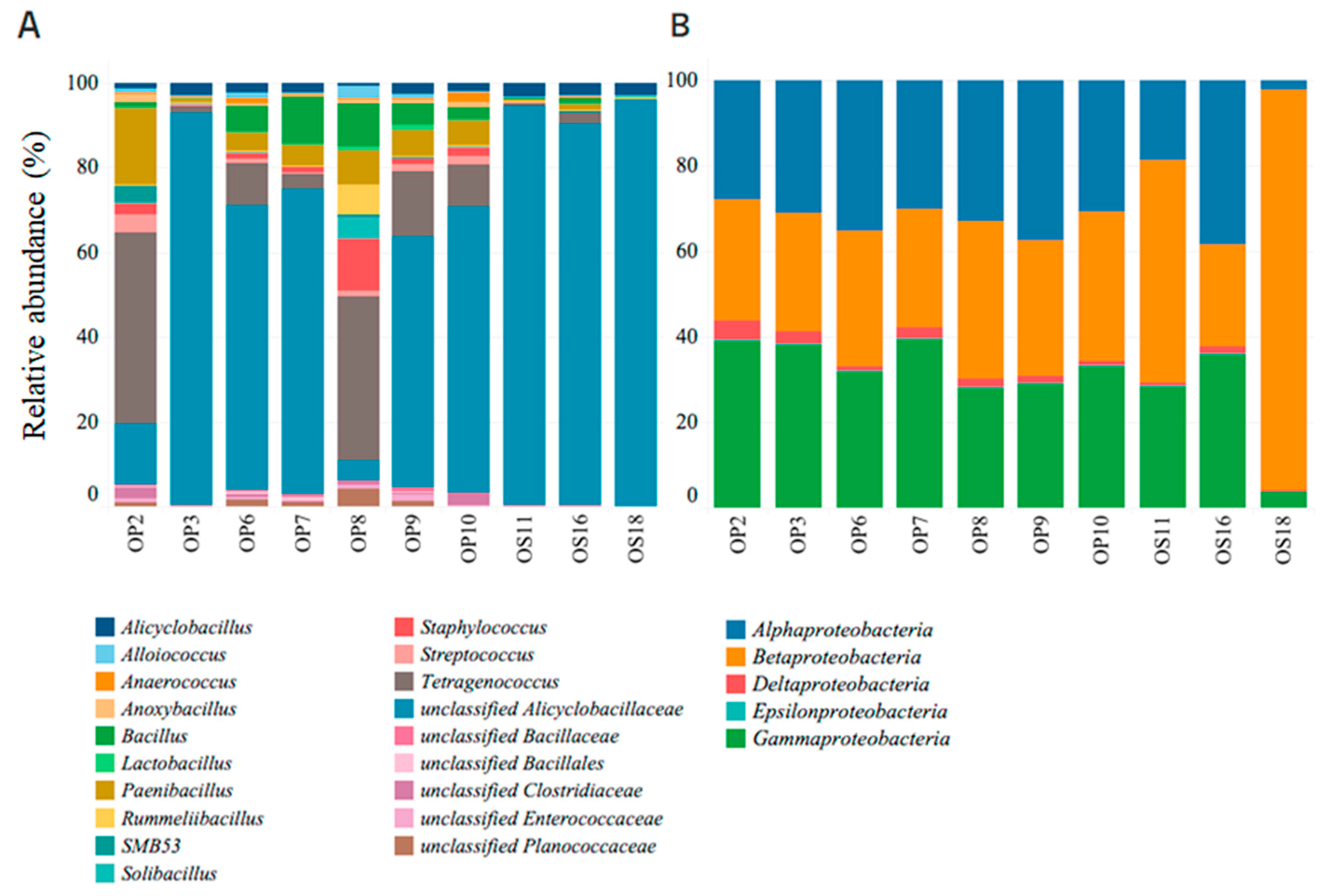
Within NID soil sites, Firmicutes and Proteobacteria were the dominant phyla, with the Firmicutes class exhibiting the highest relative abundance and diversity (refer to Figure 4 and Figure 5A). Notably, soil samples from sites OP3, OS11, OS16, and OS18 showed low diversity but were highly enriched (over 90%) in unclassified Alicyclobacillaceae. In contrast, other NID sites exhibited a more diverse array of microbial genera (see Figure 5A).
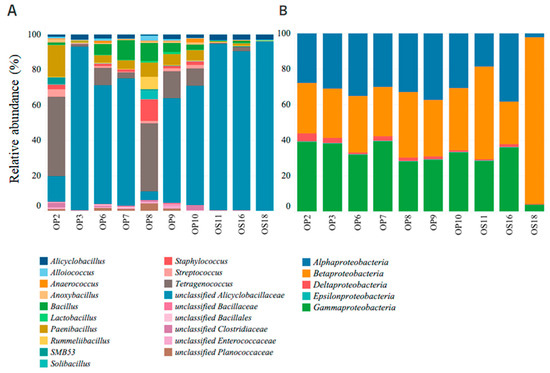
Figure 5.
Relative abundance of bacterial genera from classes Firmicutes (A) and Proteobacteria (B), identified at NID soil samples sites, with 99% similarity.
All NID soil sites exhibited the presence of the Proteobacteria phylum, ranging from approximately 8% to 60% relative abundance (see Figure 4). This phylum was primarily represented by the genera Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria, with site OS18 showing a significant enrichment in Betaproteobacteria (see Figure 5B). These findings align with previous studies emphasizing the importance of the Proteobacteria phylum and its major genera in Atacama soils containing nitrate, halite, and perchlorate [10,53,54].
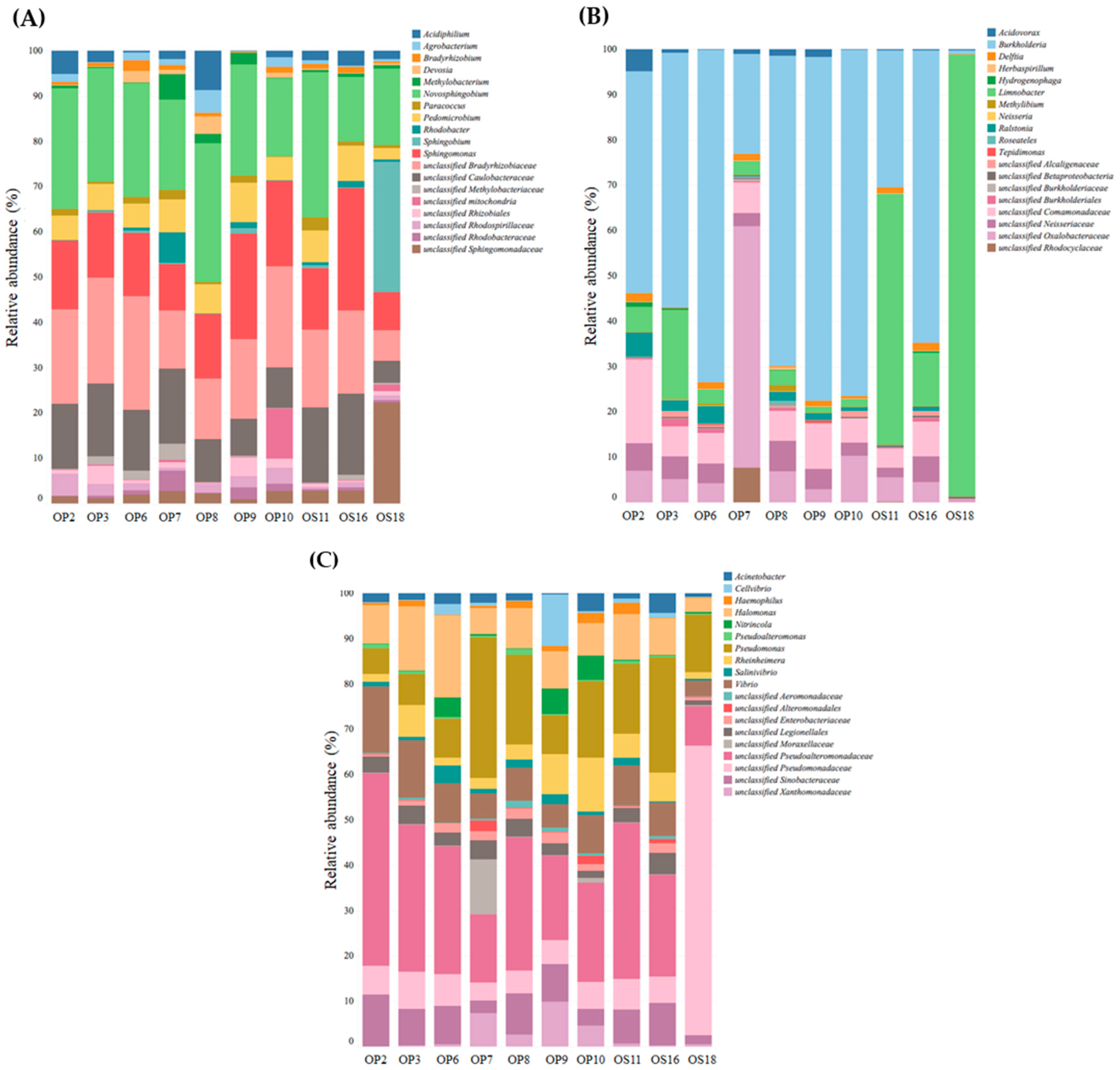
The information provided in Figure 6 presents the microbial genera identified within the Proteobacteria phylum in Atacama NID soils. Alphaproteobacteria were represented by Novosphingobium, unclassified Bradyrhizobiaceae, unclassified Rhizobiales, Sphingomonas, unclassified Sphingomonadaceae, unclassified Caulobacteraceae, and Pedomicrobium (see Figure 6A). Notably, Novosphingobium is known as a metabolically versatile bacterium found in various soil environments [55]. The Betaproteobacteria phylum in NID soils exhibited enrichment in genera such as Burkholderia, Limnobacter, unclassified Oxalobacteraceae, and unclassified Comamonadaceae (see Figure 6B). In the case of the Gammaproteobacteria phylum in Atacama NID soil samples, the dominant genera included Pseudomonas, unclassified Pseudoalteromonadaceae, Halomonas, and Vibrio (see Figure 6C).
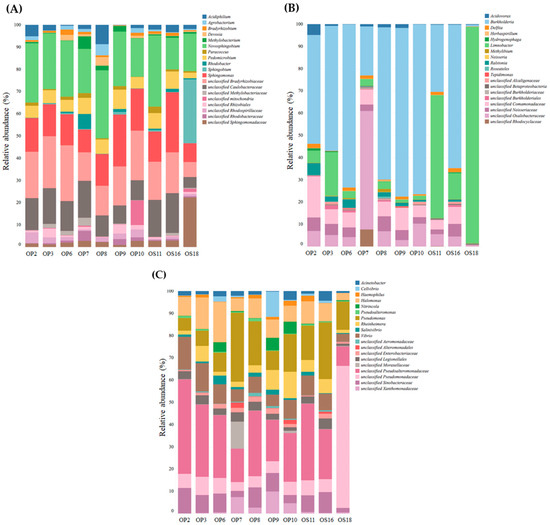
Figure 6.
Relative abundance of Alphaproteobacteria (A), Betaproteobacteria (B), and Gammaproteobacteria (C) on NID soil samples at the Domeyko District, Atacama Desert. The total number of reads was defined by 97% similarity.
Known members from the families Pseudoalteromonadaceae and Halomonadaceae have shown high metabolic versatility and adaptability to extreme environments [56,57]. Unclassified Pseudoalteromonadaceae from Gammaproteobacteria predominates in all samples tested; the family Pseudoalteromonadaceae includes many species with protein-producing members involved in the degradation and recycling of organic nitrogen, highly productive on secondary metabolites, and adaptable to various habitats [57]. Halomonas were also present in all NID samples; members of this genus can colonize and adapt to environments with high variability in temperature, salinity, and pH values [56]. These properties make them suitable for biotechnological applications, such as the production of polyhydroxyalkanoates [58].
3.3. An Insight on Nitrate/Perchlorate Reducing Bacteria in NID Samples
Soils from NIDs had a nitrate content ranging from ≤22 to 560 mg/kg, while perchlorate was below the detection limit (Table 2). This suggests that the microbial population in NIDs had adapted and grown under low concentrations of sodium chloride, nitrate, or perchlorate. Samples from sites OP6, OP8, OP9, OS14, and OS17 yielded enriched cultures capable of growing at 3 g/L NaCl under anaerobic conditions in 8 mM nitrate or 1 mM perchlorate. These enriched cultures exhibited an average nitrate reduction of 62.9 ± 6.4% and could remove up to 0.25 ± 0.06 g of nitrate. When grown with perchlorate, enriched cultures from OP6 soil showed a maximum perchlorate reduction of 18.6 ± 2.1% and removed 0.021 ± 0.01 g of nitrate.
Atacama soils have been proposed as a terrestrial analog for Martian soils [33]. Both Atacama and Mars share evidence of hygroscopic salts (such as halite, sulfate, and perchlorate) at the surface and subsurface of saline soils. Their deliquescence capabilities provide valuable insights into understanding the possibility of past or present microbial colonization on Mars.
Metagenomic analyses of NID soils revealed the presence of Betaproteobacteria and Alphaproteobacteria (specifically, the Azospirillum genus) (Figure 6). Many members of the phylum Proteobacteria are perchlorate-tolerant bacteria capable of catalyzing the reduction of perchlorate to chlorate and, subsequently, to chlorite. These substances are strong oxidizers with toxic and damaging effects on cells. However, perchlorate metabolism in bacteria and archaea may provide an alternative to environmental detoxification. This microbial activity depends on several factors, including the presence of oxygen, competing electron acceptors for anaerobic respiration, the availability of molybdenum, and the presence of chlorite dismutase, among others [13,50,59].
Nitrate serves as a potential source of biological nitrogen for microorganisms, supporting biomass production and acting as an energy source for chemotrophic metabolizers engaged in processes like denitrification, dissimilatory reduction of nitrate to ammonium (DNRA), and anaerobic ammonium oxidation [1,7]. Microbial DNRA plays a central role in nitrogen conservation within ecosystems, but its dynamics are influenced by various factors, including climate, oxygen levels, redox conditions, and organic carbon content in soils [51]. Notably, in addition to deliquescent salts, nitrate deposits have been identified at Gale Crater on Mars, with concentrations similar to those in Atacama sediments (70–1100 ppm) [1,2,36]. This discovery raises the possibility of extinct or extant chemotrophic microorganisms on Mars, adding to the realm of scientific speculation.
Finally, the enriched cultures from NID soils contain native nitrate–perchlorate-reducing bacterial populations. Once these populations are isolated and identified, the anaerobic chemotrophs from NIDs can serve as valuable experimental models to enhance our understanding of their reductive nitrate and perchlorate metabolism.
4. Conclusions
This study employs metagenomic and culturing approaches to shed light on the presence, relative abundance, and diversity of microbial life within nitrogen/iodide deposits in the Atacama Desert’s arid heart. These nitrogen/iodide deposits are considered ‘islands of fertility’ within one of our planet’s driest and oldest deserts. Here, the deliquescence of salts provides a source of liquid water, sustaining a diverse community of bacteria, with Firmicutes and Proteobacteria as the dominant phyla.
Water availability in these halite–nitrate-rich deposits, as indicated by water activity (Aw) values, hovers close to the lower limit required for cellular division. These soils are replete with hygroscopic salts and serve as a nitrogen source through darapskite and nitratine, albeit containing minimal levels of perchlorate. Notably, these nitrogen/iodide deposits house a native nitrate/perchlorate-reducing bacteria population whose activity was validated in enriched cultures. Further experiments will offer invaluable insights into these reducing bacteria’s metabolic roles, particularly in providing biological nitrogen and detoxifying perchlorate.
Author Contributions
Conceptualization, M.C., D.A. and L.V.E.; methodology, M.C., D.A. and L.V.E.; formal analysis, P.A., O.E. and D.C.; investigation, M.C., D.A. and L.V.E.; resources, C.S.-A. and D.C.A.; data curation, D.A., B.G.-S. and L.V.E.; writing—original draft preparation, M.C., D.A. and L.V.E.; writing—review and editing, N.T., D.A., B.G.-S. and L.V.E.; visualization, C.S.-A. and M.C.; supervision, D.A. and L.V.E.; project administration, L.V.E.; funding acquisition, D.A. and L.V.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agencia Nacional de Investigación y Desarrollo-ANID through the projects Fondecyt Initiation # 11150686 and Fondecyt Postdoctoral # 3210675.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
We thank C. Demergasso and G. Chong for their guidance and assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Shen, J.; Zerkle, A.L.; Stueeken, E.; Claire, M.W. Nitrates as a Potential N Supply for Microbial Ecosystems in a Hyperarid Mars Analog System. Life 2019, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Ericksen, G.E. The Chilean Nitrate Deposits. Am. Sci. 1983, 71, 366–374. [Google Scholar]
- Mpodozis, C.; Allmendinger, R.W. Extensional Tectonics, Cretaceous Andes, Northern Chile (27°S). Geol. Soc. Am. Bull. 1993, 105, 1462–1477. [Google Scholar] [CrossRef]
- Maksaev, V. Metallogeny. Geological Evolution and Thermochronology of the Chilcan Andes Between Latitudes 21° and 26° South. and the Origin of Major Porphyry Copper Deposits. Ph.D. Thesis, Dalhousie University, Halifax, NS, Canada, 1990; 544p. [Google Scholar]
- Manning, C.V.; Zahnle, K.J.; McKay, C.P. Impact Processing of Nitrogen on Early Mars. Icarus 2009, 199, 273–285. [Google Scholar] [CrossRef]
- Navarro-González, R.; Navarro, K.F.; Coll, P.; McKay, C.P.; Stern, J.C.; Sutter, B.; Archer, P.D.; Buch, A.; Cabane, M.; Conrad, P.G.; et al. Abiotic Input of Fixed Nitrogen by Bolide Impacts to Gale Crater during the Hesperian: Insights from the Mars Science Laboratory. J. Geophys. Res. Planets 2019, 124, 94–113. [Google Scholar] [CrossRef]
- Van de Graaf, A.A.; Mulder, A.; De Bruijn, P.; Jetten, M.S.M.; Robertson, L.A.; Kuenen, J.G. Anaerobic Oxidation of Ammonium Is a Biologically Mediated Process. Appl. Environ. Microbiol. 1995, 61, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Silva, B. Lithobiontic Life: “Atacama Rocks Are Well and Alive”. Antonie Van Leeuwenhoek Int. J. General. Mol. Microbiol. 2018, 111, 1333–1343. [Google Scholar] [CrossRef]
- Sajjad, W.; Ilahi, N.; Kang, S.; Bahadur, A.; Zada, S.; Iqbal, A. Endolithic Microbes of Rocks, Their Community, Function and Survival Strategies. Int. Biodeterior. Biodegrad. 2022, 169, 105387. [Google Scholar] [CrossRef]
- Parro, V.; De Diego-Castilla, G.; Moreno-Paz, M.; Blanco, Y.; Cruz-Gil, P.; Rodríguez-Manfredi, J.A.; Fernández-Remolar, D.; Gómez, F.; Gómez, M.J.; Rivas, L.A.; et al. A Microbial Oasis in the Hypersaline Atacama Subsurface Discovered by a Life Detector Chip: Implications for the Search for Life on Mars. Astrobiology 2011, 11, 969–996. [Google Scholar] [CrossRef]
- Gómez-Silva, B.; Vilo-Muñoz, C.; Galetović, A.; Dong, Q.; Castelán-Sánchez, H.G.; Pérez-Llano, Y.; Sánchez-Carbente, M.D.R.; Dávila-Ramos, S.; Cortés-López, N.G.; Martínez-ávila, L.; et al. Metagenomics of Atacama Lithobiontic Extremophile Life Unveils Highlights on Fungal Communities, Biogeochemical Cycles and Carbohydrate-Active Enzymes. Microorganisms 2019, 7, 94–113. [Google Scholar] [CrossRef]
- Gómez-Silva, B.; Batista-García, R.A. The Atacama Desert: A Biodiversity Hotspot and Not Just a Mineral-Rich Region. Front. Microbiol. 2022, 13, 812842. [Google Scholar] [CrossRef] [PubMed]
- Flores, N.; Hoyos, S.; Venegas, M.; Galetović, A.; Zúñiga, L.M.; Fábrega, F.; Paredes, B.; Salazar-Ardiles, C.; Vilo, C.; Ascaso, C.; et al. Haloterrigena sp. Strain SGH1, a Bacterioruberin-Rich, Perchlorate-Tolerant Halophilic Archaeon Isolated from Halite Microbial Communities, Atacama Desert, Chile. Front. Microbiol. 2020, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Warren-Rhodes, K.A.; Rhodes, K.L.; Pointing, S.B.; Ewing, S.A.; Lacap, D.C.; Gómez-Silva, B.; Amundson, R.; Friedmann, E.I.; McKay, C.P. Hypolithic Cyanobacteria, Dry Limit of Photosynthesis, and Microbial Ecology in the Hyperarid Atacama Desert. Microb. Ecol. 2006, 52, 389–398. [Google Scholar] [CrossRef] [PubMed]
- de los Ríos, A.; Valea, S.; Ascaso, C.; Davila, A.; Kastovsky, J.; McKay, C.P.; Gómez-Silva, B.; Ierzchos, J. Comparative Analysis of the Microbial Communities Inhabiting Halite Evaporates of the Atacama Desert. Int. Microbiol. 2010, 13, 79–89. [Google Scholar] [CrossRef]
- Gómez-Silva, B.; Rainey, F.A.; Warren-Rhodes, K.A.; Mckay, C.P.; Navarro-González, R. Atacama Desert Soil Microbiology. In Micorbiology Extrem Soils; Springer: Berlin/Heidelberg, Germany, 2008; pp. 117–132. [Google Scholar] [CrossRef]
- Orellana, G.; Gómez-Silva, B.; Urrutia, M.; Galetović, A. Uv-a Irradiation Increases Scytonemin Biosynthesis in Cyanobacteria Inhabiting Halites at Salar Grande, Atacama Desert. Microorganisms 2020, 8, 1690. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Gelsinger, D.R.; Ma, B.; Wierzchos, J.; Ravel, J.; Davila, A.; Casero, M.C.; DiRuggiero, J. Functional Interactions of Archaea, Bacteria and Viruses in a Hypersaline Endolithic Community. Environ. Microbiol. 2016, 18, 2064–2077. [Google Scholar] [CrossRef]
- Fuentes, B.; Choque, A.; Gómez, F.; Alarcón, J.; Castro-Nallar, E.; Arenas, F.; Contreras, D.; Mörchen, R.; Amelung, W.; Knief, C.; et al. Influence of Physical-Chemical Soil Parameters on Microbiota Composition and Diversity in a Deep Hyperarid Core of the Atacama Desert. Front. Microbiol. 2022, 12, 794743. [Google Scholar] [CrossRef]
- Reutter, K.J.; Scheuber, E.; Wigger, P. Tectonics of the Southern Central Andes: Structure and Evolution of an Active Continental Margin; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1994; ISBN 9783642773556. [Google Scholar]
- de Geología y Minería, C.S.N.; Marinovic, N. Carta Oficina Domeyko: Región de Antofagasta; Servicio Nacional de Geología y Minería. 2007. Available online: https://tiendadigital.sernageomin.cl/en/-basic-geology/3137-carta-oficina-domeyko-region-de-antofagasta.html (accessed on 10 September 2023).
- Schumacher, B.A.; Shines, K.C.; Burton, J.V.; Papp, M.L. Comparison of three methods for soil homogenization. Soil. Sci. Soc. Am. J. 1990, 54, 1187–1190. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Nomura, Y. A Simple Method to Determine the Water Activity of Ethanol-Containing Samples. Biotechnol. Bioeng. 1999, 62, 242–245. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; De Waal, E.C.; Uitterlinden, A.G. Profiling of Complex Microbial Populations by Denaturing Gradient Gel Electrophoresis Analysis of Polymerase Chain Reaction-Amplified Genes Coding for 16S RRNA. Appl. Environ. Microbiol. 1993, 59, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High- Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; Desantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A Flexible Tool for Aligning Sequences to a Template Alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- Clarke, K.R.; Somerfield, P.J.; Gorley, R.N. Clustering in Non-Parametric Multivariate Analyses. J. Exp. Mar. Biol. Ecol. 2016, 483, 147–155. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Krona-385.Pdf. BMC Bioinform. 2011, 12, 385. [Google Scholar]
- Herman, D.C.; Frankenberger, W.T. Bacterial Reduction of Perchlorate and Nitrate in Water. J. Environ. Qual. 1999, 28, 1018–1024. [Google Scholar] [CrossRef]
- Wierzchos, J.; Casero, M.C.; Artieda, O.; Ascaso, C. Endolithic Microbial Habitats as Refuges for Life in Polyextreme Environment of the Atacama Desert. Curr. Opin. Microbiol. 2018, 43, 124–131. [Google Scholar] [CrossRef]
- Navarro-González, R.; Rainey, F.A.; Molina, P.; Bagaley, D.R.; Hollen, B.J.; De La Rosa, J.; Small, A.M.; Quinn, R.C.; Grunthaner, F.J.; Cáceres, L.; et al. Mars-Like Soils in the Atacama Desert, Chile, and the Dry Limit of Microbial Life. Science 2003, 302, 1018–1021. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Chong, G.; Jensen, A. Neogene Evaporites in Desert Volcanic Environments: Atacama Desert, Northern Chile. Sedimentology 2001, 48, 1411–1431. [Google Scholar] [CrossRef]
- Smith, W.L.; Gadd, G.M. Reduction and Precipitation of Chromate by Mixed Culture Sulphate-Reducing Bacterial Biofilms. J. Appl. Microbiol. 2000, 88, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Walvoord, M.A.; Phillips, F.M.; Stonestrom, D.A.; Evans, R.D.; Hartsough, P.C.; Newman, B.D.; Striegl, R.G. A Reservoir of Nitrate Beneath Desert Soils. Science 2003, 302, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- McCalley, C.K.; Sparks, J.P. McCalley Abiotic Gas Formation Drives Nitrogen Loss from a Desert Ecosystem. Science 2009, 326, 837–841. [Google Scholar] [CrossRef]
- Jackson, W.A.; Böhlke, J.K.; Andraski, B.J.; Fahlquist, L.; Bexfield, L.; Eckardt, F.D.; Gates, J.B.; Davila, A.F.; McKay, C.P.; Rao, B.; et al. Global Patterns and Environmental Controls of Perchlorate and Nitrate Co-Occurrence in Arid and Semi-Arid Environments. Geochim. Cosmochim. Acta 2015, 164, 502–522. [Google Scholar] [CrossRef]
- Hallsworth, J.E. Salt Deliquescence Can Support Extraterrestrial Life. Nat. Astron. 2020, 4, 739–740. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Delgado-Baquerizo, M.; An King, P.T.; Benham, M.; Arca, V.; Power, S.A. Ecosystem Type and Resource Quality Are More Important than Global Change Drivers in Regulating Early Stages of Litter Decomposition. Soil. Biol. Biochem. 2019, 129, 144–152. [Google Scholar] [CrossRef]
- Stevenson, A.; Burkhardt, J.; Cockell, C.S.; Cray, J.A.; Dijksterhuis, J.; Fox-Powell, M.; Kee, T.P.; Kminek, G.; Mcgenity, T.J.; Timmis, K.N.; et al. Multiplication of Microbes below 0.690 Water Activity: Implications for Terrestrial and Extraterrestrial Life. Environ. Microbiol. 2015, 17, 257–277. [Google Scholar] [CrossRef]
- Rivera-Valentín, E.G.; Chevrier, V.F.; Soto, A.; Martínez, G. Distribution and Habitability of (Meta)Stable Brines on Present-Day Mars. Nat. Astron. 2020, 4, 756–761. [Google Scholar] [CrossRef]
- Davila, A.F.; Gómez-Silva, B.; de los Rios, A.; Ascaso, C.; Olivares, H.; McKay, C.P.; Wierzchos, J. Facilitation of Endolithic Microbial Survival in the Hyperarid Core of the Atacam Desert by Mineral Deliquescence. J. Geophys. Res. Biogeosci 2008, 113, G1. [Google Scholar] [CrossRef]
- Davila, A.F.; Duport, L.G.; Melchiorri, R.; Jänchen, J.; Valea, S.; De Los Rios, A.; Fairén, A.G.; Möhlmann, D.; McKay, C.P.; Ascaso, C.; et al. Hygroscopic Salts and the Potential for Life on Mars. Astrobiology 2010, 10, 617–628. [Google Scholar] [CrossRef]
- Hausrath, E.M.; Adcock, C.T.; Bechtold, A.; Beck, P.; Benison, K.; Brown, A.; Cardarelli, E.L.; Carman, N.A.; Chide, B.; Christian, J.; et al. An Examination of Soil Crusts on the Floor of Jezero Crater, Mars. J. Geophys. Res. Planets 2023, 128, e2022JE007433. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. Methods Soil. Anal. Part Chem. Methods 1996, 9, 961–1010. [Google Scholar] [CrossRef]
- Zavalaga, C.B.; Hardesty, J.; Mori, G.P.; Chávez-Villavicencio, C.; Tello, A. Current Status of Peruvian Terns Sternula Lorata in Perú: Threats, Conservation and Research Priorities. Bird. Conserv. Int. 2009, 19, 175–186. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Chong, G.; Vega, M. Mineralogía y Evolución de Las Salmueras Madres En El Yacimiento de Nitratos Pedro de Valdivia, Antofagasta, Chile. Rev. Geológica Chile 1998, 25, 3–15. [Google Scholar] [CrossRef]
- Cockell, C.S.; Raven, J.A. Zones of Photosynthetic Potential on Mars and the Early Earth. Icarus 2004, 169, 300–310. [Google Scholar] [CrossRef]
- Zhang, H.; Bruns, M.A.; Logan, B.E. Perchlorate Reduction by a Novel Chemolithoautotrophic, Hydrogen-Oxidizing Bacterium; Environ. Microbiol. 2002, 4, 570–576. [Google Scholar]
- Cheng, Y.; Elrys, A.S.; Merwad, A.R.M.; Zhang, H.; Chen, Z.; Zhang, J.; Cai, Z.; Müller, C. Global Patterns and Drivers of Soil Dissimilatory Nitrate Reduction to Ammonium. Environ. Sci. Technol. 2022, 56, 3791–3800. [Google Scholar] [CrossRef]
- Connon, S.A.; Lester, E.D.; Shafaat, H.S.; Obenhuber, D.C.; Ponce, A. Bacterial Diversity in Hyperarid Atacama Desert Soils. J. Geophys. Res. Biogeosci 2007, 112, 1–9. [Google Scholar] [CrossRef]
- Costello, E.K.; Halloy, S.R.P.; Reed, S.C.; Sowell, P.; Schmidt, S.K. Fumarole-Supported Islands of Biodiversity within a Hyperarid, High-Elevation Landscape on Socompa Volcano, Puna de Atacama, Andes. Appl. Environ. Microbiol. 2009, 75, 735–747. [Google Scholar] [CrossRef]
- Campos, V.L.; Escalante, G.; Yañez, J.; Zaror, C.A.; Mondaca, M.A. Isolation of Arsenite-Oxidizing Bacteria from a Natural Biofilm Associated to Volcanic Rocks of Atacama Desert, Chile. J. Basic. Microbiol. 2009, 49, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Comparative Genomic Analysis Reveals Habitat-Specific Genes and Regulatory Hubs within the Genus Novosphingobium Roshan. MSystems 2017, 2, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Mormile, M.R.; Romine, M.F.; Garcia, M.T.; Ventosa, A.; Bailey, T.J.; Peyton, B.M. Halomonas campisalis sp.nov., a Denitrifying, Moderately Haloalkaliphilic Bacterium. Syst. Appl. Microbiol. 1999, 22, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, M.; Huang, J.; Guo, X.; Zhang, Y.; Liu, D.; Wu, R.; He, H.; Wang, J. Diversity of the Microbial Community and Cultivable Protease-Producing Bacteria in the Sediments of the Bohai Sea, Yellow Sea and South China Sea. PLoS ONE 2019, 14, e0215328. [Google Scholar] [CrossRef]
- Thomas, T.; Elain, A.; Bazire, A.; Bruzaud, S. Complete Genome Sequence of the Halophilic PHA-Producing Bacterium Halomonas sp. SF2003: Insights into Its Biotechnological Potential. World J. Microbiol. Biotechnol. 2019, 35, 50. [Google Scholar] [CrossRef]
- Nozawa-Inoue, M.; Scow, K.M.; Rolston, D.E. Reduction of Perchlorate and Nitrate by Microbial Communities in Vadose Soil. Appl. Environ. Microbiol. 2005, 71, 3928–3934. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).