Soil Microbial Community Structures under Annual and Perennial Crops Treated with Different Nitrogen Fertilization Rates
Abstract
1. Introduction
2. Materials and Methods
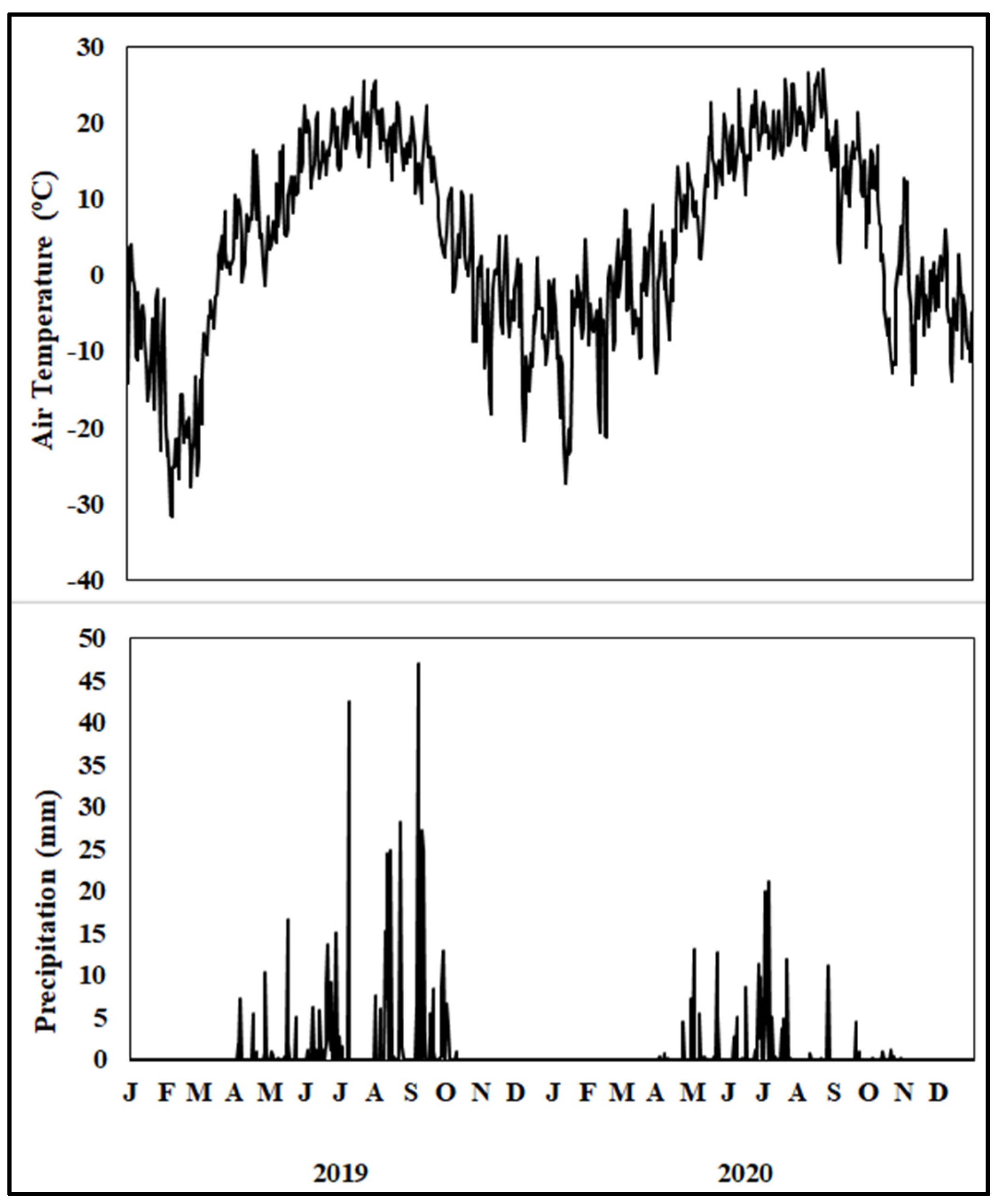
2.1. Study Site
2.2. Soil Sampling
2.3. Soil Analysis for Phospholipid Fatty Acid and Neutral Lipid Fatty Acid
2.4. Statistical Data Analysis
3. Results
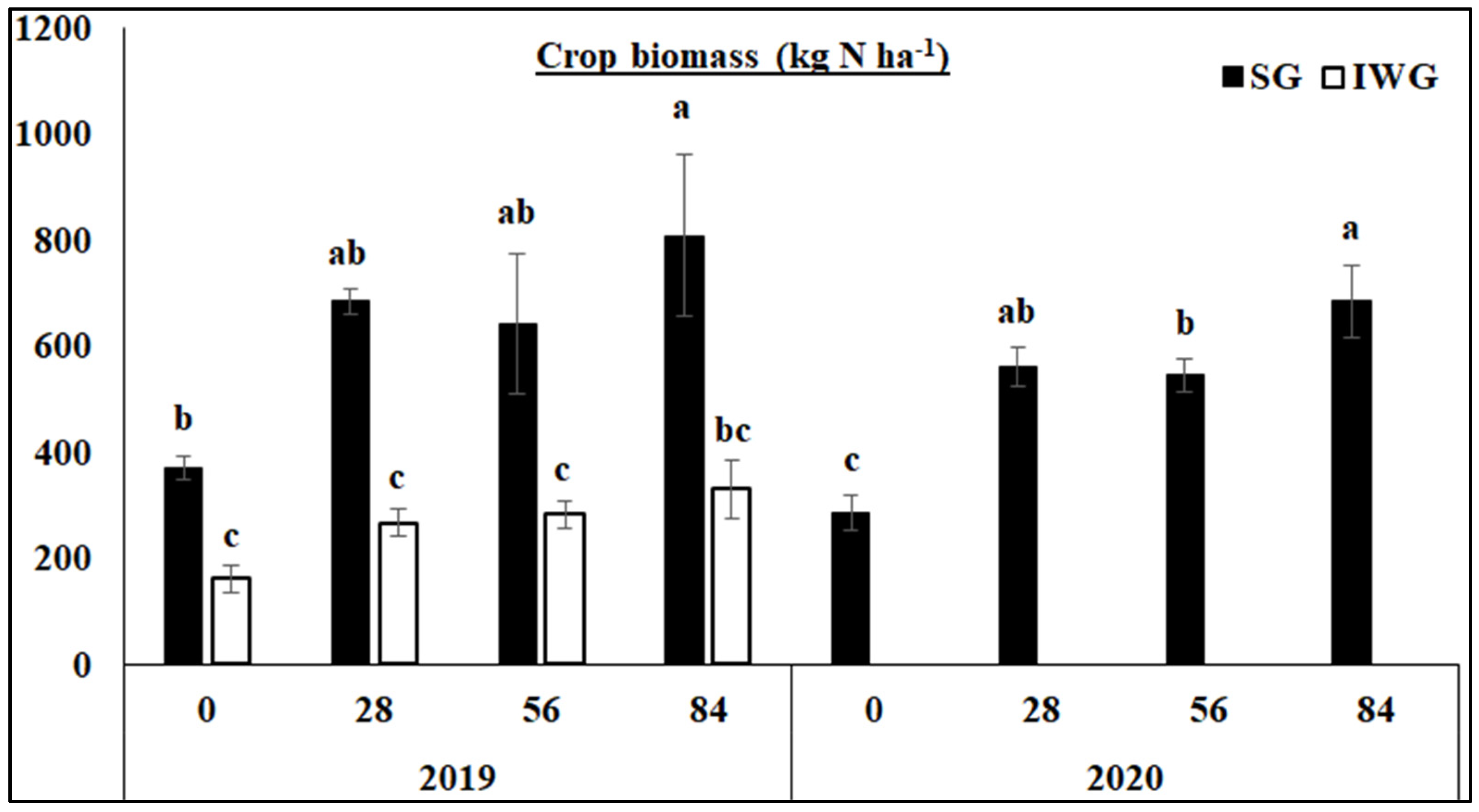
3.1. Aboveground Biomass
3.2. Soil Chemical Properties
3.3. Microbial Community Composition
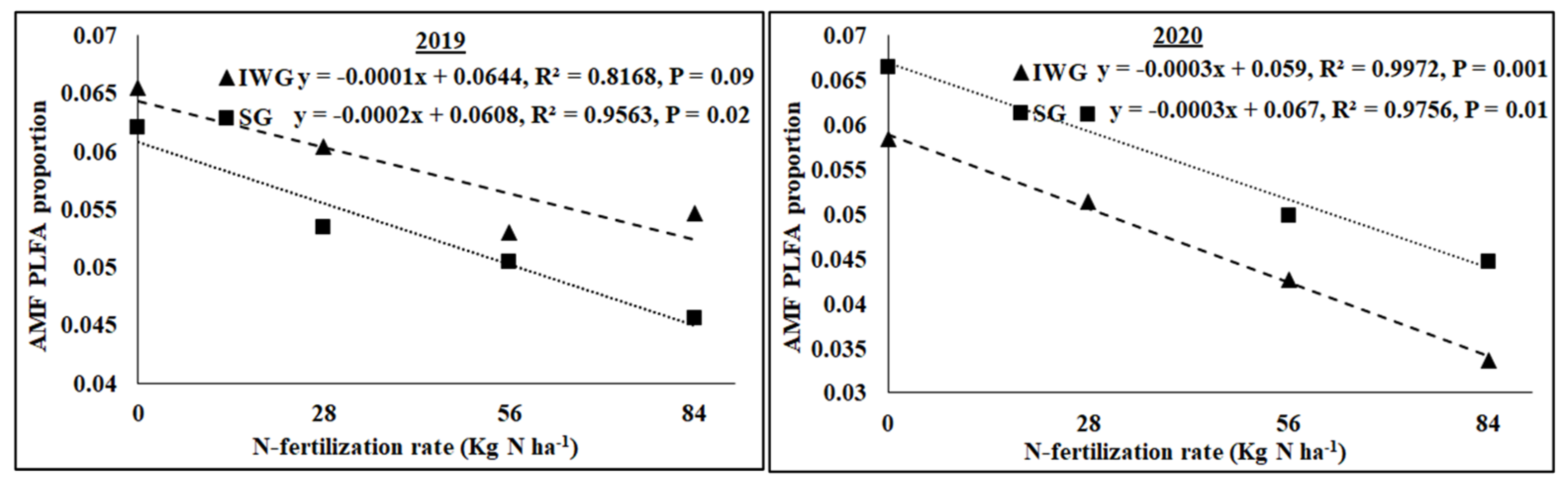
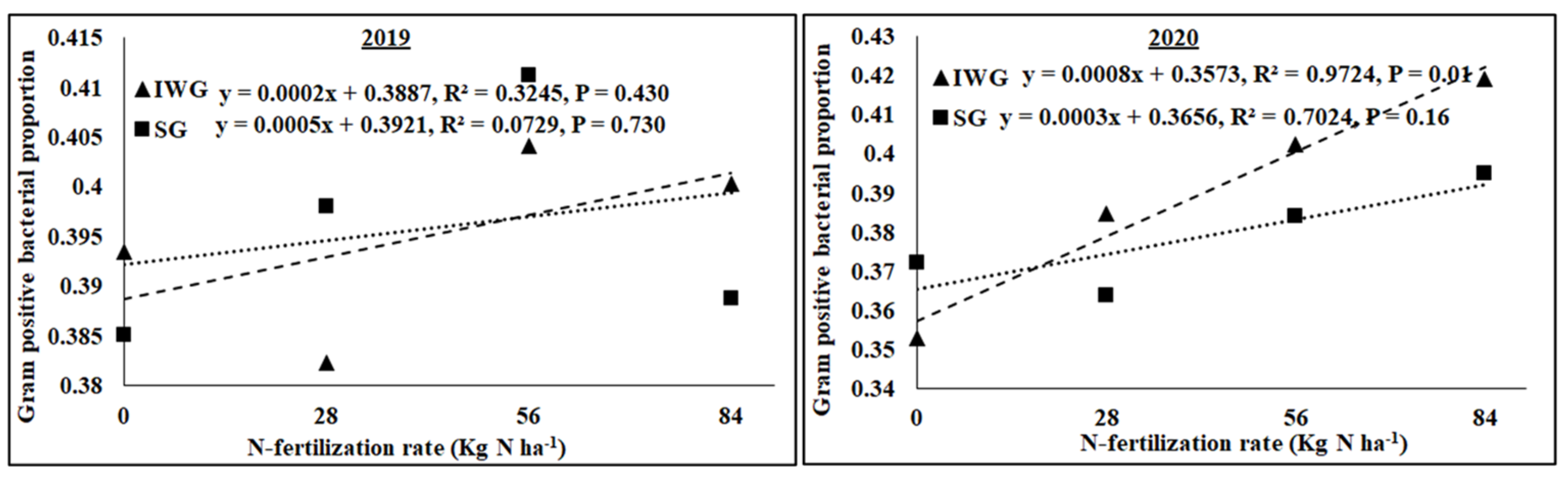
3.4. Microbial Community Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
USDA Disclaimer
References
- Robertson, G.P.; Hamilton, S.K.; Barham, B.L.; Dale, B.E.; Izaurralde, R.C.; Jackson, R.D.; Landis, D.A.; Swinton, S.M.; Thelen, K.D.; Tiedje, J.M. Cellulosic biofuel contributions to a sustainable energy future: Choices and outcomes. Science 2017, 356, eaal2324. [Google Scholar] [CrossRef]
- Sainju, U.M.; Allen, B.L.; Lenssen, A.W.; Ghimire, R.P. Root biomass, root/shoot ratio, and soil water content under perennial grasses with different nitrogen rates. Field Crops Res. 2017, 210, 183–191. [Google Scholar] [CrossRef]
- Sainju, U.M.; Allen, B.L.; Lenssen, A.W.; Mikha, M. Root and soil total carbon and nitrogen under bioenergy perennial grasses with various nitrogen rates. Biomass Bioenergy 2017, 107, 326–334. [Google Scholar] [CrossRef]
- Sekaran, U.; McCoy, C.; Kumar, S.; Subramanian, S. Soil microbial community structure and enzymatic activity responses to nitrogen management and landscape positions in switchgrass (Panicum virgatum L.). GCB Bioenergy 2019, 11, 836–851. [Google Scholar] [CrossRef]
- Smith, C.M.; David, M.B.; Mitchell, C.A.; Masters, M.D.; Anderson-Teixeira, K.J.; Bernacchi, C.J.; DeLucia, E.H. Reduced Nitrogen Losses after Conversion of Row Crop Agriculture to Perennial Biofuel Crops. J. Environ. Qual. 2013, 42, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canqui, H.; Ferguson, R.B.; Jin, V.L.; Schmer, M.R.; Wienhold, B.J.; Tatarko, J. Can cover crop and manure maintain soil properties after stover removal from irrigated no-till corn? Soil Sci. Soc. Am. J. 2014, 78, 1368–1377. [Google Scholar] [CrossRef]
- Vogel, K.P.; Mitchell, R.B. Heterosis in Switchgrass: Biomass Yield in Swards. Crop Sci. 2008, 48, 2159–2164. [Google Scholar] [CrossRef]
- Perrin, R.; Vogel, K.; Schmer, M.; Mitchell, R. Farm-Scale Production Cost of Switchgrass for Biomass. BioEnergy Res. 2008, 1, 91–97. [Google Scholar] [CrossRef]
- Liebig, M.A.; Johnson, H.A.; Hanson, J.D.; Frank, A.B. Soil carbon under switchgrass stands and cultivated cropland. Biomass Bioenergy 2005, 28, 347–354. [Google Scholar] [CrossRef]
- Sainju, U.M.; Allen, B.L.; Lenssen, A.W. Soil total carbon and nitrogen under long-term perennial bioenergy crops receiving various nitrogen fertilization rates. Agron. J. 2023, 115, 2216–2226. [Google Scholar] [CrossRef]
- Schmer, M.R.; Vogel, K.P.; Mitchell, R.B.; Perrin, R.K. Net Energy of Cellulosic Ethanol from Switchgrass. Proc. Natl. Acad. Sci. USA 2008, 105, 464–469. [Google Scholar] [CrossRef]
- Crews, T.E.; Rumsey, B.E. What agriculture can learn from native ecosystems in building soil organic matter: A review. Sustainability 2017, 9, 578. [Google Scholar] [CrossRef]
- Culman, S.W.; Snapp, S.S.; Ollenburger, M.; Basso, B.; DeHaan, L.R. Soil and water quality rapidly responds to the perennial grain Kernza wheatgrass. Agron. J. 2013, 105, 735–744. [Google Scholar] [CrossRef]
- Leff, J.W.; Jones, S.E.; Prober, S.M.; Barberán, A.; Borer, E.T.; Firn, J.L.; Harpole, W.S.; Hobbie, S.E.; Hofmockel, K.S.; Knops, J.M.; et al. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc. Natl. Acad. Sci. USA 2015, 112, 10967–10972. [Google Scholar] [CrossRef]
- Duchene, O.; Celette, F.; Barreiro, A.; Dimitrova Mårtensson, L.-M.; Freschet, G.T.; David, C. Introducing Perennial Grain in Grain Crops Rotation: The Role of Rooting Pattern in Soil Quality Management. Agronomy 2020, 10, 1254. [Google Scholar] [CrossRef]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in Agriculture: Balancing the cost of an essential resource. Annu. Rev. Environ Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Wei, C.; Yu, Q.; Bai, E.; Lü, X.; Li, Q.; Xia, J.; Kardol, P.; Liang, W.; Wang, Z.; Han, X. Nitrogen deposition weakens plant–microbe interactions in grassland ecosystems. Glob. Chang. Biol. 2013, 19, 3688–3697. [Google Scholar] [CrossRef]
- Robertson, G.P.; Hamilton, S.K.; Del Grosso, S.J.; Parton, W.J. The biogeochemistry of bioenergy landscapes: Carbon, nitrogen, and water considerations. Ecol. Appl. 2011, 21, 1055–1067. [Google Scholar] [CrossRef]
- Böhme, L.; Langer, U.; Böhme, F. Microbial biomass, enzyme activities and microbial community structure in two European long-term field experiments. Agric. Ecosys. Environ. 2005, 109, 141–152. [Google Scholar] [CrossRef]
- Miller, R.M.; Wilson, G.W.T.; Johnson, N.C. Arbuscular Mycorrhizae and Grassland Ecosystems. In Biocomplexity of Plant–Fungal Interact; Wiley Blackwell: Chichester, UK, 2012; pp. 59–84. [Google Scholar] [CrossRef]
- Lee, M.S.; Wycislo, A.; Guo, J.; Lee, D.K.; Voigt, T. Nitrogen Fertilization Effects on Biomass Production and Yield Components of Miscanthus × giganteus. Front. Plant Sci. 2017, 18, 544. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.Y.; Wu, H.M.; Zhang, F.F.; Li, C.J.; Li, X.X.; Lambers, H.; Li, L. Root exudates drive interspecific facilitation by enhancing nodulation and N2 fixation. Proc. Natl. Acad. Sci. USA 2016, 113, 6496–6501. [Google Scholar] [CrossRef]
- Buyer, J.S.; Sasser, M. High throughput phospholipid fatty acid analysis of soils. Appl. Soil Ecol. 2012, 61, 127–130. [Google Scholar] [CrossRef]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Ferti. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Frostegård, A.; Bååth, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Ferti. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Van Diepen, L.T.; Lilleskov, E.A.; Pregitzer, K.S.; Miller, R.M. Decline of arbuscular mycorrhizal fungi in northern hardwood forests exposed to chronic nitrogen additions. New Phytol. 2007, 176, 175–183. [Google Scholar] [CrossRef]
- Buyer, J.S.; Roberts, D.P.; Russek-Cohen, E. Microbial community structure and function in the spermosphere as affected by soil and seed type. Can. J. Microbiol. 1999, 45, 138–144. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models; SAS Inst. Inc.: Cary, NC, USA, 2006. [Google Scholar]
- Strickland, M.S.; Rousk, J. Considering Fungal: Bacterial Dominance in Soils—Methods, Controls, and Ecosystem Implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Liang, C.; Gutknecht, J.L.; Balser, T.C. Microbial lipid and amino sugar responses to long-term simulated global environmental changes in a California annual grassland. Front. Microb. 2015, 5, 385. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Ebblewhite, N.; Dobson, G. Soil microbial community structure: Effects of substrate loading rates. Soil Biol. Biochem. 1998, 31, 145–153. [Google Scholar] [CrossRef]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Sainju, U.M.; Allen, B.L. Carbon footprint of perennial bioenergy crop production receiving various nitrogen fertilization rates. Sci. Total Environ. 2023, 861, 160663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Miao, Y.; Zhang, T.; Wei, Y.; Qiao, X.; Miao, R.; Wang, D.; Han, S.; Yang, Z. Drought timing and primary productivity in a semiarid grassland. Land Degrad. Develop. 2020, 31, 2185–2195. [Google Scholar] [CrossRef]
- Peacock, A.G.; Mullen, M.; Ringelberg, D.; Tyler, D.; Hedrick, D.; Gale, P.; White, D. Soil microbial community responses to dairy manure or ammonium nitrate applications. Soil Biol. Biochem. 2001, 33, 1011–1019. [Google Scholar] [CrossRef]
- Sainju, U.M.; Alasinrin, S.Y. Changes in soil chemical properties and crop yields with long-term cropping system and nitrogen fertilization. Agro. Geosci. Environ. 2020, 3, e20019. [Google Scholar] [CrossRef]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Baldock, J.A. Microbial utilization of biochar-derived carbon. Sci. Total Environ. 2013, 465, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.A.; Fritschi, F.B.; Horwath, W.R.; Scow, K.M.; Rains, W.D.; Travis, R.L. Comparisons of soil microbial communities influenced by soil texture, nitrogen fertility, and rotations. Soil Sci. 2011, 176, 487–494. [Google Scholar] [CrossRef]
- Lupwayi, N.Z.; Lafond, G.P.; Ziadi, N.; Grant, C.A. Soil microbial response to nitrogen fertilizer and tillage in barley and corn. Soil Till Res. 2012, 118, 139–146. [Google Scholar] [CrossRef]
- Omar, S.A.; Ismail, M. Microbial populations, ammonification and nitrification in soil treated with urea and inorganic salts. Folia Microbiol. 1999, 44, 205–212. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms—A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Lv, F.; Xue, S.; Wang, G.; Zhang, C. Nitrogen addition shifts the microbial community in the rhizosphere of Pinus tabuliformis in Northwestern China. PLoS ONE 2017, 12, e0172382. [Google Scholar] [CrossRef] [PubMed]
- Grayston, S.; Griffith, G.; Mawdsley, J.; Campbell, C.D.; Bardgett, R. Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biol. Biochem. 2001, 33, 533–551. [Google Scholar] [CrossRef]
- Malik, A.A.; Roth, V.N.; Hébert, M.; Tremblay, L.; Dittmar, T.; Gleixner, G. Linking molecular size, composition and carbon turnover of extractable soil microbial compounds. Soil Biol. Biochem. 2016, 100, 66–73. [Google Scholar] [CrossRef]
- Rodgers, H.R.; Norton, J.B.; van Diepen, L.T. Effects of semiarid wheat agriculture management practices on soil microbial properties: A review. Agronomy 2021, 11, 852. [Google Scholar] [CrossRef]
- Bailey, V.L.; Smith, J.L.; Bolton Jr, H. Fungal-to-bacterial ratios in soils investigated for enhanced C sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Acharya, P.; Ghimire, R.; Acosta-Martinez, V. Cover crop mediated soil carbon storage and soil health in semi-arid irrigated cropping systems. Agric. Ecosyst. Environ 2023, 361, 108813. [Google Scholar] [CrossRef]
- Liang, C.; Jesus, E.D.; Duncan, D.S.; Jackson, R.D.; Tiedje, J.M.; Balser, T.C. Soil microbial communities under model biofuel cropping systems in southern Wisconsin, USA: Impact of crop species and soil properties. Appl. Soil Ecol. 2012, 54, 24–31. [Google Scholar] [CrossRef]


| Perennial Bioenergy Crop a | pH | SOM | EC | N-NO3− Conc. | K Conc. | Ca Conc. | Mg Conc. | Na Conc. | P Conc. | CEC | K Base Saturation | Ca Base Saturation | Mg Base Saturation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg N kg−1 | mg K kg−1 | mg Ca kg−1 | mg Mg kg−1 | mg Na kg−1 | mg P kg−1 | cmolc kg−1 | % | % | % | |||
| IWG | 5.49 | 2.61 | 0.08 | 3.71 | 295a b | 796 | 226 | 21.5 | 40.6 | 12.3 | 6.12a | 32.8 | 15.8 |
| SG | 5.26 | 2.55 | 0.09 | 8.03 | 241b | 691 | 209 | 17.9 | 53.8 | 12.1 | 5.18b | 29.0 | 14.5 |
| Significance p values | |||||||||||||
| G | 0.07 | 0.54 | 0.33 | 0.19 | 0.01 | 0.09 | 0.29 | 0.62 | 0.063 | 0.62 | 0.05 | 0.09 | 0.19 |
| R | <0.001 | 0.07 | 0.02 | 0.43 | 0.01 | 0.03 | <0.001 | 0.74 | 0.010 | <0.001 | <0.001 | <0.002 | <0.001 |
| G × R | 0.17 | 0.33 | 0.41 | 0.87 | 0.53 | 0.25 | 0.32 | 0.26 | 0.037 | 0.52 | 0.71 | 0.11 | 0.10 |
| RL | <0.001 | 0.19 | 0.003 | 0.16 | 0.001 | <0.005 | <0.001 | 0.68 | 0.003 | <0.001 | <0.001 | <0.001 | <0.001 |
| RQ | 0.46 | 0.02 | 0.34 | 0.45 | 0.58 | 0.37 | 0.82 | 0.37 | 0.084 | 0.08 | 0.79 | 0.78 | 0.70 |
| G × RL | <0.001 | 0.16 | 0.17 | 0.15 | 0.08 | 0.001 | <0.001 | 0.11 | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 |
| G × RQ | 0.60 | 0.10 | 0.23 | 0.58 | 0.29 | 0.79 | 0.70 | 0.85 | 0.634 | 0.51 | 0.45 | 0.74 | 0.68 |
| Parameter | AMF NLFA | AMF PLFA | Gram − Bacteria | Fungi | Gram + Bacteria | Actinomycetes | F/B | G+/G− |
|---|---|---|---|---|---|---|---|---|
| Grass a | ||||||||
| IWG | 0.339b b | 0.052 | 0.258b | 0.089 | 0.392 | 0.160a | 0.174 | 1.532a |
| SG | 0.432a | 0.054 | 0.271a | 0.088 | 0.387 | 0.152b | 0.176 | 1.437b |
| Year | ||||||||
| 2019 | 0.445a | 0.055a | 0.256b | 0.084b | 0.395a | 0.163a | 0.171 | 1.549a |
| 2020 | 0.326b | 0.051b | 0.272a | 0.094a | 0.384b | 0.149b | 0.179 | 1.420b |
| Significance | p values------------------------------------------------------------------------------------------------------------------------------ | |||||||
| Grass (G) | 0.012 | 0.323 | 0.008 | 0.842 | 0.300 | 0.052 | 0.689 | 0.021 |
| Rate (R) | 0.046 | <0.0001 | 0.254 | 0.029 | 0.001 | 0.717 | 0.567 | 0.019 |
| Year (Y) | 0.001 | 0.010 | 0.001 | 0.032 | 0.040 | 0.0002 | 0.201 | 0.002 |
| G × R | 0.731 | 0.990 | 0.966 | 0.547 | 0.425 | 0.890 | 0.436 | 0.807 |
| G × Y | 0.476 | <0.0001 | 0.323 | 0.101 | 0.284 | 0.003 | 0.991 | 0.677 |
| R × Y | 0.819 | 0.124 | 0.841 | 0.675 | 0.052 | 0.837 | 0.371 | 0.371 |
| G × R × Y | 0.176 | 0.702 | 0.987 | 0.118 | 0.140 | 0.060 | 0.097 | 0.634 |
| RL | 0.274 | <0.0001 | 0.197 | 0.005 | 0.0002 | 0.854 | 0.784 | 0.004 |
| RQ | 0.010 | 0.504 | 0.505 | 0.783 | 0.602 | 0.411 | 0.560 | 0.909 |
| G × RL | 0.159 | <0.0001 | 0.302 | 0.069 | 0.0002 | 0.886 | 0.670 | 0.007 |
| G × RQ | 0.090 | 0.608 | 0.605 | 0.675 | 0.784 | 0.434 | 0.824 | 0.860 |
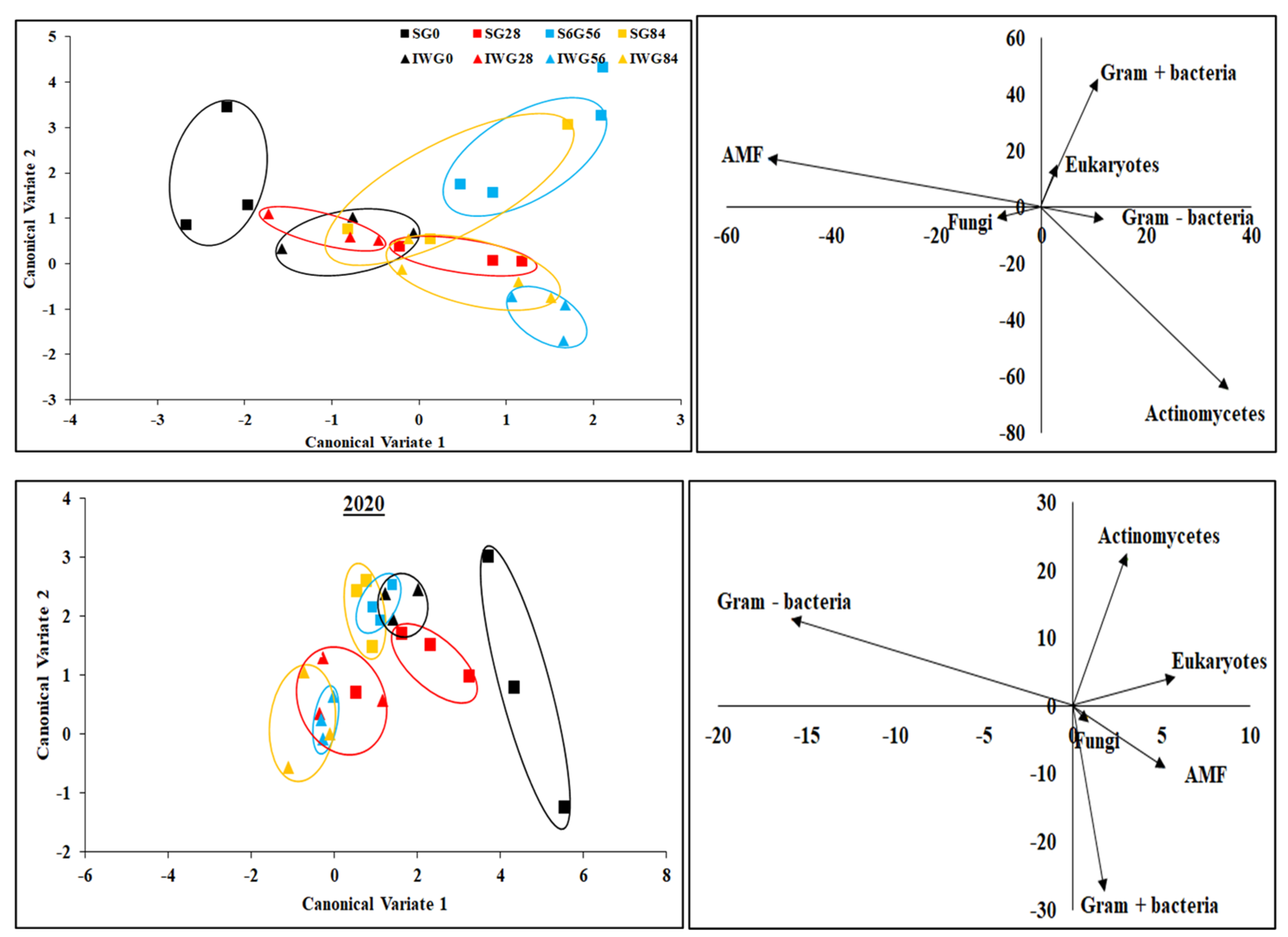
| Parameter | 2019 | 2020 | |||
|---|---|---|---|---|---|
| CV1 a | CV2 a | CV1 | CV2 | ||
| AMF | 0.16 | 0.41 | 0.15 | 0.12 | |
| Gram-negative bacteria | 0.18 | 0.41 | 0.03 | 0.14 | |
| Eukaryotes | 0.20 | 0.45 | 0.13 | 0.12 | |
| Fungi | 0.19 | 0.41 | −0.03 | 0.16 | |
| Gram-positive bacteria | 0.18 | 0.40 | −0.001 | 0.15 | |
| Actinomycetes | 0.18 | 0.40 | 0.01 | 0.20 | |
| Group centroids | |||||
| Perennial bioenergy crop b | N fertilization rate (kg N ha−1) | ||||
| IWG | 0 | −1.43 | −0.06 | 0.41 | 0.50 |
| 28 | −1.10 | −0.55 | −0.37 | −0.05 | |
| 56 | 1.09 | −1.59 | −1.18 | −0.70 | |
| 84 | 0.60 | −0.96 | −2.37 | −0.89 | |
| SG | 0 | −1.86 | 0.80 | 3.03 | −0.88 |
| 28 | 0.42 | −0.06 | 1.01 | −0.03 | |
| 56 | 1.40 | 1.92 | −0.13 | 1.27 | |
| 84 | 0.88 | 0.50 | −0.40 | 0.79 | |
| Crop a | AMF NLFA | AMF PLFA | Gram − Bacteria | Fungi | Gram + Bacteria | Actinomycetes | Total Fungi | Total Bacteria | F/B | G+/G− |
|---|---|---|---|---|---|---|---|---|---|---|
| IWG | 0.381 | 0.054 | 0.245 | 0.082 | 0.400b b | 0.171a | 0.137ab | 0.818ab | 0.169ab | 1.640ab |
| SG | 0.409 | 0.045 | 0.265 | 0.111 | 0.388b | 0.145b | 0.157a | 0.801b | 0.196a | 1.472b |
| WH | 0.282 | 0.047 | 0.248 | 0.068 | 0.435a | 0.154ab | 0.115b | 0.841a | 0.137b | 0.176a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dangi, S.R.; Sainju, U.M.; Allen, B.L.; Calderon, R.B. Soil Microbial Community Structures under Annual and Perennial Crops Treated with Different Nitrogen Fertilization Rates. Soil Syst. 2024, 8, 81. https://doi.org/10.3390/soilsystems8030081
Dangi SR, Sainju UM, Allen BL, Calderon RB. Soil Microbial Community Structures under Annual and Perennial Crops Treated with Different Nitrogen Fertilization Rates. Soil Systems. 2024; 8(3):81. https://doi.org/10.3390/soilsystems8030081
Chicago/Turabian StyleDangi, Sadikshya R., Upendra M. Sainju, Brett L. Allen, and Rosalie B. Calderon. 2024. "Soil Microbial Community Structures under Annual and Perennial Crops Treated with Different Nitrogen Fertilization Rates" Soil Systems 8, no. 3: 81. https://doi.org/10.3390/soilsystems8030081
APA StyleDangi, S. R., Sainju, U. M., Allen, B. L., & Calderon, R. B. (2024). Soil Microbial Community Structures under Annual and Perennial Crops Treated with Different Nitrogen Fertilization Rates. Soil Systems, 8(3), 81. https://doi.org/10.3390/soilsystems8030081







